Abstract
Rhesus (Rh) antigens are carried by a membrane complex that includes Rh proteins (D and CcEe), Rh-associated glycoproteins (RhAG), and accessory chains (LW and CD47) associated by noncovalent bonds. In heterologous expression systems, RhAG and its kidney orthologs function as ammonium transporters. In red blood cells (RBCs), it is generally accepted that NH3 permeates by membrane lipid diffusion. We have revisited these issues by studying RBC and ghosts from human and mouse genetic variants with defects of proteins that comprise the Rh complex. In both normal and mutant cells, stopped-flow analyses of intracellular pH changes in the presence of inwardly directed methylammonium (CH3NH+3+CH3NH2) or ammonium (NH+4+NH3) gradients showed a rapid alkalinization phase. Cells from human and mouse variants exhibited a decrease in their kinetic rate constants that was strictly correlated to the degree of reduction of their RhAG/Rhag expression level. Rate constants were not affected by a reduction of Rh, CD47, or LW. CH3NH2/NH3 transport was characterized by (i) a sensitivity to mercurials that is reversible by 2-mercaptoethanol and (ii) a reduction of alkalinization rate constants after bromelain digestion, which cleaves RhAG. The results show that RhAG facilitates CH3NH2/NH3 movement across the RBC membrane and represents a potential example of a gas channel in mammalian cells. In RBCs, RhAG may transport NH3 to detoxifying organs, like kidney and liver, and together with nonerythroid tissue orthologs may contribute to the regulation of the systemic acid–base balance.
Keywords: ammonium, mouse mutants, Rhesus deficiency
Rhesus (Rh) is a clinically significant blood group system in transfusion medicine (1). Rh blood group antigens are defined by a complex association of membrane polypeptides that includes the nonglycosylated Rh proteins (carriers of RhD and RhCcEe antigens) and Rh-associated glycoprotein (RhAG), which is strictly required for cell-surface expression of Rh antigens (2, 3). In red blood cells (RBCs), the core of the Rh complex may be a tetramer composed of two Rh and two RhAG subunits, to which accessory chains (CD47, LW, and GPB) are associated by noncovalent linkages (2, 4).
The Rhnull phenotype is an inherited condition in which various Rh antigen deficiencies result in a clinical syndrome characterized by a hemolytic anemia of varying severity, with abnormalities of the red cell shape (stomato-spherocytosis), cation transport, and membrane phospholipid organization (2, 5). Rhnull disease is caused by several different mutations that occur in either the RHAG or RH loci on chromosomes 6p12-p21 and 1p34-p36, respectively. The Rh complex is missing or severely reduced in RBCs from Rhnull individuals without alteration of the genes encoding the accessory chains (2, 5). Because of a variable expressivity, some mutations of the RHAG gene result in the total lack of RhAG (and Rh protein), defining the Rhnull of the regulator type, but others result in weak RhAG (and Rh protein) levels, defining the Rhmod phenotype (5). Mutations of the RH gene resulting in the total lack of Rh protein and only a reduced expression of RhAG (20% of normal) define Rhnull as the amorph type. Accordingly, primary defects of either RhAG or Rh protein result in defective cell surface expression and/or transport of the whole Rh complex.
The murine mutations normoblastosis (Ank1nb) and spherocytosis (Spna1sph) disrupt the RBC spectrin-based cytoskeleton through deficiencies of ankyrin and α-spectrin, respectively. In addition to the well described band 3-ankyrin–protein 4.2 and glycophorin C–protein 4.1–p55 complexes (6), the Rh complex represents a major interaction site between the membrane lipid bilayer and the spectrin-based cytoskeleton. Indeed, recent studies of the erythroid ankyrin-deficient normoblastosis mice and analysis with the yeast two-hybrid system have shown that Ank1 may interact directly with the C-terminal cytoplasmic domains of Rh protein and RhAG (7). Interestingly, in humans this interaction was abolished by a mutation found in an Rhnull patient (7).
The first indication of the biological role of Rh proteins came from the discovery of a significant homology, particularly to RhAG, with the methylammonium (MA) permease/ammonium transporter (Mep/Amt) superfamily of ammonium transporters present from bacteria to yeast but absent in vertebrates (8). This discovery was followed by the characterization of two nonerythroid orthologs, RhBG and RhCG (9, 10), and the identification of paralogs in a variety of living species, thereby suggesting the conservation of some critical function (reviewed in ref. 11). Moreover, when expressed in Mep/Amt-deficient yeast, RhAG and RhCG function as bidirectional ammonium transporters (12). Recently, functional studies of Rh glycoproteins in Xenopus laevis oocytes were performed but yielded discordant results. Some studies suggested that RhAG-mediated uptake of MA in oocytes was an electroneutral NH4+/H+ exchange (13), whereas electrophysiological studies in voltage-clamped RhBG- and RhCG-expressing oocytes suggested that transport is (14, 15) or is not (16) electrogenic. It was also speculated that Rh and Mep/Amt proteins might be facilitators for NH3 gas transport (17).
In physiological systems, such as RBCs, it is generally accepted that NH3 permeates through membrane lipid diffusion (18–20). The purpose of the present study is to revisit these findings by studying the ammonium transport in RBCs from human and mouse genetic variants with various defects of proteins that comprise or interact with the Rh complex. Accordingly, stopped-flow analysis of kinetic rate constants of intracellular pH (pHi) changes of resealed ghosts in the presence of MA or ammonium gradients as well as mercurial inhibition and protease cleavage studies were performed. The results indicate that RhAG facilitates CH3NH2/NH3 movement across the red cell membrane.
