Abstract
Introduction:
Subclinical hypothyroidism (SCH) is a condition wherein there are small elevations in thyroid stimulating hormone (TSH), yet normal circulating levels of free thyroid hormones (FT4). Alterations in thyroid function result in changes in composition and transport of lipoproteins.
Methods:
The study was undertaken in the outpatient department of Lourdes hospital, Ernakulam. 110 patients between 40-69 years were studied, of which 60 were identified to have SCH based on cross-sectional survey, 50 patients were randomly selected to represent the EU group. Data based on interview, clinical examination, thyroid function, lipid profiles. Student's t, chi-square tests used for computation of p values.
Results:
SCH were seen in females (66.66%) and in the age group of 40-49 years (61.66%). Mean cholesterol values were elevated in the subclinical hypothyroid group and in relation to age (60-69) and gender. Statistical analysis showed significant difference in total cholesterol(TC) (P<0.005) and triglycerides (P<0.05) in relation to age (40–49 yrs.) between two groups. Based on TSH, group A 5-7.49Mu/L and group B 7.5-10Mu/L were compared to the euthyroid group N<5Mu/L. Mean cholesterol values were raised in both subgroups. Statistically significant difference seen (P<0.0005) in cholesterol values between the subclinical hypothyroid group B and the euthyroid group N.
Conclusions:
SCH appears to be associated with increased mean cholesterol levels in females and of age > 60 years. The TC values were elevated in both subgroups of patients with SCH (A and B) based on TSH values.
Keywords: Hypercholesterolemia, lipid profile, subclinical hypothyroidism, thyroid function test
Introduction
Thyroid hormone is known to play a role in regulating the synthesis, metabolism, and mobilization of lipids.[1] It is well known that alterations in thyroid function result in changes in the composition and transport of lipoproteins.[2,3,4] Hence, thyroid disorders are known to influence lipid metabolism and are common in dyslipidemia patients.[5]
Thyroid dysfunction is the most commonly encountered endocrinological problem by physicians and about 3% of the populations have thyroid disease.[6] The need for providing the clinicians with guidelines for the day-to-day approach of this frequent disorder is widely recognized, but the appropriate management of subclinical thyroid disease is itself a matter of controversy.
Subclinical and overt hypothyroidism are relatively common disorders in the general population, the former being more common than overt hypothyroidism with a prevalence of 1.4%–7.8% in older individuals and even greater percentiles among women.[7,8]
The definition of subclinical hypothyroidism (SCH) is widely recognized as somewhat arbitrary. Over the years, different terms have been used for this condition: Compensated hypothyroidism, preclinical hypothyroidism, SCH, mild thyroid failure, and mild hypothyroidism. Each of these terms implies to some extent a different diagnostic, prognostic, and therapeutic attitude.
SCH expresses the concept that hypothyroidism, although not perceived, is present to a very mild degree. It is worth noting that by and large, the clinical recognition of signs and symptoms is very much dependent on the alertness of both the physicians and the patient. This is in keeping with the frequently reported retrospective recognition of hypothyroid symptoms after adequate treatment.
With regard to its therapeutic implications, this term is somewhat ambiguous because it may also indicate that in this case hypothyroidism does not reach clinical relevance deserving a therapeutic intervention.
SCH is a term used for a condition in which there are small elevations in thyroid stimulating hormone (TSH), yet normal circulating levels of free thyroid hormones (FT4 and FT3).[9]
It also includes patients who have high normal basal serum TSH concentrations but supernormal serum TSH responses to thyrotropin-releasing hormone.[10] A TSH value usually between 5 and 15 mU/L with normal FT4 and FT3 determines patients with SCH biochemically.[11] The worldwide prevalence ranges from 1% to 10%.[4] While it is uncommon in younger persons, by the age of 65 years, the overall prevalence of the disorder is about 17% in women and 7% in men.[12]
The effects of SCH on serum lipid values are less clear. In general, SCH is associated with hypercholesterolemia mainly due to increased serum levels of low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and possibly triglycerides whereas serum levels of high-density lipoprotein cholesterol (HDL-C) usually remains normal or even elevated.[4,13,14]
SCH has been shown to be a risk factor for coronary artery disease (CAD) because of increased serum levels of TC and LDL-C in most but not all studies along with a reduced level of HDL-C.[15] A number of studies have shown the presence of dyslipidemia among patients with SCH.[12,16,17]
The purpose of this study was to determine whether lipid abnormalities are common in patients with SCH when compared to lipid levels in euthyroid (EU) group.
Aim of study
To assess whether SCH is associated with abnormal lipid levels and compare it with EU group
To compare the difference in the distribution of lipid profile between the two groups of patients with respect to age, gender, and TSH values.
Materials and Methods
This was an observational, cross-sectional study conducted over a period of 1 year, March 2007–2008, in the Medical Outpatient Department of Lourdes Hospital, Kochi.
