Abstract
Asymmetrical dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase (NOS), impairs the beneficial effect of NO. The predictive value of ADMA for all-cause mortality remains controversial, though it is important in the development of cardiovascular disease (CVD) and progression to dialysis in renal disease. This systematic review and meta-analysis was conducted to investigate the association between circulating ADMA and all-cause mortality. Studies with data pertinent to the association between circulating ADMA and all-cause mortality were reviewed and OR, HR or RR with 95% CI derived from multivariate Cox’s proportional-hazards analysis were extracted. A total of 34 studies reporting 39137 participants were included in final analysis. The results demonstrated that circulating ADMA was independently associated with all-cause mortality (RR = 1.27, 95% CI: 1.20–1.34). The association was still statistically significant in patients with pre-existing renal disease (RR = 1.30, 95% CI: 1.19–1.43) and pre-existing CVD (RR = 1.26, 95% CI: 1.16–1.37). In those without pre-existing renal or CVD, ADMA also predicted all-cause mortality (RR = 1.31, 95% CI: 1.13–1.53). The present study suggests a positive association of circulating ADMA with all-cause mortality. Further studies are needed to investigate the effects of interventions on ADMA, and the value of ADMA as a biomarker.
Asymmetrical dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase (NOS), inhibits nitric oxide (NO) biosynthesis1. Therefore, increase in circulating ADMA may impair the beneficial effects of NO on endothelial function, vasodilatation, and arterial stiffness2. Elevation of ADMA has been observed to be associated with hypertension, diabetes, hypertriglyceridemia, and hyperhomocysteinemia. These are risk factors for cardiovascular and cerebrovascular diseases3,4,5. Furthermore, studies have shown that a high circulating ADMA concentration is associated with a higher incidence of cardiovascular disease (CVD) and worse cerebrovascular disease outcomes6,7.
Owing to its involvement in CVD, circulating ADMA as a predictor of risk for mortality in patients with pre-existing CVD has been investigated. The prognostic value of ADMA has been well documented in patients with dilated cardiomyopathy, diabetes mellitus, and acute ischemic stroke8,9. Additionally, in patients with chronic kidney disease, ADMA has also been shown to be an important predictor of progression to end-stage renal disease (ESRD) and all-cause mortality10. Circulating ADMA is also associated with all-cause mortality in patients with diabetes mellitus11.
Even in a healthy population, high levels of circulating ADMA may be associated with higher rates of all-cause death12. However, the predictive value of ADMA for all-cause mortality remains controversial. Some researchers have reported that ADMA is not an independent predictor of all-cause mortality after multivariate adjustment13,14. The complicated interpretation of the predictive value of ADMA might be attributed to the limited number of participants involved in each individual study and reduced mortality. Therefore, it is important to clarify the relationship between ADMA and survival for potential interventions. To investigate the association between circulating ADMA and all-cause mortality, we conducted a systematic review and meta-analysis.
Methods
Literature search
We searched the following databases: Cochrane Library, PubMed, Web of Knowledge, and Elsevier (ScienceDirect OnLine) to retrieve literature investigating the association between circulating ADMA and all-cause mortality. The terms “ADMA or asymmetrical dimethylarginine” and “mortality or survival or outcomes or prognosis or prognostic” were used as search terms. Eligible trials were identified up to June 1, 2016 through electronic searches. Hand searches of the references of the identified trials were also conducted. The meta-analysis was conducted in accordance with the PRISMA guidelines.
Inclusion and exclusion criteria
Studies were considered for inclusion if they met the following criteria: (i) written in English, (ii) reported the all-cause mortality of participants, (iii) investigated the relationship between circulating ADMA with all-cause mortality, (iv) reported hazard ratio (HR), relative risk (RR), or odds ratio (OR) with 95% confidence intervals (95% CI) for ADMA associated with all-cause mortality, and (v) used a multivariate Cox’s proportional-hazards model to analyze the HR, RR, or OR value. Studies were excluded on the basis of the following criteria: (i) not written in English, (ii) used univariate analyses alone for the correlation of plasma ADMA with all-cause mortality, and (iii) the full-text article was not available.
Data extraction
The data were extracted independently by two reviewers (Qianqian Zhu and Xiang Li) and validated by a third reviewer (Shaoli Zhou). The following information was extracted: primary author, year of publication, geographical location, number of participants, sex ratio, and baseline average age (mean, median, or range).
Statistical analysis
Meta-analysis was performed in Review Manager 5 (The Cochrane Collaboration, Oxford, UK). The pooled effect of ADMA as a predictor of all-cause mortality was calculated as RR with 95% CI. A chi-square test was used to assess the heterogeneity. I2 value <25% was defined as no heterogeneity and a random-effects model was used when heterogeneity existed among the studies analyzed. A Begg and Egger test was used to test the publication bias and analyses were performed by using Stata 12.1 (Stata Corp., College Station, TX). Differences were considered significant when the two-tailed p values were <0.05.
