Abstract
Background:
Resistance of Leishmania species to antimonial drugs has increased. Hence, in the present study Leishmania major isolates were collected from patients with resistance phenotype and the presence/absence of resistance to Glucantime was investigated.
Materials and Methods:
Samples were taken from 10 cutaneous leishmaniasis (CL) patients who had not responded to chemotherapy with Glucantime. Nested polymerase chain reaction (PCR) was performed to identify the isolated species. Stationary phase promastigotes were added to the grown, adhesive J774 macrophages. Values obtained from standard strain were compared with the test cultures after exposure to the medicine. In vivo, the effects of Glucantime were assessed by comparing the sizes and the parasite burden of the lesions on mouse model.
Results:
The results of amplified band on agarose gel demonstrated all samples were L. major. After exposure to medicine, a reduction of intracellular amastigotes to half was detected. In vivo, the parasite was eliminated in 90% of mice with lesions caused by both isolates of patients and standard L. major, and their lesions became smaller significantly.
Conclusion:
Pentavalent antimonial (SbV) salts are the main component of chemotherapy against leishmaniasis. However, the medicine has been found ineffective. In the present study, isolates from patients with no response to treatment had no significant difference from the standard L. major strain (as the sensitive strain). Therefore, in patients with resistance phenotype to Glucantime, the parasites did not actually have intrinsic resistance, i.e., environmental and host factors prevented the successful treatment of the disease.
Keywords: Glucantime, Leishmania, Leishmania major, resistance, treatment
Introduction
Cutaneous leishmaniasis (CL) refers to chronic skin lesions with different clinical forms, courses of disease, and tissue reactions. In the Old World, the disease is caused by Leishmania aethiopica, Leishmania major, Leishmania tropica, and Leishmania infantum, and is transmitted to humans by Phlebotomus sand flies. Over 12 million people have been estimated to be infected with the Leishmania parasites.[1] On the other hand, Iran, particularly Isfahan province, has been identified as an endemic area of CL caused by L. major.[2]
Despite the self-limiting nature of CL, treatment is generally preferred due to various reasons. CL treatment without delay can prevent not only scarring but also necrosis and rapid development of lesions following secondary bacterial infections. On the other hand, 10% of untreated lesions will remain unhealed and become chronic. Therefore, effective treatment of CL through chemotherapy is strongly recommended.[3]
During the past 60 years, pentavalent antimonial (SbV) compounds, such as meglumine antimoniate (Glucantime) and sodium stiboglucunate (Pentostam), have been the first-line treatment for all forms of leishmaniasis.[4] Being basically regarded as a treatment for schistosomiasis, antimonial salts were first used to treat human leishmaniasis in 1946.[5] Until 1970, the treatment involved the administration of low doses of SbV (10 mg/kg; maximum: 600 mg) for 6–10 days. However, since this method failed in 30% of the cases,[6] in 1984, the World Health Organization proposed the dosage to be increased to 20 mg/kg/day (maximum: 850 mg) for 20 days.[7] Resistance of Leishmania species to antimonial drugs has alarmingly increased during the past decades.[8,9]
SbV compounds are administered through intramuscular (IM) and intralesional (IL) injections. The compounds reach their peak values within 2 h from the IM injection and 50% of the drug is excreted through the kidneys during the first 24 h.[10]
Variation in therapeutic responses to SbV compounds in the past 50 years,[6] numerous cases of treatment failure around the world and in Iran,[11,12] and resistance to SbV therapy[13] have complicated the administration of the first-line drugs for leishmaniasis.[14]
Reduction of efficacy of SbV was reported by Lira et al., Hadighi et al., Rojas et al., and Yardely et al.[8,15,16,17] in several endemic areas. Wide differences in the sensitivity of various CL isolates to SbV compounds were discovered via amastigote–macrophage model.[18] In Guatemala, Pentostam was reported to treat successfully 96% of CL caused by Leishmania braziliensis. Nevertheless, the rate was as low as 57% when the disease was caused by L. mexicana.[19]
Iran is an endemic area for CL where the disease is considered as a regional health issue.[20,21] Since Glucantime is the first-line treatment for leishmaniasis, a proper understanding of its mechanism of action is essential. Meanwhile, besides having to deal with proven resistance of L. tropica to Glucantime, Iranian health authorities have been recently faced with high prevalence of unresponsiveness of zoonotic CL (ZCL) cases to the drug.[22] Hence, the present study collected L. major isolates from patients with resistance phenotype and investigated the presence/absence of resistance to Glucantime both in vitro and in vivo. We believe that our findings will be beneficial in future decisions to resolve patients’ problems with no response to treatment.
