Abstract
Normal blood-cell differentiation is controlled by regulated gene expression and signal transduction. Transcription deregulation due to chromosomal translocation is a common theme in hematopoietic neoplasms. AML1-ETO, which is a fusion protein generated by the 8;21 translocation that is commonly associated with the development of acute myeloid leukemia, fuses the AML1 runx family DNA-binding transcription factor to the ETO corepressor that associates with histone deacetylase complexes. Analyses have demonstrated that AML1-ETO blocks AML1 function and requires additional mutagenic events to promote leukemia. Here, we report that the loss of the molecular events of AML1-ETO C-terminal NCoR/SMRT-interacting domain transforms AML1-ETO into a potent leukemogenic protein. Contrary to full-length AML1-ETO, the truncated form promotes in vitro growth and does not obstruct the cell-cycle machinery. These observations suggest a previously uncharacterized mechanism of tumorigenesis, in which secondary mutation(s) in molecular events disrupting the function of a domain of the oncogene promote the development of malignancy.
Keywords: t(8;21), truncated protein, hematopoietic malignancy, mouse model, cell cycle
Chromosomal translocations involving transcriptional regulators and/or signaling molecules are a common theme associated with dysregulation of hematopoiesis. The transcription factor AML1 (Acute Myeloid Leukemia 1, also called RUNX1, CBFα, and PEBP2αB) and its heterodimerization partner CBFβ are frequent targets of genetic alterations or chromosomal translocations in leukemia (1–3). A common AML1 involved chromosomal translocation, t(8;21)(q22;q22), is found in 12% of all acute myeloid leukemia (AML) cases, including 40–50% of AML M2 subtype and a small portion of M0, M1, and M4 subtypes [French–American–British (FAB) classification] (4–7). The translocation brings together the DNA sequence encoding the N terminus of the AML1 with nearly all of ETO (eight–twenty–one; also called MTG8 and CBFA2T1) to generate the fusion protein AML1-ETO (8–11). AML1-ETO-involved leukemogenesis provides a challenging and excellent model to study cell proliferation and differentiation.
A critical step toward understanding many human pathological processes is to produce the human disease in animal models. Several of the mouse models of AML1-ETO expression that have been developed over the years have shown limited success in reproducing and dissecting molecular mechanisms of t(8;21)-associated AML development. Heterozygous AML1-ETO knockin mice (12, 13) died at the embryonic stage of embryonic day 12.5 (E12.5)–E13.5, failing to establish definitive hematopoiesis. Various subsequent transgenic mouse models that circumvented embryonic lethality did not develop any malignancies (14–16), indicating that AML1-ETO alone was not sufficient for leukemia development. Furthermore, by using retroviral-mediated AML1-ETO expression and bone marrow transplantation, AML1-ETO alone did not induce AML (17–19). However, treatment of MRP8-AML1-ETO transgenic mice (15) and conditional AML1-ETO knockin mice (16) with the DNA alkylating mutagen ENU resulted in the rapid development of a diseased state mimicking human t(8;21)-associated AML. In addition, bone-marrow-transplant experiments using retrovirally transduced cells with the ICSBP (Interferon Consensus Sequence Binding Protein)-deficient background or the coexpression of TEL-PDGF fusion protein cooperated with AML1-ETO in promoting AML in mice (18, 20). These results strongly support the hypothesis that AML1-ETO associated leukemogenesis requires additional genetic mutation(s).