The study was based on detailed interview and physical examination followed by biochemical investigations for thyroid function and lipid profile in 110 patients aged between 40 and 69 years, of either sex and were further divided into SCH (n = 60) and EU (n = 50) groups based on the thyroid function test. SCH was defined as TSH between 5 and 10 mU/L and normal FT4. The EU group was classified as those with normal TSH values between 0.3 and 4.9 mU/L.
Patients with a history of hypothyroidism, diabetes mellitus, CAD, hyperthyroidism on treatment, pregnancy, psychiatric illnesses, or on drug therapy such as oral contraceptives, hormone replacement therapy, amiodarone, lithium, and cholesterol-lowering agents were excluded from the study.
Blood samples for lipid profile were drawn at 8 h after 12 h of overnight fasting in either group.
Patients were considered to have hypercholesterolemia if the serum values for cholesterol was >200 mg%, LDL >130 mg%, and triglycerides >250 mg%; and HDL <35 mg%.
A total of 60 cases (40 females, 20 males) in the SCH group were evaluated against 50 cases (32 females, 18 males) of age-matched EU group. Data were expressed as the mean ± SD. Student's t-test and Chi- square test were used for consumption of P values. A P < 0.05 was considered statistically significant.
Results
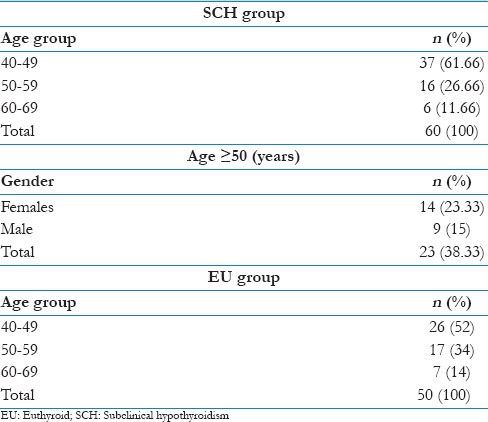
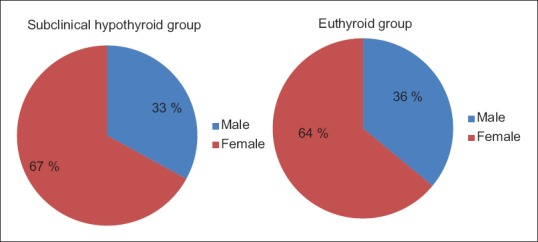
A total 110 patients of either sex had participated in this study. Both groups had predominantly female patients and prevalence of SCH in female was twice more than that in males as shown in Table 1.
Table 1.
Gender distribution

The age of patients who participated in this study were 40–69 years, with predominance in the age group of 40–49 in either group as shown in the age distribution in Table 2. The prevalence of SCH in the age 50 years and above was 23.33% in females and 15% in males.
Table 2.
Age distribution
Most patients with SCH presented with generalized weakness (38%) and lethargy (12%) whereas others presented with myalgia (9%) and weight gain (1%).
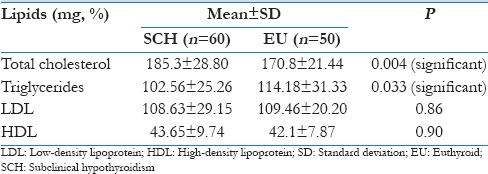
On analyzing the mean lipid values in either group, only serum TC was found to be elevated in the SCH group.
However, statistically significant difference was seen when serum TC (P < 0.005) and triglycerides (P < 0.05) were compared between the two groups using Student's t-test. However, no such difference was seen in other lipid parameters as shown in Table 3.
Table 3.
Mean lipid values in subclinical and euthyroid group
On dichotomization of these (lipid) variables into high and low [Table 4] in relation to their normal values and comparing the percentage between the two groups, patients with SCH showed elevated TC and LDL levels but no variations in serum triglyceride and HDL levels [Figure 1].
Table 4.
Comparison of lipid profiles between subclinical hypothyroid and euthyroid groups
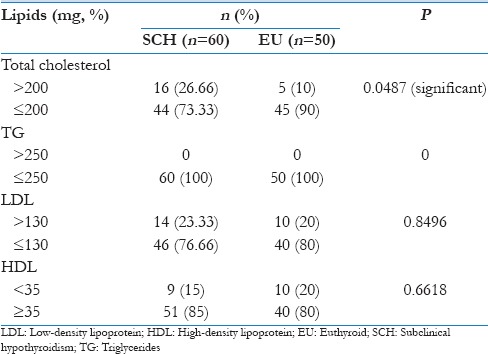
Figure 1.
