Abstract
Two-component signaling systems, in which a receptor-coupled kinase is used to control the phosphorylation level of a response regulator, are commonly used in bacteria to sense their environment. In the chemotaxis system of Escherichia coli, the receptors, and thus the kinase, are clustered on the inner cell membrane. The phosphatase of this system also is recruited to receptor clusters, but the reason for this association is not clear. By using FRET imaging of single cells, we show that in vivo the phosphatase activity is substantially larger at the cluster, indicating that the signaling source (the kinase) and the signaling sink (the phosphatase) tend to be located at the same place in the cell. When this association is disrupted, a gradient in the concentration of the phosphorylated response regulator appears, and the chemotactic response is degraded. Such colocalization is inevitable in systems in which the activity of the kinase and the phosphatase are produced by the same enzyme. Evidently, this design enables a more rapid and spatially uniform response.
Keywords: bacteria, cyan fluorescence protein, signal transduction, yellow fluorescence protein
Two-component signaling systems are commonly used in bacteria to sense and respond to changes in the environment (1). Nutrients, attractants, repellents, osmotic agents, and the like are detected by receptors that control the autophosphorylation activity of an associated histidine kinase. This enzyme, in turn, phosphorylates a response-regulator protein, which controls the appropriate downstream cellular response. In this way, each environmental parameter is continuously represented by the phosphorylation level of the corresponding response regulator. An essential condition for such a system to work is the existence of an appropriate level of phosphatase activity that prevents saturation of the response-regulator phosphorylation level.
In the Escherichia coli chemotaxis system, the kinase CheA (2) is part of a receptor complex that forms a tight cluster on the inner cell membrane, preferentially at the cell pole (3). Therefore, CheA provides a localized phosphoryl transfer source. Although the response regulator of this system, CheY, has a relatively high autophosphatase activity with a lifetime of ≈10 s, the system still utilizes an auxiliary cytoplasmic phosphatase, CheZ, that reduces the response time to ≈0.1 s. This reduction is essential for chemotaxis (4). Recently, it was found that CheZ also is recruited to receptor clusters by binding to the short form of the kinase, CheAs (5, 6). Moreover, coexpression of CheZ and CheAs is common within chemotactic members of the Enterobacteriaceae family (7). However, because CheAs is not essential for chemotaxis in standard laboratory assays (8), the role of this interaction is still not clear. In vitro measurements have shown that, under certain conditions, CheZ activity is enhanced by the binding of CheAs (9). It has been proposed that regulation of CheZ–CheAs binding at the cluster can act as an adaptation mechanism (10). It also has been argued that phosphorylated CheY (CheYp)-dependent oligomerization of CheZ tends to enhance its activity, suggesting an additional regulatory mechanism for CheZ (11).
We first monitored the changes in the association of CheZ fused to cyan fluorescence protein (CFP) or CheY fused to yellow fluorescence protein (YFP) with the cluster upon addition of attractant. Then, to map the spatial distribution of the interaction between these proteins within the cells, we carried out FRET measurements with both fusion proteins coexpressed. The consequences of disrupting the association of the phosphatase with a cluster could then be studied by using a variant of CheZ that does not localize, CheZF98S (5).
The addition of saturating levels of attractant drastically reduces the activity of the kinase (12) and thus allows the phosphatase to convert most of CheYp to nonphosphorylated CheY (CheY0). Because CheZ preferentially binds to CheYp, the addition of attractant abolishes the transfer of energy from CheZ-CFP to CheY-YFP and thus provides a baseline from which the contribution of FRET to the cyan and yellow signals can be deduced. Because diffusion is negligible on the time scale of the fluorescence lifetime, FRET imaging measures the local level of association between the labeled proteins.
Materials and Methods
Strains. VS161 [ΔcheZ], VS104 [Δ(cheY–cheZ)], and VS149 [Δ(cheR–cheZ)] were constructed by in-frame deletions of the corresponding genes in RP437 (13); VS169 [Δ(tar–cheZ) tsr::Tn5] was made from RP5723; and AV4 [Δ(cheR–cheZ) CheAs–] was made from AJW536 (a gift from Alan Wolfe, Loyola University, Chicago).