To investigate the nature of the secondary mutations required for AML1-ETO-mediated leukemogenesis, we performed bone-marrow-transplant experiments by using hematopoietic cells infected with an AML1-ETO-expressing retrovirus. Interestingly, in the absence of any artificially induced mutations, one mouse rapidly developed AML 14 weeks after the bone marrow transplantation. A surprising finding that emerged from this study was that, because of a 1-bp insertion, the leukemic cells expressed a C-terminal 200-aa truncated form of AML1-ETO lacking a critical domain for NCoR/SMRT and ETO interaction (21–23). Unlike its full-length counterpart, the expression of AML1-ETOtr resulted in a rapid onset of leukemia in transplantation-recipient mice. The associated growth-arrest function of AML1-ETO is lost with AML1-ETOtr. In addition, the regulation of cell-cycle regulatory proteins, such as cyclin D3, cyclin A, CDK4, and CDK inhibitors p21WAF1/CIP1 and p27KIP1, by AML1-ETO and C-terminal truncated AML1-ETO are opposed. These results indicated that disruption of molecular events relating to the function of the AML1-ETO C-terminal NCoR/SMRT interacting domain promotes the onset of t(8;21)-involved leukemogenesis and offers a paradigm to the study genetic events related to cancer development.
Materials and Methods
Generation of AML1-ETO Retrovirus. The blunt ended XbaI fragment of cDNA for hemagglutinin (HA)-tagged AML1-ETO (a gift from M. Ohki, National Cancer Center Research Institute, Tokyo) was subcloned into the HpaI site of MigR1 retroviral vector (24) to form Mig-A/E (Fig. 1A). For the production of retrovirus, 293T cells were cotransfected with MigR1 vector or Mig-A/E and MCV-ecopac (25) by Ca3(PO4)2 precipitation. Retroviral supernatants were harvested 48 h after transfection, filtered through 0.45-μm filters, and stored at –80°C. The quality of the viral supernatant was assessed on total bone marrow cells because Mig-A/E is toxic to NIH 3T3 cells.
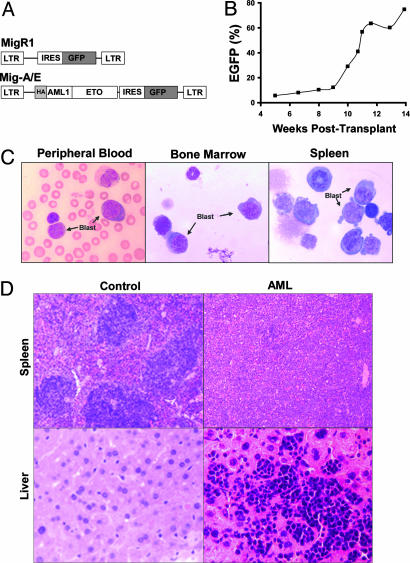
Fig. 1.
Detection of AML1-ETO-expressing cells and analysis of leukemia. (A) The structure of MigR1 and Mig-A/E retroviruses. (B) The percentage of GFP+ (AML1-ETO-expressing) cells in the peripheral blood was monitored regularly by flow cytometry. (C) Morphological analysis of hematopoietic cells from the AML mouse. Blood smear and cytocentrifugation of bone marrow and spleen cells were stained by Wright–Giemsa staining. (D) Histological analysis of spleens and livers of control and disease mice. The tissue sections were stained by hematoxylin and eosin.
Bone Marrow and Fetal Liver Cells Isolation, Retroviral Transduction, and Transplantation. Bone marrow cells were harvested from MF-1, BALB/c, or C57 mice 5 days after intraperitoneal injection with 100 mg of 5-fluorouracil (5-FU) per kg of body weight (Sigma). Alternatively, fetal liver cells were harvest from E14.5 embryos. Subsequently, 5 × 105 cells were infected with retroviruses by centrifugation (spinoculation) at 1,400 × g for 90 min at 25°C in 2 ml of retroviral supernatant supplemented with 8 μg/ml of polybrene (Sigma). The supernatant was then removed and the cells were resuspended in RPMI medium 1640 (Invitrogen) with 10% FBS (Sigma)/10 ng/ml IL-3 (PeproTech, Rocky Hill, NJ)/10 ng/ml IL-6 (Peprotech)/100 ng/ml stem cell factor (Peprotech). The cells were incubated overnight at 37°C in 5% CO2 before a second round of infection, followed by an overnight incubation. The efficiency of transduction by the various MigR1-based virus was determined on the basis of green fluorescence by flow cytometry at 24 h after the second round of spinoculation. The recipient mice were lethally irradiated with 1,000 rad in a split dose separated by 4 h. At 24 h after the second round of infection, bone marrow cells or fetal liver cells (4 × 105 cells) were transplanted into recipient mice by tail-vein injection. Mice were maintained in sterilized cages for 3 weeks and given acidified water (pH 2.7). The transplantability of the neoplasms was determined by injecting 4 × 105 bone marrow cells from the primary diseased mice into sublethally irradiated mice.
