Abstract
The arrival of invasive species can devastate natural ecosystems, but the long-term effects of invasion are less clear. If native organisms can adapt to the presence of the invader, the severity of impact will decline with time. In Australia, invasive cane toads (Bufo marinus) are highly toxic to most snakes that attempt to eat them. Because snakes are gape-limited predators with strong negative allometry for head size, maximum relative prey mass (and thus, the probability of eating a toad large enough to be fatal) decreases with an increase in snake body size. Thus, the arrival of toads should exert selection on snake morphology, favoring an increase in mean body size and a decrease in relative head size. We tested these predictions with data from specimens of four species of Australian snakes, collected over >80 years. Geographic information system layers provided data on the duration of toad exposure for each snake population, as well as environmental variables (latitude, precipitation, and temperature). As predicted, two toad-vulnerable species (Pseudechis porphyriacus and Dendrelaphis punctulatus) showed a steady reduction in gape size and a steady increase in body length with time since exposure to toads. In contrast, two species at low risk from toads (Hemiaspis signata and Tropidonophis mairii) showed no consistent change in these morphological traits as a function of the duration of toad exposure. These results provide strong evidence of adaptive changes in native predators as a result of the invasion of toxic prey.
Keywords: adaptation, conservation, contemporary evolution, coevolution
Human-induced environmental change is the greatest threat to global biodiversity. Such processes include global climate change, invasive species, habitat removal, overharvesting, and altered biogeochemical cycles (1–3). These changes have caused many extinctions (local and global) and will lead to many more, but whenever the impact is nonrandom (i.e., selective), there is the potential for adaptive evolution. Under the right circumstances, adaptive evolution can happen very rapidly in wild populations. Such “contemporary evolution” (see ref. 4) occurs as a consequence of selection during natural events (e.g., refs. 5–7). Importantly, however, it has also been documented from “unnatural” (human-mediated) events. The classic example of industrial melanism in peppered moths is the most celebrated case (8, 9); however, there is also clear evidence of adaptive evolution in populations as a consequence of overfishing (10), global warming (11), and heavy-metal pollution (12).
These studies highlight the importance of examining the potential for adaptive change in impacted populations. Doing so can clarify both the nature of the impact and the response of the affected population. Clearly, a population exhibiting an adaptive response is more likely to persist in the face of an environmental change than one that fails to adapt. Invasive species are of particular interest in this respect because they are believed to constitute a major threat to global biodiversity (3, 13, 14). Although invasive species have caused extinctions (e.g., refs. 15 and 16) they may also exert nonrandom selection upon impacted species, such that natives can adapt to their presence. Although much evolutionary research has been directed toward invasive species themselves and how they adapt to new environments, much less research has been conducted on counteradaptations by native species (17, 18).
Many species of Australian snake have been severely impacted by the invasion of highly toxic cane toads (Bufo marinus), a conservation problem that also offers an ideal situation to explore the possibility of an adaptive response by natives to an invader. Cane toads were introduced into Australia in 1935. Since then, they have spread throughout large areas of Queensland and have entered the Northern Territory and New South Wales, currently occupying a range of ≈1 million km2 (19). The ecological impact of toads on the native fauna has been poorly elucidated, mainly because of logistical difficulties and a lack of baseline data for comparison (20). Nevertheless, there is a clear inference that the invasion of the toad has had a massive impact on species of Australian snakes. Toads are highly toxic, and most Australian snakes attempting to eat toads will die. A recent study suggests that 49 species of snake are potentially impacted by the toad and that the majority of these species are unable to deal with a likely dose of toad toxin (21).
However, snakes are gape-limited predators and, thus, their ability to poison themselves by consuming a toad depends on their head size (HS) relative to their body mass. Thus, within any given population, a snake with a small head relative to its body mass will be at less risk of ingesting a toad large enough to kill it than will a conspecific capable of ingesting a relatively larger toad. At an intraspecific level, two major factors influence the size of a snake's head relative to body mass: the snake's absolute size (because smaller individuals have relatively larger heads, as is generally true in most vertebrates; ref. 22) and relative HS (because even at the same body length, some individuals will have larger heads than will others). Thus, the risk of a snake consuming a toad large enough to be fatal will depend on snake body size and relative HS (unpublished data). Accordingly, we expect that the arrival of toads will impose selection on the morphology of snakes, favoring individuals with larger-than-average body sizes and smaller-than-average relative HS.