Plasmids. cheZ-cfp (pVS54), cheZF98S-cfp (pAV6), cheZ (pAV3), and cheZF98S (pAV7) were cloned in pBAD33, which is chloramphenicol-resistant (CamR) and inducible by arabinose (14). cheY-yfp (pVS18), cheZF98S-yfp (pAV11), cheZ-cfp-cheY-yfp (pVS88), and cheZF98S-cfp-cheY-yfp (pAV8) were cloned in pTrc99A (Pharmacia), which is ampicillin-resistant (AmpR) and inducible by isopropyl β-d-thiogalactopyranoside. cheY-cfp (pAV14) was cloned in pKG110 (a gift from Sandy Parkinson, University of Utah, Salt Lake City), which is CamR and inducible by sodium salicylate.
Cell Preparation. Cells were grown as described in ref. 12 and resuspended in motility medium (buffer): 10 mM potassium phosphate/0.1 mM EDTA/67 mM NaCl/1 μM methionine/10 mM lactic acid, pH 7. Unless noted otherwise, the inducers used were 50 μM isopropyl β-d-thiogalactopyranoside for pTrc99A-based plasmids, 0.01% arabinose for pBAD33, and 1 μM sodium salicylate for pKG110. Low levels of expression, mentioned in the text for the pBAD33 plasmid, refer to arabinose concentrations <0.0003%.
FRET Imaging. A Nikon Optiphot microscope equipped with a ×100 plan fluor 1.3 numerical aperture oil-immersion objective and Chroma filters (Chroma Technology, Brattleboro, VT) was used. The fluorescence was excited by a 75-W xenon lamp using an HQ440/20 excitation filter and a 455 DCLP dichroic mirror. The emitted light was split into two channels, cyan and yellow, by using a 515 DCXR mirror with HQ485/40 and HQ535/30 emission filters, respectively. Half of each image was blocked, and two identical halves were recombined by using a 515 DCSP mirror and projected side-by-side onto the face of a charge-coupled device camera (DV887ECS-BV, Andor Technology, South Windsor, CT). By setting an additional phase-contrast channel at 600–640 nm, extracted with a 580 DCSP mirror above the excitation cube, a “live” image of the cells could be observed in parallel by using a second video camera (BP550; Panasonic, Secaucus, NJ). This channel was used to monitor the focus; it had no measurable effect on the cyan and yellow channels. To follow the YFP fluorophore directly, the CFP excitation cube was replaced with a red-shifted YFP cube by using an HQ515/5 excitation filter, a Q525LP dichroic mirror, and an HQ530LP emission filter. A counterweight was added to the microscope stage to reduce its drift.
The optical parameters of the system were measured by using a purified CFP–YFP fusion protein (a gift from Victor Sourjik, ZMBH, Heidelberg) with a trypsin-sensitive site in the middle that enables separation of these fluorescent proteins by using trypsin. With excitation at 440 nm, trypsin digestion increased the cyan signal by 32% and decreased the yellow signal (after subtracting the leakage of CFP emission; see below) by 70% of their maximal levels. However, with excitation at 515 nm, the YFP emission was not affected by digestion. After digestion, both fluorophores were present at identical concentrations, and the cyan signal from CFP with excitation at 440 nm was four times stronger then the yellow signal from YFP with excitation at 515 nm (4-fold attenuated). These were the excitation conditions used in vivo.
When cells expressing only CFP were excited at 440 nm, the yellow signal was 30% of the cyan signal. The leakage of YFP emission into the cyan channel was within the noise, and the signal from cells that did not express either CFP or YFP was <100 photons per pixel, with 1-s exposure time. Therefore, with excitation at 440 nm, the cyan signal represents only the CFP emission. However, the yellow signal represents (i) the YFP emission due to FRET, (ii) direct excitation of YFP, and (iii) leakage of the CFP emission. Changes in FRET affect contributions i and iii by an amount ΔYi and ΔYiii, respectively. Redistribution of YFP during a response affects contribution ii by an amount ΔYii. ΔYi is a measure of the concentration of YFP fluorophores excited through FRET with cells in buffer. We have ΔYi = ΔYtot – 0.3·ΔC – α·ΔY515, where ΔYtot is the total response in the yellow channel, ΔC is the response in the cyan channel, and ΔY515 is the response observed when only YFP is excited at 515 nm. The second term is the correction because of the 30% leakage of the cyan emission to the yellow channel (ΔYiii). Because both ΔYii and ΔY515 represent the redistribution of YFP during a response, ΔY515 could be used to correct for ΔYii. However, because different excitation conditions were used in these cases, the corresponding emissions, Yii and Y515, were different. To correct for that, we introduced the factor α = Yii/Y515 in the third term. Yii was extracted from the image in serine (which eliminates the FRET contribution) by subtracting the CFP contribution, and Y515 was directly extracted from the corresponding image in serine. All corrections were done after alignment of the corresponding images.