Hematological and Histological Analyses, Flow Cytometry, and Southern Blot Analyses. These studies were performed as reported (15). The levels of peripheral blood hemoglobin were measured with an Hb-Meter hemoglobinmeter (Leica, Deerfield, CT) according to the manufacturer's instructions.
Western Blot Analysis. The blots were incubated with a 1:1,000 dilution of mouse anti-HA (Covance, Princeton); a 1:1,000 dilution of rabbit anti-cyclin D3, anti-cyclin A, anti-p27, and anti-CDK4; a 1:500 dilution of rabbit anti-p21Waf1 (Santa Cruz Biotechnology); or a 1:500,000 dilution of mouse anti-mouse tubulin (Sigma).
Identification and Subcloning of the AML1-ETO Truncation. The AML1-ETO DNA was isolated from leukemia cells of AML mice transplanted with Mig-A/E by using PCR. PCR products were subcloned into pGEM-T Easy vector (Promega) and sequenced. Several independent clones were used to characterize the mutation. No mutations were identified other than the reported 1-bp insertion. The C-terminal truncated AML1-ETO expression retroviral construct was created by inserting the BamHI and blunted XbaI fragment of 3′ end of mutated HA-AML1-ETO cDNA from the AML mouse into BamHI and blunt-ended EcoRI sites in Mig-A/E to form Mig-A/Etr.
Analysis of Cell Proliferation and Differentiation. Retroviral infection and cell-cycle analysis were performed essentially as described (26). The percentage of EGFP+ cells was counted every 2 or 3 days. Amphotropic retroviruses were prepared by transfecting 293T cells in a 10-cm plate with 15 μg pCL-10A1 (27) and 10 μg MigR1 based DNA by using the Ca3(PO4)2-precipitation method. Retroviruses were harvested 60 h after the transfection. For retrovirus infection, 5 × 105 cells were resuspended in 1 ml of retrovirus-containing medium with 16 μg/ml polybrene and spun at 2,250 × g for 2 h. EGFP+ cells were sorted 48 h after the initial infection. Cells were harvested 18 h after the sorting for cell-cycle and protein expression analyses. FDCPMix cell culture and differentiation were performed as described by Spooncer et al. (28).
Results
Leukemia Development in One of the Mice Transplanted with AML1-ETO-Expressing Bone Marrow Cells. To identify and investigate the role of cooperating mutations in the development of the t(8;21) leukemia, we established a retroviral-mediated AML1-ETO expression and bone-marrow-transplant mouse model by using Mig-A/E, which expresses HA-tagged AML1-ETO (29) in MigR1 retrovirus vector (24) (Fig. 1 A). Expression of AML1-ETO alone did not induce leukemogenesis. Most bone-marrow-transplanted mice (>30 mice in all) had a low percentage of EGFP+ cells in peripheral blood. However, surprisingly, after a latency period of only 10 weeks after transplantation, one mouse showed a very rapid increase of EGFP+ cells (i.e., AML1-ETO-expressing cells in the peripheral blood, especially at the later stage of its life; Fig. 1B). The mouse eventually developed symptoms of anemia (<4 g/dl hemoglobin) and labored breathing, and it showed increased numbers of immature cells in the peripheral blood. The mouse was killed when it became moribund at 4 weeks after the onset of the disease.