In this study, we examine morphological variation in four species of Australian snakes. Two of these taxa (one colubrid, one elapid) are predicted to face little to no impact from toads, either because they are too small to ingest a fatal dose or because they have high physiological resistance to toad toxins. The other two taxa (again, one colubrid and one elapid) are predicted to be much more vulnerable to toad invasion. We examine variation in body size and relative HS with reference to environmental variables and time since exposure to toads. We predict that mean body sizes and/or relative HSs will have changed through time since toad arrival in the toad-vulnerable species, but not in the other taxa.
Methods
Study Species and Collection of Morphological Data. To ensure phylogenetic independence, we selected distantly related taxa within each of our two categories (vulnerable and nonvulnerable species). All four study species feed primarily or exclusively on anurans, and are widely distributed through the parts of Queensland invaded by cane toads since 1935. The two highly vulnerable taxa comprised red-bellied blacksnakes (Pseudechis porphyriacus, Elapidae, n = 99) and green tree snakes (Dendrelaphis punctulatus, Colubridae, n = 242). Like most Australian snake species, these snakes are highly susceptible to toad toxins, and will die if they ingest even a relatively small toad. To consume a fatal dose of toxin, Pseudechis needs only to consume a toad whose head width is 43% of its own, and Dendrelaphis needs only consume a toad whose head width is 49% of its own (21). The two relatively nonvulnerable species were swamp snakes (Hemiaspis signata, Elapidae, n = 158) and keelbacks (Tropidonophis mairii, Colubridae, n = 124). These two taxa are less likely to be severely impacted by cane toads. Hemiaspis is a small species with an unusually small head, and hence consumes only very small anurans, even relative to its own body mass; to consume a fatal dose it would need to ingest a toad that is 108% of its head-width (21). In contrast, Tropidonophis is a larger species with a normal-sized head, but has an unusually high physiological tolerance for toad toxins; to consume a fatal dose, this species would need to consume a toad that is 185% of its head-width, and so feeds readily on toads without severe ill effects (21, 23).
We collected morphological data from preserved specimens held at the Queensland Museum. Toads have populated >60% of Queensland, and all sampled animals came from areas where toads have colonized. We sampled the entire specimen collection for each of our species, except Tropidonophis (for which we took a random sample amounting to ≈50% of the entire collection for the species). Thus, our sampling spanned the entire known geographic range of each species in Queensland. We selected these taxa based on their wide phylogenetic separation, abundance, and differential vulnerability to toads. Each specimen was measured for snout-vent length (SVL), head length (angle of jaw to tip of snout), head width (across the head at the junction of supraoculars and parietals), and gape width (across head at last supralabial). SVL was measured to the nearest centimeter with a flexible tape, and all head measurements were taken to the nearest 0.1 mm by using dial callipers. Data on the date and locality of collection for each specimen were taken from the museum register.
Collection of Climatic Data. To minimize the chance of a spurious correlation as a consequence of spatial autocorrelation and to increase our chance of detecting an effect by reducing error variances, we included the effect of climate and latitude on snake morphology in our analyses. We derived two climatic layers for Australia by using the program anuclim (24) and a digital elevation model of Australia with 0.05° grid cells. Snake locality data were laid over the resultant climate grids in arcview. The climatic data for each locality were extracted by using the arcview extension bioclimav (25). We used two climatic variables that are likely to influence snake morphology: annual mean temperature and annual precipitation.
Collection of Data on Duration of Exposure to Toads. More than 2,000 records of toad locality and date were available from the Queensland Museum and from the data set collected by Floyd et al. (26). Sabath et al. (27) and Easteal et al. (28) used the latter data set to map the spread of toads in Australia. However, the results were hand-drawn maps of the toad distribution at 5-year intervals. Improvements in mapping tools since then (i.e., geographic information systems, GIS) allow us to create a single digital map of far greater accuracy, which can be used to provide information on the toad's range expansion at yearly intervals.
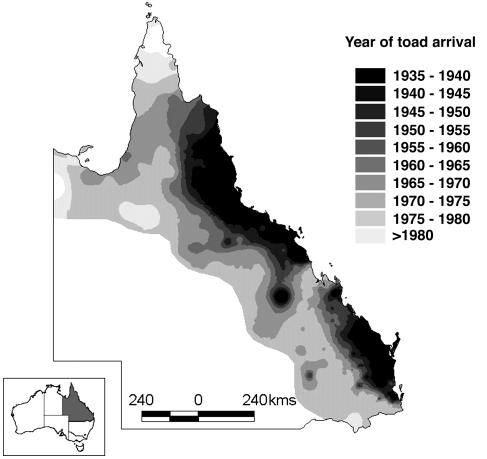
We used linear interpolation of locality dates in arcview to derive a layer describing the arrival date of toads. To do this, we plotted toad locality data and identified the earliest record of toads at each site by cumulatively stepping through the data set at 2-year intervals beginning at 1935 (the year that toads were released in Queensland). Minimum area polygons were drawn around records selected at each step, and records with a later date inside each of these polygons were deleted. After this process, we used a linear spline to create a surface describing the timing of toad arrival throughout Queensland. The resulting surface is shown in Fig. 1.