Results
Search
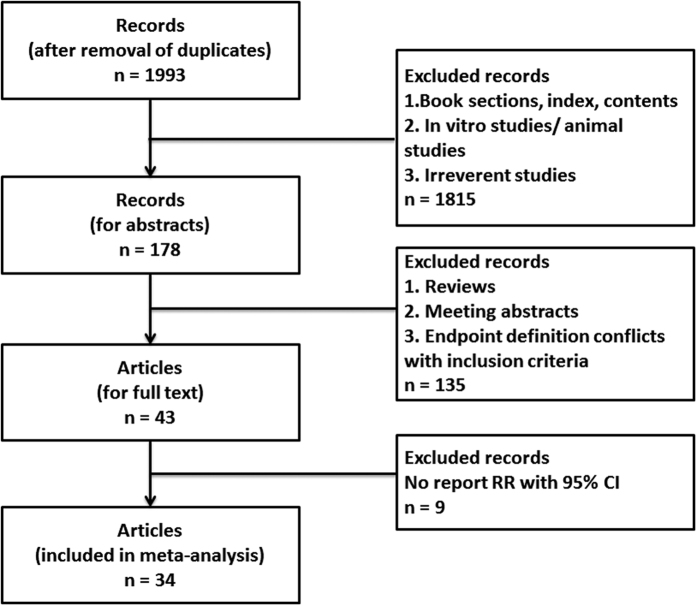
The search strategy yielded a total of 1993 non-duplicated entries. After screening the titles, the type of entries, and the abstracts, 43 articles were chosen for full review. We identified 34 eligible studies for the final analysis. The inclusion and exclusion process of the studies is shown in Fig. 1.
Figure 1. The flow chart of inclusion and exclusion.
Characteristics of the included studies
A total of 34 studies reporting a total of 39137 participants were included in the final analysis (Table 1)7,9,11,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43. Eleven studies were based on 9319 participants with pre-existing renal diseases including 2533 renal transplant recipients7,15,19,21,22,25,26,28,38,39,43. Nineteen studies included participants at high cardiovascular events risk9,11,13,17,18,20,26,27,29,30,31,32,35,36,37,40,41,42,44. Of these 19 studies, 14 studies involved 15584 participants with pre-existing cardiovascular disease9,13,18,26,27,29,30,32,35,36,37,40,41,42.
Table 1. Characteristics of studies included in meta-analysis.
| Author, year | Country | Baseline characteristics | No. of participants | Mean age (y) | Male (%) | Follow-up time |
|---|---|---|---|---|---|---|
| Abedini, Meinitzer et al.7 | Norway | renal transplant recipients | 2102 | 40.7 | N/A | 6 y (max) |
| Aucella, Maas et al.15 | Puglia | haemodialysis and dialysis | 288 | 58 | 57% | 56 m (mean) |
| Boeger, Endres et al. 2011 | Germany | Framingham Offspring | 2447 | 73.0 | 43.7% | 5 y (max) |
| Boger, Sullivan et al. 2009 | UAS | Framingham Off spring | 3320 | 59 | 45.7% | 10.9 y (mean) |
| Borgeraas, Hertel et al.18 | Norway | suspected stable angina pectoris undergoing coronary angiography | 4122 | 62 | 72% | 4.7 y (mean) |
| Cavusoglu, Ruwende et al.9 | USA | acute coronary syndrome referred for coronary angiography | 182 | 64.8 | 100% | 2 y (max) |
| Cavusoglu, Ruwende et al.11 | USA | diabetes mellitus referred for coronary angiography | 162 | 65.9 | 100% | 2 y (max) |
| Frenay, van den Berg et al.19 | Netherlands | Renal transplant recipients | 686 | 53.0 | 57% | 3.1y (mean) |
| Gore, Luneburg et al.20 | UK | Dallas Heart Study | 3523 | 43 | 44% | 7.4 y (mean) |
| Ignjatovic, Cvetkovic et al.21,22 | Serbia | dialysis patients | 153 | 58 | 68.6% | 3y (max) |
| Ignjatovic, Cvetkovic et al.21,22 | hemodialysis | 162 | N/A | N/A | 14 m (N/A) | |
| Koch, Weiskirchen et al.23 | Germany | critically ill patients | 255 | 63(median) | 58.4% | 3y (max) |
| Leong, Zylberstein et al.24 | Norway | women in the Population Study (helath) | 880 | N/A | 0% | 24 y (max) |
| Levin, Rigatto et al.25 | Canadian | chronic kidney disease | 2544 | 68.1 | 63% | 1y (mean) |
| Lu, Chung et al.26,27 | Taiwan | stage 3 to 4 CKD | 298 | 73 | 85.9% | 2.7y (mean) |
| Lu, Chung et al.26,27 | Taiwan | referred for coronary angiography | 997 | 66.9 | 79% | 2.4 y (mean) |
| Mallamaci, Tripepi et al.28 | Italy | end-stage renal disease | 224 | 54.9 | 60 | 42.3 (mean) |
| Meinitzer, Kielstein et al.29 | Germany | referred for coronary angiography | 3229 | N/A | N/A | 7.7 y (mean) |
