Abstract
Aims
Although cardiac resynchronization therapy (CRT) is effective in patients with systolic heart failure (HF) and a wide QRS interval, a substantial proportion of patients remain non-responsive. The SonR contractility sensor embedded in the right atrial lead enables individualized automatic optimization of the atrioventricular (AV) and interventricular (VV) timings. The RESPOND-CRT study investigated the safety and efficacy of the contractility sensor system in HF patients undergoing CRT.
Methods and results
RESPOND-CRT was a prospective, randomized, double-blinded, multicentre, non-inferiority trial. Patients were randomized (2:1, respectively) to receive weekly, automatic CRT optimization with SonR vs. an Echo-guided optimization of AV and VV timings. The primary efficacy endpoint was the rate of clinical responders (patients alive, without adjudicated HF-related events, with improvement in New York Heart Association class or quality of life), at 12 months. The study randomized 998 patients. Responder rates were 75.0% in the SonR arm and 70.4% in the Echo arm (mean difference, 4.6%; 95% CI, −1.4% to 10.6%; P < 0.001 for non-inferiority margin −10.0%) (Table 2). At an overall mean follow-up of 548 ± 190 days SonR was associated with a 35% risk reduction in HF hospitalization (hazard ratio, 0.65; 95% CI, 0.46–0.92; log-rank P = 0.01).
Conclusion
Automatic AV and VV optimization using the contractility sensor was safe and as effective as Echo-guided AV and VV optimization in increasing response to CRT.
ClinicalTrials.gov number
Keywords: SonR, AV and VV optimization, CRT optimization, Cardiac contractility, RESPOND, HF hospitalization, Adaptive CRT
Introduction
Cardiac resynchronization therapy (CRT) is an effective and established therapy for patients with medically refractory heart failure (HF), left ventricular (LV) systolic dysfunction (ejection fraction ≤ 35%), and a wide QRS complex.1 CRT improves the functional class and quality of life (QoL) and remodels the heart favourably, while reducing the rates of HF hospitalization and overall mortality in this large subgroup of HF patients.2,3 Despite these salutary effects of CRT, a significant minority (∼30%) of patients remain non-responsive to this therapeutic modality.4 Amongst several factors that could adversely affect response, suboptimal optimization of the atrioventricular (AV) and interventricular (VV) timings of the CRT device constitute the commonest and most supposed correctable variable.5 Several studies have demonstrated the acute haemodynamic benefits of Echo-guided AV and VV timings optimization.6
Of note, Echo-guided optimization remains a logistically challenging and a resource-intensive process, with the programming parameters measured at rest and in the supine position.7 There is an unmet need for an individualized, device-based strategy that can automatically optimize the AV and VV electrical timings, on a repetitive basis during rest and exercise. Novel technology using the SonR contractility sensor enables this automatic optimization.8 Several studies have shown that cardiac contraction generates mechanical vibrations that propagate through the entire heart. The sensor records these as SonR signals that correlate strongly with LV dP/dtmax, a measure of cardiac contractility.9,10
A pilot study (CLEAR) assessing this contractility sensor within the right ventricular lead of a CRT-pacemaker implant showed a trend to improvement in clinical outcomes.8 Built off this preliminary data, a new dedicated right atrial lead with an embedded sealed sensor was evaluated in this large, prospective, double-blind, randomized non-inferiority clinical trial in patients eligible for implantation of a CRT-D device.11 The RESPOND-CRT study investigated the safety and efficacy of the contractility sensor system in HF patients undergoing CRT.
Methods
Study design and oversight
The Clinical Trial of the SonRtip lead and automatic AV-VV optimization (RESPOND-CRT) was a prospective, multicentre, randomized, double-blind, non-inferiority trial. The study was designed by a Steering Committee and the sponsor (LivaNova), approved by the Food and Drugs Administration (FDA) as an Investigational Device Exemption (IDE) study, and has been described previously.11 An independent Clinical Events Committee reviewed all relevant events and an independent Data Safety Monitoring Board regularly reviewed the safety data (see Supplementary material online).
The last author (J.P.S.) wrote the first draft of the manuscript, which was revised and approved by all co-authors. The authors accept full responsibility for the accuracy and completeness of the data and all analyses, and for the fidelity of this report of the trial protocol.
Study population and intervention
The trial enrolled patients with clinical indications for implantation of a de novo CRT defibrillator (with or without a pre-existing implantable cardioverter-defibrillator or pacemaker), according to current guidelines.12,13 The study was approved by local ethics committees (investigational review boards and health authorities). Patients provided written informed consent.
The main inclusion criteria were New York Heart Association functional class III or ambulatory IV, LV ejection fraction ≤ 35%, sinus rhythm, and QRS complex ≥ 120 ms of left bundle-branch block (LBBB) morphology, or QRS complex > 150 ms in non-LBBB. The complete list of study criteria is provided in the Supplementary material online.
Patients underwent full echocardiographic evaluation, and a global clinical status (NYHA) assessment and quality-of-life (QoL) questionnaire using the Kansas City Cardiomyopathy Questionnaire14. Patients were then implanted with a CRT-defibrillator device (PARADYM RF SonR 9770, LivaNova), connected to a straight, active fixation bipolar atrial pacing lead (SonRtip, LivaNova). Within 14 days of a successful implant, patients were randomized (2:1, respectively) to weekly automatic AV and VV delay optimization with SonR or to Echo-guided optimization (Echo), using a mandatory standardized protocol (AV timing was optimized using the iterative method, and the VV timing was set up through the aortic velocity time integral measurement). Further details on the optimization procedures are provided in the Supplementary material online. Clinical status evaluation and QoL questionnaire administration were performed 3, 6, and 12 months postimplant by clinicians blinded to the randomization assignment. A second echocardiogram was performed at 12 months. Measurements were reviewed by a centralized independent core laboratory (see Supplementary material online).