Fig. 1.
GIS layer describing the timing of the cane toad invasion in Queensland, Australia. The extreme western edge of the distribution follows the extent of distribution records in Queensland and may not accurately reflect the actual invasion extent. Data are from Floyd et al. (26) and the specimen register of the Queensland Museum.
After the derivation of this surface, snake locality records were plotted and the year of toad arrival at each site was extracted by using a spatial join. For each measured snake, we subtracted the year of toad arrival (from the GIS layer) from the collection year (from the Queensland Museum database) to yield exposure time (ET), that is, the number of years a population of snakes had been exposed to toads at the time a snake was collected. Negative values for ET (populations that were toad-naïve at the time of collection) were converted to zero values. Fortuitously, toads were introduced at three separate localities along the Queensland coast. This fact, coupled with haphazard collection by the museum, resulted in very low spatial autocorrelation in the ET variable (e.g., the magnitude of the correlation between ET and latitude was <0.23 in all instances).
Data Analysis. Our primary interest lay in determining the effect of the presence of toads on body size and relative HS in snakes. However, other variables doubtless also influence snake morphology and, thus, we need to incorporate them into our analyses to reduce spurious correlations and so that we can focus on the residual variance, that potentially explicable by the time since toad arrival. We predicted that latitude, annual mean temperature, and annual precipitation may all influence snake morphology, and so we included these, along with ET, as variables in a multiple regression. However, climatic and latitudinal variables were correlated to varying extents, so for each species we calculated the first two principal components of climatic and latitudinal variables (PC1 and PC2) and used these as independent variables in our analysis. Two analyses were run for each species. The first used SVL as the dependent variable and the second used snake HS. HS was calculated as the first principal component of the three HS variables we measured (head length, head width, and gape width). The multiple regression for snake HS also included snake body size (SVL) as a fixed independent variable because we were interested in changes in relative HS. In all cases, correlations between independent variables in the multiple regression were low. We log-transformed all morphometric variables and the exposure time variable was mean-centered (y′ = y – ȳ) before analysis (principal components are already mean-centered). Mean-centering (such that the new mean is zero) ensures that estimated coefficients are informative even in the presence of interactions; this method also reduces colinearity between variables and their interaction terms (29).
With three independent, nonfixed variables, we had seven combinations of primary variables that could produce a model (ignoring interaction terms). Because we had no a priori knowledge about how each variable would affect snake morphology and because the total number of models was small, we ran each of these combinations as a full model and deleted interaction terms if P values indicated they were not significant (i.e., P > 0.05). To make model exploration and interpretation tractable, we only considered first-order interactions. Thus, for each combination of primary variables, we derived the most parsimonious reduced model and we calculated the Akaike information criterion (AIC) value and Akaike weight (wi) for this model. We collected the best set of models for each species and each independent variable based upon these AIC values, with models <2 units from the best model (i.e., Δi < 2) retained within the best set (30). All statistical analysis was performed in jmp version 5.01 (31).
Some of the models thus selected contained interaction terms. Our primary interest was whether exposure time to toads was an important influence on snake morphology and, if so, the direction of the effect. The presence of interaction terms complicates the interpretation of main effects because the partial coefficient for the main effect of interest depends on the values of other variables. Mean-centering causes the main effect coefficient to be calculated for the mean value of interacting variables. However, in all models with interaction terms affecting exposure time, we also calculated a range of coefficients for exposure time by using values for the interacting variables that were two standard deviations above and below their mean.
Results
Principal Components. Principal components of climate and latitude for each species are shown in Table 1. Although results varied between specific data sets, PC1 appears to capture most of the variation caused by latitude with PC2, accounting for most of the residual variation in precipitation and temperature. PC1 and PC2 accounted for between 89% and 97% of the variance in the three input variables.