Data Acquisition. Cells were immobilized by using anti-FliC antibody on a 12-mm coverslip and mounted in a flow cell (15). A series of images were acquired with 1-s exposure time under continuous flow at 600 μl/min, alternating between buffer and attractant, with ≈2 min between exposures. The responses reported here were reproducible between every pair of successive images in the series.
Results
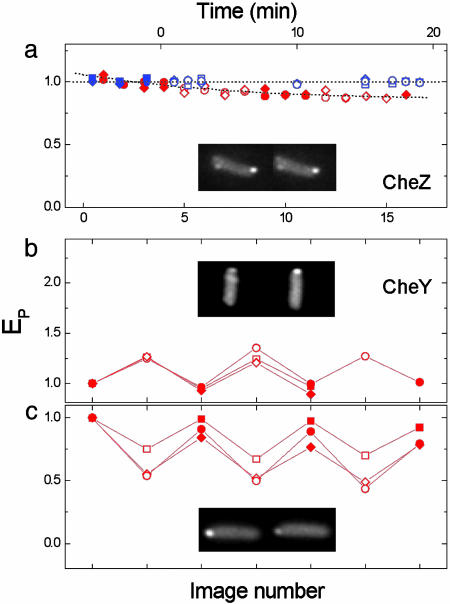
Changes in the Association of the Phosphatase or the Response Regulator with the Cluster, Generated by Addition of Chemoattractant. In these experiments, CheZ-CFP or CheY-YFP was expressed separately in cells, and fluorescence images of the same cells were acquired in the absence or presence of a saturating level of attractant. As noted in refs. 5 and 6, both proteins appear to localize to receptor clusters. Fig. 1 shows the peak fluorescence of CheZ-CFP or CheY-YFP at the cluster in the absence (filled symbols) or presence (open symbols) of an attractant. The association of CheZ with the clusters did not change during a chemotactic response to either α-methylaspartate or serine (Fig. 1a). Note that cells adapt to the addition of α-methylaspartate over several minutes, but they do not completely adapt to serine. The association of CheY with the cluster, however, changed upon the addition of attractant in a way that depended upon CheZ. If either wild-type CheZ or its variant, CheZF98S, was poorly expressed, the association of CheY with the cluster increased in attractant (Fig. 1b). In contrast, if wild-type CheZ was fully expressed, the association of CheY with the cluster decreased in attractant (Fig. 1c). These results are consistent with the in vitro observations that CheZ binds preferentially to CheYp (16), whereas CheA binds preferentially to CheY0 (17). In the absence of CheZ at the cluster, binding of CheY0 to CheA dominates and increases in attractant. But when CheZ is present at the cluster, binding of CheYp to CheZ dominates and decreases in attractant.
Fig. 1.
Changes in the association of CheZ and CheY with the clusters generated by addition of attractant. The cells were cycled between buffer and attractant, and fluorescence images were recorded. Plots show the peak emission intensity at the cluster (EP), normalized to the first image, for cells in buffer (filled symbols, left images) or in attractants (open symbols, right images). Different symbols correspond to different cells. Attractants used were serine (300 μM, red) or α-methylaspartate (100 μM, blue). (a) cheZ cells expressing CheZ-CFP (pVS54), excited at 440 nm. The upper abscissa is for the data in blue and indicates the time since the addition of attractant; the lower abscissa is for the data in red and indicates successive images. (b and c) cheY cheZ cheR cheB cells expressing CheY-YFP (pVS18), excited at 515 nm, with wild-type CheZ (pAV3) or the CheZF98S mutant (pAV7) poorly induced (b), or with wild-type CheZ (pAV3) fully induced (c). The abscissa indicates successive images.