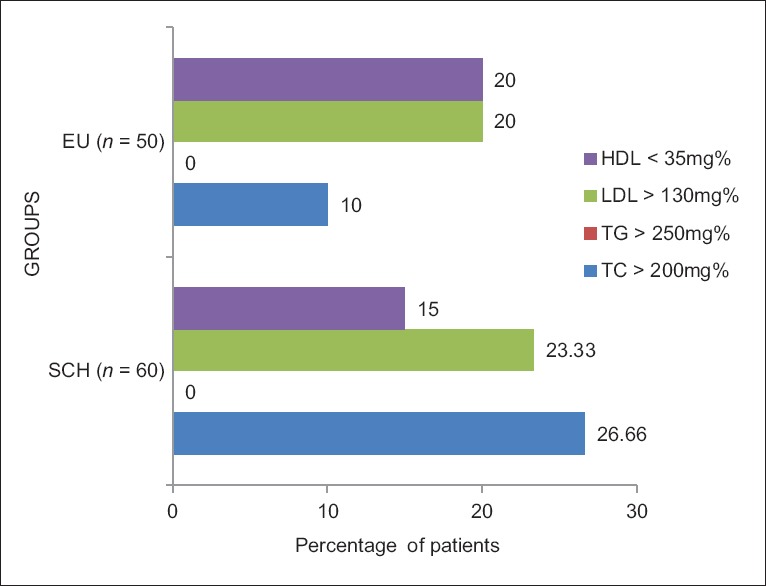
Distribution of abnormal lipid values – comparison between the two groups
Statistically significant difference, using Chi-square test, was found between SCH and EU groups in the percentage of patients with abnormal TC levels (P < 0.05). No such difference was seen in triglyceride, LDL, or HDL levels [Table 4].
The entire study group was further subdivided into three subgroups according to their age, as shown in Table 5 and analyzed statistically using Student's t-test for any significant differences in their lipid profiles.
Table 5.
Comparison of lipid profiles in subclinical hypothyroid and euthyroid control in various age groups
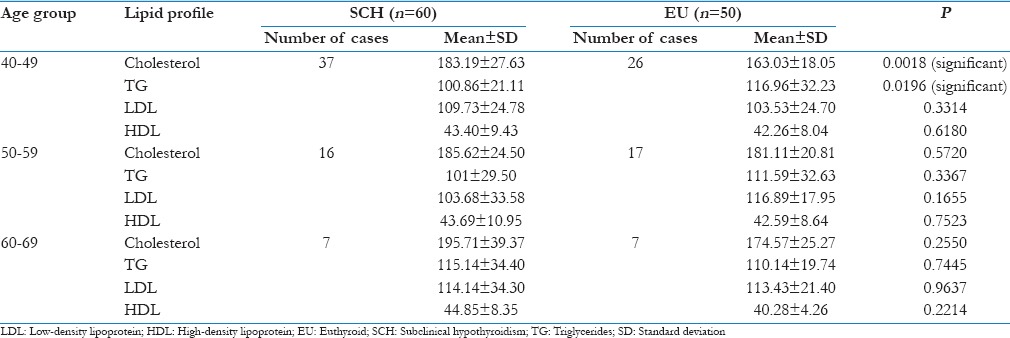
The average TC values were found to be elevated in all the three age groups of subjects with SCH, with slightly higher values in the age group of 60–69 years. No other lipid parameters showed any significant elevation in their mean values in either group.
When statistically compared using Student's t-test, a significant difference was seen in the serum TC (P < 0.005) and triglyceride (P < 0.05) levels between the two age group of 40–49 years. However, no significant change was seen in any of the lipid parameters, between SCH and EU subjects in the age groups of 50–59 and 60–69 years [Table 5].
On comparing the lipid profile between the two groups in relation to gender, the mean serum cholesterol values were found to be elevated in both male (186.55 ± 22.83) and female (184.67 ± 31.29) patients of the SCH group, but no other lipid parameters in either group were found to be elevated.
By comparing the lipid profiles in male patients between SCH and EU groups, statistically significant changes were seen in serum TC (P < 0.05) and triglycerides (P < 0.0001) as shown in Table 6. Student's t-test was used to determine the P value.
Table 6.
Comparison of lipid profile in subclinical hypothyroid and euthyroid control in relation to gender
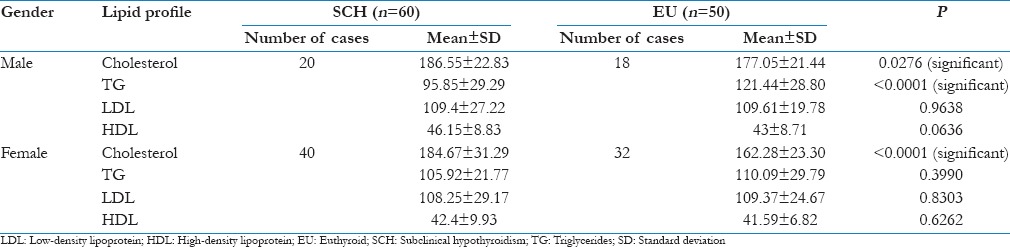
Table 7 shows the division of SCH group according to their serum TSH values.
Table 7.
Division of subclinical hypothyroid group in relation to their thyroid stimulating hormone value and comparison with euthyroid group
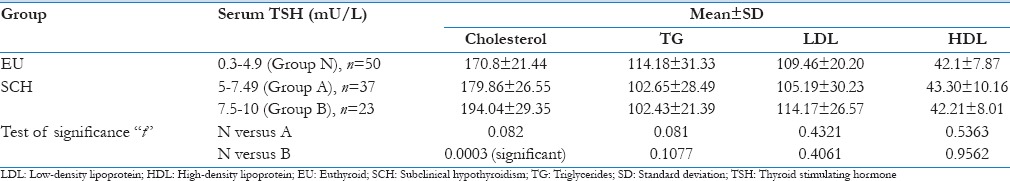
Based on this, they were categorized into two groups – Group A, serum TSH values between 5 and 7.49 mU/L and Group B, serum TSH values between 7.5 and 10 mU/L.