Table 1. The first two principal components of climatic and latitudinal variables for each snake species.
| Principal component
|
Cumulative percent
|
Eigenvectors
|
||||
|---|---|---|---|---|---|---|
| Species | Eigenvalue | AMT | Aprecip | DecLat | ||
| Hemiaspis | PC1 | 1.88 | 62.69 | 0.49 | 0.57 | -0.66 |
| PC2 | 0.81 | 89.83 | 0.79 | -0.60 | 0.07 | |
| Pseudechis | PC1 | 2.14 | 71.35 | 0.55 | 0.58 | -0.60 |
| PC2 | 0.53 | 88.86 | 0.80 | -0.58 | 0.17 | |
| Dendrelaphis | PC1 | 2.03 | 67.63 | 0.66 | 0.32 | -0.68 |
| PC2 | 0.89 | 97.15 | -0.28 | 0.94 | 0.18 | |
| Tropidonophis | PC1 | 1.99 | 66.17 | 0.66 | 0.30 | -0.69 |
| PC2 | 0.92 | 96.71 | -0.29 | 0.95 | 0.13 | |
Principal components were constructed from three raw variables: annual mean temperature (AMT), annual precipitation (Aprecip), and decimal latitude (DecLat). Cumulative percent describes the cumulative percentage of the total variance captured by the principal components.
Snake Body Size (SVL). Our models of SVL suggest that, in most species, this trait was influenced by all three of the independent variables that we used (Table 2). At least one independent variable had a significant effect on snake body size for all species examined except Tropidonophis. Models for Tropidonophis exhibited low Akaike weights and very low r2 values, indicating that our independent variables explained negligible variance in body size for this species.
Table 2. Parameter estimates for best model sets for multiple regression analyses of body size (SVL) in four species of Australian snake.
| Species | r2 | Δi | wi | Intercept | ET | ET range | PC1 | PC2 | Interactions |
|---|---|---|---|---|---|---|---|---|---|
| Pseudechis | 0.196 | 0 | 0.49 | 0.007 | 0.016 | 0.004-0.028 | -0.092 | ET*PC2, 0.0083 | |
| 0.153 | 0.85 | 0.32 | 0.001 | 0.014 | |||||
| Dendrelaphis | 0.077 | 0.00 | 0.64 | -0.003 | 0.006 | 0.048 | |||
| 0.077 | 1.95 | 0.24 | -0.003 | 0.006 | 0.048 | 0.007 | |||
| Hemiaspis | 0.054 | 0.00 | 0.46 | 0.002 | -0.089 | ||||
| 0.058 | 1.38 | 0.23 | 0.002 | -0.016 | -0.090 | ||||
| 0.054 | 1.96 | 0.17 | 0.003 | 0.002 | -0.029 | -0.092 | |||
| Tropidonophis | 0.014 | 0.00 | 0.27 | 0.007 | -0.042 | ||||
| 0.023 | 0.85 | 0.18 | 0.006 | -0.004 | -0.049 | ||||
| 0.006 | 0.85 | 0.18 | 0.005 | -0.003 | |||||
| 0.000 | 1.53 | 0.13 | 0.006 | 0.008 | |||||
| 0.014 | 1.97 | 0.10 | 0.007 | -0.042 | 0.008 |
Three independent variables were used: time since exposure to toads (ET) and two principal components (PC1 and PC2) incorporating data on latitude, annual mean temperature, and annual precipitation. Species are listed in order of decreasing susceptibility to toads. Parameter estimates significantly different from zero are shown in bold. wi is the Akaike weight of each model, and Δi refers to the change in Akaike information criterion value from the best model.
Time since exposure to toads appeared in the best model set for all species, but only indicated a significant effect in Pseudechis and Dendrelaphis. In both of these cases, the effect of ET on snake body size was positive, indicating that these species (the two most vulnerable to toads) increase in average size with increasing ET. An interaction between ET and PC2 acts as a modifier to the partial coefficient of ET for Pseudechis, but within the bounds defined by 0 ± 2σPC2, the partial coefficient did not change sign, remaining positive.