Device under investigation
During cardiac contraction the myocardium generates mechanical vibrations that propagate throughout the heart. The SonR sensor records an endocardial acceleration signal corresponding to these vibrations. The highest amplitude of the signal occurs during the isovolumetric contraction phase of the cardiac cycle and corresponds to the cardiac contractility.15 The correlation between the amplitude of the recorded signal and LV dP/dtmax, as a surrogate of the contractile function of the heart, has been demonstrated.16 The signal amplitude corresponds to the first heart sound and is an index of systolic function.10 The sensor is embedded in the tip of a bipolar, active fixation, right atrial pacing lead (SonRtip) (Figure 1). The system composed of the lead and the CRT-D device automatically adjusts the AV and VV delays, on a weekly basis, at rest and exercise.17 Concordance between echocardiographic methods and this device-based method has been demonstrated.18
Figure 1.
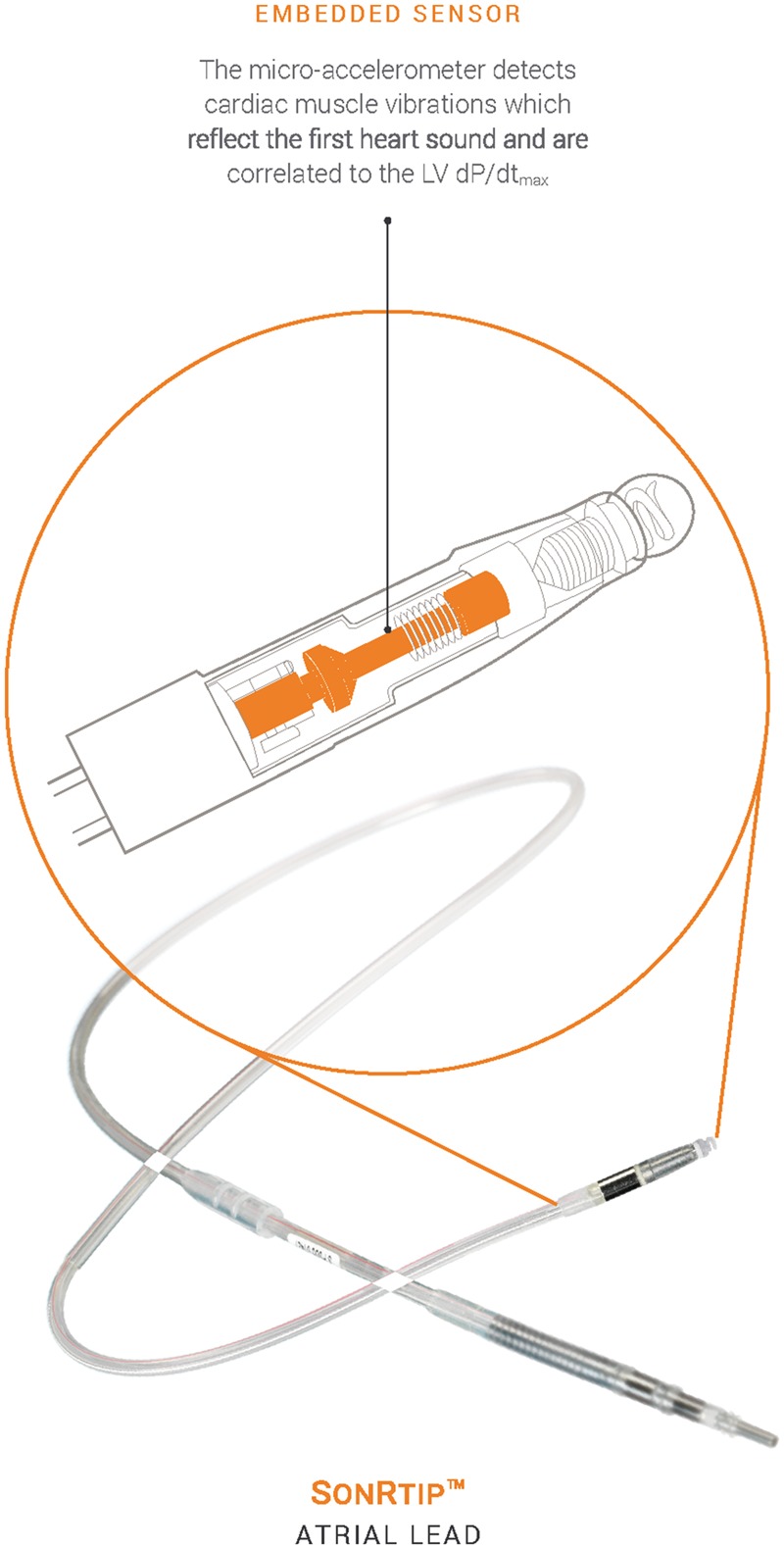
SonRtip lead.
Study outcomes
The primary efficacy endpoint was the rate of clinical responders (comprising a nested composite of patients alive, without adjudicated HF-related events, with improvement in New York Heart Association (NYHA) class improvement of ≥1 level or QoL improvement of at least 5 points, at 12 months). NYHA and QoL were assessed at 3, 6 and 12 months. Primary safety endpoints were freedom from acute (0–3 months) and chronic (3–12 months) atrial lead-related complications. All events (including HF-related events and lead-related complications, and other serious adverse events) were adjudicated by the blinded Clinical Events Committee. Secondary endpoints included the rate of clinically worsened patients and the composite of all-cause mortality or HF hospitalization. Pre-specified ancillary analysis was conducted on all data available at the time of database lock on all-cause mortality or HF hospitalization and its component, HF hospitalization. Left ventricular ejection fraction and LV end-systolic volumes were ancillary endpoints. Full details on endpoint definition are provided in the Supplementary material online.