The EU group whose serum TSH values were in the normal range was classified as Group N.
Lipid abnormalities in the different groups (A and B) of SCH patients were compared with the EU group (N) using Student's t-test.
Except for an increased mean TC value in both Group A (176.86 ± 26.55) and Group B (194.04 ± 29.35) of the SCH group, no changes were seen in other mean lipid parameters when compared to EU group (N) along the entire spectrum of raised TSH. Statistically significant difference (P < 0.0005) was seen in the serum cholesterol values between SCH (Group B; TSH 7.5–10 mU/L) and EU group (Group N).
Discussion
Most of the clinical trials focused on the prevalence of SCH in the general population or dyslipidemic patients and whether screening and treatment of SCH individuals, especially in association with dyslipidemia could improve or worsen the lipid profile. However, there are few population-based studies that have compared lipid levels in patients who have SCH with lipid levels in EU individuals.
The results of this study showed that SCH, predominantly a disease of females 66.66% (40/60) [Figure 2], is mostly found in the age group of 40-49 years 61.66% (37/60) [Figure 3] and presents with nonspecific symptoms such as generalized weakness, lethargy, and myalgia.
Figure 2.
Gender distribution
Figure 3.
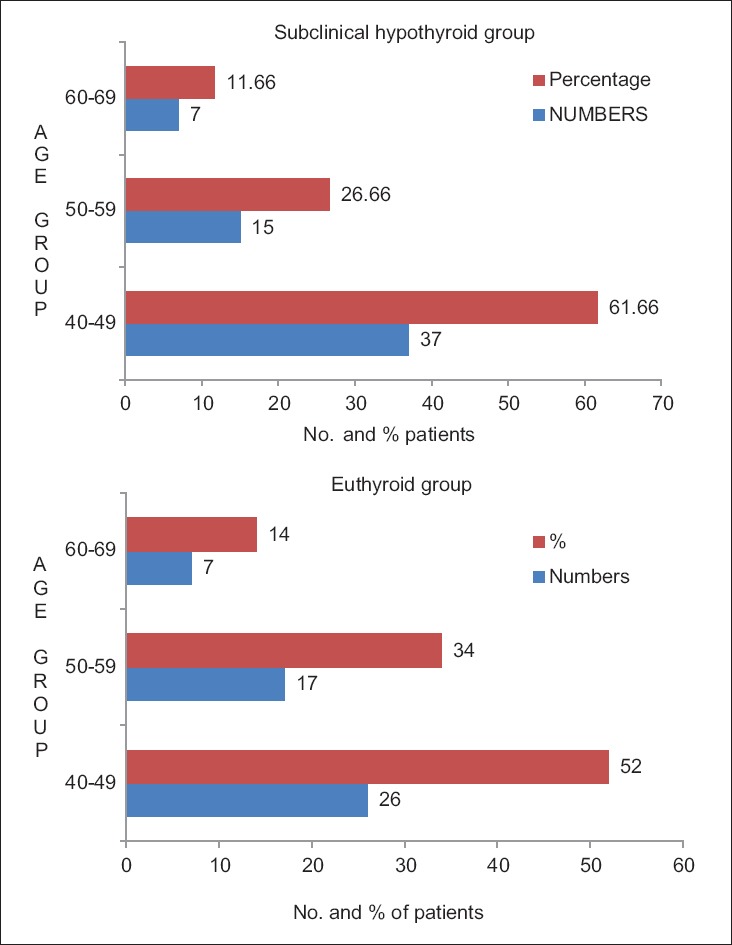
Age distribution
In this observational cross-sectional survey, a significant elevation from normal was seen only in the mean TC value of the SCH group [Figure 4], but no changes were seen in the mean values of other lipid parameters in either group. However, statistically significant difference was seen when serum TC and triglycerides were compared between the two groups [Table 3].
Figure 4.