Postmortem examination showed signs of nonlymphoid leukemia, including pale femurs and massive hepatosplenomegaly (0.74 g spleen and 1.96 g liver. The normal spleen and liver are ≈0.14 and 1.34 g, respectively). The lymphoid organs (thymuses and lymph nodes) were not affected by the disease. Histological examination revealed extensive infiltrations of immature blast cells in all hematopoietic organs (peripheral blood, spleen, and bone marrow) as well as liver, which destroyed the normal architectures of these tissues (Fig. 1 C and D). To assess transplantability of the hematopoietic neoplasm, we injected the leukemic cells from the diseased mouse into secondary recipient mice. These recipient mice also rapidly developed leukemia similar to the primary disease (data not shown).
Flow cytometric analysis showed that 40.9% of bone marrow cells and 75.7% of spleen cells were positive for EGFP and negative for the lymphoid and myeloid differentiation markers CD127, CD3, B220, CD11b, Gr-1, and TER119 (data not shown). The proportion of myeloid progenitors (Lin–/Sca-1–/c-Kit+) was increased greatly in EGFP+ cells (Fig. 6, which is published as supporting information on the PNAS web site). In the bone marrow, 27.8% of EGFP+ cells compared with 0.39% of EGFP– cells were Lin–/Sca-1–/c-Kit+. EGFP– cells in this leukemic mouse exhibited an almost normal distribution of three myeloid progenitor populations (Fig. 6) (30). In contrast, EGFP+ fraction in this leukemic mouse lost the typical distribution of these three populations and displayed homogenous expansion of FcγRII/IIImedCD34– cells. The features of the hematopoietic neoplasm suggest the classification of this leukemia as AML without maturation according to the classification of the hematopathology subcommittee of the Mouse Models of Human Cancers Consortium (MMHCC) (31).
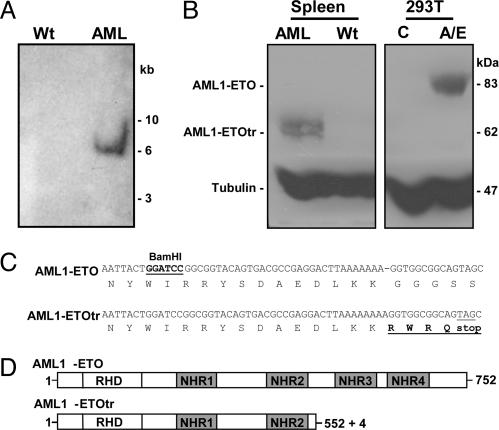
A C-Terminal Truncated AML1-ETO Is Expressed in the Leukemic Mouse. Genomic DNA was isolated from the spleen cells and analyzed by Southern blot analysis with an ETO probe to analyze the integration of the retrovirus (Fig. 2A). One major band was detectable in the leukemic mouse, suggesting an expansion of a single proviral clonal population. Surprisingly, Western blot analysis of the spleen extract and antibodies against the HA tag showed a truncated protein (62 kDa) instead of full-length AML1-ETO (84 kDa) (Fig. 2B). Furthermore, secondary bone-marrow-transplanted AML mice displayed the same 62-kDa AML1-ETO protein as in the primary sample (data not shown). Because of the N-terminal location of the HA tag in AML1-ETO, this result suggested a possible mutation in AML1-ETO that led to the early termination of protein translation. Sequencing of genomic PCR fragments from spleen DNA of both primary and secondary recipient leukemia mice revealed a single-nucleotide insertion in the AML1-ETO DNA sequence (Fig. 2C). This mutation resulted in a frame shift and the introduction of a stop codon 9 bp downstream of the insertion. These results demonstrate that the production of a shorter C-terminal truncated AML1-ETO protein in the leukemic mouse is due to a 1-bp insertion in the AML1-ETO sequence, and we named this truncated form AML1-ETOtr (A/Etr) in the following description. The predicted AML1-ETOtr protein includes AML1-ETO amino acids 1–552, plus four additional amino acids, and it does not include the NHR3 and NHR4 domains that are critical for the repressor activity of ETO and AML1-ETO (Fig. 2D) (21–23, 32).