Statistical analysis
The sample size determination is detailed in the Supplementary material online.
For the analysis of the primary efficacy endpoint, the status of the patient towards hard endpoints (mortality or HF-related events) had to be known at 12 months. For NYHA and QoL, 3- or 6-month evaluations would be used if the 12-month assessment was not available (Lost Observation Carried Forward method).
Non-inferiority on primary CRT efficacy endpoint was tested using a one-sided Z-test of two binomial proportions with an alpha level of 0.025, and a −10.0% pre-defined absolute non-inferiority margin.8
All efficacy analyses were conducted on the modified intention-to-treat (mITT) population which included all randomized patients successfully implanted with the full entire system with an evaluable primary efficacy endpoint data.
A method of worst case data imputation was used to address missing values. The patients lost to follow-up or with missing data at 12 months were imputed as non-responders.
The primary lead safety endpoints: rate of freedom from acute and rate of freedom from chronic lead complications were compared with 91%19 and 94%20 pre-specified values, respectively, using a one-sided exact test of one binomial proportion at an alpha level of 0.025.
The safety analyses were performed on the safety population which included all patients successfully implanted with SonRtip lead and patients with SonRtip lead fracture, insulation breach or perforation occurring during the implant procedure, regardless successful implantation.
If all statistical tests were passed, the secondary CRT efficacy endpoints, rate of clinically worsened patients, and the rate of composite of all-cause mortality or HF hospitalization were tested using a −10.0% absolute non-inferiority margin.
The pre-specified long-term ancillary time-to-event analyses were conducted using Kaplan–Meier statistics on all follow-up data available at the time of the 12 month database lock. The log-rank test and hazard ratios with 95% confidence intervals (CIs) were estimated from the Cox proportional hazard model.
Echocardiographic parameters were assessed on patients with data at implant and at 1 year follow-up. Mean intra-patient changes from baseline to 12 months in LV ejection fraction and LV end-systolic volume were tested in each group using a paired t-test or Wilcoxon test. Mean changes were compared between groups using one-sided non-inferiority test, with an alpha level of 0.025 and a pre-defined absolute non-inferiority margin of −2.5% in LV ejection fraction and 15 mL in LV end-systolic volume, respectively. A logistic regression model was used to estimate odds ratios with 95% CIs in pre-defined subgroup analyses. For each of the pre-specified subgroups, interactions between the subgroup and the randomly assigned group were tested without adjusting for multiple comparisons. Categorical variables are reported as frequencies and percentages, and continuous variables as mean ± standard deviation. P-values for non-inferiority are one-sided in nature, whereas all other tests are two-sided. Analyses were performed using the SAS software version 9.4.
Results
Patients
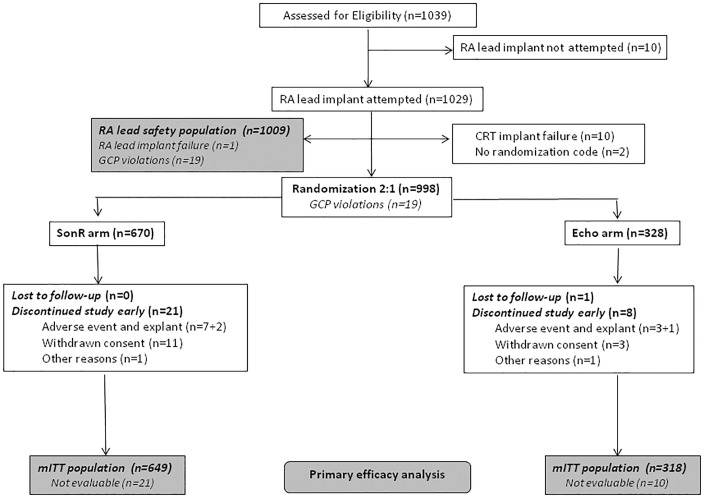
A total of 1039 patients were assessed for eligibility from 125 sites in 12 countries in Europe, Australia, and North America, from 13 January 2012 through 29 October 2014. Of these patients, 670 were randomly assigned to SonR and 328 to Echo (Figure 2). One patient was lost to follow-up. Nineteen patients were excluded from the analysis before unblinding due to violations of Good Clinical Practice guidelines at two sites, making the data unreliable. Mean follow-up (of up to 12 months) was 358.1 ± 61.3 days (range, 7.0–543.0).
Figure 2.
Patient flow. GCP, Good Clinical Practice; mITT, modified intention to treat; RA, right atrial.
The patients’ baseline characteristics were generally well matched between groups (Table 1). Mean age was 67.0 ± 10.2 years and 68.8% were men; 96.2% of patients were in NYHA class III; mean LV ejection fraction was 29.5 ± 8.3%; mean QRS width was 160.5 ± 22.7 ms, and 85.5% had LBBB. Most patients were on beta-blockers (90.3%) and on angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (89.5%). Medications at baseline are summarized in the Supplementary material online, Table S1. Successful implantation of the SonRtip lead was achieved in 1027/1029 (99.8%) patients (one patient experienced a complication during the procedure and in the second patient, an acceptable position could not be reached).
Table 1.