Materials and Methods
Blood Samples. Four Rhnull patients of the regulator type [P1(TB), P2(YT), P3(AL), and P4(AC)], one Rhmod patient [P5(CB)], and one Rhnull patient of the amorph type [P6(DR)] have been described (2, 5). Control RBCs from RhD-positive, RhD-negative, and LWnull individuals [P7(Big) and P8(Mil)] were provided by the Centre de Référence pour les Groupes Sanguins (Paris). RBCs from two individuals [P9(Sar) and P10(Fer)] of the AQP1null phenotype lacking AQP1 (also called COnull because AQP1 carries Colton antigens) have been described (21, 22). A blood sample from a 4.2-deficient patient [P11(Rem)] was provided by O. Agulles (Etablissement Français du Sang, Nancy, France). RBCs from mouse models of hereditary hemolytic anemia, normoblastosis (nb/nb) (ankyrin-deficient) and spherocytosis (sph/sph) (spectrin-deficient), and from wild-type mice (WBB6F1, C57BL/6J) were from The Jackson Laboratory. Before analysis, cryopreserved or fresh RBCs were washed twice with PBS.
Flow Cytometry Analysis. Indirect immunofluorescence of human and mouse RBCs for cell-surface or intracellular epitope analysis was performed with a FACScalibur flow cytometer (Pharmingen) by using the following monoclonal antibodies: mouse anti-human Rh protein BRIC69 (D. Anstee, International Blood Group Reference Laboratory, Bristol, U.K.), mouse anti-human CD47 (clone 3E12, Bioatlantique, Nantes, France), rat antimouse CD47 (clone miap 301, Pharmingen), mouse anti-LW BS46 (H. H. Sonneborn, Offenbach, Germany), human anti-Band 3 HIRO-58/Dib (M. Uchikawa, Japanese Red Cross Central Blood Center, Tokyo); mouse anti-AQP1 (clone 1/A5F6, Serotec, Cergy, France), and rabbit polyclonal antibodies to human Rh protein (MPC8) and mouse Rhag (mCTRhAG). The antibodies were used at saturating conditions as described in refs. 3, 7, and 23. In control studies, a human anti-Colton a antigen (Centre de Référence pour les Groupes Sanguins, Paris) and a rabbit polyclonal antibody to AQP3 protein were used with intact and fixed permeabilized RBCs as described in refs. 24 and 25.
RBC Ghost Preparation. All preparation steps, except resealing (37°C), were carried out at 4°C, and assays were performed the same day (26). One volume of blood was washed three times in resealing buffer (140 mM NaCl/2.5 mM KCl/10 mM Hepes/NaOH, pH 7.4) and resuspended in 80 volumes of hypotonic lysis buffer (7 mM NaCl/10 mM Hepes/NaOH, pH 7.4) for 40 min on ice folllowed by resealing in resealing buffer containing 1 mM MgSO4 and 0.15 mM pyranine, the fluorescent pH-sensitive dye (1-hydroxypyrene-3,6,8-trisulfonic acid, Sigma–Aldrich) at 37°C for 1 hr. After three washes in resealing buffer, ghosts were equilibrated in buffer A (130 mM NaCl/5 mM KCl/10 mM Hepes/NaOH, pH 7.0) and kept on ice before assays.
pHi Determination by Stopped-Flow Analysis. Ammonium or MA (ammonium and MA refer to the sum of protonated and unprotonated forms) transport was measured in isoosmotic conditions. Membrane ghosts equilibrated in buffer A containing 5 mM p-xylene-bis(N-pyridinium bromide) (Sigma), a fluorescence quencher of external pyranine, were mixed with an equal volume of buffer B (65 mM NaCl/5 mM KCl/65 mM NH4Cl or MA chloride/10 mM Hepes/NaOH, pH 7.0), generating an inwardly directed 32.5 milliequivalent gradient. In preliminary experiments, pHi changes were measured with a stopped-flow instrument (SFM3, Biologic, Grenoble, France) that was modified by addition of the titrator device (Biologic), which consists of replacing the 30-μl cuvette (see below) with a thermostated (15°C) and vortex-mixed cuvette (4 ml), allowing the use of the equipment as an ordinary spectrofluorometer. The excitation wavelength was 460 nm, and the emitted light was filtered with a 520-nm cut-on filter. The pH-dependent fluorescence changes were followed and analyzed, because a fluorescence increase corresponded to a pH elevation and a fluorescence decrease corresponded to a pH reduction (27). Over the pH range used (7.0–7.8), the relative fluorescence of the dye was proportional to pH, as determined by titration on ghosts incubated in 2 ml of buffer A containing 5mM p-xylene-bis(N-pyridinium bromide) and 5 μM nigericin (Sigma) and submitted to pH change by step additions of 2 μl of KOH (1 M). Subsequently, ghost alkalinization and acidification kinetics were performed in the stopped-flow mode (30-μl FC-15 cuvette; dead time, 7.8 ms). Preliminary experiments showed that ammonium transport was similar for ghosts prepared from fresh and cryopreserved RBCs. Data from five to eight time courses were averaged, and the alkalinization phase was fitted to a single exponential function by using the Simplex procedure of the biokine software (Biologic) to calculate kinetic rate constants, k, ins–1. In some experiments, apparent permeabilities (P′) were determined without unstirred layers correction according to Priver et al. (28) by using the following equation:
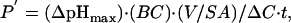 |
where ΔpHmax is the maximum pH change value, BC is the buffer capacity, V/SA is the volume-to-surface-area ratio (in cm) of ghosts, ΔC is the MA/ammonium gradient (mM), and t = 1/k (s), which is the reciprocal of the exponential kinetic rate constant k.