Fig. 2.
Detection of C-terminal truncated AML1-ETO. (A) Southern blot analysis for clonality of retroviral integration in the leukemic mouse. We digested 10 μg of spleen genomic DNA from wild-type control (Wt) and AML mice with BamHI, and they were hybridized with a 32P-labeled human 5′ ETO probe. (B) A shorter version of AML1-ETO (AML1-ETOtr) was detected in the leukemic mouse. Protein extracts from the spleen of leukemic (AML) and wild-type control (Wt) mice from untransfected (C) and Mig-A/E transfected (A/E) 293T cells were used in Western blotting to analyze AML1-ETO expression with antibodies to HA epitope. Antibodies to tubulin were used to show relative protein loading. (C) A 1-bp insertion in AML1-ETO cDNA was detected in the leukemia sample. Sequence comparison of wild-type AML1-ETO and truncated AML1-ETO (AML1-ETOtr) from AML mouse is shown. The coding amino acid sequence is given under the DNA sequence. The stop codon generated in truncated AML1-ETO is underlined. The four amino acids in bold are derived from the insertional mutation. (D) Schematic representation of human AML1-ETO protein and its truncated form. The full-length protein has 752 aa. AML1 contributes amino acids 1–177; ETO gives amino acids 178–752. RHD, the runt homology domain. NHR, nervy homology region.
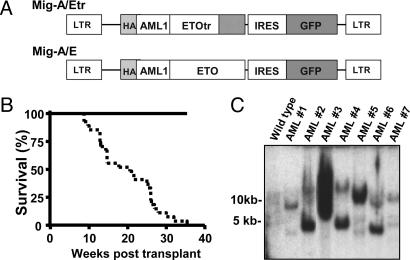
High Penetrance of AML Development with AML1-ETOtr Expression. We recreated AML1-ETOtr in the MigR1 retroviral vector (Fig. 3A) and confirmed its expression by Western blot analysis (data not shown). Transplantation experiments were performed with MigR1-, Mig-A/E-, or Mig-A/Etr-infected bone marrow cells or fetal liver cells adjusted to engraft mice with the same percentage of EGFP+ cells. At 3 weeks after transplantation, we monitored the mice for signs of disease, such as elevated EGFP+ cells and immature cells, in the peripheral blood. Mice transplanted with cells infected with Mig-A/Etr rapidly developed leukemia with phenotypes similar to the original diseased mouse (Fig. 3B and data not shown). Also, similar differential counts of the original leukemic mouse and mice transplanted with Mig-A/Etr-infected bone marrow or fetal liver cells were observed (Tables 1 and 2, which are published as supporting information on the PNAS web site). Most of their EGFP+/Lin–/Sca-1–/c-Kit+ splenocytes are FcγRII/IIImedCD34–, as in the original leukemic mouse shown in Fig. 2 (data not shown). The first Mig-A/Etr mouse became moribund with leukemia 9 weeks after the transplantation. The median survival time of Mig-A/Etr-transplanted mice (n = 27) was 20 weeks. The weak oncogenic potential of full-length AML1-ETO was emphasized further because none of the fetal liver-AML1-ETO-transplanted mice (n = 14) developed leukemia. Aside from the lineage markers used in the analysis of the original AML mouse, we found no expression of the megakaryocytic lineage marker CD41 in these AML mice (data not shown). Furthermore, Southern blot analysis with DNA prepared from spleens of seven of these AML mice showed that there was oligoclonal retroviral integration in each sample (Fig. 3C), indicating there is no association of preferential selection of retroviral integration site and AML1-ETOtr expression in leukemogenesis. However, because the median survival time is 20 weeks, additional mutations may still be necessary for leukemogenesis.
