Abstract
Changes in neural activity caused by exposure to drugs may trigger homeostatic mechanisms that attempt to restore normal neural excitability. In Drosophila, a single sedation with the anesthetic benzyl alcohol changes the expression of the slo K+ channel gene and induces rapid drug tolerance. We demonstrate linkage between these two phenomena by using a mutation and a transgene. A mutation that eliminates slo expression prevents tolerance, whereas expression from an inducible slo transgene mimics tolerance in naïve animals. The behavioral response to benzyl alcohol can be separated into an initial phase of hyperkinesis and a subsequent phase of sedation. The hyperkinetic phase causes a drop in slo gene expression and makes animals more sensitive to benzyl alcohol. It is the sedative phase that stimulates slo gene expression and induces tolerance. We demonstrate that the expression level of slo is a predictor of drug sensitivity.
Keywords: drug abuse, potassium channel, transcription regulation
Inhalation of organic solvents or volatile anesthetics causes dramatic changes in neural excitability. Moderate exposure can have excitatory effects, whereas higher levels of exposure produce sedation and anesthesia. The euphoria and intoxication associated with solvent inhalation has led to their abuse as drugs (1, 2).
The changes in neural activity caused by exposure to these drugs may trigger homeostatic mechanisms that attempt to restore normal neural excitability. Because the electrical character of a neuron is an emergent property of the channels that it expresses, a likely target of these homeostatic changes are ion channel proteins and genes. Changes in gene expression that alter the density or ratios of channels can have a strong effect on cellular electrical properties (3) and, in the case of drug-induced changes in excitability, could contribute to drug tolerance and addiction.
We have used Drosophila and a channel gene to study the response of the nervous system to agents that alter excitability, such as volatile solvents. The slo gene encodes a BK-type Ca2+-activated K+ channel. This channel integrates two of the most basic methods of neural signaling: It responds both to increases in free Ca2+ and to changes in membrane potential. Null mutations in the slo gene cause subtle changes in fly behavior. Drosophila lacking slo expression have a reduced capacity for flight, show a stimulus-induced “sticky-feet” phenotype, have an unusual mating song, are arrhythmic with regard to circadian rhythms, and are more sensitive to sedation by volatile anesthetics (4–7). In Caenorhabditis elegans, the channel encoded by the slo homologue is directly modulated by ethanol, and genetic studies have shown that it plays a role in the mechanism of intoxication (8).
In flies, the slo gene is expressed in neurons, muscles, midgut, and trachea and has a very complex transcriptional control region (9). Expression of slo has been shown to be controlled by five tissue-specific promoters (10, 11). Here, we show that slo expression is dynamically modulated in an experience-dependent manner and that the level of slo expression is a predictor of drug sensitivity. Furthermore, sedation-induced changes in neural slo expression contribute to a form of drug tolerance.
Methods
Fly Stocks. Flies were raised on standard cornmeal/molasses/agar medium. Flies that emerged from pupae were collected over a 2-day period, transferred to fresh food, and studied between 5 and 7 days later. Genotype of mutant stocks were eag1 ShKS133, seits1, hk1,Dppara, and slo4. The genotype of the B52H transgenic stock is w1118, B52H, slo4. In the B52H transgene, an inducible hsp70 promoter drives expression of a slo cDNA whose splice pattern is found in the nervous systems and muscles (12). Wild types used were Canton S and w1118 (tested because the B52H transgene is a w1118 background). The behavioral and molecular responses of Canton S and w1118 were not distinguishable (data not shown).
Pharmacological Manipulations. Benzyl alcohol exposure was performed by coating 30-ml glass vials with 200 μl of a solution of benzyl alcohol in acetone (0.15, 0.3, or 0.4%). These experiments included an acetone control. The vials were continuously rotated for 45 min at 22°C to evaporate the acetone, leaving a thin coat of evenly distributed benzyl alcohol. Fifteen flies were placed in each vial and exposed to the benzyl alcohol until sedation (10–15 min).
Exposure to chloroform, toluene, and trichloroethylene was achieved by inverting a glass tube containing 15 flies over 0.5 ml of 100% solvent in a 1.5-ml microfuge tube. A Kimwipe was placed between the two tubes to prevent flies from falling into the solvent. After a 15-min exposure, the flies were transferred to food for recovery. For CO2 anesthesia, flies were placed in a constant stream of the gas for 15 min. Vials were placed for 15 min in ice for cold anesthesia or in a 37°C incubator for heat shock. Treatment with tetrodotoxin was performed by intraabdominal injections of 100 nl of 10 μM tetrodotoxin dissolved in a vehicle solution of 0.1M Na2HPO4 and 5 mM KCl (pH 6.8). Vehicle alone was used as a control.