Membrane ghosts are crenated spheres in isotonic solution (29). Determination of ghost diameter by light and confocal microscopy confirmed their spheric shape and that ghosts are smaller than intact RBCs. When ghosts from normal and human variant RBCs were compared, we found no significant difference (4.95 ± 0.2 versus 5.07 ± 0.38 μm). The volume calculated from the measured mean diameter corresponds to a sphere of ≈7 × 10–14 liter, as expected (30). The buffer capacity of ghosts in buffer A containing 5 mM p-xylene-bis(N-pyridinium bromide) was measured by a single addition of 20 mM acetate as in ref. 27, but with the stopped-flow mode. The mean value was within the same range for normal and variant ghosts (82.57 ± 9.83 versus 82.18 ± 3.71 mM per pH unit). In some experiments, ghosts were incubated for 5 min at 15°C in the presence of 0.1 mM HgCl2 before the application of the MA gradient. In other experiments, ghosts were prepared from protease-treated RBCs that were obtained by incubating washed RBCs (10% hematocrit) for 30 min at 37°C in the presence or absence of 3 mg/ml bromelain (product no. B4882, Sigma) in PBS. Then, cysteine proteinase inhibitor transepoxysuccinyl-l-leucylamido-(-4-guanidino)butane (E-64, Calbiochem) was added to a 10 μM final concentration, and RBCs were washed three times at 4°C in PBS containing 10 μM E64.
Water and Glycerol Permeabilities and Western Blot Analysis. Water and glycerol osmotic permeabilities were determined by RBC volume change analysis in the stopped-flow spectrophotometer by measuring 90° scattered-light intensity with 600-nm excitation light, as described in ref. 31. Western blot analyses of native and protease-treated ghosts were performed with a murine monoclonal anti-RhAG (clone LA 18-18, gift from A. van dem Borne, University of Amsterdam, Amsterdam), the reactivity of which depends on the presence of the carbohydrate moiety, and with rabbit antisera against the C-ter of human RhAG (anti-C-ter) and MPC8 (12, 23).
Results and Discussion
CH3NH2/NH3 Transport in Normal RBC Ghosts. Studies of ammonium permeability by 14N and 15N saturation transfer NMR spectroscopy have demonstrated that NH3 very rapidly enters RBCs by passive lipid diffusion (20). To further investigate this question, we have studied RBC and ghosts from human and mouse genetic variants with defects of proteins that comprise the Rh complex. Because ammonium is a weak base (pKa = 9.56 at 15°C), cell uptake results in pHi changes, either alkalinization or acidification, depending on whether the neutral form (NH3) or charged form (NH+4) is taken up. Because of the high buffer capacity of RBCs, pHi changes were determined on membrane ghosts resealed on a 10 mM Hepes buffer (pH 7.0) containing pyranine as a fluorescent pH-sensitive dye. Because all ghosts preparations, including those from controls from human variant RBCs analyzed below, exhibited a similar size and buffer capacity (see Materials and Methods), the kinetic rate constants of CH3NH2/NH3 influx measured in transport studies strictly reflected differences in permeabilities to CH3NH2/NH3.
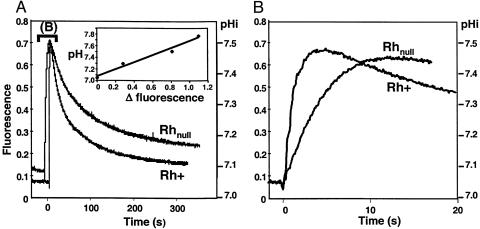
By using ghosts from control RhD-positive RBCs with normal Rh, RhAG, LW, and CD47 expression (Table 1) submitted to a 32.5 mM inwardly directed MA or ammonium gradient (pH 7.0) in isoosmotic conditions, we found that the fluorescence intensity curve exhibited a biphasic response, consisting first of a rapid phase of alkalinization (ΔpH ≈ 0.5 unit), followed by a slow acidification phase to return to external pH (pH0) of the incubation medium (Fig. 1A). The alkalinization can be accounted for by the uptake of CH3NH2 or NH3, thereby trapping protons within the cells until the equilibrium values of these neutral forms were reached (20). The kinetic rate constant of alkalinization of ghosts from RhD-positive cells (Fig. 1B) was 0.95 ± 0.1 s–1 for CH3NH2 and 4.95 ± 0.28 s–1 for NH3, and there was no significant difference between ghosts from RhD-positive and RhD-negative RBCs. RhD-positive and RhD-negative RBCs exhibiting normal levels of RhAG, LW, CD47, and RhCE proteins but differing by the presence or absence of the RhD protein, suggests that, by itself, the RhD protein has no significant effect on CH3NH2/NH3 transport. From these data, the calculated apparent permeabilities (P′) of RhD-positive ghosts for CH3NH2 and NH3 at 15°C are 0.88 ± 0.09 10–4 and 4.46 ± 0.25 10–4 cm·s–1, respectively, without the correction of the unstirred layers (Table 2). A value in the same range has been published for NH3 permeability of RBCs at 21°C (20). Although most experiments were done with a 32.5 mM gradient, some were also performed with 2.5–40 mM MA (pH 7.0) gradients, and we found a linear relationship with fluorescence variation (data not shown).
Table 1. Antigen and protein expression of human RBCs.
| Control
|
Variants
|
||||||
|---|---|---|---|---|---|---|---|
| Membrane protein | RhD-positive (n = 5) | Rhnull regulator of P1-P4 | Rhmod of P5 | Rhnull amorph of P6 | LWnull of P7/P8 | 4.2null* of P11 | AQP1null of P9/P10 |
| Rh | 126 ± 10 | 0 | 7.6 | 0.1 | 90/100 | 112 | 131/136 |
| RhAG | 81 ± 7 | <0.2 | 8.9 | 17.6 | 65/75 | 108 | 85/95 |
| CD47 | 17 ± 1 | 1.9 ± 0.2 | 3 | 2.3 | 13/15 | 4.1 | 17/16 |
| LW | 3 ± 1 | 0 | 0 | 0 | 0/0 | 4 | n.t. |
| Band 3 | 2,129 ± 60 | 2,076 ± 46 | 1,987 | 2,139 | 1,927/1,762 | 1,853 | 2,029 |
| AQP1 | 298 ± 15 | 270 ± 24 | 243 | 318 | 350/380 | 297 | <0.2 |
| AQP3 | 172 ± 10 | 151 ± 26 | 152 | 169 | 133/157 | 199 | 119 |
Values indicate the copy number of membrane protein per red cell (×10-3), except for Band 3, AQP1 (anti-Colton a antigen), and AQP3, which were measured as mean fluorescence intensity. n.t., not tested.