| Meinitzer, Seelhorst et al.30 | Germany | angiographic coronary artery disease | 3238 | 62.7 | 69.7% | 5.45 y (mean) |
| Mittermayer, Krzyzanowska et al.31 | Austria | advanced peripheral artery disease | 496 | 70 | 56.3% | 19 m (mean) |
| Mommersteeg, Schoemaker et al.32 | Netherlands | heart failure | 104 | 65.7 | 72% | 6.1 y (mean) |
| Pilz, Putz-Bankuti et al.33 | Austria | chronic liver disease | 94 | 59 | 69.1% | 3.5 y (mean) |
| Pizzarelli, Maas et al.34 | Italy | elderly | 1025 | 75 | 44% | 110 m (mean) |
| Schulze, Carter et al.13 | UK | acute ischemic stroke | 394 | 69.9 | 53.5% | 7.4 y (mean) |
| Schwedhelm, Wallaschofski et al.14 | Germany | study of Health in Pomerania | 3952 | 51 | 49% | 10.1 y (mean) |
| Sen, Ozlu et al.35,46 | Turkey | acute myocardial infarction patients | 168 | 57.4 | 70% | 1 y (max) |
| Siegerink, Maas et al.36 | Germany | stable coronary heart disease | 1148 | 58.7 | 84.6% | 8.1 y (mean) |
| Tang, Tong et al.37 | Cleveland | chronic systolic heart failure | 132 | 57.8 | 77% | 33 m (mean) |
| Tripepi, Mattace Raso et al.38 | Germany | hemodialysis patients | 225 | 60 | 55% | 13 y (max) |
| Young, Terrin et al.39 | USA | stages 3 to 4 chronic kidney disease | 820 | 52 | 60% | 9.5 y (mean) |
| Zairis, Patsourakos et al.40 | Greece | chronic heart failure | 651 | 73 | 64.1% | 1 y (max) |
| Zeller, Korandji et al.41 | France | acute myocardial infarction | 249 | 68.7 | 78% | 1 y (max) |
| Zhang, Blasco-Colmenares et al.42 | USA | heart failure (PROSE-ICD) | 402 | 60.1 | 73.6% | 5.5y (mean) |
| heart failure (GRADE) | 240 | 62.5 | 77.1% | 3.7y (mean) | ||
| Zoccali, Bode-Boger et al.43 | Germany | hemodialysis patients | 225 | 59.9 | 54.7% | 33.4 m (mean) |
Circulating ADMA concentration and all-cause mortality for all participants
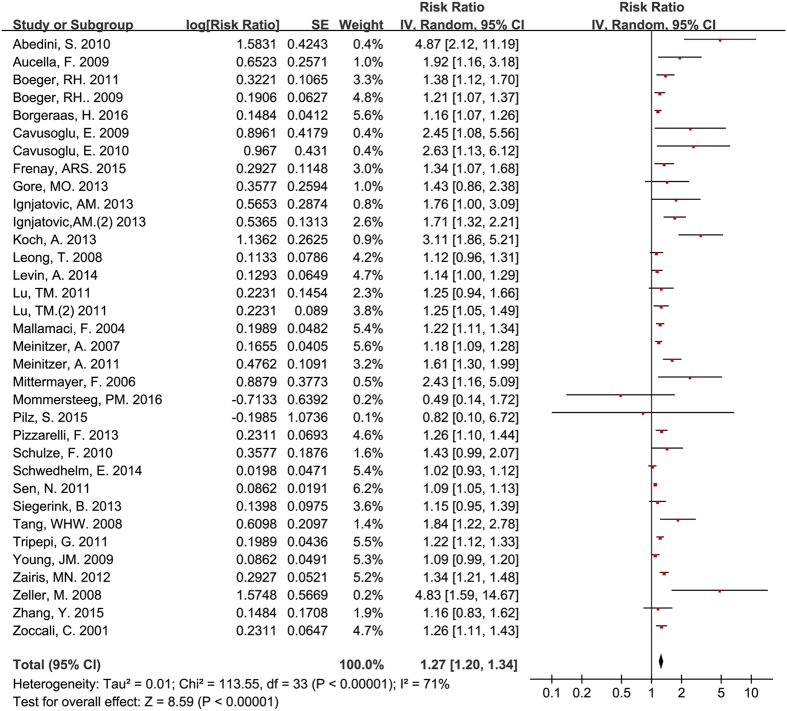
All of the 34 studies included in the meta-analysis provided a multivariate HR, RR, or OR with a 95% CI for the pooled predictive effect of ADMA for all-cause mortality. Our results demonstrated that ADMA was independently associated with all-cause mortality (RR = 1.27, 95% CI: 1.20–1.34, Fig. 2). Moderate heterogeneity (I2 = 71%) existed for these studies. After excluding the four studies11,21,26,29 with fewer participants that might have come from the same study group used in four other studies9,22,27,30, circulating ADMA was still associated with all-cause mortality (RR = 1.25, 95% CI: 1.18–1.31), and the level of between-study heterogeneity was moderate (I2 = 71%).
Figure 2. Circulating ADMA concentration and all-cause mortality for all participants.
Circulating ADMA concentration and all-cause mortality for participants with pre-existing renal diseases
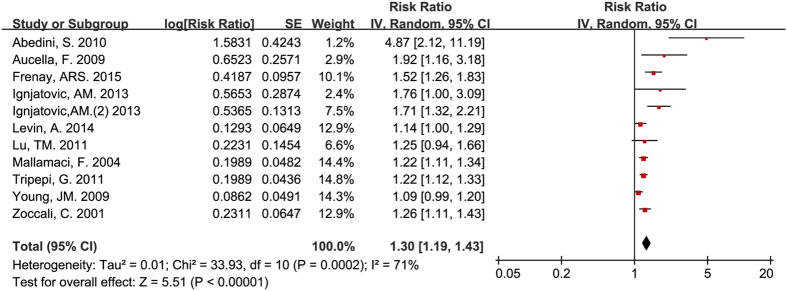
After analyzing the data from 11 studies based on participants with pre-existing renal diseases7,15,19,21,22,25,26,28,38,39,43, we found that circulating ADMA still showed a predictive value for mortality (RR = 1.30, 95% CI: 1.19–1.43, Fig. 3). The value remaining after excluding one of the two studies that used patients from the same study group was almost identical (RR = 1.27, 95% CI: 1.16–1.39)21,22. However, the heterogeneity in both the 11 (I2 = 71%) and the 10 studies was significant (I2 = 67%).