Benzyl Alcohol Behavioral Assay. In the first exposure, flies were treated in triplicate with benzyl alcohol (0.3%) or vehicle (100% acetone, evaporated to dryness) as described above. Twenty-four hours later, treated and control flies were simultaneously exposed to benzyl alcohol. Snapshots were taken every 20 sec during the course of exposure and recovery and stored as a stop-motion movie. Recovery from anesthesia was scored as the return of geotactic behavior. Flies on the walls of the tube were scored as recovered. Values for recovered flies then were plotted as a percentage of the population in each tube (average of three tubes) against time at 20-sec intervals. The data and statistics shown in the figures are derived from a protocol in which each assay is performed in triplicate. The entire protocol has been repeated, in triplicate, a minimum of three times. Each repetition yielded the same results.
The log-rank test for equality of survival was used to determine the significant difference between recovery curves because survival analysis is best suited for data in which one is measuring the time to a specific event (13). In all of the tolerance assays, we measured the time that it took for each fly to recover from sedation. The statistic evaluates whether entire recovery curves are statistically different (as opposed to individual data points comprised by the curve). However, in the behavioral assays, we have also included error bars that represent the SEM for each individual data point.
Quantitative Real-Time PCR Analysis. Total RNA was extracted from 50 flies 6 h after the start of the treatment by using a single-step RNA isolation tissue protocol (14). Contaminating DNA was removed with RNase-free DNase I (Ambion, Austin, TX).
First-strand cDNA was synthesized from 100 ng of total RNA, primed with 200 nM each of gene-specific primers for slo and for cyclophilin 1 transcripts with Superscript II reverse transcriptase (Invitrogen). The cDNA was amplified by real-time PCR in an ABI Prism 7700 Sequence Detection System (Applied Biosystems) in the presence of gene-specific dual-labeled single-stranded probes. The primers used to detect exon C1 were 5′-aaacaaagctaaataagttgtgaaagga-3′ and 5′-gatagttgttcgttcttttgaatttga-3′, whereas the primers 5′-accaaccacaacggcactg-3′ and 5′-tgcttcagctcgaagttctcatc-3′ were used to detect the cyclophilin 1 message. These primers flanked the TaqMan oligonucleotide probes 5′-[6-carboxyfluorescein (FAM)]agaaactgcgcttagtcacactgctcatgt[6-carboxytetramethylrhodamine (TAMRA)]-3′ and 5′-(FAM)cggcaagtccatctacggcaacaagtt(TAMRA)-3′, respectively. The primers used to detect exon C3 were 5′-ttggccgacgatccaaca-3′ and 5′-accagtacttgcgcaccttga-3′. For quantification of the C1 exon, PCR was performed by using the TaqMan probes and the TaqMan Universal PCR Master mix (Applied Biosystems), whereas for the C3 exon PCR was performed by using SYBR Green I nucleic acid stain (Molecular Probes). Each PCR was performed in triplicate, and the yields thereof were expressed as an average. mRNA abundance was quantified by using the standard curve method. Significance was calculated by using Student's t test.
β-Gal Assay. Transcriptional activity of the transgenic slo promoter was determined by measuring β-gal specific activity. Protein extract was prepared from P3 flies between 11 and 14 h after solvent exposure, and β-gal activity was determined (15). Total protein concentration was determined by using the Bradford-based Bio-Rad Protein Assay Kit. Wild-type (WT) flies have a small level of endogenous β-gal activity that was accounted for by subtraction. Significance was calculated by using Student's t test.
Results
Benzyl Alcohol Intoxication Increases slo mRNA Abundance. We altered neural excitability by exposing groups of flies to the anesthetic benzyl alcohol, a solvent that has very low toxicity to flies. Upon exposure to an intoxicating dose of benzyl alcohol, flies entered a brief hyperkinetic phase characterized by spinning and shaking. This phase was followed by a period of deep sedation in which the flies showed no signs of movement. Once all members of the group entered the sedative phase, they were removed to benzyl alcohol-free vials and allowed to recover. Within 30–45 min, the animals resumed normal behavior and appeared to have recovered completely.
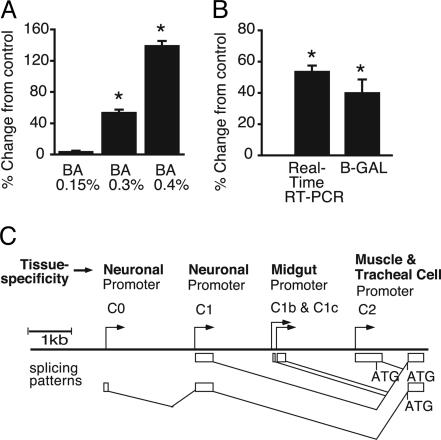
We investigated the effects of benzyl alcohol on slo message abundance. To allow time for changes in gene expression, total RNA was extracted 6 h after solvent exposure. The level of slo mRNA in the nervous system was determined by using real-time RT-PCR to quantify the neural-specific slo exon called C1 (11). To account for variability in purification efficiency, the abundance of slo mRNA was expressed relative to the abundance of mRNA from the cyclophilin 1 gene. cyclophilin 1 mRNA was chosen as an internal control because its abundance was not affected by the experimental paradigm. The validity of this control was confirmed by demonstrating that normalization against total RNA yielded a slo expression profile that was essentially the same as that obtained by using the cyclophilin 1 internal control (data not shown). After a single 15-min exposure to benzyl alcohol, a dose-dependent change in the abundance of slo mRNA was observed (Fig. 1A). The intermediate dose (0.3%), which produced a 53% change in slo message abundance, was chosen for subsequent experiments because it had the largest effect on slo mRNA abundance without compromising viability. This treatment did not cause significant changes in the abundance of the Shaker or seizure voltage-gated K+ channel mRNAs or slo muscle-specific transcript (data not shown).