Materials and Methods
In this experimental study, 10 non-healing CL patients despite receiving at least one full course of treatment with Glucantime were selected. Used L. major strain (MRHO/IR/75/ER) was the drug-sensitive strain, that isolated from naturally infected Rhombomys opimus, and has no human origin, therefore had not been exposed to any anti-leishmaniasis drugs.
Direct microscopy was used as a tool for detecting parasites of the smeared slides from the lesions of cases. Small amounts of the samples were then added to vials containing 0.5 ml brain-heart infusion (BHI) medium and transferred to the parasitology laboratory of Isfahan University of Medical Sciences (Isfahan, Iran) to isolate the parasite.
Culture of the parasites
The isolated samples were cultured in modified Novy-MacNeal-Nicolle (NNN) medium with 100 µg/ml streptomycin and 100 IU/ml penicillin G at 24 ± 1°C. They were transferred to RPMI 1640 medium supplemented with 20% inactivated fetal calf serum (FCS) with 100 µg/ml streptomycin and 100 IU/ml penicillin G at 24 ± 1°C. L. major (MRHO/IR/75/ER) and L. tropica (MRHO/IR/02/Mash10) strains were also cultured by a similar process and considered as standard strains.
Species identification
Nested polymerase chain reaction (PCR) was performed to identify the isolated species. DNA of the cultured promastigotes was extracted using a commercial kit (GeNet Bio, Seoul, Korea) and according to the manufacturer's instructions. DNA extraction was confirmed through spectrophotometry. Finally, the first and second stages of nested PCR were conducted to detect the kinetoplastic DNA (kDNA) by using CSB7XR and CSB2XF as first primers and 13Z and LiR as second primers[23] [Table 1].
Table 1.
Primers used in nested PCR
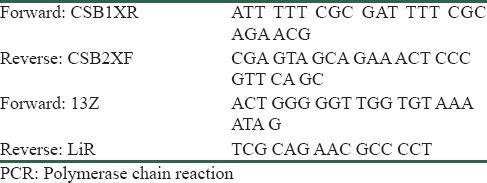
L. major (MRHO/IR/75/ER) and L. tropica (MHOM/IR/02/Mash10) were used as standards in nested PCR.
In vitro experiments
J744 cell line (purchased from Pasteur Institute of Iran, Tehran, Iran) was incubated in cell culture flasks containing RPMI 1640 medium supplemented with decomplemented FCS at 37°C and in the presence of 5% CO2. After cell growth, a scraper was utilized to detach cells from the plates. Subsequent to Neubauer chamber cell counting, 2 × 106 cells/well were transferred to a six-well plate with a 22 × 22 mm2 coverslip on the bottom. RPMI 1640 medium (2 ml) supplemented with inactivated FCS 20%, penicillin, and streptomycin was added to the cells and the plates were incubated at 37°C for 5–6 days. In order to count intracellular amastigotes before and after exposure to the drug (control and case, respectively), two wells were dedicated to each sample.
Stationary phase promastigotes from each patient and the standard L. major strain (MRHO/IR/75/ER) were separately added to the grown, adhesive J774 macrophages at a 7:1 ratio. After 4 h of incubation at 34°C, the extracellular parasites were washed with phosphate buffered saline (PBS) and the medium was replaced with fresh RPMI 1640 supplemented with inactivated FCS 15%. The plates were incubated at 37°C in the presence of 5% CO2 for an additional 24 h.