Figure 3. Circulating ADMA concentration and all-cause mortality for participants with pre-existing renal diseases.
Circulating ADMA concentration and all-cause mortality for participants with pre-existing cardiovascular disease
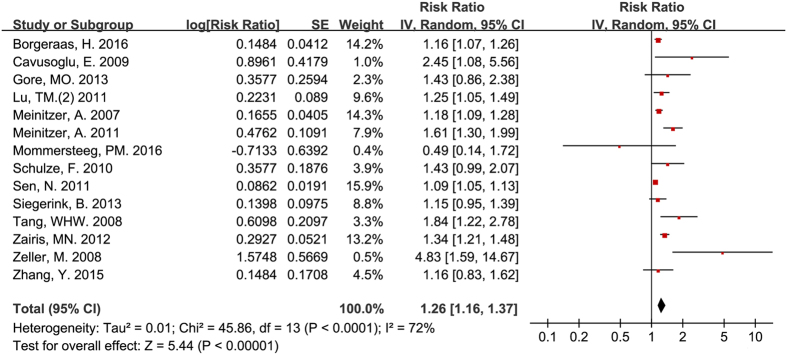
Fourteen studies included patients with pre-existing cardiovascular disease9,13,18,26,27,29,30,32,35,36,37,40,41,42. Circulating ADMA was also an independent predictor for all-cause mortality for participants with pre-existing cardiovascular disease (RR = 1.26, 95% CI: 1.16–1.37, Fig. 4). There was moderate heterogeneity among studies (I2 = 72%).
Figure 4. Circulating ADMA concentration and all-cause mortality for participants with pre-existing cardiovascular disease.
After excluding one of the two studies using patients from the same study group, circulating ADMA was still associated with all-cause mortality (RR = 1.23, 95% CI: 1.13–1.33)29,30.
Circulating ADMA concentration and all-cause mortality for participants without pre-existing renal diseases or CVD
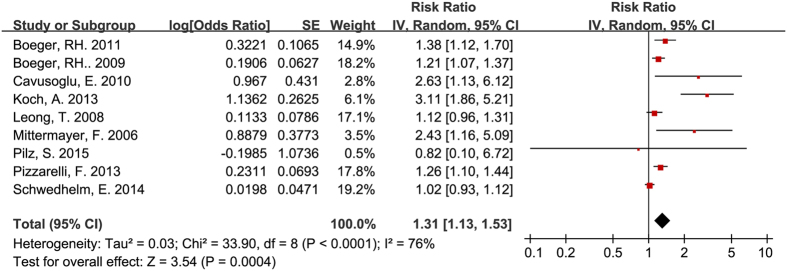
Consistent with the results of studies involving participants with CVD or renal disease, circulating ADMA was independently associated with all-cause mortality for participants without CVD or renal disease (RR = 1.31, 95% CI: 1.13–1.53, Fig. 5). The heterogeneity among studies was moderate (I2 = 76%).
Figure 5. Circulating ADMA concentration and all-cause mortality for participants without pre-existing renal diseases or CVD.
Circulating ADMA concentration and cardiac events for all participants
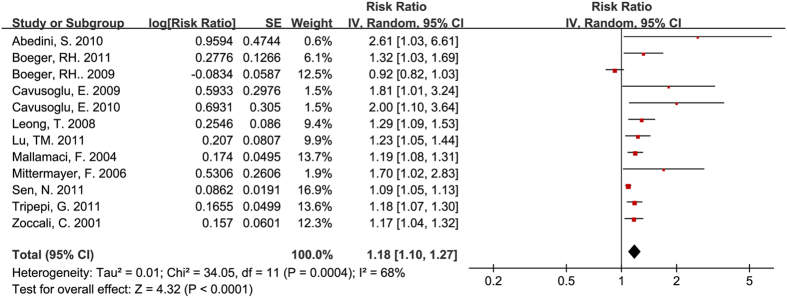
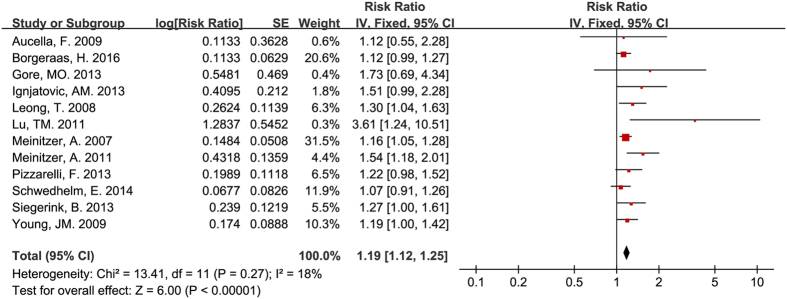
Statistically significant associations existed between circulating ADMA and major cardiovascular events (RR = 1.18, 95% CI: 1.10–1.27, Fig. 6) and cardiovascular death (RR = 1.19, 95% CI: 1.12–1.25, Fig. 7).