Fig. 1.
Benzyl alcohol-induced increase in slo transcriptional activity. (A) Abundance of slo message relative to the cyclophilin 1 internal control as measured by real-time RT-PCR after treatment with 200 μl of a 0.15, 0.3, or 0.4% benzyl alcohol solution (for each, n = 3). (B) The percentage change in slo expression in response to 0.3% benzyl alcohol sedation measured by real-time RT-PCR and by a change in the specific activity of β-gal expressed from the P3 slo transgene. The abscissa shows the percentage change relative to control animals (not exposed to drug but otherwise treated identically). *, Significant difference from control (P < 0.01; for each, n = 3). (Error bars are SEM.) (C) Map of the slo transcriptional control region. The horizontal line represents genomic DNA, the arrowheads are transcriptional promoters, and the boxes below the line are the alternative 5′ exons generated by each tissue-specific promoter. The connecting lines identify the splicing pattern. ATG identifies translation start sites (11). Real-time PCR primer pairs were within exons C1 and C2. Exon C1 begins mRNAs produced by promoter C1 and is also part of mRNAs produced by promoter C0. Exon C2 begins mRNAs expressed from muscle/tracheal cell promoter C2.
To determine whether the increase in slo mRNA abundance arises from transcriptional activation of the gene or from changes in mRNA stability, we measured the response of the P3 β-gal reporter transgene to benzyl alcohol. The P3 transgene contains the portion of the slo transcriptional control region responsible for neural expression and reproduces the neural expression pattern (10, 11, 16). The endogenous slo gene and the reporter gene share the same transcriptional control regions; however, they express distinct transcripts whose stability is unlikely to be coregulated. Therefore, a coincident increase in slo mRNA and in slo driven β-gal expression is likely to reflect an increase in promoter activity rather than a change in mRNA stability. P3 flies treated with benzyl alcohol showed a 41.5% increase in β-gal-specific activity 11–14 h after treatment, relative to untreated flies (Fig. 1B). The increased incubation time, with regard to the previous experiment, was included to ensure translation of the reporter. This response is similar to the change observed in mRNA abundance from the endogenous gene. Therefore, the benzyl alcohol-induced boost in expression is largely or completely attributable to a change in the transcription rate of the gene.
Increased mRNA Abundance Is a Response to Sedation. When exposed to an incapacitating dose of benzyl alcohol, flies pass through a hyperkinetic phase before entering the sedative phase. We used a combination of drugs and mutants to determine whether the increase in slo mRNA is a response to the hyperactive or the sedative phase.
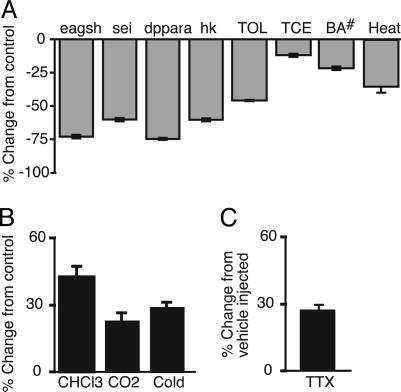
To examine the effect of hyperactivity on slo mRNA abundance, we used ion channel mutations to produce animals that were constitutively hyperactive. The mutations used were eag1, ShKS133, seits1, Hk1, and Dppara (17–21). The first four are mutations in voltage-gated K+ channel genes or K+ channel accessory subunits and produce hyperexcitability by perturbing K+ channel activity. The fifth mutation (Dppara) is a duplication of the para Na+ channel gene and is believed to produce hyperexcitability by increasing the production of para voltage-gated Na+ channels (22). In all of these animals, slo mRNA abundance is reduced with respect to the WT (Fig. 2A).
Fig. 2.