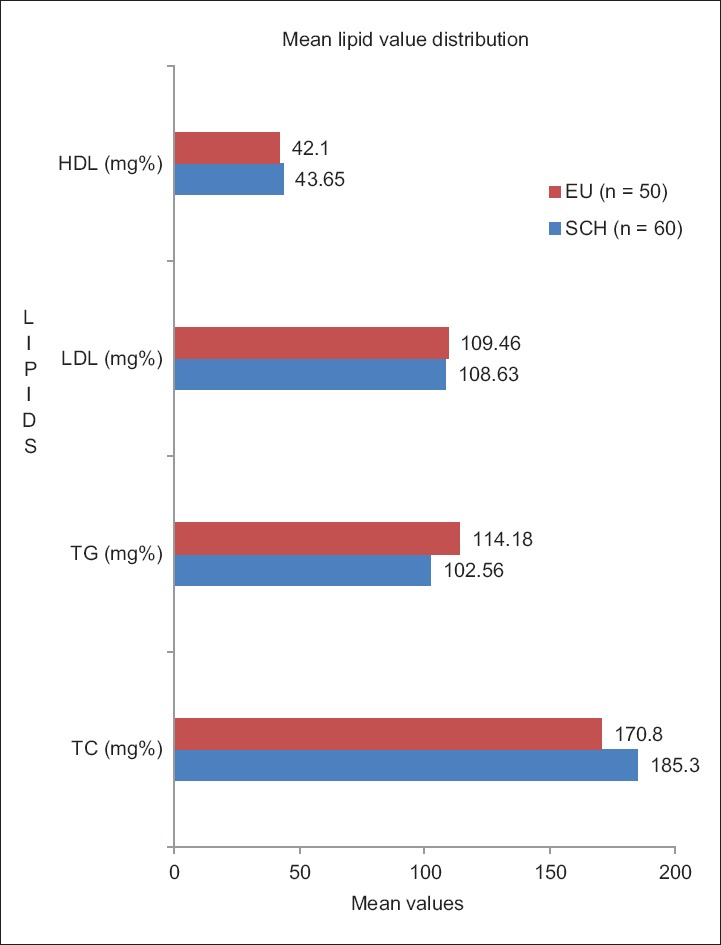
Mean lipid values in subclinical hypothyroidism and euthyroid groups
By showing an association with increased prevalence of atherogenic lipids (TC and triglycerides), SCH is shown to be related to dyslipidemia. This association possibly suggests that the hypothyroid state may be responsible for dyslipidemia.
A relationship between dyslipidemia and atherosclerosis is well established in overt hypothyroidism.[18] Early clinical and autopsy studies have suggested an association between SCH and CAD.[15] In a recent population-based survey, SCH emerged as an independent risk factor for aortic atherosclerosis and myocardial infarction.[19] However, the association of SCH with a change in serum lipid levels is still an open question, despite the fact that several clinical trials have addressed this issue.
On analyzing the difference in the distribution of lipid profile between the SCH and EU control in relation to various age groups and gender [Figures 5 and 6], only the mean cholesterol values [Table 5] showed an increase in all age groups and males and females [Table 6] of the SCH group.
Figure 5.
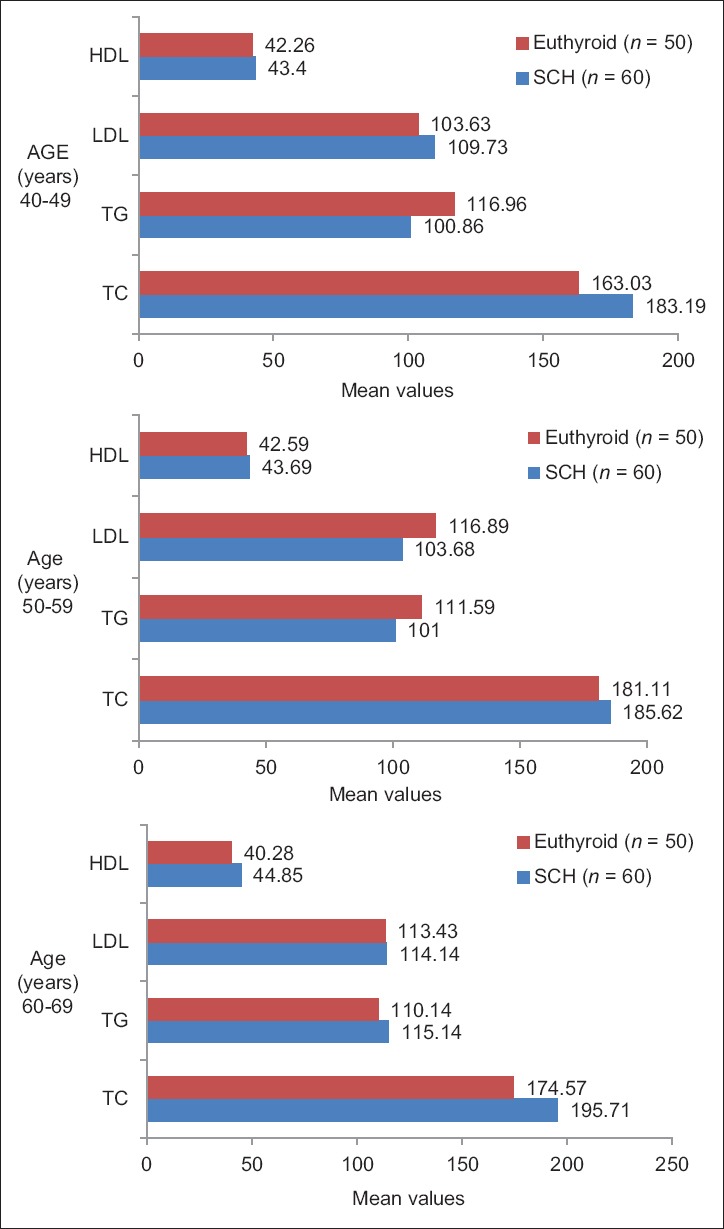
Distribution of lipid profile between subclinical hypothyroidism and euthyroid in relation to various age groups
Figure 6.