Figure 6. Circulating ADMA concentration and major cardiovascular events.
Figure 7. Circulating ADMA concentration and cardiovascular death.
Publication bias
The funnel plot showed that there might be publication bias (Fig. 8). A Begg and Egger test was then used to test the publication bias for all studies and the studies enrolling participants with pre-existing diseases (p < 0.001 for both).
Figure 8. Funnel plot for all publication.
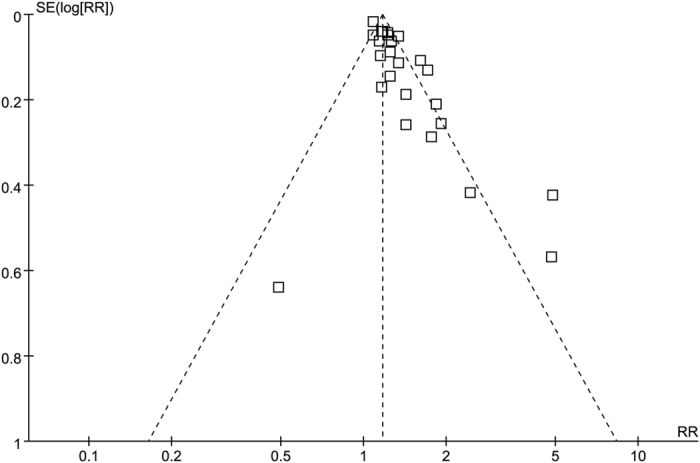
There was no significant publication bias for the studies that enrolled participants with pre-existing renal diseases (p = 0.306). However, publication bias was observed for studies enrolling participants with pre-existing cardiovascular diseases (p = 0.017).
Discussion
We performed a systematic review and meta-analysis to investigate the association between circulating ADMA and all-cause mortality. Our analysis showed that high circulating ADMA was independently associated with all-cause mortality, and the association remained in patients with or without pre-existing renal disease and pre-existing CVD. In addition, high circulating ADMA was also associated with major cardiovascular events or cardiovascular death.
An elevation of circulating ADMA in patients with CVD and pre-existing renal disease has been reported9,18. A previous study reported that high ADMA was associated with CVD events, consistent with the results of the present subgroup analyses that high circulating ADMA was associated with both major cardiovascular events and cardiovascular death24. Additionally, previous studies also indicated that a high circulating ADMA concentration was inversely related to glomerular filtration rate and positively correlated with progression to dialysis10,26. Furthermore, ADMA could predict mortality in patients with CVD or chronic renal disease18,21.The predictive value of ADMA for CVD outcomes remained significant even in participants without pre-existing CVD or kidney disease at baseline6.
However, studies investigating the relationship between ADMA and all-cause mortality reported inconsistent results. Although some studies reported that ADMA was significantly associated with all-cause mortality11,16,18, other studies did not find that ADMA was associated with all-cause mortality32,33,42. The different results might be because of the limited number of participants involved in each single-center study in addition to the complicated role of ADMA in vivo.
Our present study included data from 34 studies with more than 35000 participants and our results suggest that increased levels of circulating ADMA are an independent predictor for all-cause mortality after multivariate Cox’s proportional-hazards model adjustment. The association was similar in patients with or without pre-existing chronic renal disease and pre-existing CVD.
The main results of our present study are consistent with a recent published meta-analysis by Schlesinger and colleagues that showed that ADMA was an independent risk marker for all-cause mortality and CVD45. However, we searched more databases than they did and included as many studies as possible. We also tested for publication bias.
Furthermore, Review Manager was used to carry out the meta-analysis evaluating the weight of each study to determine which studies influenced the final results more than the others did. In our present study, after excluding the studies ranked in the top three weights, significance remained in every meta-analysis. For example, after excluding the top three weighted studies18,30,46, ADMA was still independently associated with all-cause mortality (RR = 1.31, 95% CI: 1.23–1.40). In addition, although the studies included heterogeneous populations with different conditions at baseline or different interventions, we only included the HR, RR, or OR value from multivariate Cox’s proportional-hazards model analyses, which reduced the bias.
For both CVD and chronic renal disease, the relationship between ADMA and outcomes might involve endothelial dysfunction. The endothelial dysfunction might be because of disturbed NO regulation, which would lead to impaired biological activity of NO2,47. NO deficiency is attributed to two possible causes, substrate (L-arginine) limitations and increased levels of circulating endogenous inhibitors of NOS, particularly ADMA. Therefore, the elevation of circulating ADMA might be partly reflecting the imbalance of arginine and ADMA ratio that affects NO production and has been shown to be related to changes in microcirculation of major organs and increased hospital mortality48,49. In addition to its important role in endothelial regulation, NO is also involved in vascular smooth muscle cell growth, platelet aggregation, and leukocyte adhesion, which play important roles in microcirculation50. The reduction of NO production in glomerular endothelia might cause vascular damage and enhance endothelial adhesion of leukocytes and platelets, which would subsequently increase the mortality of patients with renal disease21.