Baseline characteristics
| Baseline characteristics | SonR (N=670) | Echo (N=328) |
|---|---|---|
| Demographic | ||
| Age, years | 67.2 ±10.2 | 66.6 ±10.2 |
| Men (%) | 70.4 (472) | 65.5 (215) |
| BMI, kg/m2 | 28.5 ±5.6 | 27.9 ±5.0 |
| NYHA class | ||
| II | 1.5 (10) | 0.3 (1) |
| III | 96.6 (647) | 95.4 (313) |
| IV | 1.9 (13) | 4.3 (14) |
| Cardiac risk factors | ||
| Atrial fibrillation | 14.8 (99) | 16.5 (54) |
| Diabetes | 37.3 (250) | 41.8 (137) |
| Current smoker | 33.0 (221) | 32.3 (106) |
| Systemic hypertension | 62.1 (416) | 61.6 (202) |
| Renal dysfunction | 22.8 (153) | 24.7 (81) |
| Chronic obstructive pulmonary disease | 13.1 (88) | 13.7 (45) |
| Cause of heart failure | ||
| Ischaemic | 45.5 (300) | 42.5 (138) |
| Non-ischaemic | 54.5 (360) | 57.5 (187) |
| Electrocardiographic finding | ||
| QRS duration, ms | 160.7 ±23.1 | 160.0 ±21.9 |
| LBBB | 84.0 (563) | 88.4 (290) |
| Non-LBBB | 16.0 (107) | 11.6 (38) |
| Heart rate, b.p.m. | 70.7 ±13.4 | 70.9 ±13.6 |
| PR interval, ms | 188.1 ±44.9 | 188.3 ±42.7 |
| Systolic blood pressure, mmHg | 125.7 ±19.8 | 124.5 ±20.2 |
| Diastolic blood pressure, mmHg | 72.7 ±12.0 | 71.8 ±11.0 |
| Echocardiographic finding | ||
| Left ventricular ejection fraction | ||
| ≤25% | 33.6 (225) | 30.5 (100) |
| >25% | 66.4 (445) | 69.5 (228) |
| Left ventricular end-systolic volume, mL | 162.0 ±72.5 | 159.8 ±75.0 |
| Left ventricular end-diastolic volume, mL | 226.2 ±88.0 | 225.6 ±94.3 |
| Concomitant cardiac medications | ||
| Beta-blocker | 89.4 (599) | 92.1 (302) |
| ACE inhibitor, substitutes, or ARB | 89.9 (602) | 88.7 (291) |
| Ivabradine | 9.0 (60) | 10.4 (34) |
| Diuretic | 79.6 (533) | 84.5 (277) |
| Spironolactone | 57.9 (388) | 56.7 (186) |
There were no significant differences between groups, except for the NYHA class distribution (P < 0.05). Values are mean ± SD or % (n).
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker, BMI, body mass index; LBBB, left bundle branch block; NYHA, New York Heart Association functional class.
Outcomes
Responder rates were 75.0% in the SonR arm and 70.4% in the Echo arm (mean difference, 4.6%; 95% CI, –1.4% to 10.6%; P < 0.001 for non-inferiority margin −10.0%) (Table 2). As the lower bound of the confidence interval did not exceed 0%, superiority was not demonstrated. The primary endpoint remained statistically significant when adjusted for New York Heart Association class (mean difference, 4.9%; 95% CI, −1.1% to 11.0%; P < 0.001 for non-inferiority margin −10.0%), and was confirmed in the worst case sensitivity analysis (mean difference, 4.3%; 95% CI, −1.9% to 10.4%; P < 0.001 for non-inferiority margin −10.0%). Similar rates of death were reported between SonR and Echo arms (5.5% and 6.0%, respectively).
Table 2.
Clinical outcomes
| Outcome | SonR (N=649) | Echo (N=318) | Mean % difference (95% CI) |
P-value |
|
|---|---|---|---|---|---|
| % (n) | Non-inferiority | Superiority | |||
| Clinical respondersa | 75.0 (487) | 70.4 (224) | 4.6 (−1.4, 10.6) | <0.001 | 0.13 |
| NYHA improved | 65.6 (426) | 61.9 (197) | |||
| Stable NYHA, improved quality of life | 9.4 (61) | 8.5 (27) | |||
| Clinical non-respondersb | 25.0 (162) | 29.6 (94) | |||
| Clinically stable | 4.0 (26) | 4.4 (14) | |||
| Clinically worsened: secondary endpoint | 21.0 (136) | 25.2 (80) | 4.2 (−1.5, 9.9) | <0.001 | 0.15 |
| Death from any cause | 5.5 (36) | 6.0 (19) | |||
| If no death, HF-related event | 10.2 (66) | 12.9 (41) | |||
| Worsened NYHA class | 0.9 (6) | 0.3 (1) | |||
| Worsened quality of life; stable NYHA stable | 4.3 (28) | 6.0 (19) | |||
| Death or HF hospitalization | 14.2 (92) | 17.6 (56) | 3.4 (−1.5, 8.4) | <0.001 | 0.18 |
P-value for non-inferiority is based on one-sided Z-test of two binomial proportions at 0.025 alpha level with 10.0% non-inferiority margin; P-value for superiority is based on two-sided Z-test.
CI, confidence interval; HF, heart failure; NYHA, New York Heart Association functional class.
Patients who are clinically improved according to the primary efficacy endpoint.
Patients who are either stable or deteriorated according to the primary efficacy endpoint.
In the safety evaluation, the rates of freedom from acute and chronic lead complications were 98.5% (95% CI, 97.3–99.2%; P < 0.001) and 99.8% (95% CI, 99.1–100.0%; P < 0.001), respectively. The complications are detailed in the Supplementary material online, Table S2.
As the primary endpoints were met, the secondary endpoints were tested for non-inferiority. In the SonR group, 21.0% of patients were worsened vs. 25.2% in the Echo group (mean difference, 4.2%; 95% CI, –1.5% to 9.9%; P < 0.001 for non-inferiority margin −10%). The rates of death or HF hospitalization were 14.2% with SonR and 17.6% with Echo (mean difference, 3.4%; 95% CI, –1.5% to 8.4%; P < 0.001 for non-inferiority margin −10%) (Table 2).