Fig. 3.
The C-terminal truncated AML1-ETO causes a rapid onset of AML. (A) Structure of Mig-A/E and Mig-A/Etr retroviruses. (B) Kaplan–Meier survival curves of MF-1 mice transplanted with retrovirus-infected fetal liver cells. The solid line represents mice that received MigR1 vector-infected cells (n = 12) or Mig-A/E-infected cells (n = 14). The dotted line represents mice that received Mig-A/Etr-infected cells (n = 27). (C) Southern blot analysis of the clonality of retroviral integration in seven leukemic mice with AML1-ETOtr. We analyzed 10 μg of spleen genomic DNA from each sample, as shown in Fig. 2 A.
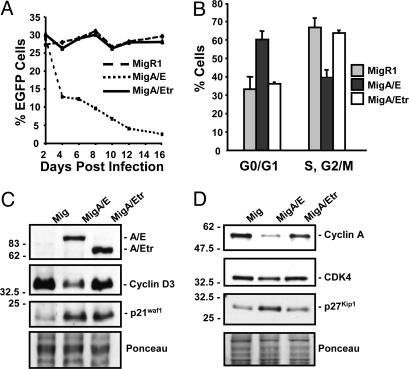
Differential Effects of AML1-ETO and AML1-ETOtr on the Cell Cycle. Multiple studies in hematopoietic cell lines have indicated the varying effects of AML1-ETO on cell cycle, apoptosis, and differentiation (33). To compare the effects of AML1-ETO and AML1-ETOtr on cell proliferation, hematopoietic K562 cells were infected with MigR1, MigA/E, and MigA/Etr. The frequency of EGFP+ cells was used to measure the relative proliferation of retrovirally infected cells to noninfected cells. A decrease of the percentage of EGFP+ cells in MigA/E-infected cells was observed (Fig. 4A). In contrast, retroviral vector MigR1- and MigA/Etr-infected cells did not show such a negative effect on proliferation (Fig. 4A). EGFP+ K562 cells were isolated by fluorescence-activated cell sorting, and they underwent cell-cycle analysis (Fig. 4B). Only MigA/E-infected cells showed a much higher percentage of cells in the G0/G1 phase of the cell cycle, indicating the blockage of G1- to S-phase transition. These data indicate that, in contrast to AML1-ETOtr, AML1-ETO causes growth arrest.
Fig. 4.
Differential effect of AML1-ETO and AML1-ETOtr on cell cycle and cell-cycle proteins. (A) At 2 days after retroviral infection, the percentage of EGFP+ K562 cells was adjusted to the same level among MigR1-, MigA/E-, and MigA/Etr-infected samples with K562 cells that also went through the infection procedure in the absence of viral supernatant. The percentage of EGFP+ cells was analyzed by fluorescence-activated cell sorting. (B) Cell-cycle analyses on sorted EGFP+ cells. The cell-cycle distribution was determined by propidium iodide staining. (C) Western blot analysis of cyclin D3 and p21WAF1/CIP1 expression. Protein samples (20 μg) prepared from sorted EGFP+ cells were used in Western blotting and probed with HA, cyclin D3, and p21WAF1/CIP1 antibodies. Ponceau staining of the blot showed the relative amount of proteins in each lane. All assays were repeated at least three times with similar results. (D) Analysis of cyclin A, CDK4, and p27KIP1 by Western blotting using samples as prepared in A.