In the next stage, each culture plate was exposed to 7.2 µM pure Glucantime powder equal to ED50 of the drug.[23] For this purpose, the supernatant was discarded, 2 ml of fresh culture medium containing Glucantime was added, and the plates were incubated for 72 h. Then, the coverslips were removed, fixed with methanol, and stained with Giemsa.
In microscopic examination, the number of intracellular amastigotes in coverslips containing cells and parasites with and without the drug (case and control, respectively) was calculated. This process involved the assessment of 100 macrophages/plate, counting the existing amastigotes, and calculating the average of obtained values. The final numbers of amastigotes in the isolates of patients before and after exposure to the drug were compared with the values obtained from standard strains.
In vivo experiments
Female BALB/c mice (4–6 weeks old) were purchased from Pasteur Institute of Iran (Tehran, Iran) and were divided into 11 groups, with 10 mice in each group and housed in the animal facility of School of Medicine, Isfahan University of Medical Sciences (Isfahan, Iran). A total of 2 × 106 promastigote isolates of patients and standard L. major, which had been cultured and had reached the stationary phase, were intradermally injected to the base of the mice's tail.
The mice were treated with daily IL injections of 60 mg/kg home-prepared solution of Glucantime for 2 weeks.[24] In order to determine the size of the lesions, two perpendicular diameters of the lesions were measured using a caliper and the averages of obtained values were calculated. The measurements were repeated at baseline, on days 7 and 14, and 4 weeks after the cessation of treatment.
The parasite burden of the lesions before and after treatment was evaluated by smearing lesion secretions on slides, Giemsa staining, and parasite counting based on the following method.
The mean numbers of amastigotes in 10 microscopic fields with oil-immersion lens were computed and scored as + 1 to + 4 if the number of amastigotes was 1, 2–10, 11–100, and 101–1000, respectively. If no parasite was observed on the slide, the sample was considered as negative.
Ultimately, the therapeutic effect of Glucantime was assessed by comparing the sizes and parasite burden of the lesions caused by isolates from the patients and the standard strains. Paired and independent t-tests were applied to analyze the data. All analyses were performed in SPSS version 20 and at P value < 0.05.
Results
Species identification of the isolates
The product of the second stage of nested PCR of all isolates of patients with resistance phenotype on agarose gel 1% showed amplified band of 560 bp. Comparison of the results with reference bands of L. major (MRHO/IR/75/ER) and L. tropica (MHOM/IR/02/Mash 10) suggested all samples to be L. major. In fact, L. tropica shows the 750 bp band [Figure 1].
Figure 1.
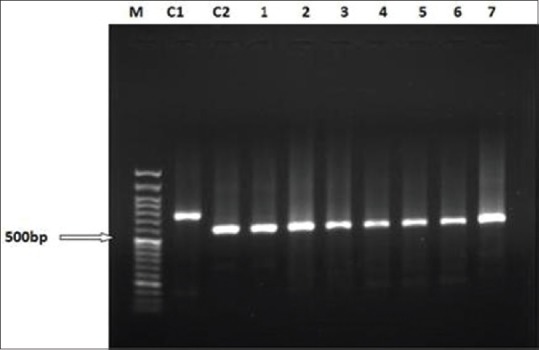
Agarose gel electrophoresis of second phase of nested PCR product of amastigotes genomic DNA isolated from patients with resistance phenotype. Lane M is a 50 bp DNA ladder (fermentase), C1: DNA from L. tropica (MHOM/IR/02/Mash10), C2: DNA from L. major (MRHO/IR/75/ER), lanes 1–7 are samples from non-healing individuals
Results of in vitro experiments
After the termination of the test phase, the coverslips were removed from the case and control plates, stained with Giemsa, and the mean number of the intracellular amastigotes was calculated in 100 macrophages [Figure 2].
Figure 2.