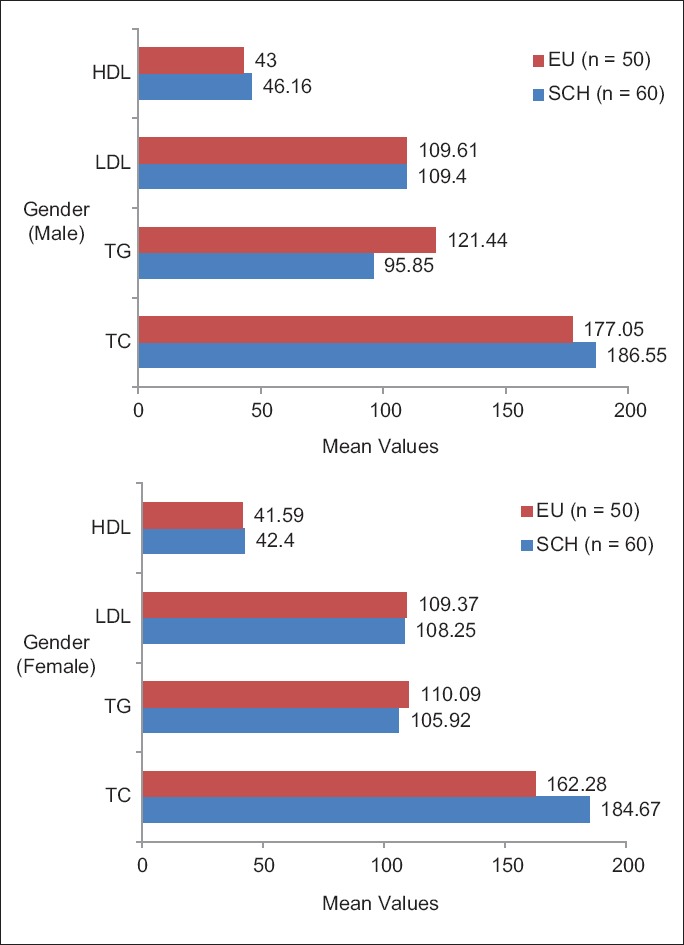
Lipid profile distribution between subclinical hypothyroidism and euthyroid groups in relation to gender
Our findings were consistent with that of Bandyopadhyay et al.,[20] who showed similar elevations in TC, triglycerides and LDL-C in two subgroup analyses: age between 40 and 50 years as well as in female patients. This suggests that females in the perimenopausal years were possibly more at risk of having dyslipidemia.
In a subgroup analysis of patients with SCH based on the serum TSH values [Figure 7], the mean cholesterol values were found to be elevated in Group A (TSH = 5.0–7.49 mU/L) and in group B (TSH = 7.5–10.0 mU/L) when compared to EU group N (TSH = 0.3–4.9 mU/L). This was found to be statistically significant (P < 0.0005) when compared between the SCH (Group B) and EU group N [Table 7]. These findings were consistent with the various cross-sectional observational surveys mentioned in literatures.
Figure 7.
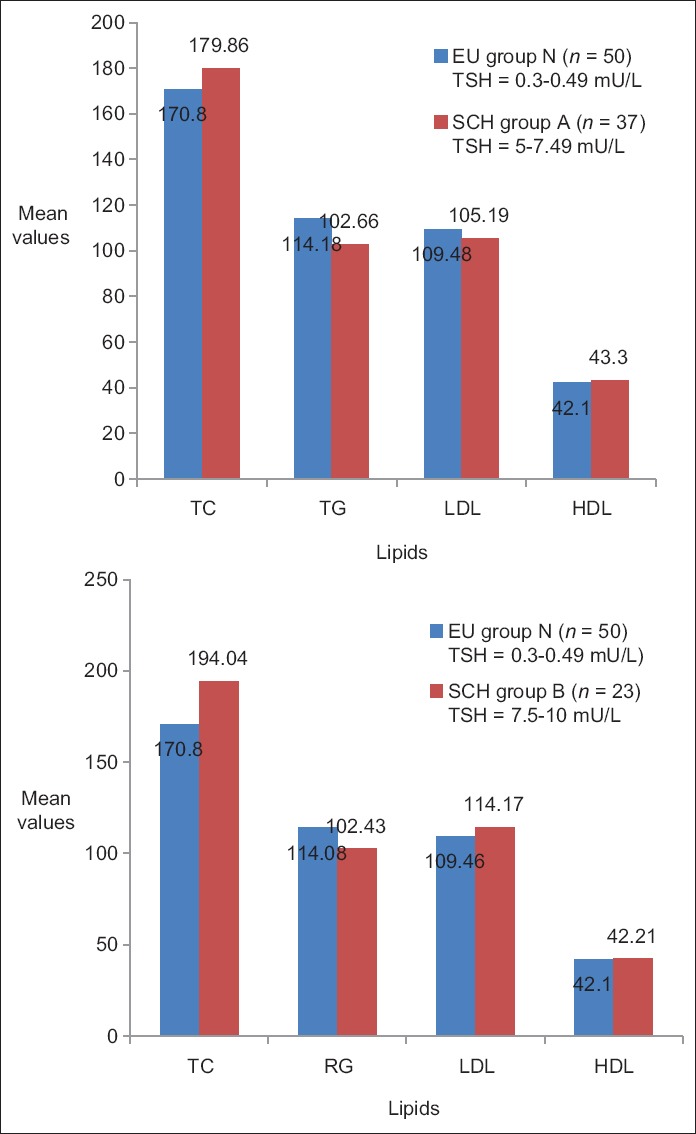
Comparison of lipid profile between euthyroid (Group N) and subclinical hypothyroidism subgroups (A and B) in relation to thyroid stimulating hormone values
Some surveys showed that SCH subjects manifest moderately (mostly up to 10%) higher average TC than controls.[4,21,22,23,24] Such evidence is far from decisive because more than half of these noninterventional cross-sectional studies report either no statistically significant differences between EU and SCH subjects[21,22] or lower TC in SCH subjects.