Only a single patient was tested.
Fig. 1.
Time course of fluorescence and pHi changes in ghosts. (A) Ghosts from RhD-positive (Rh+) and Rhnull regulator (P1) were submitted to a 32.5 mM MA inwardly directed gradient (pH 7.0) at 15°C, and pHi changes were followed by fluorescence change of a pH-sensitive dye in the stopped-flow spectrofluorometer by using the titrator device (see Materials and Methods). The response was biphasic. Experiments were repeated three times with different Rhnull of the regulator type (P2, P3, and P4) and yielded the same results. (Insert) Over the pH range used (7.0–7.8), the relative fluorescence of the dye was proportional to pH. (B) Expanded scale of the rapid alkalinization phase.
Table 2. Rate constants and permeability to CH3NH2/NH3 of human RBC ghosts.
| Controls
|
Variants
|
||||||
|---|---|---|---|---|---|---|---|
| Substrate | RhD-positive (n = 3) | RhD-negative (n = 3) | Rhnull regulator of P1-P4 | Rhmod of P5 | Rhnull amorph of P6 | LWnull of P7/P8 | 4.2null of P11 |
| CH3NH2 | |||||||
| k, s-1 | 0.95 ± 0.1 | 0.81 ± 0.03 | 0.14 ± 0.03* | 0.26 | 0.38 | 0.8/0.8 | 0.74 |
| P′, cm·s-1 | 0.88 ± 0.09 | 0.71 ± 0.03 | 0.12 ± 0.03* | 0.23 | 0.36 | 0.8/0.7 | 0.68 |
| NH3 | |||||||
| k, s-1 | 4.95 ± 0.28 | 5.4 ± 0.28 | 2.48 ± 0.09* | 2.6 | 2.9 | 3.9/4.1 | 4.56 |
| P′, cm·s-1 | 4.46 ± 0.25 | 3.91 ± 0.21 | 2.54 ± 0.08* | 2.63 | 2.53 | 4.1/4.3 | 4.11 |
Figures indicate alkalinization rate constants (k) and permeability (P′ × 10-4) to CH3NH2/NH3 of ghosts submitted to 32.5 mM MA or ammonium gradient.
, P < 0.001 versus RhD-positive and RhD-negative.
The phase of slow acidification after the alkalinization step corresponds to a return to pH0, which is a complex process, because the ghost membrane carries several transporters, such as Band 3 and specific or nonspecific ammonium/MA transporter(s), including the Na+/H+ antiport, that could participate in pH regulation. This process was not investigated further. Because the acidification phase is 25 or 45 times slower than the alkalinization phase (as estimated by t1/2 values for CH3NH2 and NH3, respectively), we assume that it does not significantly interfere with the calculation of first-phase rate constants.
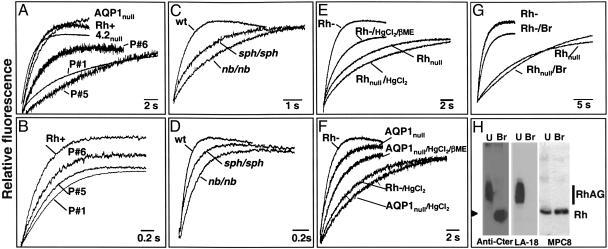
CH3NH2/NH3 Transport of Ghosts from Human Variants. We next determined pHi changes of ghosts from Rhnull cells of the regulatory type (no Rh/RhAG/LW; low CD47) (Table 1), and we found also a biphasic response (Fig. 1). However, the rate constants of alkalinization were much slower (0.14 ± 0.03 s–1) compared with control RBCs, because of a 6-fold reduction of apparent permeability to CH3NH2 (Table 2). Rhmod and Rhnull (amorph) ghosts that express only 10% and 20% of RhAG, respectively (Table 1), exhibited intermediate values (Fig. 2A and Table 2), suggesting that CH3NH2 transport was correlated to RhAG expression level. pHi changes were faster in the presence of the NH4Cl gradient at pH 7.0 (Fig. 2B), but there was still an ≈2-fold reduction of alkalinization rate constants and P′ values for Rhnull regulators compared with controls, again with Rhmod and Rhnull (amorph) exhibiting intermediate values (Table 2). The fact that the rates change in the same-sized ghosts with or without Rh proteins indicates that the unstirred layers do not play a significant role. Therefore, although a “basal” CH3NH2/NH3 entry occurs by lipid diffusion in Rhnull cells, our data strongly suggest that RhAG may accelerate this process. As shown in Table 2, we also found a normal alkalinization rate constant of ghosts from LWnull RBCs (normal Rh/RhAG/CD47; no LW) (Table 1), indicating no role of LW in CH3NH2/NH3 transport. In addition, the alkalinization rate constant of 4.2null ghosts, which have a severe reduction of CD47 but normal levels of Rh, RhAG, and LW (Table 1) (23, 32), was only slightly lower in the single patient (P11) investigated (0.74 s–1 for CH3NH2), indicating that CD47 does not play a significant role. We found, however, that AQP1null (COnull) ghosts with a normal content of RhAG (Table 1) showed a small but significant (P < 0.01) reduction of CH3NH2 transport (k = 0.51 s–1 for P9 and 0.59 s–1 for P10 versus k = 0.95 ± 0.1 s–1 for control RBCs), suggesting that AQP1 might partly contribute to NH3 transport, as suggested in ref. 33.