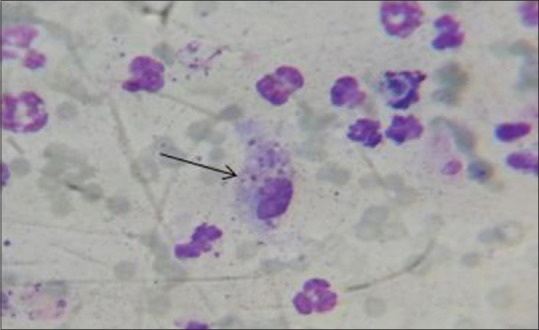
Macrophages containing phagocytosed parasites that transformed to amastigotes from promastigotes. Magnification 100×
Comparison of the number of parasites in these two stages revealed that this amount of drug had almost halved the number of parasites. Similar reductions were detected in plates containing isolates of the patients and standard L. major (MRHO/IR/75/ER) [Figure 3].
Figure 3.
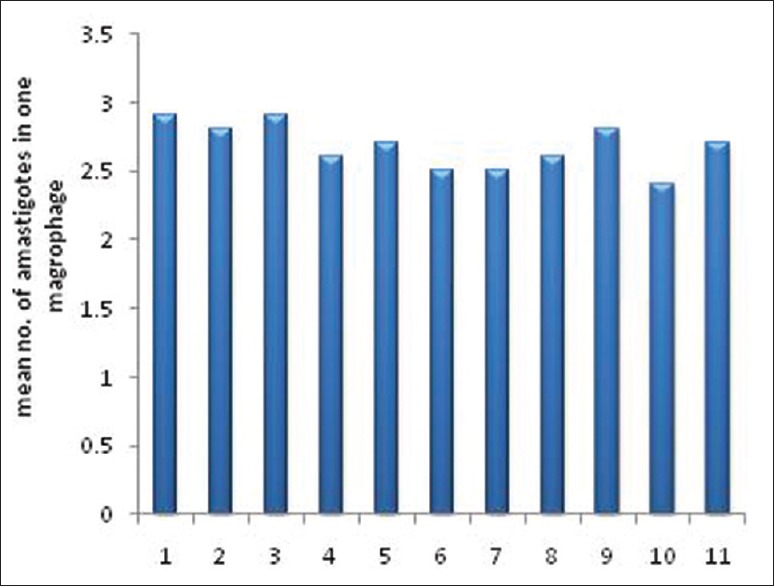
The mean number of amastigotes in 100 macrophages of the standard strain (11th sample) and isolates of patients (1–10 samples) after exposure to drug
Results of in vivo experiments
Results of parasite burden assessments
A same decline in parasite burden was detected among all treated groups. In fact, at the end of the treatment course, the parasite was eliminated in 90% of mice with lesions caused by both isolates of patients and standard L. major [Table 2].
Table 2.
Percent of parasite burden in the groups of BALB/c mice that received isolates from individuals (group 1) and standard L. major (MRHO/IR/75/ER) (group 2), before and after treatment
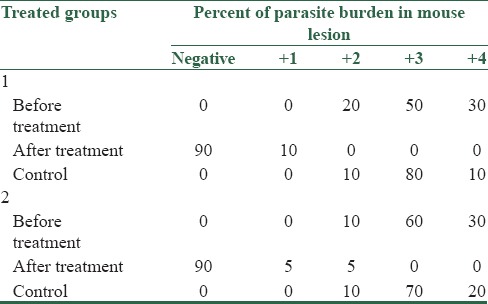
At the end of the treatment in groups 1 and 2, independent t-test showed significant differences between the case and control groups. Paired t-test showed no significant difference between the test and standard in groups 1 and 2 (P < 0.5).
Results of lesion size measurements
Lesion size measurements at baseline and on days 7 and 14 after the cessation of treatment were done. A significant reduction in lesion size was observed after treatment in both groups [Table 3] and there was no difference in treatment responses between the two groups. At the end of the course of treatment, the lesion size of both groups was diminished and the same response was observed on comparing the two groups.
Table 3.