Long-term follow-up
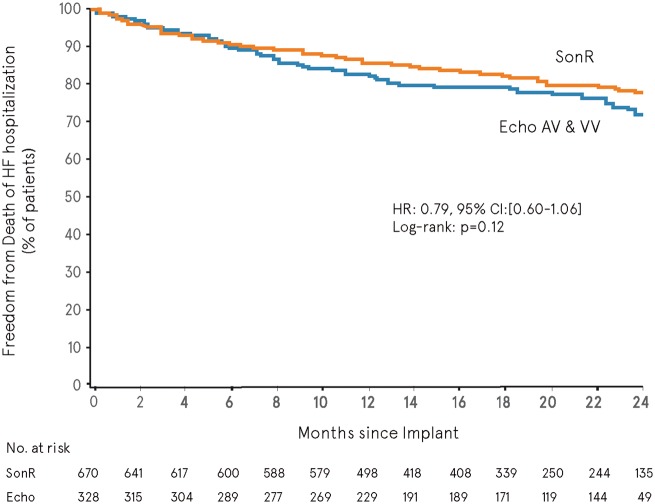
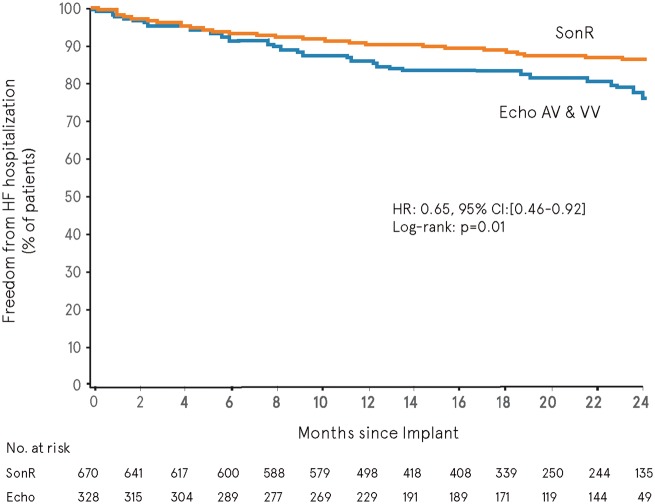
When using all adjudicated data available in the database at the time of the primary analysis, the overall mean follow-up was 548.2 ± 190.3 days (range, 0.0–867.0). The composite of death or HF hospitalization (pre-specified ancillary analysis) showed no significant difference in freedom from event between SonR and Echo over time (hazard ratio, 0.79; 95% CI, 0.60–1.06; log-rank P = 0.12, two-sided) (Figure 3). A 35% risk reduction in HF hospitalization was associated with SonR (hazard ratio, 0.65; 95% CI, 0.46–0.92; log-rank P = 0.01, two sided) (Figure 4).
Figure 3.
Freedom from all-cause mortality or heart failure hospitalization. CI, confidence interval; HR, hazard ratio.
Figure 4.
Freedom from heart failure hospitalization. CI, confidence interval; HR, hazard ratio.
Ancillary and subgroup analyses
Left ventricular ejection fraction increased significantly from baseline to 12 months in both arms (P < 0.001 two-sided). The mean increase was similar between groups (10.8 ± 12.3% in the SonR arm vs. 11.5 ± 12.0% in the Echo arm; mean difference, −0.6%; lower bound 97.5% −2.1%; P = 0.006 for non-inferiority margin −2.5%). Similarly, the LV end-systolic volumes decreased significantly from baseline to 12 months in both arms (P < 0.001, two-sided), with a mean decrease of −44.1 ± 54.3 mL in the SonR arm vs. −50.2 ± 61.0 mL in the Echo arm (mean difference, 6.0; upper bound 97.5% CI, 14.9; P = 0.023 for non-inferiority margin 15.0 mL).
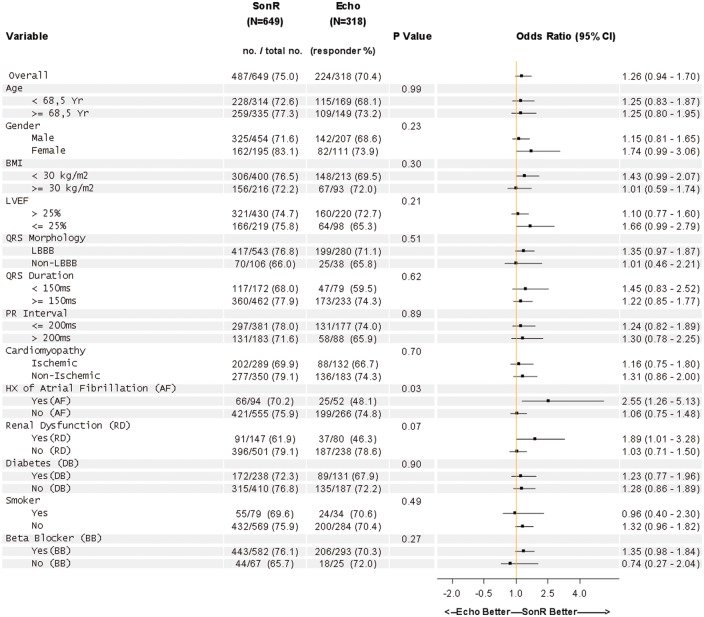
The pre-specified analysis on the likelihood of an improved response rate according to baseline characteristics showed consistency towards benefit in SonR across most of the pre-specified subgroups (Figure 5). Significant interactions (P < 0.15 two-sided) were found in patients with a history of atrial fibrillation (n = 146), in whom 70.2% were responders with SonR vs. 48.1% with Echo (odds ratio, 2.6; 95% CI, 1.3–5.1), and in patients with renal dysfunction (n = 227), in whom 61.9% were responders with SonR vs. 46.3% with Echo (odds ratio, 1.9; 95% CI, 1.0–3.3).