Cyclin D3 and p21WAF1/CIP1 are two critical regulators of the cell cycle, and their promoters are known targets of AML1 (34–36). Therefore, we studied their expression in sorted EGFP+ cells. Compared with MigR1 cells, AML1-ETO cells showed a decrease of cyclin D3 expression (Fig. 4C). AML1-ETOtr cells had a similar level of cyclin D3 to MigR1 cells (Fig. 4C). Both AML1-ETO and AML1-ETOtr cells showed a similar increase in p21WAF1/CIP1 expression (Fig. 4C). We further analyzed other cell-cycle-related proteins by Western blot analysis in these sorted K562 cells. Cyclin A and CDK4 levels were decreased, and p27KIP1 was increased, in AML1-ETO cells (Fig. 4D). Cyclin D1 expression was the same in MigR1-, MigA/E-, and MigA/Etr-infected cells (data not shown). These observations suggest that the increased levels of both CDK inhibitors (p21WAF1/CIP1 and p27KIP1) and the decreased levels of cyclin D3, cyclin A, and CDK4 contribute to the growth arrest induced by AML1-ETO.
To analyze the effect of AML1-ETO and AML1-ETOtr on cell differentiation, MigR1-, MigA/E-, and MigA/Etr-infected FDCPMix cells (IL-3-dependent multipotent hematopoietic stem cell line) were stimulated for neutrophil differentiation (28). Both AML1-ETO- and AML1-ETOtr-expressed cells showed similar block of differentiation (Fig. 7, which is published as supporting information on the PNAS web site), indicating that AML1-ETO and AML1-ETOtr have a similar capacity to delay myeloid cell differentiation, although they have different effects on cell proliferation.
Discussion
Analyses of various AML1-ETO-expression mouse models indicate that AML1-ETO is necessary but not sufficient, requiring additional mutations for leukemogenesis. Here, we report that AML1-ETO, without its C-terminal 200 aa, strongly promotes leukemia development. The short time interval required for leukemia development with AML1-ETOtr indicates that the C-terminal region of AML1-ETO has negative effects on cell transformation. Additional mutations required for AML1-ETO leukemogenesis could either directly or indirectly block such negative effects of the AML1-ETO C-terminal region. This hypothesis is further supported by the negative effect of AML1-ETO C-terminal region on cell proliferation, in which AML1-ETO and AML1-ETOtr differentially regulate cell-cycle protein levels and cell-cycle progression. Additional studies did not reveal any obvious difference of AML1-ETO and AML1-ETOtr in nuclear localization and oligomer formation (data not shown). The substantial different effects of full-length and truncated AML1-ETO on leukemogenesis and cell proliferation indicate that the ETO portion of AML1-ETO fusion protein can be separated into different functional domains regarding hematopoietic cell proliferation and differentiation. This article provides an angle to study AML1-ETO in leukemia development by using additional biochemical approaches.
We reported (15) that transgenic mice with myeloid-specific MRP8-directed AML1-ETO expression and additional mutations induced by ENU developed AML. Increased numbers of CD11b+Gr-1– cells were detected in both blood and bone marrow cells from these mice. Furthermore, we also observed a block of myeloid progenitors at the transition between CMP to GMP with FcγRII/IIImedCD34+ myeloid progenitor cells (Lin–/ScaI–/c-Kit+). The AML cells in the current AML1-ETOtr model are not exactly the same as the previous MRP8-AML1/ETO leukemic cells. The EGFP+ cells from these AML1-ETOtr AML mice are mainly Lin–/ScaI–/c-Kit+ and FcγRII/IIImedCD34–. Such a pattern of surface-marker expression is characteristic of myeloid progenitors but does not exactly define typical CMP, GMP, or MEP. Because the EPO receptor but not the G-CSF receptor was detected in these cells and MPO expression was detected only in some of the leukemia samples (data not shown), the leukemia blasts may have more characteristics of MEP. In fact, AML1-ETO has been reported to block erythroid differentiation of K562 cells and primary human erythroid cells (37, 38). The different experimental strategies used to create these two AML mouse models may explain the phenotypic variations observed between them. This study used the murine stem cell virus as a vehicle, targeting AML1-ETOtr expression in early stem cells and progenitor cells, whereas in the previous study, MRP8-AML1/ETO expression occurred later in the hematopoietic pathway. Also, the mutations induced by the treatment of ENU in the MRP8 study could also account for the differences in leukemic phenotype observed between the two models. Furthermore, in the MRP8-AML1/ETO model, the induced mutations may block the function of the AML1-ETO C-terminal domain only partially, whereas in the current model, the whole C-terminal domain of AML1-ETO is missing.