The mean of lesions diameters (mm) after treatment in group 1 of BALB/c mice that received isolates from individuals and group 2 that received L. major (MRHO/IR/75/ER)

Discussion
Despite extensive research throughout the world, leishmaniasis, a disease with complex dimensions, keeps affecting people's lives and causes dramatic mortality and disability. SbV compounds [e.g., meglumine antimoniate (Glucantime)] are the main components of chemotherapy against leishmaniasis. Notwithstanding its toxicity, Glucantime is the treatment of choice for leishmaniasis in many parts of the world, including Iran.[25] However, the drug has been found ineffective in increasing numbers of cases, even in the presence of healthy immune systems.[16]
Unresponsiveness to treatment has been reported in patients with CL caused by L. major in Iran. In 2002, an epidemic of anthroponotic CL (ACL) occurred in Mashhad (a northeastern city of Iran). In a cross-sectional study in the region, Hadighi et al. indicated 94.2% of the isolates were L. tropica and 12% of them did not respond to SbV therapy. They further evaluated and followed patients in Mashhad and concluded that chemotherapy with Glucantime was unsuccessful in CL cases caused by both L. tropica and L. major.[8]
Resistance to glucantime has been proven in several Leishmania species. For instance, in 1999, Lira and Sundar detected intrinsic resistance of L. donovani to this drug.[15] Moreover, in other species such as L. infantum, relationships have been identified between clinical characteristics of the disease and the parasite's sensitivity to treatment.[15,26]
Gradual mutations of the parasite might have been responsible for the decreased sensitivity of the phenotype to treatment. Evaluations with restriction fragment length polymorphism (RFLP) and conformational sensitive gel electrophoresis (CSGE) have actually clarified mutations in drug resistance genes of L. tropica samples from endemic areas of Iran.[27]
Since Isfahan is an endemic area of L. major and previous studies have mainly focused on other Leishmania species, the present research only studied L. major samples. Species identification was performed through nested PCR, which possesses high sensitivity and specificity in DNA sequencing.
Recent studies have adopted the amastigote–macrophage model to describe drug resistance mechanisms in vitro. The results of these studies have been consistent with clinical findings. In addition, the amastigote–macrophage model has been proposed as the gold standard to evaluate anti-leishmaniasis effects of different drugs.[8,15,17] This model was used with samples isolated from patients with and without response to treatment by researchers in Bihar (India). They found the first group of patients to be three times more sensitive to the medicine compared to the second group, while there was not such a difference between promastigote stage of the isolates.[15] In a study in Sudan, the clinical response of field isolates to Pentostam was similar to the results of the amastigote–macrophage model but differed from the results of the promastigote model.[28] It can thus be concluded that our in vitro findings using the amastigote–macrophage model are highly reliable.
In the present study, isolates from patients with no response to treatment had no significant difference with the standard L. major strain (as the sensitive strain) in terms of sensitivity to Glucantime. Therefore, in patients with resistance phenotype to Glucantime, the parasites did not actually have intrinsic resistance and probably environmental and host factors prevented the successful treatment of the disease.
In vivo assessments were done to make sure that factors affecting the phenotype, which are caused by host–parasite interactions during parasite growth, have been included. We inoculated BALB/c mice with parasites isolated from selected patients. According to our findings, the treatment could successfully reduce both lesion size and parasite load in BALB/c mice model. Identical results were observed in lesions produced by the standard strain. Consequently, the absence of intrinsic resistance to Glucantime among the parasites isolated from patients with no response to treatment (resistance phenotype) can be reemphasized.
The multifactorial nature of therapeutic responses has complicated the relation between drug resistance and clinical responses. Differences in immune systems of hosts, pharmacological factors, different drug formulations and manufacturers, pharmacokinetics of the drug and its effects on drug distribution in the body, drug metabolism and excretion in different hosts should necessarily be taken into account. We used pure Glucantime powder without pharmaceutical additives. In other words, exposure of the parasites to pure drug and minimizing the effects of drug formulation can justify the greater efficacy of treatment in the current study compared to treatment modalities in health centers.