Figure 5.
Subgroup analysis.
Discussion
The RESPOND-CRT study was a multicentre, double-blind, randomized controlled clinical trial, which demonstrated that contractility sensor-guided automatic optimization of CRT was safe and non-inferior to the AV and VV Echo-guided approach. The primary efficacy endpoint of non-inferiority was met with a patient responder rate of 75.0% in the sensor arm vs. 70.4% in the Echo arm (mean difference, 4.6%; 95% CI, –1.4% to 10.6%; P < 0.001 for non-inferiority margin −10.0%). Clinical response for most subgroups favoured the SonR group, especially in patients with a history of atrial fibrillation or renal dysfunction. Notably, the contractility sensor also had a favourable effect on long-term HF hospitalization.
An ideal optimization approach is one that is automated, where the device can self-adjust and provides resynchronization therapy over a spectrum of physiological states. To enable this requires a dedicated atrial lead with an embedded sensor, which does not compromise the success of its implantation, pacing and sensing functions. In this study, the rate of successful implantation was 99.8% and the freedom from acute and chronic complications was 98.5% and 99.8%, respectively. Complications were very rare, with a 1% right atrial lead dislodgement rate.
Effective CRT is dependent on the appropriate selection of patients, suitable placement of the LV pacing lead, and individualized programming of the AV and VV timings for the delivery of the pacing therapy.4 There is considerable heterogeneity within patients, based on the presence of ventricular scar or fibrosis, extent of cardiac dilatation, electrical activation within the heart, along with a myriad of anatomical variations in the location of the atrial and ventricular leads.21,22 Such a variance between patients emphasizes the need for a patient-centric approach to program the AV and VV timings, to help maximize cardiac synchrony and the consequent increase in cardiac contractility.7 The optimal AV interval is integral to enhancing response to CRT, and a suboptimal AV interval may contribute to a decline in cardiac output, by up to 20%.23,24 Even though tailoring the delivery of CRT with AV and VV optimization is considered best practice, this has neither been adopted as a part of routine clinical care nor supported by the ESC 2013 guidelines.1 Prior reports from world-wide surveys have suggested that less than half of implanting physicians optimize the programming at implant, and that <10% of clinicians continue to systematically optimize the AV and VV delays during follow-up.25 Even though several studies have demonstrated the value of Echo-guided optimization in reducing the number of non-responders to CRT,5,26 clinicians do not perform this because of the lack of precision, availability of skilled staff, resources, and logistical challenges.7 This in turn has created a need for a simpler, automated approach to individualize the optimization of AV and VV intervals within patients.
Optimization approaches using measures of electrical activity within the heart and between the right and LV pacing leads have been tested prospectively in other studies. The SMART-AV trial was a study designed to compare a device-based algorithm with Echo-guided optimization and a fixed nominal AV delay in a randomized pattern.27 The primary endpoint in this study was LV end-systolic volume and secondary endpoints included NYHA class, quality of life score, and 6 min walk test at 6 month follow up. The study concluded that neither the device-based algorithm (SmartDelay™) nor Echo-guided optimization was superior to a fixed AV delay of 120 ms. It does appear that this study was underpowered and the definition of a responder may have been too stringent. Also, the optimization method in the SMART-AV study used a static variable that was manually programmed into the device at a clinic visits.27 Like the Echo-guided approach, this algorithm is limited by its inability to adapt to exercise and progressive remodelling of the heart.
The Adaptive CRT (aCRT™) study recently examined an algorithm enabling RV-synchronized LV and bi-ventricular pacing, in the setting of intact intrinsic AV conduction.28 This report demonstrated non-inferiority of the algorithm on the overall population when compared with Echo-guided optimization at 6 months of follow-up.28 aCRT seems to benefit to patients with normal AV interval at baseline while being potentially suboptimal for patients with prolonged AV.29 Unlike any of the above optimization strategies, the signal recorded by the SonR sensor reflects global ventricular contractility. Previous work has suggested that the signal recorded on the atrial lead is stable and reproducible during atrial fibrillation, exercise, and sensitive to pacing conditions.30
Of note, the clinical characteristics of patients in RESPOND-CRT were comparable to those in other studies.2,3 Majority of the patients were NYHA class III, with an even distribution of ischaemic and non-ischaemic cardiomyopathy. Notably current practice in CRT implantations includes a substantial percentage of NYHA class II patients (30–50%), as opposed to the population of the RESPOND CRT trial. Also noteworthy, the percentage of LBBB patients was higher in the RESPOND-CRT trial as compared with historical trials on HF patients, most likely due to the evolution of the guidelines. Defining response to CRT is complex and there remains a high degree of variance between responder rates, when one examines functional endpoints vs. that of anatomical remodelling.31 We used a composite of functional as well as hard endpoints, inclusive of HF and all-cause mortality in defining responders. Heart failure hospitalization was a pre-specified endpoint and examined separately. Notably, the overall response in the SonR arm was higher (75%) than that reported in the Echo arm (70.4%), as well in historical controls from other previous studies that used optimization. The SonR arm was observed to have reduced HF hospitalization rate. This could potentially have cost implications, as HF hospitalization is a major component of the overall cost related to the management of this cohort of patients.
Importantly the sensor-strategy was notably better in patients with a past history of atrial fibrillation and renal dysfunction. We could speculate that this subgroup of patients is sicker and haemodynamically more tenuous, and therefore benefit from more frequent CRT optimization. The ability of the sensor strategy to frequently optimize and adjust for exercise periods may result in benefit in these sicker patients, especially during the augmented stress of exercise and over the course of remodelling. In this study, the trend to benefit from continuous optimization emerged early in the course with a reduction of HF hospitalization that continued to improve over the extended follow-up period.