Fig. 2.
CH3NH2/NH3 transport in ghosts from human and mouse variants. (A–G) Time course of fluorescence change of human (A, B, and E–G) and murine (C and D) ghosts subjected to a 32.5 mM MA (A, C, and E–G) or ammonium (B and D) inwardly directed gradient (pH 7.0) at 15°C were followed by stopped-flow analysis (see Materials and Methods). Only the rapid initial alkalinization phase is shown. (A and B) Human samples: RhD-positive (Rh+), Rhnull regulator (P1), Rhmod (P5), Rhnull amorph (P6), 4.2null (P11), and AQP1null (P9). (C and D) Mouse samples: wild type (wt), nb/nb, and sph/sph.(E and F) Thiol sensitivity. Ghosts from RBCs were preincubated with 0.1 mM HgCl2, followed by a treatment with or without 5 mM 2-mercaptoethanol (β-ME). (E) Effect on RhD-negative (Rh–) and Rhnull (P1) ghosts and reversibility by 2-mercaptoethanol. (F) Effect on AQP1null (P9) ghosts. (G and H) Effect of proteolytic degradation. (G) MA transport was reduced in Rh-negative but not Rhnull (P1) ghosts prepared from RBCs digested with bromelain (Br). (H) Western blot analysis of ghosts prepared from untreated (U) or bromelain-digested (Br) Rh-negative RBCs. Blots were immunostained with a rabbit serum against human RhAG (anti-C-ter), the mouse monoclonal LA18-18 (anti-human RhAG), and MPC8 (anti-human Rh protein). The arrowhead indicates digested RhAG product.
The differences of alkalinization rate constants and permeabilities seen between CH3NH2 and NH3 with Rh control samples (≈5-fold) might reflect the different molecular structure of these substrates (Table 2). These differences are even larger with Rhnull samples (≈20-fold), in which the transport is most likely limited to lipid diffusion. Interestingly, 4.2null ghosts, which derived from RBCs with the same morphological characteristics as Rhnull cells (34), have a normal RhAG protein content (Table 1) and exhibit a transport activity virtually identical to that of controls (Fig. 2 A and Table 2). Moreover, Rhnull of the regulator and amorph types also have the same morphological characteristics (2), but they exhibit different rate constant values (Table 2) that follow their difference in RhAG content (Table 1). As a further test of whether cell volume variation affects the comparative analysis of rate constants, RBC permeabilities to water and glycerol under hyperosmotic conditions (100 and 150 mM sucrose and glycerol gradients, respectively) were determined. No significant differences were observed between control and Rh-variant cells (Table 3A), except for the water permeability of AQP1null cells that, as expected (21), was severely reduced (data not shown).
Table 3. Volume change rate constants of human and mouse RBCs.
| Human RBCs
|
Mouse RBCs
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Solute | RhD-positive (n = 3) | RhD-negative (n = 1) | Rhnull regulator | Rhmod | Rhnull amorph | WBB6F1 (n = 6) | C57BL/6J (n = 6) | nb/nb (n = 5) | sph/sph (n = 7) |
| Sucrose | 3.29 ± 0.2 | 3.14 | 3.67 ± 0.46 (P1-P4) | 3.29 (P5) | 2.73 (P6) | 7.73 ± 0.27 | 6.67 ± 0.27 | 5.15 ± 0.23* | 3.51 ± 0.09* |
| Glycerol | 0.14 ± 0.016 | n.t. | 0.14 ± 0.01 (P1 and P2) | n.t. | n.t. | 0.091 ± 0.01 | 0.062 ± 0.002 | 0.1 ± 0.007 | 0.07 ± 0.003 |
Values indicate volume change rate constants of RBCs submitted to 100 mM sucrose or 150 mM glycerol gradients. Rate constants (in s-1) were calculated from shrinkage for water movement and swelling for glycerol uptake. n.t., not tested. *, P < 0.001.
Our results show that Rhnull cells exhibit reduced permeabilities to CH3NH2 and NH3 that may be related to their protein defects. However, it is known that the membrane lipid composition and the leaflet bilayer asymmetry may affect NH3 permeability (35, 36). The composition and content of red cell lipids and phospholipids in Rhnull RBCs has been examined in only a few cases and was found to be normal (37, 38). However, there is an abnormal membrane distribution of phosphatidylethanolamine and an enhanced transbilayer movement of phosphatidylcholine (39). The membrane fluidity of Rhnull cells is poorly documented but has been found to be either normal (40, 41) or slightly increased (42), suggesting that such small variations cannot account for the large reduction of alkalinization rate constants of Rhnull ghosts (Table 2).
Our results showing that RhAG accelerates NH3 import contradict the conclusion of a recent report based on a method inappropriate to measure fast kinetics (43).
CH3NH2/NH3 Transport of Ghosts from Mutant Mice. Further studies were carried out by using mouse models of hereditary hemolytic anemia. These mice have primary defects in Ank1 (nb/nb) (44) or α-spectrin (sph/sph) (45). RBCs from nb/nb mice exhibit a sharp reduction of Rh and Rhag proteins but normal levels of CD47 (7), which is also true for RBCs from sph/sph mice (Table 4). Accordingly, we hypothesized that, similarly to Rhnull cells, these cells would exhibit reduced CH3NH2 or NH3 transport activity. Indeed, the alkalinization rate constants in the presence of a MA gradient (pH 7.0) were high in wild type (1.55 s–1) and much lower, although correlated to their respective level of Rhag protein, in nb/nb (0.38 s–1) and sph/sph (0.66 s–1) mice (Fig. 2C and Table 4). The rate constants were much faster with an ammonium gradient at pH 7.0 (Fig. 2D), but there was still a difference between wild type (12.8 s–1) and mutant mice (7.6 and 5.5 s–1). These results support the view that the Rhag protein plays a role in CH3NH2/NH3 transport of mouse RBCs. In control experiments, although mouse RBCs lack AQP3 (25, 46), we found no significant differences in the passive lipid diffusion of glycerol. However, the water permeability of mutant mice RBCs was reduced compared with wild type (Table 3) but was correlated to an apparent lower level of AQP1 (Table 4).