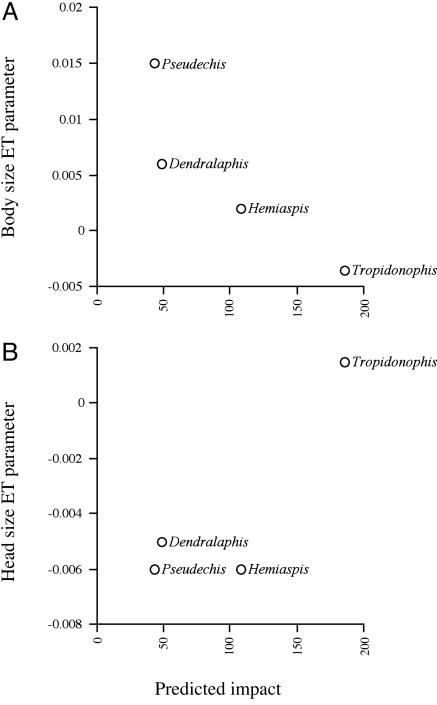
Across all four species, the predicted impact from toads (i.e., the relative size of toad that each species would need to consume to ingest a lethal dose, see Methods) was negatively correlated with the mean coefficient for the effect of ET on SVL (r = –0.89, n = 4, not significant; or r = –1.00, n = 3, P = 0.005 if Pseudechis is excluded because of the interaction between ET and PC1; Fig. 2). This result indicates that snake species at greater risk from toads showed more rapid shifts in body size after toad arrival.
Fig. 2.
Parameter estimates describing the rate of change in body and HS for each snake species, plotted against the predicted impact from toads (from ref. 21). See text for statistical tests.
Snake Relative HS. ET and PC1 contributed significantly to variation in snake relative HS in several of our study species (Table 3). Models for Tropidonophis again showed very low Akaike weights (none of the eight models were notably better than the others), suggesting that none of the independent variables explained much variation in relative HS in this species. In the remaining three species, ET had a significant negative effect on relative HS. However, for Hemiaspis, this negative effect became positive at low values of PC1, as a consequence of the interaction between these terms. Thus, we are left with an unequivocally negative effect for only two species, Pseudechis and Dendrelaphis, the two facing the highest impact from toads. For these species, relative HS decreased with time since exposure to toads.
Table 3. Parameter estimates for best model sets for multiple regression analyses of HS in four species of Australian snake.
| Species | r2 | Δi | wi | Intercept | SVL | ET | ET Range | PC1 | PC2 | Interactions |
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudechis | 0.9569 | 0.00 | 0.26 | 0.040 | 3.040 | -0.005 | -0.081 | |||
| 0.9556 | 0.46 | 0.20 | 0.041 | 3.049 | -0.005 | |||||
| 0.9575 | 0.83 | 0.17 | 0.033 | 3.034 | -0.007 | 0.036 | -0.087 | |||
| 0.956 | 1.69 | 0.11 | 0.039 | 3.045 | -0.007 | 0.030 | ||||
| 0.9548 | 1.91 | 0.10 | 0.042 | -0.079 | ||||||
| Dendrelaphis | 0.9233 | 0.00 | 0.77 | 0.016 | 3.937 | -0.005 | -0.194 | 0.012 | PC1*PC2, 0.0526 | |
| Hemiaspis | 0.9402 | 0.00 | 0.71 | -0.295 | 5.267 | -0.006 | -0.017-0.006 | 0.030 | ET*PC1, 0.0042 | |
| 0.9402 | 1.97 | 0.26 | -0.293 | 5.263 | -0.006 | -0.016-0.006 | 0.031 | -0.007 | ET*PC1, 0.0041 | |
| Tropidonophis | 0.9485 | 0.00 | 0.19 | -0.049 | 3.201 | 0.043 | 0.034 | PC1*PC2, -0.0655 | ||
| 0.9455 | 0.07 | 0.18 | -0.051 | 3.175 | ||||||
| 0.9464 | 0.35 | 0.16 | -0.052 | 3.187 | 0.035 | |||||
| 0.9491 | 0.56 | 0.14 | -0.033 | 3.193 | 0.002 | -0.013-0.017 | 0.046 | 0.007 | ET*PC1, -0.0056 | |
| 0.9477 | 1.55 | 0.09 | -0.001 | 3.176 | 0.001 | -0.012-0.016 | 0.012 | ET*PC1, -0.0050 | ||
| 0.9467 | 1.67 | 0.08 | -0.050 | 3.193 | 0.002 | 0.039 | ||||
| 0.9457 | 1.76 | 0.08 | -0.049 | 3.178 | 0.001 | |||||
| 0.9456 | 1.99 | 0.07 | -0.051 | 3.175 | 0.011 |
Snake body size (SVL) is included as a fixed independent variable as we are concerned with relative HS. Three independent variables were used: time since exposure to toads (ET) and two principal components (PC1 and PC2) incorporating data on latitude, annual mean temperature, and annual precipitation. Species are listed in order of decreasing susceptibility to toads. Parameter estimates significantly different from zero are shown in bold. wi is the Akaike weight of each model, and Δi refers to the change in Akaike information criterion value from the best model.