Moreover, unequal sensitivity of different Leishmania species to the medicine, alone or in combination with other factors, can influence treatment outcome.[30,31] In a study on ZCL in Saudi Arabia, the amastigote–macrophage model did not reveal any difference in the sensitivity of L. major isolates to medicine. However, the patients had different responses to SbV compounds. These findings are exactly comparable to our results.[6]
In addition to all of the above-mentioned factors, noncompliance with treatment and incomplete treatment courses will expose the parasites to drug pressure and increase the chance of SbV resistance.[32]
Conclusion
Despite the proven intrinsic resistance of some Leishmania species (e.g. L. infantum, L. tropica, and L. donovani) to Glucantime, unresponsiveness of L. major to Glucantime is, in most cases, not due to its intrinsic resistance. Therefore, appropriate administration of the medicine under the supervision of skilled professionals, along with policies to ensure complete courses of treatment will lead to healing of the involved cases.
Financial support and sponsorship
The financial support of this research was received from Deputy of Faculty of Medicine of the Isfahan University of Medical Science.
Conflicts of interest
There are no conflicts of interest.
References
- 1.van der Meide WF, Jensema AJ, Akrum RA, Sabajo LO, Lai A, Fat RF, Lambregts L, et al. Epidemiology of cutaneous leishmaniasis in Suriname: A study performed in 2006. Am J Trop Med Hyg. 2008;79:192–7. [PubMed] [Google Scholar]
- 2.Yaghoobi-Ershadi MR, Jafari A, Hanafi-Bojd AA. A new epidemic focus of zoonotic cutaneous leishmaniasis in central Iran. Ann Saudi Med. 2004;24:98–101. doi: 10.5144/0256-4947.2004.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh AK, Papadopoulou B, Ouellette M. Gene amplification in amphotericin B-resistant leishmania tarentolae. Exp Parasitol. 2001;99:141–7. doi: 10.1006/expr.2001.4663. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira FM, Castro RA, Batista MA, Rossi FM, Silveira-Lemos D, Frézard F, et al. Association of water extract of green propolis and liposomal meglumine antimoniate in the treatment of experimental visceral leishmaniasis. Parasitol Res. 2014;113:533–43. doi: 10.1007/s00436-013-3685-8. [DOI] [PubMed] [Google Scholar]
- 5.Beheshti M, Ghotbi S, Amirizade S. Therapeutic and adverse effects of glucantime used for treatment of cutaneous leishmaniasis. Shiraz E-Medical Journal. 2007:8155–161. [Google Scholar]
- 6.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–26. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakur CP, Sinha GP, Pandey AK, Kumar N, Kumar P, Hassan SM, et al. Do the diminishing efficacy and increasing toxicity of sodium stibogluconate in the treatment of visceral leishmaniasis in Bihar, India, justify its continued use as a first-line drug? An observational study of 80 cases. Ann Trop Med Parasitol. 1998;92:561–9. doi: 10.1080/00034989859258. [DOI] [PubMed] [Google Scholar]
- 8.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3:e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mary C, Faraut F, Deniau M, Dereure J, Aoun K, Ranque S, et al. Frequency of drug resistance gene amplification in clinical leishmania strains. Int J Microbiol 2010. 2010 doi: 10.1155/2010/819060. pii: 819060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salaün P, Frézard F. Unexpectedly high levels of antimony (III) in the pentavalent antimonial drug Glucantime: Insights from a new voltammetric approach. Anal Bioanal Chem. 2013;405:5201–14. doi: 10.1007/s00216-013-6947-5. [DOI] [PubMed] [Google Scholar]
- 11.Bryceson A. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop Med Int Health. 2001;6:928–34. doi: 10.1046/j.1365-3156.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 12.Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health. 2001;6:849–54. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 13.Chawla B, Madhubala R. Drug targets in leishmania. J Parasit Dis. 2010;34:1–13. doi: 10.1007/s12639-010-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashutosh, Sundar S, Goyal N. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol. 2007;56:143–53. doi: 10.1099/jmm.0.46841-0. [DOI] [PubMed] [Google Scholar]