Table 4. Antigen and protein expression of mouse RBCs.
| Controls
|
Variants
|
|||
|---|---|---|---|---|
| Membrane component | WBB6F1 (n = 3) | C57BL/6J (n = 3) | nb/nb (n = 3) | sph/sph (n = 3) |
| Rh | 505 ± 15 | n.t. | 31 ± 1 | 181 ± 4 |
| RhAG | 272 ± 4 | 264 ± 18 | 7 ± 2 | 117 ± 5 |
| CD47 | 104 ± 2 | 103 ± 2 | 104 ± 5 | 111 ± 2 |
| Band 3 | 2,200 ± 30 | 2,139 ± 33 | 773 ± 40 | 1,088 ± 8 |
| AQP1* | 504 ± 31 | 519 ± 17 | 356 ± 20 | 321 ± 12 |
| AQP3 | 24 ± 1 | 30 ± 1 | 25 ± 1 | 30 ± 2 |
Values indicate expression level of membrane components measured as mean fluorescence intensity. n.t., not tested.
Monoclonal antibody 1/A5F6.
CH3NH2/NH3 Transport Function Is Mediated by a Protein-Dependent Pathway. Altogether, the kinetic studies with human and mouse variants indicate either more rapid diffusion of CH3NH2/NH3 across membrane lipids in normal ghosts or accelerated movement mediated by the presence of RhAG. To provide further evidence for protein-mediated transport, we examined the effect of HgCl2, which is known to interact with thiol groups of proteins on the alkalinization rate constants of ghosts from control RBCs (either RhD-positive or RhD-negative) and from RBCs of the Rhnull regulator type. Preincubation of RBCs with 1 mM HgCl2 severely reduced the rate constants of RhD-negative ghosts (and RhD-positive; data not shown) to the value typical of untreated Rhnull cells, and this effect was reversible with 5 mM 2-mercaptoethanol (Fig. 2E). Moreover, pretreatment of Rhnull cells with HgCl2 did not further reduce their transport activity (data not shown), whereas AQP1null ghosts (no AQP1; normal RhAG) (Table 1) behaved as control cells before and after incubation with HgCl2 (and 2-mercaptoethanol treatment) (Fig. 2F). As additional proof that permeability to CH3NH2/NH3 is facilitated by a membrane protein, transport experiments were performed with ghosts prepared from protease-digested RBCs. Because bromelain cleaves RhAG and RhD, but not the RhCE protein (47), the experiments were carried out with RhD-negative cells. Ghosts from bromelain-digested RBCs exhibited a significant reduction of the alkalinization rate constant compared with the untreated control (Fig. 2G), whereas protease digestion of Rhnull (regulator) cells had no effect. Western blot analysis confirmed that RhAG but not the Rh protein of ghosts prepared from enzyme-treated RBCs was cleaved by bromelain (Fig. 2H).
Altogether, our results favor a critical role of the RhAG protein in CH3NH2/NH3 transport. Moreover, the results showing that RhAG facilitates CH3NH2/NH3 movement across the red-cell membrane point to RhAG as a potential example of a gas channel in mammalian cells. There are ≈2 × 105 copies of RhAG per RBC and presumably 2 RhAG molecules per Rh complex (2), which leaves us with ≈105 copies of functional Rh complexes. From these data and transport studies (Table 2), we tentatively calculate that for a 1 M ammonium gradient (pH 7.0), there is an approximate conductance of 2 × 106 NH3 molecules per second per Rh complex.
Our results support previous speculation (17) that Rh proteins may facilitate diffusion of NH3. However, it was also proposed recently that the Rh1 protein paralog of the green alga Chlamydomonas reinhardtii might be regarded as a gas channel for CO2, because mutants lacking this protein grow only very slowly when cultured in high CO2 conditions (48). That Rh proteins might transport NH3 and/or CO2 is a possibility that should be further explored (32). Although still a matter of debate (49, 50), current studies suggest that the water channel AQP1 may represent another example of protein permeable to gases, because it also substantially increases the permeability of the membrane to CO2 (50, 51) and NH3 (33). Moreover, NtAQP1-related CO2 permeability in plants plays a physiological role in photosynthesis and stomatal opening, particularly under low CO2 gradient conditions (52).
Most interestingly, after this article was submitted, Khademi et al. (53,53) resolved the crystallographic structure of the bacterial NH3 transport channel AmtB and showed by reconstitution into vesicles that AmtB conducts uncharged NH3, which is fully consistent with our present studies on the RhAG protein.
In RBCs, RhAG may act to carry NH3 to detoxifying organs, like kidney and liver (12). However, the transport function with regard to the substrate transported may be different according to which cells or tissues are examined and which Rh protein homologue (RhAG, RhBG, or RhCG) is present. These findings should stimulate further investigation of the physiological role of RhAG orthologs in nonerythroid tissues to understand their role in the regulation of systemic acid–base balance. These proteins might also play a role as ammonium/NH3 sensors in the regulation of various cellular functions, such as ion transports, in response to variations in extracellular ammonium level (54–56).
Acknowledgments
We thank A. Sentenac and J. M. Verbavatz (Commissariat á l'Energie Atomique/Saclay, Gif sur Yvette, France) for the stopped-flow equipment and Dr. O. Agulles for the 4.2-deficient sample.
Author contributions: P.R., O.B., and P.G. performed research; P.R. and J.-P.C. analyzed data; P.R. and J.-P.C. wrote the paper; O.B., P.G., C.B., and Y.C. contributed new reagents/analytic tools; and J.-P.C. designed research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Rh, Rhesus; RhAG, Rh-associated glycoprotein; RBC, red blood cell; MA, methylammonium; pHi, intracellular pH.