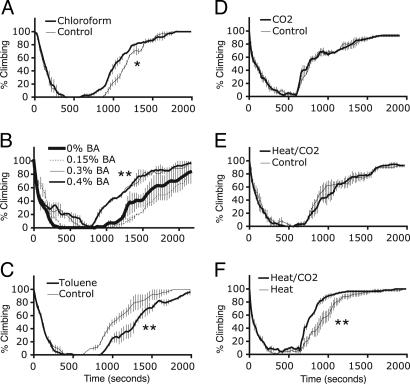
slo gene expression and activity levels are inversely related. slo transcript abundance relative to cyclophilin 1 as measured by real-time RT-PCR from mutant flies and flies exposed to drugs that induce hyperactivity (gray bars) or sedation (black bars). (A) Relative slo expression in the hyperexcitable mutants eag1Sh120b, seits1, Hk1, and Dppara and in animals treated with toluene (TOL), trichloroethylene (TCE), 0.3% benzyl alcohol (BA#; flies were removed from the benzyl alcohol before entering anesthesia), and 37°C heat shock (Heat). (B) Relative slo expression levels 6 h after sedation with chloroform (CHCl3), CO2, or 0°C cold anesthesia (Cold). (C) Relative slo expression levels 6 h after tetrodotoxin (TTX) injection. All values are significantly different from control (P < 0.01).
Hyperexcitability without sedation also was environmentally induced. In flies, toluene and trichloroethylene, except at extremely high doses, produced only a hyperkinetic response. Flies that were exposed to a low dose of benzyl alcohol showed hyperkinesis but not sedation. Elevated temperature (37°C) also was used to produce populations of hyperkinetic flies. Each treatment reduced the abundance of slo mRNA (Fig. 2 A).
Sedation without hyperactivity was induced by using chloroform, CO2, and cold anesthesia (0°C) and by injecting flies with a sublethal dose of tetrodotoxin. Each treatment caused an increase in slo mRNA abundance (Fig. 2 B and C).
Benzyl Alcohol-Induced Tolerance Depends on slo Expression. We began this study intending to test whether perturbation of excitability by solvent sedation would induce homeostatic changes in channel gene expression. A response of this kind should counter the effects of the solvent and, if the response was significantly large, would reduce drug responsiveness. The term “tolerance” is used to identify reduced drug responsiveness that results from prior drug exposure (23). Many different drug responses have been used to demonstrate tolerance. The length of time that the animals are sedated is a direct response to the drug. Thus, a drug-induced reduction in the duration of sedation fits the definition of tolerance. Tolerance produced by a single brief exposure is sometimes termed “rapid tolerance” (24). The term “resistance” will be reserved for instances in which responsiveness has been reduced in the absence of prior drug exposure. Animals that show heightened drug responsiveness, for any reason, will be referred to as having increased sensitivity or as being sensitized.
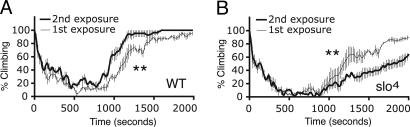
We noticed that a single benzyl alcohol exposure was sufficient to induce tolerance to the drug. Tolerance was said to have been induced if the animals recovered more rapidly from their second benzyl alcohol sedation than from their first sedation (reduced drug responsiveness). To measure the recovery time from anesthesia, we used a variation of the distribution test (25). This test takes advantage of the negative geotactic reflex, which causes flies to climb the walls of a container. When flies in a vial are exposed to a benzyl alcohol, they become uncoordinated, lose their ability to climb, and fall to the bottom of the vial. They eventually succumb to anesthesia and remain on the bottom of the vial. We recorded recovery from anesthesia by monitoring the return of the negative geotactic behavior.
Fig. 3A shows that a population previously treated with benzyl alcohol recovered ∼5 min earlier than naïve flies. The coincident increase in slo mRNA levels with the induction of tolerance prompted us to determine whether the increase in slo expression contributes to tolerance. For this experiment, we examined the responses of the slo4 mutant. The slo4 mutation is a chromosomal rearrangement that has a breakpoint in the slo gene. This mutation eliminates the production of channels from the gene (9, 26). Surprisingly, slo4 homozygotes are healthy and fecund (4). The slo4 flies were subjected to the tolerance test described above. Fig. 3B shows that in the absence of slo expression, prior benzyl alcohol sedation does not induce drug tolerance. Instead, the previously treated slo4 flies appeared more sensitive and showed a slower recovery from anesthesia when exposed to benzyl alcohol a second time.
Fig. 3.
Behavioral response to serial benzyl alcohol exposure. Knockdown and recovery curve of WT (A) and slo4 mutant (B) flies after one (thin line) and two (thick line) exposures to 0.3% benzyl alcohol. Time between exposures is 24 h. Loss of climbing indicates sedation. Values are plotted as a percentage of climbing flies against time at 20-sec intervals. **, Significant difference from control as determined by the log-rank test (n = 3, P < 0.01). (Error bars are SEM for each data point.)
Expression Level of slo Is a Predictor of Drug Sensitivity. To determine whether the level of slo expression affects benzyl alcohol responsiveness, we manipulated slo expression and measured drug responsiveness. Expression was increased by using an inducible slo transgene or with drugs, and expression was reduced with environmental treatments.