In addition to its role in endothelial dysfunction and microcirculation, various studies have reported that ADMA is consistently associated with biomarkers of inflammation in chronic conditions including diabetes, renal disease, and hypertension38,51,52. ADMA can induce TNF-α and IL-8 production via oxidative stress due to generation of reactive oxygen species/NF-κB-dependent pathway in vitro53. Therefore, circulating ADMA might be a potential pro-inflammatory factor in addition to inhibiting NOS.
Furthermore, the prognostic role of ADMA in all-cause mortality might involve additional underlying mechanisms. ADMA belongs to a family of amino acid methylation derivatives including N-mono-methylarginine (MMA), the immediate precursor to ADMA, and symmetric dimethylarginine (SDMA), a stereoisomer of ADMA54. Low plasma MMA is the most potent NOS inhibitor and has been found to be inversely related to cardiovascular disease outcomes55. Unlike ADMA, SDMA lacks NOS inhibitory activity. However, SDMA is a weak inhibitor of arginine transporters54. Studies have also reported that SDMA is associated with an increased prevalence of major adverse cardiac events, renal dysfunction, and all-cause mortality55,56. These derivatives mentioned above also show predictive value for mortality in patients with pre-exiting CVD or renal disease55,56. Furthermore, some findings suggest that ADMA may directly promote vascular disease57. Therefore, these findings raised the possibility that the underlying mechanisms of ADMA in predicting all-cause mortality might be partly independent of NOS inhibition and NO production.
Various studies have explored the predictive value of some biomarkers in patients with cardiac failure and those undergoing hemodialysis58,59,60. However, none have had satisfactory high sensitivity and specificity. Since elevated plasma ADMA concentrations independently predict CVD and renal disease outcomes, ADMA may have potential utility as a clinical biomarker. Ideal biomarkers should also exhibit pharmacologic responses to a therapeutic intervention. Some drugs such as statins and angiotensin converting enzyme inhibitors and angiotensin receptor blockers can affect ADMA61. A meta-analysis reported a significant reduction in plasma ADMA concentrations following statin therapy62. However, the cut-off value of ADMA in prediction, whether the absolute increase in ADMA is clinically relevant, and whether earlier or more aggressive intervention can improve clinical outcomes remains unclear. Therefore, studies exploring the above-mentioned unclear problems are needed in the future.
Several limitations of our present study should be considered. First, not all of the studies were prospective, which might lead to biases. Second, the substantial heterogeneity of the included studies might decrease the power of the results. Third, we only included published studies written in English. This might lead to publication bias. However, the large number of participants included in the present meta-analysis provided sufficient data for calculation of the pooled effect of ADMA on all-cause mortality. Furthermore, we only used the OR, RR, or HR with 95% CI from multivariate Cox’s proportional-hazards analysis to calculate the pooled predictive value of ADMA on mortality, which should reduce any potential bias.
In summary, the present study suggests a positive association of circulating ADMA with all-cause mortality. Further studies are needed to investigate the associations in the general population, the effects of interventions on ADMA, and the value of ADMA as a biomarker, especially in patients with CVD and those with renal diseases.
Additional Information
How to cite this article: Zhou, S. et al. Asymmetric dimethylarginine and all-cause mortality: a systematic review and meta-analysis. Sci. Rep. 7, 44692; doi: 10.1038/srep44692 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The authors thank the contributions made by Zhengwen Liu form First Affiliated Hospital of Xi’an Jiaotong University, for careful proofreading of this manuscript. The present study was supported by Science and Technology Planning Project of Guangdong Province, China (No. 2013B021800185).
Footnotes
The authors declare no competing financial interests.
Author Contributions Shaoli Zhou, Qianqian Zhu and Xiang Li helped data collection, data analysis; Chaojin Chen prepared figures and table; Jiping Liu, Yuping Ye, and Ying Ruan helped check correctness of data, figures and tables; Qianqian Zhu and Ziqing Hei helped design the study and prepare the manuscript; All of the authors helped revised the manuscript.