References
- 1.Mollison, P. L., Engelfriet, C. P. & Contreras, M. (1997) Blood Transfusion in Clinical Medicine (Blackwell, London), 10th Ed., pp. 152–184.
- 2.Cartron, J. P. (1999) Baillieres Best Pract. Res. Clin. Haematol. 12, 655–689. [DOI] [PubMed] [Google Scholar]
- 3.Mouro-Chanteloup, I., D'Ambrosio, A. M., Gane, P., Le Van Kim, C., Raynal, V., Dhermy, D., Cartron, J. P. & Colin, Y. (2002) Blood 100, 1038–1047. [PubMed] [Google Scholar]
- 4.Avent, N. D. & Reid, M. E. (2000) Blood 95, 375–387. [PubMed] [Google Scholar]
- 5.Huang, C. H., Liu, P. Z. & Cheng, J. G. (2000) Semin. Hematol. 37, 150–165. [DOI] [PubMed] [Google Scholar]
- 6.Lux, S. E. & Palek, J. (1995) in Blood: Principles and Practice of Hematology, eds. Handlin, S. E., Lux, S. E. & Stossel, T. P. (Lippincott, Philadelphia), pp. 1701–1818.
- 7.Nicolas, V., Le Van Kim, C., Gane, P., Birkenmeier, C., Cartron, J. P., Colin, Y. & Mouro-Chanteloup, I. (2003) J. Biol. Chem. 278, 25526–25533. [DOI] [PubMed] [Google Scholar]
- 8.Marini, A. M., Urrestarazu, A., Beauwens, R. & Andre, B. (1997) Trends Biochem. Sci. 22, 460–461. [DOI] [PubMed] [Google Scholar]
- 9.Liu, Z., Chen, Y., Mo, R., Hui, C., Cheng, J. F., Mohandas, N. & Huang, C. H. (2000) J. Biol. Chem. 275, 25641–25651. [DOI] [PubMed] [Google Scholar]
- 10.Liu, Z., Peng, J., Mo, R., Hui, C. & Huang, C. H. (2001) J. Biol. Chem. 276, 1424–1433. [DOI] [PubMed] [Google Scholar]
- 11.Huang, C. H. & Liu, P. Z. (2001) Blood Cells Mol. Dis. 27, 90–101. [DOI] [PubMed] [Google Scholar]
- 12.Marini, A. M., Matassi, G., Raynal, V., Andre, B., Cartron, J. P. & Cherif-Zahar, B. (2000) Nat. Genet. 26, 341–344. [DOI] [PubMed] [Google Scholar]
- 13.Westhoff, C. M., Ferreri-Jacobia, M., Mak, D. O. & Foskett, J. K. (2002) J. Biol. Chem. 277, 12499–12502. [DOI] [PubMed] [Google Scholar]
- 14.Nakhoul, N. L. DeJong, H., Abdulnour-Nakhoul, S. M., Boulpaep, E. L., Hering-Smith, K. & Hamm, L. L. (2004) Am. J. Renal. Physiol., in press. [DOI] [PubMed]
- 15.Bakouh, N., Benjelloun, F., Hulin, P., Brouillard, F., Edelman, A., Cherif-Zahar, B. & Planelles, G. (2004) J. Biol. Chem. 279, 15975–15983. [DOI] [PubMed] [Google Scholar]
- 16.Ludewig, U. (2004) J. Physiol. (Paris) 559, 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soupene, E., Ramirez, R. M. & Kustu, S. (2001) Mol. Cell. Biol. 21, 5733–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klocke, R. A., Andersson, K. K., Rotman, H. H. & Forster, R. E. (1972) Am. J. Physiol. 222, 1004–1013. [DOI] [PubMed] [Google Scholar]
- 19.Aubert, L. & Motais, R. (1975) J. Physiol. (Paris) 246, 159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labotka, R. J., Lundberg, P. & Kuchel, P. W. (1995) Am. J. Physiol. 268, C686–C699. [DOI] [PubMed] [Google Scholar]
- 21.Preston, G. M., Smith, B. L., Zeidel, M. L., Moulds, J. J. & Agre, P. (1994) Science 265, 1585–1587. [DOI] [PubMed] [Google Scholar]
- 22.Chrétien, S., De Figueiredo, M. & Cartron, J. P. (1999) Blood 93, 4021–4022. [PubMed] [Google Scholar]
- 23.Mouro-Chanteloup, I., Delaunay, J., Gane, P., Nicolas, V., Johansen, M., Brown, E. J., Peters, L. L., Le Van Kim, C., Cartron, J. P. & Colin, Y. (2003) Blood 101, 338–344. [DOI] [PubMed] [Google Scholar]
- 24.Trinh-Trang-Tan, M. M., Lasbennes, F., Gane, P., Roudier, N., Ripoche, P., Cartron, J. P. & Bailly, P. (2002) Am. J. Physiol. Renal. Physiol. 283, F912–F922. [DOI] [PubMed] [Google Scholar]
- 25.Roudier, N., Ripoche, P., Gane, P., Le Pennec, P. Y., Daniels, G., Cartron, J. P. & Bailly, P. (2002) J. Biol. Chem. 277, 45854–45859. [DOI] [PubMed] [Google Scholar]
- 26.Mohandas, N., Clark, M. R., Health, B. P., Rossi, M., Wolfe, L. C., Lux, S. E. & Shohet, S. B. (1982) Blood 59, 768–774. [PubMed] [Google Scholar]
- 27.Damiano, E., Bassilana, M., Rigaud, J. L. & Leblanc, G. (1984) FEBS Lett. 166, 120–124. [DOI] [PubMed] [Google Scholar]
- 28.Priver, N. A., Rabon, E. C. & Zeidel, M. L. (1993) Biochemistry 32, 2459–2468. [DOI] [PubMed] [Google Scholar]