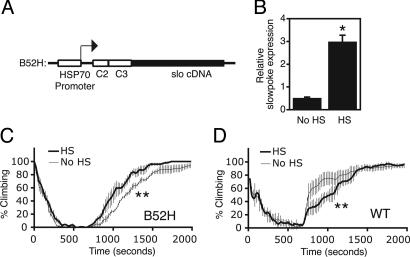
First, we tested the benzyl alcohol response of flies carrying a heat-inducible slo transgene. These animals are homozygous for the slo4 null allele and carry the B52H transgene. B52H has an hsp70 promoter that drives expression of a slo cDNA (12) (Fig. 4A). In this paradigm, heat shock (37°C for 30 min) was used to induce the transgene and was substituted for the first benzyl alcohol treatment. Twenty-four hours later, the animals were sedated with benzyl alcohol, and their recovery time was compared with age-matched B52H siblings that were not heat-shocked but that were otherwise treated identically. Heat-shocked flies were more resistant to benzyl alcohol and recovered faster than their non-heat-shocked siblings (Fig. 4C). Real-time RT-PCR demonstrated that the heat pulse induces expression from B52H (Fig. 4B).
Fig. 4.
Effects of slo induction on recovery from benzyl alcohol sedation. (A) Map of the B52H transgenic construct (12) in which the HSP70 promoter drives expression of a slo cDNA. C2 and C3 are two exons that also are shown in Fig. 1D. (B) Relative slo expression levels in B52H transgenic flies, 6 h after treatment with heat shock (HS) or control (No HS). *, Significant difference from control (P < 0.01). (C and D) Recovery curve of WT and the HSP70-slo transgenic flies (B52H) after exposure to 0.3% benzyl alcohol. Effects of slo induction on the recovery were determined by treating the transgenic and WT flies with a heat shock (thick line) 24 h before the benzyl alcohol treatment or with no heat shock (thin line). Loss of climbing indicates sedation. **, Significant difference from control as determined by the log-rank test (P < 0.01). (Error bars are SEM for each data point.)
To determine whether inducible resistance was merely a heat-shock response that was unassociated with the B52H transgene, we monitored, in parallel, the effect of heat shock on WT flies that do not carry the transgene. In WT flies, heat shock did not induce resistance but slowed the recovery from anesthesia (Fig. 4D).
In a second test we determined the effects of chloroform-induced slo expression on the recovery rate from benzyl alcohol sedation. Chloroform, which sedates animals without causing them to pass through a hyperactive phase, also produces an increase in slo expression (Fig. 2B). A single chloroform sedation was sufficient to induce benzyl alcohol resistance. Fig. 5A shows that a population previously treated with chloroform recovered from benzyl alcohol sedation more rapidly than naïve flies.
Fig. 5.
Treatments that change slo expression alter drug responsiveness. (A) Recovery of WT flies from 0.3% benzyl alcohol sedation after pretreatment with chloroform compared with nonpretreated control flies. (B) Recovery of WT flies from 0.3% benzyl alcohol after pretreatment with 0.15, 0.3, or 0.4% benzyl alcohol compared with nonpretreated control flies. * indicates that the 0.3 and 0.4% pretreatments are significantly different (**, P < 0.01) from control (whereas the 0.15% pretreatment is not). (C) Recovery of WT flies from 0.3% benzyl alcohol sedation after pretreatment with toluene compared with nonpretreated control flies. (D and E) Recovery of WT flies from 0.3% benzyl alcohol sedation after pretreatment with CO2 (D) or heat followed by CO2 (E) compared with nonpretreated control flies. (F) Recovery of WT flies from benzyl alcohol after pretreatment with heat and CO2 compared with flies pretreated with heat. In A, C, and F, * indicates that the recovery curves are significantly different from control (*, P < 0.05; **, P < 0.01). (Error bars are SEM calculated for each data point.)
A working hypothesis that accounts for these data is that sedation induces slo expression and increased slo expression results in tolerance (inducible-resistance). In concert with this hypothesis are the results of a dose-response study using benzyl alcohol. Low doses that fail to sedate the animals (0.15%) and do not cause an increase in slo expression (Fig. 1 A) also fail to induce drug tolerance (Fig. 5B). Conversely, higher doses that induce sedation and slo expression also cause tolerance.
To determine the effect of decreasing slo expression, we relied on environmental treatments that reduce slo mRNA levels. Hyperkinetic behavior induced with increased temperature (37°C) or by exposure to a nonsedating dose of toluene was used to reduce slo expression (Fig. 2 A). The benzyl alcohol sensitivity of the animals was measured 24 h after exposure. Both the heat pulse (Fig. 4D) and toluene exposure (Fig. 5C) not only reduced slo mRNA abundance but also caused increased sensitivity to benzyl alcohol.