References
- Vallance P., Leone A., Calver A., Collier J. & Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339, 572–575 (1992). [DOI] [PubMed] [Google Scholar]
- Boger R. H. et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98, 1842–1847 (1998). [DOI] [PubMed] [Google Scholar]
- Perticone F. et al. Endothelial dysfunction, ADMA and insulin resistance in essential hypertension. Int J Cardiol 142, 236–241 (2010). [DOI] [PubMed] [Google Scholar]
- Lundman P. et al. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol 38, 111–116 (2001). [DOI] [PubMed] [Google Scholar]
- Stuhlinger M. C. et al. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation 104, 2569–2575 (2001). [DOI] [PubMed] [Google Scholar]
- Willeit P. et al. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. J Am Heart Assoc 4, e001833 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedini S. et al. Asymmetrical dimethylarginine is associated with renal and cardiovascular outcomes and all-cause mortality in renal transplant recipients. Kidney International 77, 44–50 (2010). [DOI] [PubMed] [Google Scholar]
- Anderssohn M. et al. The L-Arginine-asymmetric dimethylarginine ratio is an independent predictor of mortality in dilated cardiomyopathy. J Card Fail 18, 904–911 (2012). [DOI] [PubMed] [Google Scholar]
- Cavusoglu E. et al. Relationship of baseline plasma ADMA levels to cardiovascular outcomes at 2 years in men with acute coronary syndrome referred for coronary angiography. Coronary Artery Disease 20, 112–117 (2009). [DOI] [PubMed] [Google Scholar]
- Ravani P. et al. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. Journal of the American Society of Nephrology 16, 2449–2455 (2005). [DOI] [PubMed] [Google Scholar]
- Cavusoglu E. et al. Relation of baseline plasma ADMA levels to cardiovascular morbidity and mortality at two years in men with diabetes mellitus referred for coronary angiography. Atherosclerosis 210, 226–231 (2010). [DOI] [PubMed] [Google Scholar]
- Maas R. et al. Asymmetric dimethylarginine, smoking, and risk of coronary heart disease in apparently healthy men: Prospective analysis from the population-based Monitoring of Trends and Determinants in Cardiovascular Disease/Kooperative Gesundheitsforschung in der Region Augsburg study and experimental data. Clinical Chemistry 53, 693–701 (2007). [DOI] [PubMed] [Google Scholar]
- Schulze F. et al. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis 208, 518–523 (2010). [DOI] [PubMed] [Google Scholar]
- Schwedhelm E. et al. Incidence of all-cause and cardiovascular mortality predicted by symmetric dimethylarginine in the population-based study of health in pomerania. Plos One 9, e96875 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucella F. et al. Methylarginines and mortality in patients with end stage renal disease: A prospective cohort study. Atherosclerosis 207, 541–545 (2009). [DOI] [PubMed] [Google Scholar]
- Boeger R. H. et al. Plasma Asymmetric Dimethylarginine and Incidence of Cardiovascular Disease and Death in the Community. Circulation 119, 1592–U1565 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger R. H. et al. Asymmetric dimethylarginine as an independent risk marker for mortality in ambulatory patients with peripheral arterial disease. J Intern Med 269, 349–361 (2011). [DOI] [PubMed] [Google Scholar]
- Borgeraas H. et al. Association between Body Mass Index, Asymmetric Dimethylarginine and Risk of Cardiovascular Events and Mortality in Norwegian Patients with Suspected Stable Angina Pectoris. Plos One 11, e0152029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenay A.-R. S. et al. Plasma ADMA associates with all-cause mortality in renal transplant recipients. Amino Acids 470, 1941–1949 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore M. O. et al. Symmetrical dimethylarginine predicts mortality in the general population: observations from the Dallas heart study. Arterioscler Thromb Vasc Biol 33, 2682–2688 (2013). [DOI] [PubMed] [Google Scholar]
- Ignjatovic A. M. et al. Endothelial dysfunction, inflammation and malnutrition markers as predictors of mortality in dialysis patients: multimarker approach. Int Urol Nephrol 45, 1715–1724 (2013). [DOI] [PubMed] [Google Scholar]
- Ignjatovic A. M. et al. ADMA and C-reactive protein as mortality predictors in dialysis patients. Central European Journal of Medicine 8, 346–353 (2013). [Google Scholar]
- Koch A. et al. Elevated asymmetric dimethylarginine levels predict short- and long-term mortality risk in critically ill patients. J Crit Care 28, 947–953 (2013). [DOI] [PubMed] [Google Scholar]
- Leong T. et al. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women - 24-year follow-up of the population study of women in Gothenburg. Arterioscler Thromb Vasc Biol 28, 961–967 (2008). [DOI] [PubMed] [Google Scholar]
- Levin A. et al. Biomarkers of inflammation, fibrosis, cardiac stretch and injury predict death but not renal replacement therapy at 1 year in a Canadian chronic kidney disease cohort. Nephrol Dial Transplant 29, 1037–1047 (2014). [DOI] [PubMed] [Google Scholar]
- Lu T. M., Chung M. Y., Lin C. C., Hsu C. P. & Lin S. J. Asymmetric dimethylarginine and clinical outcomes in chronic kidney disease. Clin J Am Soc Nephrol 6, 1566–1572 (2011). [DOI] [PubMed] [Google Scholar]
- Lu T. M., Chung M. Y., Lin M. W., Hsu C. P. & Lin S. J. Plasma asymmetric dimethylarginine predicts death and major adverse cardiovascular events in individuals referred for coronary angiography. Int J Cardiol 153, 135–140 (2011). [DOI] [PubMed] [Google Scholar]
- Mallamaci F. et al. Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J Am Soc Nephrol 15, 435–441 (2004). [DOI] [PubMed] [Google Scholar]
- Meinitzer A. et al. Symmetrical and asymmetrical dimethylarginine as predictors for mortality in patients referred for coronary angiography: the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem 57, 112–121 (2011). [DOI] [PubMed] [Google Scholar]