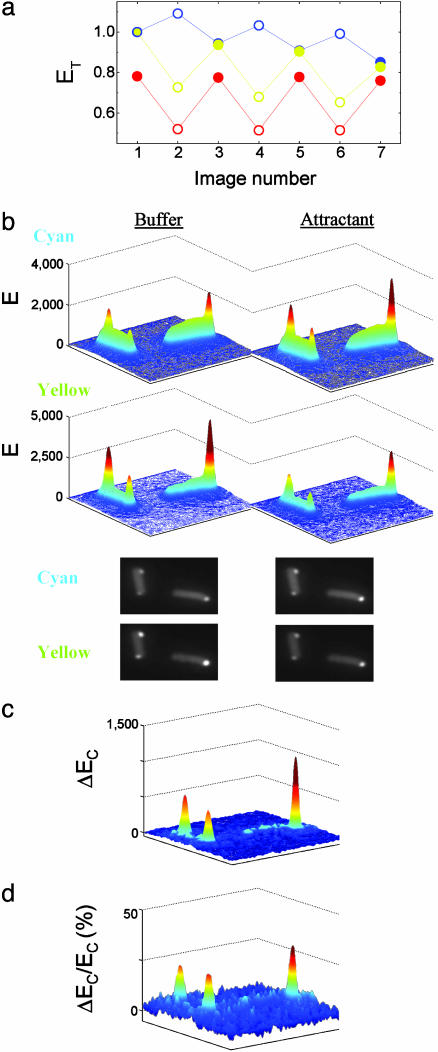
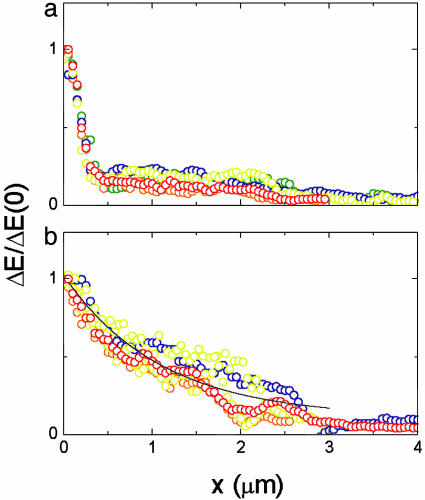
FRET Response with a Localized Phosphatase. The spatial distribution of the CheY–CheZ interaction was mapped by using FRET imaging. In these experiments, CheZ-CFP and CheY-YFP were coexpressed in cells and excited at 440 nm. During the experiment, the cyan and yellow emissions were imaged in parallel alternately in buffer and attractant (300 μM serine). A typical example, with two adjacent cells, is shown in Fig. 2. In attractant, the emission integrated over the area subtended by the plots increased in the cyan channel but decreased in the yellow channel (Fig. 2a). This effect is expected, because the addition of a saturating amount of attractant abolishes FRET (12). The spatial distribution of the emission intensity in cyan (Upper) and yellow (Lower) channels is plotted in Fig. 2b for cells in buffer (Left) or attractant (Right). The corresponding images are shown below. An increase in the cyan emission and a corresponding decrease in the yellow emission upon addition of attractant are apparent primarily at the clusters. The spatial distribution of the increase in the cyan emission, ΔEC, is shown in Fig. 2c. The corresponding decrease in the yellow emission due to FRET (see Materials and Methods) has a similar spatial distribution (data not shown). These changes in the fluorescence due to FRET represent the spatial distribution of the association of CheY with CheZ. However, because of the selective interaction of CheZ with the phosphorylated form of CheY, these changes represent predominantly the association of CheYp with CheZ. Several examples for the profile of this association along the cell are shown in Fig. 3a. It is clear that the largest change in the fluorescence occurred at the clusters (at x = 0), indicating that the association of CheYp with CheZ is much larger there.
Fig. 2.
FRET response with a localized phosphatase. cheY cheZ cheR cheB cells expressing CheY-YFP (pVS18) and CheZ-CFP (pVS54) were cycled between buffer and attractant (300 μM serine). Excitation was at 440 nm, and the spatial distribution of the emission intensity (E) in the cyan and yellow channels was measured by imaging. (a) The total emission (ET) in the cyan (blue) and the yellow (yellow) channels, and their ratio 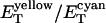 (red) in buffer (filled symbols) or attractant (open symbols). The ET values were obtained by integrating over the area of the images shown in b and were normalized to the values at the first image. (b) Three-dimensional representations (in false colors) of the spatial distributions of the emission intensity in the cyan (Upper) and yellow (Lower) channels for the same cells in buffer (Left) or attractant (Right), derived from the corresponding images, shown below. (c) The spatial distribution of the change in the cyan emission (ΔEC), i.e., the emission in attractant minus that in buffer. (d) The relative increase in the cyan emission ΔEC/EC, where ΔEC is the change shown in c, and EC is the cyan emission in attractant (b Upper Right). The numbers on the ordinates in b and c are photons per pixel per s.