We wished to know whether nonsolvent-induced sedation also could induce benzyl alcohol resistance. CO2 and cold sedation both induced slo expression in the absence of a hyperactive phase. But neither CO2 (Fig. 5D) nor cold anesthesia (data not shown) changed benzyl alcohol sensitivity. These treatments induce less slo expression than benzyl alcohol or chloroform (Fig. 2B). They may not be sufficiently strong inducers of slo expression to cause benzyl alcohol resistance in normal animals. Therefore, we asked whether any evidence of CO2-induced benzyl alcohol resistance could be detected in flies sensitized to benzyl alcohol anesthesia. Flies heat-shocked to increase sensitivity were sedated immediately with CO2. Interestingly, the CO2 treatment eliminated all evidence of heat-induced sensitization (Fig. 5 E and F). We propose that CO2 sedation does increase benzyl alcohol resistance but that this effect can be detected only in a sensitized background.
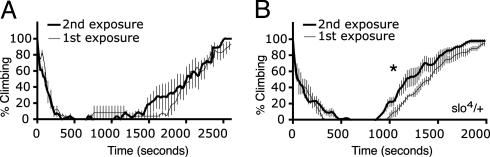
Neural-Specific Expression of slo Is Necessary for Drug Tolerance. We have shown that up-regulation of a neural-specific slo transcript is correlated with the appearance of benzyl alcohol tolerance and that a slo loss-of-function mutation prevents the acquisition of tolerance. However, this loss-of-function mutation eliminated slo expression in all tissues (neural, muscle, and epithelial). To demonstrate that tolerance depended only on neural expression of slo, we used ash218/slo4 transheterozygotes. The ash218 allele is a chromosomal deletion that removes the two transcriptional promoters that drive slo neural expression. The promoter that drives expression in muscle is still present (4). The ash218 allele is a recessive lethal mutation because it also removes the ash2 (developmental) gene. Therefore, we used ash218/slo4 transheterozygotes. Atkinson et al. (4) have shown that this double mutant has specifically lost expression in the nervous system. These flies were unable to acquire benzyl alcohol resistance (Fig. 6A), whereas the slo4/+ heterozygotes did acquire resistance (Fig. 6B).
Fig. 6.
Neural-specific expression of slo is necessary for induced drug resistance. Recovery curve of the ash218/slo4 transheterozygous (A) and slo4/+ heterozygous (B) flies after one (thin line) and two (thick line) exposures to 0.3% benzyl alcohol (24 h between exposures). Values are plotted as a percentage of climbing flies against time at 20-sec intervals. *, Significant difference from control (n = 3, P < 0.05). (Error bars are SEM calculated for each data point.)
Discussion
The term tolerance refers to reduced drug responsiveness that results from prior drug exposure. Tolerance produced by a single brief exposure is termed rapid tolerance (24). Largeconductance Ca2+-activated K+ (BK) channels, encoded by slo, participate in regulating neuronal firing patterns and neurotransmitter release. In flies, anesthetization with benzyl alcohol induces both rapid tolerance and increased expression from the slo Ca2+-activated K+ channel gene. Benzyl alcohol sedation does not evoke this response from all ion channel genes. Neither the Shaker nor seizure voltage-gated K+ channel mRNAs nor the slo muscle-specific transcript shows a significant increase in expression after sedation. Our data indicate that the sedation-induced increase in slo expression contributes to acquired benzyl alcohol tolerance.
Scholz et al. (24) have studied Drosophila rapid tolerance as induced by ethanol sedation. They characterized tolerance by measuring the time or dose necessary to sedate the animals. Sedation was defined as the point at which flies lost postural control in an inebriometer or when the flies ceased movement. By using these measures, they showed that a single bout of ethanol sedation induces rapid tolerance. They also demonstrated that a mutation interfering with octopamine biosynthesis reduced the acquisition of rapid tolerance by 50%.
In our study, we chose to use benzyl alcohol as a model solvent because it was extremely easy to deliver and was extremely well tolerated by flies. With this anesthetic, flies could be repetitively sedated without a loss of viability. For our measurements of rapid tolerance, we used a variation of the distribution test (25) in which a human observer records not the knockdown time but the period of sedation. The specific hallmark of recovery that we scored was the resumption of wall climbing. This activity means that the flies are not merely “conscious” but that they are sufficiently coordinated to implement a rather complex behavior.
We postulate that the benzyl alcohol-induced changes in slo expression reflect a homeostatic mechanism that resists untoward changes in net cellular excitability. If hyperexcitability is induced, the proposed mechanism alters channel expression to reduce this excitability, whereas if cellular excitability is suppressed, channel gene expression changes to enhance excitability. This hypothesis does not require that the activity of the slo channel be affected directly by this solvent. However, it has been shown that anesthetics and ethanol directly affect slo channel activity (27, 28). In some cells, the solvents inhibit channel activity, whereas in others they potentiate the current.
Benzyl alcohol exposure results in an initial hyperkinetic phase followed by a sedative phase. We have shown that the hyperkinetic phase reduces slo mRNA abundance and causes the drug sensitization. Conversely, the sedative phase stimulates an increase in slo expression and induces drug tolerance. We propose that decreased slo expression reduces the net excitability of the nervous system, whereas increased slo expression enhances neural excitability. Consistent with these interpretations are the observations that mutations that prevent slo expression interfere with the acquisition of tolerance, whereas artificial enhancement of slo expression with a transgene causes a tolerant-like phenotype. This observation indicates that the changes in slo channel expression make a significant contribution to behavioral tolerance to benzyl alcohol intoxication.