Evered et al.,[25] graded thyroid gland failure and defined four stages of hypothyroidism, based on which two striking themes emerged from these observational surveys: (1) Stage B (TSH elevation between 5 and 10 mU/L) SCH subjects manifest much milder degrees of dyslipidemia than those with Stage C (prominent TSH elevation >10 mU/L),[4,19,20,24] with only two,[4,24] of these seven reports showing significantly elevated TC or LDL in stage B SCH compared with EU individuals; (2) all three studies that separately considered men with SCH reported an elevation of 1% or less of TC in Stage B SCH compared with EU controls.[4,22,26]
The findings of this study must be interpreted within the limitations of the study design. Because of the cross-sectional nature of this analysis, it is difficult to ascribe causality to any associations that have been found. Because it is not known whether thyroid test abnormalities preceded elevations in cholesterol levels, it cannot be definitely stated that one leads to the other. Further evaluation of this relationship with other data would be necessary to support a causal link.
Since this study involved assessing a small number of individuals over a limited duration subsequent clinical and biochemical evaluation to analyze the progress of their lipid profiles and thyroid function tests were not possible.
There is an absolute need for large studies designed to assess the risk of developing overt hypothyroidism and subsequent cardiovascular health risks among patients with SCH.[5]
The measurement of antithyroid peroxidase (TPO) antibodies is a valuable adjunct in the evaluation of patients with SCH, because it predicts a higher risk of developing overt hypothyroidism (4.3% per year vs. 2.1% per year in antibody-negative individuals)[27] and has been recommended by the consensus statement (American Association of Clinical Endocrinologists, American Thyroid Association and the Endocrine Society) as a diagnostic tool in deciding whether to treat a patient with SCH.[28]
Certain studies have indicated that SCH has been associated with increased risk of coronary disease, especially in women with TPO antibodies as well as in smokers, the SCH-induced lipid abnormalities offering the most obvious explanation for this association.[19,29]
The TPO antibody test could not be performed due to its unavailability and due to financial constraints in our settings.
Further, literature reviews suggest a strong association between thyroid disease and cigarette smoking, which is a major risk factor for vascular disease. Smoking may deteriorate the lipid profile in women with SCH and aggravate the degree of thyroid failure, thus contributing to the development of atherosclerosis.[30] However, smokers were not excluded from this study, especially among the males in our setting.
Conclusion
SCH appears to be associated with increased mean TC levels in females and individuals of various age groups, especially those above the age of 60 years. No significant elevations in other lipid parameters were found.
Thus, by showing its association with increased the prevalence of atherogenic lipids, mainly TC, SCH state was demonstrated to be associated with dyslipidemia.