Across all species, the predicted impact from toads was correlated with the coefficient for the effect of ET on relative HS (r = 0.73, n = 4, not significant; or r = 0.99, n = 3, P = 0.055 if Hemiaspis is excluded because fo the interaction between ET and PC2; Fig. 2), indicating that the relative impact of toads also affects the rate of response in relative HS.
Discussion
The results of our modeling strongly support the prediction that Australian snakes will display morphological adaptations that reduce their vulnerability to cane toads. The duration of exposure to toads was significantly associated with changes in mean body size and relative HS in the two snake species that were identified (from previous work) as being extremely vulnerable to toads. Importantly, the changes occurring since toads arrived were in the directions predicted by our hypothesis of size-dependent vulnerability (i.e., mean body sizes have increased, and relative HSs have decreased). In contrast, the two taxa that we identified as being less vulnerable to toads showed fewer (or no) significant changes in morphology associated with the presence of these toxic anurans. Our modeling suggested that exposure to toads may influence HS for one of these species, but the exact nature of any such effect remains obscure. There was much less ambiguity about associations between morphology and the duration of exposure to toads in the two toad-vulnerable species.
Furthermore, the rate of change in morphology as a consequence of exposure to toads appears to be correlated with the predicted level of impact for each species. This is exactly what we would expect if the rate of response to toads was driven by the strength of selection imposed by toads, all else being equal.
In the two toad-vulnerable species (Dendrelaphis and Pseudechis), there has been a significant increase in mean body size in populations sympatric with toads. Because small individuals face a much higher risk of fatal poisoning by toads, these shifts through time likely result from an ongoing loss of small individuals from toad-exposed populations. At its simplest, this effect may be the product of consistently high mortality rates among juvenile snakes in each generation, such that the population structure in toad-exposed areas is shifted toward larger, older animals. This would imply strong selection against small body size, but not necessarily longer-term adaptation. Another possibility is that the presence of toads elicits a developmental response (such as increased growth rate through increased food availability) leading to fewer small individuals. The final possibility is that toads have exerted significant selection on life-history tactics of the snakes, such that populations in toad-exposed areas now produce larger (and presumably, fewer) offspring per clutch, or the young snakes (independent of changes food availability) grow more rapidly to a size at which they become less vulnerable to toads.
Similar ambiguity in interpretation also occurs with the causal processes responsible for changes in relative HS. Both the “vulnerable” species (Dendrelaphis and Pseudechis), as predicted, showed a significant decrease in HS associated with time since exposure to toads. This decrease could be due either to an ongoing impact and an adaptive response to that impact or, alternatively, to developmental changes in head growth associated with dietary change subsequent to the arrival of toads. Although early studies reported that relative HS in snakes were not developmentally plastic with respect to temperature (32, 33), recent studies provide evidence that relative HS in snakes can shift as a consequence of differences in mean prey size (34–36).
Although we have no direct data to distinguish between these two scenarios (selection versus plasticity), our data argue against an indirect environmental effect. Because the effect of ET across species is strongly related to the likelihood of a species ingesting a toad large enough to kill, we can be confident that the observed effect is a consequence of a direct interaction between toads and snakes. In other words, the morphological effect is not driven by changes in prey abundance, prey size, or other indirect environmental effects. Therefore, the morphological changes must be a consequence of (and probably also a response to) selection against small bodies and large heads.
If we accept that the morphological changes observed are a consequence of selection, then the obvious corollary is whether this selection is resulting in evolutionary change. Although there are a number of reasons why populations might not respond to selection (37), the simplest is that there is insufficient heritable variation at the traits of interest. To be confident that the effect observed here is an evolved response to selection we would need to understand the heritability of HS and body size (particularly offspring size) in our species. Although no such estimates are available, there is almost always heritable variance for life-history and morphological traits within populations (ref. 38, although see ref. 39 for an exception). For example, recent work by Sinervo and Doughty (40) showed a very high heritability (0.62) for egg size (i.e., offspring size) in a species of lizard, and egg size in birds typically shows high levels of heritability also (>0.5, ref. 41). Morphological traits (e.g., HS) tend to have heritabilities of ≈0.4 (38). Thus, it seems unlikely that snake populations would fail to respond to this selection due to a lack of heritable variation.