- 15.Hadighi R, Boucher P, Khamesipour A, Meamar AR, Roy G, Ouellette M, et al. Glucantime-resistant leishmania tropica isolated from Iranian patients with cutaneous Leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol Res. 2007;101:1319–22. doi: 10.1007/s00436-007-0638-0. [DOI] [PubMed] [Google Scholar]
- 16.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J Infect Dis. 2006;193:1375–83. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- 17.Berman JD, Chulay JD, Hendricks LD, Oster CN. Susceptibility of clinically sensitive and resistant Leishmania to pentavalent antimony in vitro. Am J Trop Med Hyg. 1982;31:459–65. doi: 10.4269/ajtmh.1982.31.459. [DOI] [PubMed] [Google Scholar]
- 18.Navin TR, Arana BA, Arana FE, Berman JD, Chajón JF. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis. 1992;165:528–34. doi: 10.1093/infdis/165.3.528. [DOI] [PubMed] [Google Scholar]
- 19.Sharifi I, Fekri AR, Aflatonian MR, Nadim A, Nikian Y, Kamesipour A. Cutaneous leishmaniasis in primary school children in the south-eastern Iranian city of Bam, 1994-95. Bull World Health Organ. 1998;76:289–93. [PMC free article] [PubMed] [Google Scholar]
- 20.Momeni AZ, Jalayer T, Emamjomeh M, Khamesipour A, Zicker F, Ghassemi RL, et al. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1999;17:466–72. doi: 10.1016/s0264-410x(98)00220-5. [DOI] [PubMed] [Google Scholar]
- 21.Sadeghian G, Ziaei H, Bidabadi LS, Baghbaderani AZ. Decreased effect of glucantime in cutaneous leishmaniasis complicated with secondary bacterial infection. Indian J Dermatol. 2011;56:37–9. doi: 10.4103/0019-5154.77549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noyes HA, Reyburn H, Bailey JW, Smith D. A nested-PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J Clin Microbiol. 1998;36:2877–81. doi: 10.1128/jcm.36.10.2877-2881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esmaeili J, Mohebali M, Edrissian GH, Rezayat SM, Ghazi-Khansari M, Charehdar S. Evaluation of mil tefosine against leishmania major (MRHO/IR/75/ER):In vitro and in vivo studies. Acta Medica Iranica. 2008;46:191–6. [Google Scholar]
- 24.Mohammadzadeh M, Behnaz F, Golshan Z. Efficacy of glucantime for treatment of cutaneous leishmaniasis in central Iran. J Infect Public Health. 2013;6:120–4. doi: 10.1016/j.jiph.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, et al. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180:564–7. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- 26.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, et al. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: A study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:827–30. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alizadeh R, Hooshyar H, Bandehpor M, Arbabi M, Kazemi F, Talari A, et al. Detection of drug resistance gene in cutaneous leishmaniasis by PCR in some endemic areas of Iran. Iran Red Crescent Med J. 2011;13:863–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Decuypere S, Rijal S, Yardley V, De Doncker S, Laurent T, Khanal B, et al. Gene expression analysis of the mechanism of natural Sb (V) resistance in Leishmania donovani isolates from Nepal. Antimicrob Agents Chemother. 2005;49:4616–21. doi: 10.1128/AAC.49.11.4616-4621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdo MG, Elamin WM, Khalil EA, Mukhtar MM. Antimony-resistant Leishmania donovani in eastern Sudan: Incidence and in vitro correlation. East Mediterr Health J. 2003;9:837–43. [PubMed] [Google Scholar]
- 30.Robledo SM, Valencia AZ, Saravia NG. Sensitivity to Glucantime of Leishmania Viannia isolated from patients prior to treatment. J Parasitol. 1999;85:360–6. [PubMed] [Google Scholar]
- 31.Croft SL, Barrett MP, Urbina JA. Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol. 2005;21:508–12. doi: 10.1016/j.pt.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Sundar S, Jha TK, Thakur CP, Mishra M, Singh VP, Buffels R. Single-dose liposomal amphotericin B in the treatment of visceral leishmaniasis in India: A multicenter study. Clin Infect Dis. 2003;37:800–4. doi: 10.1086/377542. [DOI] [PubMed] [Google Scholar]