(red) in buffer (filled symbols) or attractant (open symbols). The ET values were obtained by integrating over the area of the images shown in b and were normalized to the values at the first image. (b) Three-dimensional representations (in false colors) of the spatial distributions of the emission intensity in the cyan (Upper) and yellow (Lower) channels for the same cells in buffer (Left) or attractant (Right), derived from the corresponding images, shown below. (c) The spatial distribution of the change in the cyan emission (ΔEC), i.e., the emission in attractant minus that in buffer. (d) The relative increase in the cyan emission ΔEC/EC, where ΔEC is the change shown in c, and EC is the cyan emission in attractant (b Upper Right). The numbers on the ordinates in b and c are photons per pixel per s.
Fig. 3.
The profiles of CheY–CheZ association along the cell. The profile of the FRET interaction along the cell for cells expressing wild-type CheZ (a) or nonlocalized CheZF98S (b), extracted from the data sets of Figs. 2c and 4c, respectively (red). Other colors represent data from other cells. The receptor clusters are at x = 0, where the plots were normalized. The distance x was estimated by assuming a cell width of 1 μm. The fit to the data in b is ΔE/ΔE(0) = (0.15 + e–x/1.4)/1.15.
The spatial distribution of the normalized increase in the cyan emission, i.e., the change in the cyan emission normalized by the cyan emission in attractant, ΔEC/EC(attractant), is shown in Fig. 2d. Because the presence of a saturating amount of attractant abolishes FRET, the cyan emission in attractant, EC(attractant), represents the CheZ-CFP distribution. Therefore, this normalization accounts for the nonhomogeneous distribution of CheZ-CFP due to clustering. It is clear that, even after normalization, the major increase in cyan emission, due to FRET, occurs at the cluster. On average, the relative change at the cluster, 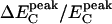 (attractant), was ≈25%, and thus comparable with the change seen when hydrolyzing a YFP–CFP fusion (see Materials and Methods), indicating that the occupation of CheZ by CheYp at the cluster is significant.
(attractant), was ≈25%, and thus comparable with the change seen when hydrolyzing a YFP–CFP fusion (see Materials and Methods), indicating that the occupation of CheZ by CheYp at the cluster is significant.
Taken together, the increased association of CheYp with CheZ at the cluster (Fig. 2c) and the enhanced catalytic activity of CheZ in the presence of CheAs (9) suggest that by recruitment of CheZ to the cluster, the local phosphatase activity is considerably enhanced. Thus, even though the phosphatase of this system is an auxiliary cytoplasmic enzyme, phosphorylation and dephosphorylation still tend to occur at the same place in the cell.
FRET Response with a Uniformly Distributed Phosphatase. The amino acid substitution F98S in CheZ disrupts its association with CheAs and thus with receptor clusters (5). In experiments with cells expressing CheZF98S-CFP and CheY-YFP, the integrated FRET response was similar to the equivalent response for wild-type CheZ (Fig. 2a); however, the spatial distribution of the response was different (Fig. 4). The spatial distribution of the emission intensity in cyan (Upper) and yellow (Lower) channels is plotted in Fig. 4a for cells in buffer (Left) or attractant (Right). The corresponding images are shown below. The cyan emission increased upon addition of attractant in most of the cell area, but it decreased somewhat at the cluster. These changes are shown more clearly in Fig. 4b (Left), which plots the difference in the cyan emission between attractant and buffer. The yellow emission decreased upon addition of attractant, markedly toward the cluster. However, if protein distributions remained fixed during the response, the increase in the cyan signal and the decrease in the yellow signal due to FRET would be expected to have a similar spatial distribution. Therefore, redistribution of CheY and/or CheZF98S must have occurred during the attractant response. Migration of CheY from the end of the cell near the cluster to the opposite end was verified by using excitation at 515 nm to probe the YFP fluorophore directly (Fig. 4b Right). Redistribution of CheZ also was observed in similar experiments using cells expressing CheZF98S-YFP and CheY-CFP, when excited at 515 nm (data not shown).
Fig. 4.