On a cursory consideration, these responses appear not to be homeostatic in nature but to exacerbate the changed excitability of the nervous system. That is, generically, one assumes that increased K+ channel activity would hyperpolarize the cell and reduce excitability, whereas reduced K+ channel activity would interfere with the ability of the cell to terminate an electrical impulse and thereby enhance electrical excitability. This role of slo channels is well documented (29–31). However, there is substantial evidence that slo channel activity also can affect neural excitability in the opposite manner.
Warbington et al. (32) have shown that loss of the slo (BK) current in flies leads to reduced motoneuron excitability and neurotransmitter release. It was proposed that these changes occurred because the normal role of the slo current was to ensure rapid repolarization of the nerve terminal, and, in the absence of the slo current, the increase in depolarization led to inactivation of voltage-gated Ca2+ channels. In a study in mammalian anterior pituitary cells, it has been shown that BK channel activation prolongs action potential duration, thus facilitating extracellular Ca2+ entry (33). In that study, Van Goor et al. (33) show that fast activation of BK channels by Ca2+ prevents the complete repolarization of the cell by limiting the activation of voltage-gated K+ channels. This reaction generates a plateau potential that results in high-amplitude Ca2+ transients. Additionally, Lovell and McCobb (34) have shown that an increase in the open-probability of BK channels enhances repetitive firing in chromaffin cells, presumably by limiting activity-dependent inactivation of Ca2+ and Na+ channels. Finally, Pattillo et al. (35) have found that toxin blockade of BK channels in Xenopus nerve-muscle synapse cultures significantly decreased transmitter release. They propose that this decrease occurs because of the effect of the BK current on the driving force for Ca2+ (35). In all of these examples, an increase in the instantaneous excitability of the cell leads to a reduction in long-term or net excitability. Clearly, an increase in Ca2+-activated K+ channel expression could elicit a positive rather than a negative effect on synaptic excitability, and, conversely, a reduction in slo expression could reduce neural excitability.
Regardless of the mechanism, our data clearly suggest a role for the modulation of slo gene expression in the homeostatic regulation of excitability. We do not suggest that slo expression is responsible for all facets of tolerance. Our data indicate only that, in flies, slo expression is required for the acquisition of tolerance and that increased slo expression can result in a degree of benzyl alcohol resistance. It is likely that slo is one component of an orchestrated response of transcriptional changes and posttranscriptional modifications that involves many genes.
Recently, Davies et al. (8) demonstrated that, in C. elegans, slo loss-of-function mutations cause ethanol resistance, and slo overexpression produces a phenotype that mimics ethanol intoxication. It is important to note that slo may play an opposing role in C. elegans with regard to its response to drugs. We do not believe that the source of the difference is the choice of drugs. We propose that, in Drosophila, the tolerance mounted to benzyl alcohol and ethanol are related phenomena because exposure to ethanol induces crosstolerance to benzyl alcohol (data not shown). The work in C. elegans focuses on resistance, per se, and not on acquired tolerance. It seems unlikely that the origins of resistance and acquired tolerance are sufficiently different to be influenced in opposing manners by the same gene. A study by Leibovitch et al. (6) also has suggested that there exists a positive correlation between slo expression and solvent anesthetic sensitivity. This group demonstrated that a null mutation in slo increased the sensitivity of flies to halothane, chloroform, and trichloroethylene. It is likely, therefore, that the apparent differences in the role of slo in the C. elegans and Drosophila drug response has to do with the fundamentally distinct organization of the nervous system of these animals. The most important consideration is not the differences in how C. elegans and Drosophila respond to changes in slo expression but the fact that studies in two very different organisms implicate slo as having an important role in the response to sedation.
Acknowledgments
We thank Moon Draper, Harish Krishnan, and Roshani Cowmeadow for insightful comments and criticisms. This work was supported by National Institute on Drug Abuse Grant R21 DA14560-01 and National Science Foundation Grant IBN-0114716 (both to N.S.A.) and a grant from the Waggoner Center (to A.G.).