- Meinitzer A. et al. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (The Ludwigshafen Risk and Cardiovascular Health Study). Clinical Chemistry 53, 273–283 (2007). [DOI] [PubMed] [Google Scholar]
- Mittermayer F. et al. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol 26, 2536–2540 (2006). [DOI] [PubMed] [Google Scholar]
- Mommersteeg P. M. et al. Depression and markers of inflammation as predictors of all-cause mortality in heart failure. Brain Behav Immun (2016). [DOI] [PubMed] [Google Scholar]
- Pilz S. et al. Association of homoarginine and methylarginines with liver dysfunction and mortality in chronic liver disease. Amino Acids 47, 1817–1826 (2015). [DOI] [PubMed] [Google Scholar]
- Pizzarelli F. et al. Asymmetric dimethylarginine predicts survival in the elderly. Age 35, 2465–2475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N. et al. Elevated plasma asymmetric dimethylarginine level in acute myocardial infarction patients as a predictor of poor prognosis and angiographic impaired reperfusion. Atherosclerosis 219, 304–310 (2011). [DOI] [PubMed] [Google Scholar]
- Siegerink B. et al. Asymmetric and symmetric dimethylarginine and risk of secondary cardiovascular disease events and mortality in patients with stable coronary heart disease: the KAROLA follow-up study. Clin Res Cardiol 102, 193–202 (2013). [DOI] [PubMed] [Google Scholar]
- Tang W. H. W. et al. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. European Heart Journal 29, 2506–2513 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripepi G. et al. Inflammation and asymmetric dimethylarginine for predicting death and cardiovascular events in ESRD patients. Clin J Am Soc Nephrol 6, 1714–1721 (2011). [DOI] [PubMed] [Google Scholar]
- Young J. M. et al. Asymmetric Dimethylarginine and Mortality in Stages 3 to 4 Chronic Kidney Disease. Clin J Am Soc Nephr 4, 1115–1120 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zairis M. N. et al. Plasma asymmetric dimethylarginine and mortality in patients with acute decompensation of chronic heart failure. Heart 98, 860–864 (2012). [DOI] [PubMed] [Google Scholar]
- Zeller M. et al. Impact of asymmetric dimethylarginine on mortality after acute myocardial infarction. Arteriosclerosis Thrombosis and Vascular Biology 28, 954–960 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Serum amine-based metabolites and their association with outcomes in primary prevention implantable cardioverter-defibrillator patients. Europace (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccali C. et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 358, 2113–2117 (2001). [DOI] [PubMed] [Google Scholar]
- Boeger R. H., Maas R., Schulze F. & Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality-An update on patient populations with a wide range of cardiovascular risk. Pharmacol Res 60, 481–487 (2009). [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Sonntag S. R., Lieb W. & Maas R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS One 11, e0165811 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N. et al. Elevated plasma asymmetric dimethylarginine level in acute myocardial infarction patients as a predictor of poor prognosis and angiographic impaired reperfusion. Atherosclerosis 219, 304–310 (2011). [DOI] [PubMed] [Google Scholar]
- Boger R. H. & Bode-Boger S. M. The clinical pharmacology of L-arginine. Annu Rev Pharmacol Toxicol 41, 79–99 (2001). [DOI] [PubMed] [Google Scholar]
- Richir M. C. et al. Low arginine/asymmetric dimethylarginine ratio deteriorates systemic hemodynamics and organ blood flow in a rat model. Crit Care Med 37, 2010–2017 (2009). [DOI] [PubMed] [Google Scholar]
- Visser M. et al. Imbalance of arginine and asymmetric dimethylarginine is associated with markers of circulatory failure, organ failure and mortality in shock patients. Br J Nutr 107, 1458–1465 (2012). [DOI] [PubMed] [Google Scholar]
- Pacher P., Beckman J. S. & Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87, 315–424 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konukoglu D., Firtina S. & Serin O. The relationship between plasma asymmetrical dimethyl-L-arginine and inflammation and adhesion molecule levels in subjects with normal, impaired, and diabetic glucose tolerance. Metabolism 57, 110–115 (2008). [DOI] [PubMed] [Google Scholar]
- Tsioufis C. et al. ADMA, C-reactive protein, and albuminuria in untreated essential hypertension: a cross-sectional study. Am J Kidney Dis 55, 1050–1059 (2010). [DOI] [PubMed] [Google Scholar]
- Chen M. F. et al. Role of asymmetric dimethylarginine in inflammatory reactions by angiotensin II. J Vasc Res 44, 391–402 (2007). [DOI] [PubMed] [Google Scholar]
- Leiper J. & Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 43, 542–548 (1999). [DOI] [PubMed] [Google Scholar]
- Wang Z., Tang W. H., Cho L., Brennan D. M. & Hazen S. L. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler Thromb Vasc Biol 29, 1383–1391 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode-Boger S. M. et al. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol 17, 1128–1134 (2006). [DOI] [PubMed] [Google Scholar]
- Achan V. et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23, 1455–1459 (2003). [DOI] [PubMed] [Google Scholar]
- Kotani K., Serban M. C., Penson P., Lippi G. & Banach M. Evidence-based assessment of lipoprotein(a) as a risk biomarker for cardiovascular diseases - Some answers and still many questions. Crit Rev Clin Lab Sci 53, 370–378 (2016). [DOI] [PubMed] [Google Scholar]
- Gluba A. et al. An update on biomarkers of heart failure in hypertensive patients. J Hypertens 30, 1681–1689 (2012). [DOI] [PubMed] [Google Scholar]
- Hogas S. et al. Predictive Value for Galectin 3 and Cardiotrophin 1 in Hemodialysis Patients. Angiology 67, 854–859 (2016). [DOI] [PubMed] [Google Scholar]
- Maas R. Pharmacotherapies and their influence on asymmetric dimethylargine (ADMA). Vasc Med 10 Suppl 1, S49–57 (2005). [DOI] [PubMed] [Google Scholar]
- Serban C. et al. A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Sci Rep 5, 9902 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]