FRET response with a nonlocalized phosphatase. cheY cheZ cheR cheB cells expressing CheY-YFP and CheZF98S-CFP (pAV8) were cycled between buffer and attractant (300 μM serine). Excitation was at 440 nm, and the spatial distribution of the emission intensity (E) in the cyan and yellow channels was measured by imaging. (a) Three-dimensional representations (in false colors) of the spatial distributions of the emission intensity in the cyan (Upper) and yellow (Lower) channels for the same cells in buffer (Left) or attractant (Right) derived from the corresponding images, shown below. (b) The spatial distribution of the changes in the emission intensity (ΔE, attractant minus buffer) in the cyan channel (Left), derived from the corresponding plots in a, and in the yellow channel (Right), with excitation at 515 nm to probe directly the YFP distribution. Note the inverted ordinate. The Inset on the right is a plot of the image in attractant, confirming the position of the cluster (arrow). (c) The spatial distribution of the changes in the CheY-YFP emission due to FRET (ΔE, attractant minus buffer). This plot was derived from the data for the yellow channel shown in a and corrected by using the data in b for direct YFP excitation at 440 nm and leakage of the CFP emission into the yellow channel, as described in Materials and Methods. Note the inverted ordinate. The numbers on the ordinates in a–c are photons per pixel per s.
The FRET contribution to the change in the yellow signal, shown in Fig. 4c, could be deduced from these data (see Materials and Methods) to reveal the spatial distribution of the association between CheZ and CheYp that occurs with cells in buffer. Note that this plot is inverted for clarity, and blue represents a decrease of the signal in attractant. The profile of this association along the cell is plotted in Fig. 3b, together with data from four other cells. By comparing these data with the corresponding change observed upon hydrolysis of a YFP–CFP fusion (see Materials and Methods), we estimate that the concentration of CheYp bound to CheZ at either end of the cell is 40% and 10% of the total CheY concentration, respectively.
Dose–Response Curves for Cell Populations. To assess the effect of CheZ localization on the actual chemotactic response, we measured FRET responses from fields of ≈100 cells to increasing doses of attractant (Fig. 5). Cells expressing wild-type CheZ were more sensitive to serine than were cells expressing CheZF98S, with an ≈2- to 3-fold difference in the concentration at half-maximal response (Fig. 5 a and c). However, no differences were observed with cells missing CheAs (Fig. 5b), as expected, because in this case, neither form of CheZ binds to the cluster. Differences in responses also were apparent when α-methylaspartate was used as an attractant with cheR cheB cells expressing only the aspartate receptor, Tar. However, no differences were detected when these experiments were repeated with either aspartate or α-methylaspartate in wild-type cells. Similarly, with swarms in tryptone soft agar, the serine ring moved ≈10% faster with wild-type CheZ than with CheZF98S, whereas the aspartate ring changed by <2% (data not shown). Thus, localization of CheZ to the cluster tends to enhance the chemotactic response in both cheR cheB and wild-type cells.
Fig. 5.
Dose–response curves for cell populations exposed to serine. (a) cheY cheZ cheR cheB cells expressing CheY-YFP (pVS18) together with either CheZ-CFP (pVS54) or CheZF98S-CFP (pAV6), filled and open symbols, respectively, excited at 440 nm. N/Npre is the number of CheY–CheZ pairs normalized by the prestimulus value (12). (b) The same experiment for cells also missing CheAs. (c) The same experiment for CheAs+ CheR+ CheB+ cells. Note the change in scale.
Discussion
The addition of attractant, or adaptation after this addition, did not change the association of the phosphatase, CheZ, with receptor clusters (Fig. 1a). However, it did change the association of the response regulator, CheY, with the clusters, reflecting interactions of CheY with both CheA and CheZ, with the latter interaction dominating under wild-type conditions (Fig. 1 b and c). With wild-type CheZ, the CheY–CheZ interaction was enhanced 5- to 10-fold at the clusters relative to the cytoplasm (Figs. 2 and 3a). Thus, recruitment of CheZ to the cluster leads to a considerable enhancement of the local phosphatase activity. Replacement of wild-type CheZ by CheZF98S disrupts the association of the phosphatase with the cluster (5). Under these conditions, spatial gradients in the association of CheYp with CheZ were apparent (Figs. 3b and 4), indicating that a gradient in the concentration of CheYp is formed, with the highest concentration near the cluster. Gradients in the concentration of total CheY and CheZF98S of the same general form as that of CheYp also were formed (Fig. 4b Right). Finally, cells expressing wild-type CheZ were more sensitive to chemotactic stimuli than were cells expressing CheZF98S (Fig. 5), so the localization of CheZ appears to enhance the chemotactic response.