Of the 1039 patients recruited 19 patients from two sites were excluded from the analysis prior to unblinding. This decision was made due to violations of good clinical practice from that centre. Also, a larger proportion of patients in this study were NYHA class III, and generalizing the results of this study beyond this subgroup of patients must be done cautiously. The Echo-guided arm did not repeatedly undergo optimization. This cohort of patients had their initial programming performed soon after the implant, which was then repeated based on clinical grounds and physician discretion. This is congruent with current clinical practice guidelines for CRT.
In summary, RESPOND-CRT is the first double-blind randomized controlled clinical trial examining the efficacy and safety of a contractility sensor-guided CRT optimization approach. This non-inferiority study met its primary endpoints of safety and efficacy. Clinical response for most subgroups was in favour of the automatic optimization arm using the SonR sensor, especially in patients with a prior history of atrial fibrillation or renal dysfunction. Notably, the contractility sensor approach was also associated with a reduction in HF hospitalization.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank the trial participants and investigators who made the study possible; the independent members of the Core Laboratory, the Clinical Event Committee, and the Data Safety Monitoring Board for their contribution. Anne Rousseau-Plasse, PhD, and Françoise Tondu, for editorial and statistical assistance, respectively.
Funding
The RESPOND-CRT study has been funded by Sorin CRM SAS (LivaNova), Clamart, France.
Conflict of interest: J.B. has received personal fees from LivaNova. P.P.D. has received personal fees to support steering committee activities and non-financial support from LivaNova. J.Bra. has received grants and personal fees from Livanova; personal fees from Biotronik, Medtronic, St. Jude Medical, Bayer, Boehringer Ingelheim, Pfizer, and Daichii Sankyo, outside the submitted work. D.R. has received personal fees from LivaNova; grants and personal fees from Medtronic, outside the submitted work. L.P. has received grants and personal fees from Livanova; personal fees from Biotronik, Medtronic, St. Jude Medical, Livanova, Boston Scientific, outside the submitted work. G.N. has received personal fees from LivaNova; Medtronic; personal fees from St. Jude Medical, grants from LivaNova, grants from Biotronik, grants from CVRX, grants from St. Jude Medical, outside the submitted work. C.K. and J.M.R.L. have no relationship relevant to the content of this study to disclose. W.D. has received personal fees from LivaNova; personal fees from Boston Scientific, Biotronik and St Jude and non-financial support from Medtronic, outside the submitted work. A.B.-B. has received a salary from LivaNova. L.V. has received a salary from LivaNova. J.P.S. has received personal fees from LivaNova; personal fees from Biotronik Inc., Boston Scientific, and Medtronic, grants and personal fees from St. Jude Medical, personal fees from Respicardia, personal fees from Impulse Dynamics, personal fees from American College of Cardiology, outside the submitted work.
References
- 1. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM.. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the european society of cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 2. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J.. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 3. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM.. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 4. Auricchio A, Prinzen FW.. Non-responders to cardiac resynchronization therapy. Circulation 2011;75:521–527. [DOI] [PubMed] [Google Scholar]
- 5. Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, Tang WH.. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol 2009;53:765–773. [DOI] [PubMed] [Google Scholar]
- 6. Houthuizen P, Bracke FA, van Gelder BM.. Atrioventricular and interventricular delay optimization in cardiac resynchronization therapy: Physiological principles and overview of available methods. Heart Fail Rev 2011;16:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorcsan J III, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, Martin R, Steinberg JS, Sutton MS, Yu CM.. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting–a report from the American society of echocardiography dyssynchrony writing group endorsed by the heart rhythm society. J Am Soc Echocardiogr 2008;21:191–213. [DOI] [PubMed] [Google Scholar]
- 8. Ritter P, Delnoy PP, Padeletti L, Lunati M, Naegele H, Borri-Brunetto A, Silvestre J.. A randomized pilot study of optimization of cardiac resynchronization therapy in sinus rhythm patients using a peak endocardial acceleration sensor vs. standard methods. Europace 2012;14:1324–1333. [DOI] [PubMed] [Google Scholar]
- 9. Rickards AF, Bombardini T, Corbucci G, Plicchi G.. An implantable intracardiac accelerometer for monitoring myocardial contractility. The multicenter PEA study group. Pacing Clin Electrophysiol 1996;19:2066–2071. [DOI] [PubMed] [Google Scholar]
- 10. Tassin A, Kobeissi A, Vitali L, Rouleau F, Ritter P, Gaggini G, Dupuis JM.. Relationship between amplitude and timing of heart sounds and endocardial acceleration. Pacing Clin Electrophysiol 2009;32 (Suppl 1):S101–S104. [DOI] [PubMed] [Google Scholar]
- 11. Brugada J, Brachmann J, Delnoy PP, Padeletti L, Reynolds D, Ritter P, Borri-Brunetto A, Singh JP.. Automatic optimization of cardiac resynchronization therapy using SonR-rationale and design of the clinical trial of the sonrtip lead and automatic AV-VV optimization algorithm in the paradym RF SonR CRT-D (respond CRT) trial. Am Heart J 2014;167:429–436. [DOI] [PubMed] [Google Scholar]