- 29.Sheetz, M. P. & Singer, S. J. (1977) J. Cell Biol. 73, 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doberstein, S. K., Wiegand, G., Machesky, L. M. & Pollard, T. D. (1995) Cytometry 20, 14–18. [DOI] [PubMed] [Google Scholar]
- 31.Roudier, N., Verbavatz, J. N., Maurel, C., Ripoche, P. & Tacnet, F. (1998) J. Biol. Chem. 273, 8407–8412. [DOI] [PubMed] [Google Scholar]
- 32.Bruce, L. J., Ghosh, S., King, M. J., Layton, D. M., Mawby, W. J., Stewart, G. W., Oldenborg, P. A., Delaunay, J. & Tanner, M. J. (2002) Blood 100, 1878–1885. [DOI] [PubMed] [Google Scholar]
- 33.Nakhoul, N. L., Hering-Smith, K. S., Abdulnour-Nakhoul, S. M. & Hamm, L. L. (2001) Am. J. Physiol. Renal. Physiol. 281, F255–F263. [DOI] [PubMed] [Google Scholar]
- 34.Beauchamp-Nicoud, A., Morle, L., Lutz, H. U., Stammler, P., Agulles, O., Petermann-Khder, R., Iolascon, A., Perrotta, S., Cynober, T., Tchernia, G., et al. (2000) Haematologica 85, 19–24. [PubMed] [Google Scholar]
- 35.Lande, M. B., Donovan, J. M. & Zeidel, M. L. (1995) J. Physiol. (Paris) 106, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill, W. G., Rivers, R. L. & Zeidel, M. L. (1999) J. Gen. Physiol. 114, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturgeon, P. (1970) Blood 36, 310–320. [PubMed] [Google Scholar]
- 38.Ballas, S. K., Clark, M. R., Mohandas, N., Colfer, H. F., Caswell, M. S., Bergren, M. O., Perkins, H. A. & Shohet, S. B. (1984) Blood 63, 1046–1055. [PubMed] [Google Scholar]
- 39.Kuypers, F., van Linde-Sibenius-Trip, M., Roelofsen, B., Tanner, M. J. A., Anstee, D. J. & Op Den Kamp, J. A. F. (1984) Biochem. J. 221, 931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, J. A., Lucas, F. V., Jr., Martin, A. P, Senhauser, D. A. & Vorbeck, M. L. (1973) Biochem. Biophys. Res. Commun. 54, 1015–1023. [DOI] [PubMed] [Google Scholar]
- 41.Thulborn, K. R., Sawyer, W. H. & Smith, J. A. (1977) Blood 50, 964–965. [PubMed] [Google Scholar]
- 42.Dorn-Zachertz, D. & Zimmer, G. (1981) Z. Naturforsch. 36, 988–996. [DOI] [PubMed] [Google Scholar]
- 43.Hemker, M. B., Cheroutre, G., van Zwieten, R., Maaskant-van Wijk, P. A., Roos, D., Loos, J. A., van der Schoot, E. & von dem Borne, A. E. G. (2003) Br. J. Haematol. 122, 333–340. [DOI] [PubMed] [Google Scholar]
- 44.Birkenmeier, C. S., Gifford, E. J. & Barker, J. E. (2003) Hematol. J. 4, 445–449. [DOI] [PubMed] [Google Scholar]
- 45.Wandersee, N. J., Birkenmeier, C. S., Gifford, E. J., Mohandas, N. & Barker, J. E. (2000) Hematol. J. 1, 235–242. [DOI] [PubMed] [Google Scholar]
- 46.Yang, B., Ma, T. & Verkman, A. S. (2001) J. Biol. Chem. 276, 624–628. [DOI] [PubMed] [Google Scholar]
- 47.Avent, N. D., Liu, W., Warner, K. M., Mawy, W. J., Jones, J. W., Ridgwell, K. & Tanner, M. J. A. (1996) J. Biol. Chem. 271, 14233–14239. [DOI] [PubMed] [Google Scholar]
- 48.Soupene, E., Inwood, W. & Kustu, S. (2004) Proc. Natl. Acad. Sci. USA 101, 7787–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper, G. J., Zhou, Y., Bouyer, P., Grichtchenko, I. I. & Boron, W. (2002) J. Physiol. (Paris) 542, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang, W., Yang, B., Matthay, M. A. & Verkman, A. S. (2002) J. Physiol. (Paris) 542, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad, G. V., Coury, L. A., Finn, F. & Zeidel, M. L. (1998) J. Biol. Chem. 273, 33123–33126. [DOI] [PubMed] [Google Scholar]
- 52.Uehlein, N., Lovisolo, C., Siefritz, F. & Kaldenhoff, R. (2003) Nature 425, 734–737. [DOI] [PubMed] [Google Scholar]
- 53.Khademi, S., O'Connel, J., Remis, J., Robles-Colmenares, Y., Miercke, L. J. W. & Stroud, R. M. (2004) Science 305, 1587–1594. [DOI] [PubMed] [Google Scholar]
- 53.Frank, A. E. & Weiner, I. D. (2001) J. Am. Soc. Nephrol. 12, 1607–1614. [DOI] [PubMed] [Google Scholar]
- 55.Chernova, M. N., Stewart, A. K., Jiang, L., Friedman, D. L., Kunes, Y. Z. & Alper, S. L. (2003) Am. J. Physiol. Cell Physiol. 284, C1235–C1246. [DOI] [PubMed] [Google Scholar]
- 56.Javelle, A., Severi, E., Thornton, J. & Merrick, M. (2004) J. Biol. Chem. 279, 8530–8538. [DOI] [PubMed] [Google Scholar]