Additionally, the relatively short generation time of these snake species (<3 years, ref. 42) allows >20 generations to have elapsed since initial exposure to toads in some areas. These facts suggest that offspring size and HS are likely to have had both the time and lability to exhibit evolved change. Further research on possible life-history shifts in toad-exposed predator populations would be of great interest.
The general approach outlined here, using a combination of museum time-series and spatial data, could be used to assess morphological change in any species provided that it is adequately represented in collections and the spatial timing of the change can be mapped. This observation highlights not only the relative ease with which impacts of and responses to environmental change can be assessed when the relevant data are available, but also the importance of museums as storehouses for specimen series that can be used to examine temporal processes.
The data presented here indicate an adaptive response by populations impacted by an invasive species. As such, these results can be added to a growing list of studies suggesting rapid adaptation associated with environmental change. Furthermore, this study demonstrates adaptive change in response to impacts from an invasive species (to our knowledge, ref. 43 is the only other study to report this). Clearly, the potential for impacted populations to adapt needs to be considered when assessing long-term impacts of environmental change. Assessing the possibility and extent of an impact associated with an environmental change is a useful first step (e.g., ref. 44), but the next logical step is to assess the potential for impacted species to adapt. Without such information, we cannot predict the long-term consequences of environmental change.
Acknowledgments
We thank Patrick Couper, Andrew Amey, and Heather Janetski at the Queensland Museum for access to specimens and many cups of tea. We are also grateful for the GIS advice and technical assistance provided by Adnan Moussalli and Michael Kearney. Previous drafts were improved by comments from Ary Hoffmann, Greg Brown, and Jon Webb. This work was supported by the Australian Research Council.
Author contributions: B.L.P. designed research; B.L.P. performed research; B.L.P. and R.S. analyzed data; and B.L.P. and R.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HS, head size; SVL, snout-vent length; GIS, geographic information system; ET, exposure time; PCn, principal component n.
References
- 1.Chapin, F. S., Zavaleta, E. S., Eviner, V. T., Naylor, R. L., Vitousek, P. M., Reynolds, H. L., Hooper, D. U., Lavorel, S., Sala, O. E., Hobbie, S. E., et al. (2000) Nature 405, 234–242. [DOI] [PubMed] [Google Scholar]
- 2.Novacek, M. J. & Cleland, E. E. (2001) Proc. Natl. Acad. Sci. USA 98, 5466–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond, J. M. (1989) in Conservation for the 21st Century, eds. Western, D. & Pearl, M. C. (Oxford Univ. Press, Oxford), pp. 37–41.
- 4.Stockwell, C. A., Hendry, A. P. & Kinnison, M. T. (2003) Trends Ecol. Evol. 18, 94–101. [Google Scholar]
- 5.Reznick, D., Shaw, F., Rodd, F. & Shaw, R. (1996) Science 275, 1934–1937. [DOI] [PubMed] [Google Scholar]
- 6.Higgie, M., Chenoweth, S. & Blows, M. W. (2000) Science 290, 519–521. [DOI] [PubMed] [Google Scholar]
- 7.Grant, P. R. & Grant, B. R. (2002) Science 296, 707–711. [DOI] [PubMed] [Google Scholar]
- 8.Kettlewell, B. (1973) The Evolution of Melanism (Clarendon, Oxford).
- 9.Grant, B. S. (1999) Evolution (Lawrence, Kans.) 53, 980–984. [Google Scholar]
- 10.Olsen, E. M., Heino, M., Lilly, G. R., Morgan, M. J., Brattey, J., Ernande, B. & Dieckmann, U. (2004) Nature 428, 932–935. [DOI] [PubMed] [Google Scholar]
- 11.Bradshaw, W. E. & Holzapfel, C. M. (2001) Proc. Natl. Acad. Sci. USA 98, 14509–14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macnair, M. (1987) Trends Ecol. Evol. 2, 354–359. [DOI] [PubMed] [Google Scholar]
- 13.The World Conservation Union (2001) Aliens 12, lift-out booklet, www.issg.org/booklet.pdf.
- 14.Williamson, M. (1999) Ecography 22, 5–12. [Google Scholar]
- 15.Ogutu-Ohwayo, R. (1999) in Invasive Species and Biodiversity Management, eds. Sandlund, O. T., Schei, P. J. & Viken, A. (Kluwer Academic, Boston), pp. 47–64.