- 12. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A.. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 13. Stevenson WG, Hernandez AF, Carson PE, Fang JC, Katz SD, Spertus JA, Sweitzer NK, Tang WH, Albert NM, Butler J, Westlake Canary CA, Collins SP, Colvin-Adams M, Ezekowitz JA, Givertz MM, Hershberger RE, Rogers JG, Teerlink JR, Walsh MN, Stough WG, Starling RC, Heart Failure Society of America Guideline C. Indications for cardiac resynchronization therapy: 2011 update from the heart failure society of America guideline committee. J Cardiac Fail 2012;18:94–106. [DOI] [PubMed] [Google Scholar]
- 14. Green CP, Porter CB, Bresnahan DR, Spertus JA.. Development and evaluation of the Kansas city cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 15. Sacchi S, Contardi D, Pieragnoli P, Ricciardi G, Giomi A, Padeletti L.. Hemodynamic sensor in cardiac implantable electric devices: the endocardial acceleration technology. J Healthc Eng 2013;4:453–464. [DOI] [PubMed] [Google Scholar]
- 16. Bordachar P, Garrigue S, Ritter P, Ploux S, Labrousse L, Casset C, Haissaguerre M, Dos Santos P.. Contributions of a hemodynamic sensor embedded in an atrial lead in a porcine model. J Cardiovasc Electrophysiol 2011;22:579–583. [DOI] [PubMed] [Google Scholar]
- 17. Duncker D, Delnoy PP, Nagele H, Mansourati J, Mont L, Anselme F, Stengel P, Anselmi F, Oswald H, Leclercq C.. First clinical evaluation of an atrial haemodynamic sensor lead for automatic optimization of cardiac resynchronization therapy. Europace 2016;18:755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliveira M, Branco L, Galrinho A, da Silva N, Cunha P, Valente B, Feliciano J, Pimenta R, Delgado A, Ferreira R.. Hemodynamic device-based optimization in cardiac resynchronization therapy: concordance with systematic echocardiographic assessment of AV and VV intervals. Res Rep Clin Cardiol 2015;6:1–7. [Google Scholar]
- 19. Johansen JB, Nielsen JC, Sandgaard NCF.. Longevity of implantable cardioverter-defibrillators: still a long way to go. Europace 2016;18:1285–1286. [DOI] [PubMed] [Google Scholar]
- 20. Glikson M, Von Feldt LK, Suman VJ, Hayes DL.. Short- and long-term results with an active-fixation, bipolar, polyurethane-insulated atrial pacing lead. Pacing Clin Electrophysiol 1996;19:1469–1473. [DOI] [PubMed] [Google Scholar]
- 21. Shetty AK, Duckett SG, Ginks MR, Ma Y, Sohal M, Bostock J, Kapetanakis S, Singh JP, Rhode K, Wright M, O'Neill MD, Gill JS, Carr-White G, Razavi R, Rinaldi CA.. Cardiac magnetic resonance-derived anatomy, scar, and dyssynchrony fused with fluoroscopy to guide LV lead placement in cardiac resynchronization therapy: a comparison with acute haemodynamic measures and echocardiographic reverse remodelling. Eur Heart J Cardiovasc Imaging 2013;14:692–699. [DOI] [PubMed] [Google Scholar]
- 22. Bose A, Kandala J, Upadhyay GA, Riedl L, Ahmado I, Padmanabhan R, Gewirtz H, Mulligan LJ, Singh JP.. Impact of myocardial viability and left ventricular lead location on clinical outcome in cardiac resynchronization therapy recipients with ischemic cardiomyopathy. J Cardiovasc Electrophysiol 2014;25:507–513. [DOI] [PubMed] [Google Scholar]
- 23. Meisner JS, Nikolic S, Tamura T, Tamura K, Frater RW, Yellin EL.. Development and use of a remote-controlled mitral valve. Ann Biomed Eng 1986;14:339–349. [DOI] [PubMed] [Google Scholar]
- 24. Inoue N, Ishikawa T, Sumita S, Nakagawa T, Kobayashi T, Matsushita K, Matsumoto K, Ohkusu Y, Taima M, Kosuge M, Uchino K, Kimura K, Umemura S.. Long-term follow-up of atrioventricular delay optimization in patients with biventricular pacing. Circulation 2005;69:201–204. [DOI] [PubMed] [Google Scholar]
- 25. Gras D, Gupta MS, Boulogne E, Guzzo L, Abraham WT.. Optimization of AV and VV delays in the real-world CRT patient population: an international survey on current clinical practice. Pacing Clin Electrophysiol 2009;32 (Suppl 1):S236–S239. [DOI] [PubMed] [Google Scholar]
- 26. Altman RK, Parks KA, Schlett CL, Orencole M, Park MY, Truong QA, Deeprasertkul P, Moore SA, Barrett CD, Lewis GD, Das S, Upadhyay GA, Heist EK, Picard MH, Singh JP.. Multidisciplinary care of patients receiving cardiac resynchronization therapy is associated with improved clinical outcomes. Eur Heart J 2012;33:2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, Lemke B, Singh JP, Spinale FG, Van Eyk JE, Whitehill J, Weiner S, Bedi M, Rapkin J, Stein KM.. Primary results from the smartdelay determined av optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (smart-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 2010;122:2660–2668. [DOI] [PubMed] [Google Scholar]
- 28. Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, Gasparini M, Starling RC, Milasinovic G, Rogers T, Sambelashvili A, Gorcsan J III, Houmsse M, Adaptive CRT. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm 2012;9:1807–1814. [DOI] [PubMed] [Google Scholar]
- 29. Birnie D, Lemke B, Aonuma K, Krum H, Lee KL, Gasparini M, Starling RC, Milasinovic G, Gorcsan J III, Houmsse M, Abeyratne A, Sambelashvili A, Martin DO.. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm 2013;10:1368–1374. [DOI] [PubMed] [Google Scholar]
- 30. Gras D, Kubler L, Ritter P, Anselme F, Delnoy PP, Bordachar P, Renesto F, Mabo P.. Recording of peak endocardial acceleration in the atrium. Pacing Clin Electrophysiol 2009;32 (Suppl 1):S240–S246. [DOI] [PubMed] [Google Scholar]
- 31. Birnie DH, Tang AS.. The problem of non-response to cardiac resynchronization therapy. Curr Opinion Cardiol 2006;21:20–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.