It has been suggested that regulation of the binding of CheAs to the cluster, and thus of the CheAs–CheZ interaction at the cluster, might provide an additional adaptation mechanism (10). This does not appear to be the case, because the amount of CheZ bound to CheAs at the cluster did not change in response to addition of attractant or during adaptation (Fig. 1a). Similar results were found in experiments that monitored the localization of CheZ in cheR cheB cells with receptors in different modification states (18). Note, however, that these observations do not preclude the possibility that CheZ activity is regulated at the cluster by an as yet unknown mechanism.
Given the enhancement of the phosphatase activity at the clusters (Figs. 2 and 3a), it appears that the signaling source (the kinase) and the signaling sink (the phosphatase) of this system tend to be located at the same place in the cell. If phosphorylation and dephosphorylation occur at the same place, a steadystate solution for the corresponding reaction diffusion equation predicts a uniform distribution for the concentration of CheYp throughout the cell. Such distribution is consistent with the apparent uniform FRET response in the cytoplasm, e.g., for Fig. 3a in the range x > 0.5 μm. A uniform distribution of CheYp was found recently also in computer simulations of E. coli cells (19). Interestingly, physical association between the kinase and phosphatase inevitably emerges in the design of two-component systems in which the kinase itself also acts as a phosphatase (20).
The time required for the system to reach steady state, limited by diffusion, is of order t = L2/D, where L is the length of the cell and D is the diffusion coefficient of CheY. For L = 1 μm and D = 10 μm2/s, we get t = 0.1 s. This time scale is comparable with the observed signal processing times (4, 21), implying that the phosphatase activity is high enough that changes in the concentration of CheYp occur on a similar (or smaller) time scale as that for diffusion.
Disruption of the physical association between the kinase and the phosphatase creates a situation in which CheYp is generated at one end of the cell and hydrolyzed throughout the cytoplasm. As shown in Figs. 3b and 4, such an arrangement leads to formation of a spatial gradient in the association between CheYp and CheZF98S, with the highest concentration of the CheYp – CheZF98S complex near the cluster. Although redistribution of both CheZF98S and CheY was observed (Fig. 4), the difference in their concentration at either end of the cell is only ≈20–30%. Therefore, most of the gradient in the concentration of the CheYp–CheZ complex is due to a gradient in the concentration of CheYp. A well known problem in one-dimensional reaction diffusion is of a substance created at the origin (x = 0) that diffuses with coefficient D and decays at rate kC. If the concentration C is fixed at the origin at C0, the steady-state concentration in a semi-infinite medium is C(x) = C0e–x/l, with the decay length l = (D/k)1/2. For D = 10 μm2/s and k = 10 s–1, we get l = 1 μm, which roughly agrees with the observed gradients (Fig. 3b). The possibility that such gradients might appear was noted earlier (21) and has been shown to be plausible by computer simulations (19).
The gradient observed in the concentration of CheY and CheZF98S, which has the same general form as that of CheYp (Fig. 4), can form by binding of CheZF98S to CheYp. This binding interaction is significant, as demonstrated in Figs. 1c and 2, and is the reason for the occurrence of FRET.
The dose–response curves deduced from the FRET experiments with cell populations (Fig. 5) indicate that delocalization of CheZ degrades the chemotactic response. The FRET signal in the experiments of Fig. 5 reflects the level of interaction between CheZ and CheYp, integrated over the entire cell, and hence, in steady state, the total phosphoryl transfer rate. Localization of CheZ can act to enhance the efficiency of phosphoryl transfer from CheA to CheY0 by increasing the local concentration of CheY0 near the cluster.
A gradient in CheYp concentration, combined with the sensitive response of the motors to CheYp (22), implies that motors near the cluster will tend to spin clockwise, whereas motors farther away will tend to spin counterclockwise. However, motors are still expected to switch stochastically and independently. Moreover, not all of them are required to spin in the same direction to maintain smooth swimming behavior (23). Therefore, chemotaxis should still be possible.
The idea that gradients of chemicals might be generated by release (or synthesis) at one point and capture (or destruction) at another is well established in developmental biology (24). In phosphoryl transfer systems, the kinase and the phosphatase serve as the source and the sink for the phosphorylated response regulator and thus are prone to formation of gradients (25–28). However, locating both at the same place seems to provide a way for the cell to maintain high phosphatase activity, enabling fast responses while minimizing the formation of gradients in the distribution of the phosphorylated signaling protein, promoting a spatially uniform response.
Acknowledgments
We thank V. Sourjik, J. S. Parkinson, and A. J. Wolfe for strains, plasmids, and useful discussions. We also thank D. Bray, K. Lipkow, and T. Shimizu for useful discussions. This work was supported by National Institutes of Health Grant AI16478.