- 16.Fritts, T. H. & Rodda, G. H. (1998) Annu. Rev. Ecol. Syst. 29, 113–140. [Google Scholar]
- 17.Lee, C. E. (2002) Trends Ecol. Evol. 17, 386–391. [Google Scholar]
- 18.D'Antonio, C. M. & Kark, S. (2002) Trends Ecol. Evol. 17, 202–204. [Google Scholar]
- 19.Lever, C. (2001) The Cane Toad: The History and Ecology of a Successful Colonist (Westbury Academic and Scientific, Yorkshire, U.K.).
- 20.van Dam, R. A., Walden, D. J. & Begg, G. W. (2002) A Preliminary Risk Assessment of Cane Toads in Kakadu National Park (Supervising Scientist, Darwin, Northern Territory, Australia), Scientist Report 164.
- 21.Phillips, B. L., Brown, G. P. & Shine, R. (2003) Conserv. Biol. 17, 1738–1747. [Google Scholar]
- 22.Calder, W. A., III (1984) Size, Function and Life History (Harvard Univ. Press, Cambridge, MA).
- 23.Covacevich, J. & Archer, M. (1975) Mem. Queensland Mus. 17, 305–310. [Google Scholar]
- 24.Hutchinson, M. F., Houlder, D. J., Nix, H. A. & McMahon, J. P. (1999) anuclimVersion 5.1 User's Guide (Centre for Resource and Environmental Studies, Australian National University, Canberra).
- 25.Moussalli, A. (2003) bioclimavVersion 1.0 User's Guide (Cooperative Research Centre for Tropical Rainforest Ecology and Management, Brisbane, Australia).
- 26.Floyd, R. B., Boughton, W. C., Easteal, S., Sabath, M. D. & van Beurden, E. K. (1981) The Distribution Records of the Marine Toad (Bufo marinus) (Griffith University, Brisbane, Australia), Vol. 1.
- 27.Sabath, M. D., Boughton, W. C. & Easteal, S. (1981) Copeia 1981, 676–680. [Google Scholar]
- 28.Easteal, S., Van Beurden, E. K., Floyd, R. B. & Sabath, M. D. (1985) J. Herpetol. 19, 185–188. [Google Scholar]
- 29.Jaccard, J. & Turrisi, R. (2003) Interaction Effects in Multiple Regression (Sage, Thousand Oaks, CA).
- 30.Burnham, K. P. & Anderson, D. R. (2001) Wildlife Res. 28, 111–119. [Google Scholar]
- 31.SAS Institute (2002) jmp (SAS Institute, Cary, IN), Version 5.01.
- 32.Forsman, A. (1996) Evolution (Lawrence, Kans.) 50, 2536–2542. [DOI] [PubMed] [Google Scholar]
- 33.Arnold, S. J. & Peterson, C. R. (1989) Physiol. Zool. 62, 1316–1333. [Google Scholar]
- 34.Bonnet, X., Shine, R., Naulleau, G. & Thiburce, C. (2001) J. Zool. 255, 341–351. [Google Scholar]
- 35.Queraz-Regal, A. & King, R. B. (1998) Copeia 1998, 423–429. [Google Scholar]
- 36.Aubret, F., Shine, R. & Bonnet, X. (2004) Nature 431, 261–262. [DOI] [PubMed] [Google Scholar]
- 37.Merila, J., Sheldon, B. C. & Kruuk, L. E. B. (2001) Genetica 112, 199–222. [PubMed] [Google Scholar]
- 38.Roff, D. A. (1997) Evolutionary Quantitative Genetics (Chapman and Hall, New York).
- 39.Hoffmann, A. A., Hallas, R. J., Dean, J. A. & Schiffer, M. (2003) Science 301, 100–102. [DOI] [PubMed] [Google Scholar]
- 40.Sinervo, B. & Doughty, P. (1996) Evolution (Lawrence, Kans.) 50, 1314–1327. [DOI] [PubMed] [Google Scholar]
- 41.Christians, J. K. (2002) Biol. Rev. 77, 1–26. [DOI] [PubMed] [Google Scholar]
- 42.Shine, R. (1978) Herpetologica 34, 73–79. [Google Scholar]
- 43.Kiesecker, J. M. & Blaustein, A. M. (1997) Ecology 78, 1752–1760. [Google Scholar]
- 44.Thomas, C. D., Cameron, A., Green, R. E., Bakkenes, M., Beaumont, L. J., Collingham, Y. C., Erasmus, B. F. N., Ferreira de Siquera, M., Grainger, A., Hannah, L., et al. (2004) Nature 427, 145–148. [DOI] [PubMed] [Google Scholar]