The TC levels were also found to be elevated in both subgroups of patients with SCH (A and B) based on their TSH values.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pearce EN. Hypothyroidism and dyslipidemia: Modern concepts and approaches. Curr Cardiol Rep. 2004;6:451–6. doi: 10.1007/s11886-004-0054-3. [DOI] [PubMed] [Google Scholar]
- 2.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–93. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 3.Friis T, Pedersen LR. Serum lipids in hyper- and hypothyroidism before and after treatment. Clin Chim Acta. 1987;162:155–63. doi: 10.1016/0009-8981(87)90447-5. [DOI] [PubMed] [Google Scholar]
- 4.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 5.Liberopoulos EN, Elisaf MS. Dyslipidemia in patients with thyroid disorders. Hormones (Athens) 2002;1:218–23. doi: 10.14310/horm.2002.1170. [DOI] [PubMed] [Google Scholar]
- 6.Singh SK, Reddy DV. Indian consensus for management of hypothyroidism. Medicine update. JAPI. 2002;12:389–94. [Google Scholar]
- 7.Samuels MH. Subclinical thyroid disease in the elderly. Thyroid. 1998;8:803–13. doi: 10.1089/thy.1998.8.803. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS. Subclinical thyroid disease: A clinician's perspective. Ann Intern Med. 1998;129:135–8. doi: 10.7326/0003-4819-129-2-199807150-00016. [DOI] [PubMed] [Google Scholar]
- 9.Hueston WJ, Pearson WS. Subclinical hypothyroidism and the risk of hypercholesterolemia. Ann Fam Med. 2004;2:351–5. doi: 10.1370/afm.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adlin V. Subclinical hypothyroidism: Deciding when to treat. Am Fam Physician. 1998;57:776–80. [PubMed] [Google Scholar]
- 11.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 12.Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: Effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: A quantitative review of the literature. J Clin Endocrinol Metab. 2000;85:2993–3001. doi: 10.1210/jcem.85.9.6841. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien T, Dinneen SF, O’Brien PC, Palumbo PJ. Hyperlipidemia in patients with primary and secondary hypothyroidism. Mayo Clin Proc. 1993;68:860–6. doi: 10.1016/s0025-6196(12)60694-6. [DOI] [PubMed] [Google Scholar]
- 14.Muls E, Rosseneu M, Blaton V, Lesaffre E, Lamberigts G, de Moor P. Serum lipids and apolipoproteins A-I, A-II and B in primary hypothyroidism before and during treatment. Eur J Clin Invest. 1984;14:12–5. doi: 10.1111/j.1365-2362.1984.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 15.Kinlaw WB. Atherosclerosis and the thyroid. Thyroid Today. 1991;14:1–8. [Google Scholar]
- 16.Luboshitzky R, Aviv A, Herer P, Lavie L. Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Thyroid. 2002;12:421–5. doi: 10.1089/105072502760043512. [DOI] [PubMed] [Google Scholar]
- 17.Tanis BC, Westendorp GJ, Smelt HM. Effect of thyroid substitution on hypercholesterolaemia in patients with subclinical hypothyroidism: A reanalysis of intervention studies. Clin Endocrinol (Oxf) 1996;44:643–9. doi: 10.1046/j.1365-2265.1996.739560.x. [DOI] [PubMed] [Google Scholar]
- 18.Bastenie PA, Vanhaelst L, Bonnyns M, Neve P, Staquet M. Preclinical hypothyroidism: A risk factor for coronary heart-disease. Lancet. 1971;1:203–4. doi: 10.1016/s0140-6736(71)90947-0. [DOI] [PubMed] [Google Scholar]
- 19.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: The Rotterdam Study. Ann Intern Med. 2000;132:270–8. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Bandyopadhyay SK, Basu AK, Pal SK, Roy P, Chakrabarti S, Pathak HS, et al. A study on dyslipidaemia in subclinical hypothyroidism. J Indian Med Assoc. 2006;104:622–4, 626. [PubMed] [Google Scholar]
- 21.Lindeman RD, Schade DS, LaRue A, Romero LJ, Liang HC, Baumgartner RN, et al. Subclinical hypothyroidism in a biethnic, urban community. J Am Geriatr Soc. 1999;47:703–9. doi: 10.1111/j.1532-5415.1999.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 22.Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: The Whickham survey. Clin Endocrinol (Oxf) 1977;7:481–93. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 23.Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160:1573–5. doi: 10.1001/archinte.160.11.1573. [DOI] [PubMed] [Google Scholar]
- 24.Elder J, McLelland A, O’Reilly DS, Packard CJ, Series JJ, Shepherd J. The relationship between serum cholesterol and serum thyrotropin, thyroxine and tri-iodothyronine concentrations in suspected hypothyroidism. Ann Clin Biochem. 1990;27(Pt 2):110–3. doi: 10.1177/000456329002700204. [DOI] [PubMed] [Google Scholar]
- 25.Evered DC, Ormston BJ, Smith PA, Hall R, Bird T. Grades of hypothyroidism. Br Med J. 1973;1:657–62. doi: 10.1136/bmj.1.5854.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdemarsson S, Hansson P, Hedner P, Nilsson-Ehle P. Relations between thyroid function, hepatic and lipoprotein lipase activities, and plasma lipoprotein concentrations. Acta Endocrinol (Copenh) 1983;104:50–6. doi: 10.1530/acta.0.1040050. [DOI] [PubMed] [Google Scholar]
- 27.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 28.Col NF, Surks MI, Daniels GH. Subclinical thyroid disease: Clinical applications. JAMA. 2004;291:239–43. doi: 10.1001/jama.291.2.239. [DOI] [PubMed] [Google Scholar]
- 29.Kahaly GJ. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid. 2000;10:665–79. doi: 10.1089/10507250050137743. [DOI] [PubMed] [Google Scholar]
- 30.Müller B, Zulewski H, Huber P, Ratcliffe JG, Staub JJ. Impaired action of thyroid hormone associated with smoking in women with hypothyroidism. N Engl J Med. 1995;333:964–9. doi: 10.1056/NEJM199510123331503. [DOI] [PubMed] [Google Scholar]