Abbreviations: CFP, cyan fluorescence protein; YFP, yellow fluorescence protein; CheAs, short form of CheA; CheYp, phosphorylated CheY protein; CheY0, nonphosphorylated CheY protein; CheY, the total CheY protein, i.e., CheYp + Chey0.
References
- 1.Hoch, J. A. & Silhavy, T. J., eds. (1995) Two-Component Signal Transduction (Am. Soc. Microbiol., Washington, DC).
- 2.Hess, J. F., Oosawa, K., Kaplan, N. & Simon, M. I. (1988) Cell 53, 79–87. [DOI] [PubMed] [Google Scholar]
- 3.Maddock, J. R. & Shapiro, L. (1993) Science 259, 1717–1723. [DOI] [PubMed] [Google Scholar]
- 4.Segall, J. E., Manson, M. D. & Berg, H. C. (1982) Nature 296, 855–857. [DOI] [PubMed] [Google Scholar]
- 5.Cantwell, B. J., Draheim, R. R., Weart, R. B., Nguyen, C., Stewart, R. C. & Manson, M. D. (2003) J. Bacteriol. 185, 2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sourjik, V. & Berg, H. C. (2000) Mol. Microbiol. 37, 740–751. [DOI] [PubMed] [Google Scholar]
- 7.McNamara, B. P. & Wolfe, A. J. (1997) J. Bacteriol. 179, 1813–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanatinia, H., Kofoid, E. C., Morrison, T. B. & Parkinson, J. S. (1995) J. Bacteriol. 177, 2713–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang, H. & Matsumura, P. (1996) Mol. Microbiol. 19, 695–703. [DOI] [PubMed] [Google Scholar]
- 10.Almogy, G., Stone, L. & Ben-Tal, N. (2001) Biophys. J. 81, 3016–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blat, Y., Gillespie, B., Bren, A., Dahlquist, F. W. & Eisenbach, M. (1998) J. Mol. Biol. 284, 1191–1199. [DOI] [PubMed] [Google Scholar]
- 12.Sourjik, V. & Berg, H. C. (2002) Proc. Natl. Acad. Sci. USA 99, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkinson, J. S. & Houts, S. E. (1982) J. Bacteriol. 151, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman, L.-M., Belin, D., Carson, M. J. & Beckwith, J. (1995) J. Bacteriol. 177, 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg, H. C. & Block, S. M. (1984) J. Gen. Microbiol. 130, 2915–2920. [DOI] [PubMed] [Google Scholar]
- 16.Blat, Y. & Eisenbach, M. (1996) J. Biol. Chem. 271, 1232–1236. [DOI] [PubMed] [Google Scholar]
- 17.Li, J., Swanson, R. V., Simon, M. I. & Weis, R. M. (1995) Biochemistry 34, 14626–14636. [DOI] [PubMed] [Google Scholar]
- 18.Liberman, L., Berg, H. C. & Sourjik, V. (2004) J. Bacteriol. 186, 6643–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipkow, K., Andrews, S. & Bray, D. (2004) J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 20.Stock, A. M., Robinson, V. L. & Goudreau, P. N. (2000) Annu. Rev. Biochem. 69, 183–215. [DOI] [PubMed] [Google Scholar]
- 21.Sourjik, V. & Berg, H. C. (2002) Proc. Natl. Acad. Sci. USA 99, 12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cluzel, P., Surette, M. & Leibler, S. (2000) Science 287, 1652–1654. [DOI] [PubMed] [Google Scholar]
- 23.Turner, L., Ryu, W. & Berg, H. C. (2000) J. Bacteriol. 182, 2793–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crick, F. (1970) Nature 225, 420–422. [DOI] [PubMed] [Google Scholar]
- 25.Brown, G. C. & Kholodenko, B. N. (1999) FEBS Lett. 457, 452–454. [DOI] [PubMed] [Google Scholar]
- 26.Kholodenko, B. N., Brown, G. C. & Hoek, J. B. (2000) Biochem. J. 350, 901–907. [PMC free article] [PubMed] [Google Scholar]
- 27.Francke, C., Postma, P. W., Westerhoff, H. V., Blom, J. G. & Peletier, M. A. (2003) Biophys. J. 85, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niethammer, P., Bastiaens, P. & Karsenti, E. (2004) Science 303, 1862–1866. [DOI] [PubMed] [Google Scholar]