Author contributions: A.G., Y.M.A.-H., R.A.B., and N.S.A. designed research; A.G., Y.M.A.-H., L.E.L., and R.A.B. performed research; A.G., Y.M.A.-H., and N.S.A. analyzed data; and A.G., Y.M.A.-H., and N.S.A. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dinwiddie, S. H. (1994) Addiction 89, 925–939. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan, R. J. & Ives, R. J. (1994) Bull. Narc. 46, 49–78. [PubMed] [Google Scholar]
- 3.Levitan, E. S. & Takimoto, K. (1998) J. Neurobiol. 37, 60–68. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson, N., Brenner, R., Chang, W.-m., Wilbur, J. L., Larimer, J. L. & Yu, J. (2000) J. Neurosci. 20, 2988–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriani, M. F., Hogenesch, J. B., Yanovsky, M., Panda, S., Straume, M. & Kay, S. A. (2002) J. Neurosci. 22, 9305–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leibovitch, B. A., Campbell, D. B., Krishnan, K. S. & Nash, H. A. (1995) J. Neurogenet. 10, 1–13. [DOI] [PubMed] [Google Scholar]
- 7.Peixoto, A. A. & Hall, J. C. (1998) Genetics 148, 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies, A. G., Pierce-Shimomura, J. T., Kim, H., VanHoven, M. K., Thiele, T. R., Bonci, A., Bargmann, C. I. & McIntire, S. L. (2003) Cell 115, 655–666. [DOI] [PubMed] [Google Scholar]
- 9.Becker, M. N., Brenner, R. & Atkinson, N. S. (1995) J. Neurosci. 15, 6250–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohm, R. A., Wang, B., Brenner, R. & Atkinson, N. (2000) J. Exp. Biol. 203, 693–704. [DOI] [PubMed] [Google Scholar]
- 11.Brenner, R., Thomas, T. O., Becker, M. N. & Atkinson, N. S. (1996) J. Neurosci. 16, 1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson, N. S., Brenner, R., Bohm, R. A., Yu, J. Y. & Wilbur, J. L. (1998) Ann. N.Y. Acad. Sci. 860, 296–305. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer, D. W., Lemeshow, S. & Kim, S. (2002) Applied Survival Analysis: Regression Modeling of Time to Event Data (Solutions Manual) (Wiley Interscience, New York).
- 14.Ausubel, F. M. (1994) Current Protocols in Molecular Biology (Wiley, New York).
- 15.Ashburner, M. (1989) Drosophila: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 16.Thomas, T., Wang, B., Brenner, R. & Atkinson, N. S. (1997) Invertebr. Neurosci. 2, 283–291. [DOI] [PubMed] [Google Scholar]
- 17.Ganetzky, B. & Wu, C.-F. (1983) J. Neurogenet. 1, 17–28. [DOI] [PubMed] [Google Scholar]
- 18.Jackson, F. R., Gitschier, J., Strichartz, G. R. & Hall, L. M. (1985) J. Neurosci. 5, 1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamb, A., Tseng-Crank, J. & Tanouye, M. A. (1988) Neuron 1, 421–430. [DOI] [PubMed] [Google Scholar]
- 20.Stern, M. & Ganetzky, B. (1989) J. Neurogenet. 5, 215–228. [DOI] [PubMed] [Google Scholar]
- 21.Stern, M., Kreber, R. & Ganetzky, B. (1990) Genetics 124, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The FlyBase Consortium (2003) Nucleic Acids Res. 31, 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao, J. & Nestler, E. J. (2004) Annu. Rev. Med. 55, 113–132. [DOI] [PubMed] [Google Scholar]
- 24.Scholz, H., Ramond, J., Singh, C. M. & Heberlein, U. (2000) Neuron 28, 261–271. [DOI] [PubMed] [Google Scholar]
- 25.Guan, Z., Scott, R. L. & Nash, H. A. (2000) J. Neurogenet. 14, 25–42. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson, N. S., Robertson, G. A. & Ganetzky, B. (1991) Science 253, 551–555. [DOI] [PubMed] [Google Scholar]
- 27.Denson, D. D., Worrell, R. T. & Eaton, D. C. (1996) Am. J. Physiol. 270, C636–C644. [DOI] [PubMed] [Google Scholar]
- 28.Namba, T., Ishii, T. M., Ikeda, M., Hisano, T., Itoh, T., Hirota, K., Adelman, J. P. & Fukuda, K. (2000) Eur. J. Pharmacol. 395, 95–101. [DOI] [PubMed] [Google Scholar]
- 29.Gribkoff, V. K., Starrett, J. E., Jr., & Dworetzky, S. I. (2001) Neuroscientist 7, 166–177. [DOI] [PubMed] [Google Scholar]
- 30.Orio, P., Rojas, P., Ferreira, G. & Latorre, R. (2002) News Physiol. Sci. 17, 156–161. [DOI] [PubMed] [Google Scholar]
- 31.Sun, Q. & Dale, N. (1998) J. Physiol. 507, 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warbington, L. C., Hillman, T., Adams, C. & Stern, M. (1996) Invertebr. Neurosci. 2, 51–60. [DOI] [PubMed] [Google Scholar]
- 33.Van Goor, F., Li, Y. X. & Stojilkovic, S. S. (2001) J. Neurosci. 21, 5902–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovell, P. V. & McCobb, D. P. (2001) J. Neurosci. 21, 3429–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pattillo, J. M., Yazejian, B., DiGregorio, D. A., Vergara, J. L., Grinnell, A. D. & Meriney, S. D. (2001) Neuroscience 102, 229–240. [DOI] [PubMed] [Google Scholar]