Also of interest, we also detected the expression of a 60-kDa form of a C-terminal truncated AML1-ETO protein as the only expressed form of AML1-ETO from another leukemic mouse. This mouse was transplanted with cells infected with full-length AML1-ETO retrovirus (data not shown). The leukemic phenotype of this mouse was similar to AML1-ETOtr mice. However, we were not able to detect any DNA mutation to explain the shorter version of AML1-ETO. It is possible that this 60-kDa protein was generated because of the abnormal expression of a protease in early hematopoietic progenitors. Such a possibility is well supported by a recent report from Lane and Ley (39) in which neutrophil elastase is involved in the cleavage and development of PML-RARα-fusion-protein-induced acute promyeloid leukemia.
The most striking characteristic of AML1-ETOtr is the lack of a C terminus of ETO containing the NHR3 and NHR4 domains. Three groups independently reported a critical role involving NHR4 in ETO interaction with the transcription corepressors NCoR and SMRT (21–23). Such interactions allow AML1-ETO to form complexes with histone deacetylase (HDAC)-blocking AML1 target-gene expression. Because AML1-ETOtr does not contain this important region for interacting with NCoR/SMRT and is more potent in leukemogenesis, it is necessary to consider additional mechanisms of leukemogenesis for AML1-ETO. The remaining ETO portion of AML1-ETOtr also interacts directly with negative regulators (40, 41). These interactions may contribute to the remaining negative effect of AML1-ETOtr on AML1 target-gene expression. Although most studies support the fact that AML1-ETO dominantly blocks AML1 function, some reports suggest that AML1-ETO also activates gene expression either in the absence or in the presence of AML1 (42–44). Furthermore, the ETO portion of AML1-ETO also interacts with PLZF, Gfi-1, and E proteins (40, 45, 46). Such interactions dysregulate the normal function of these factors and may contribute to leukemogenesis independently of AML1-ETO DNA-binding activity. Therefore, AML1-ETO properties may extend beyond dominant-negative repressive functions related to AML1 target genes in leukemogenesis.
Alternatively spliced forms of AML1-ETO transcripts have been described in t(8;21) AML-patient samples (47–50). Their involvement in leukemogenesis has been overlooked. Interestingly, Wolford and Prochazka (51) described an abundantly expressed and alternatively spliced transcript of ETO resulting from the inclusion of a 155-bp exon (designated exon 9a). The predicted AML1-ETO protein using exon 9a (AML1-ETO9a) has 575 aa and does not include the NHR3 and NHR4 domains, making it almost identical to AML1-ETOtr.
AML1-ETO suppresses both cell proliferation and differentiation (26). The negative effect of full-length AML1-ETO on the cell cycle does not favor leukemogenesis. Here, we show that AML1-ETOtr did not have a negative effect on the cell cycle (Fig. 4). Most importantly, such different effects on the cell cycle between AML1-ETO and AML1-ETOtr are linked directly to their effects on the expression of cell-cycle regulators. p21WAF1/CIP1 and p27KIP1 are potent repressors of the G1/S transition when associated with cyclin A/E-CDK2, and p21WAF1/CIP1 is a positive regulator for the G1/S transition when in complex with cyclin D/CDK4 (34). One of the important roles of cyclin D in cell-cycle regulation is to sequester p21WAF1/CIP1 away from cyclin A/E-CDK2 (34). Therefore, the combinatory effects of increased p21WAF1/CIP1and p27KIP1 accompanied by decreased cyclin D3 and cyclin A due to full-length AML1-ETO expression are indicative of the molecular events required for the observed delay of cell cycle. These changes in cell-cycle regulatory proteins are not associated with AML1-ETOtr, thereby allowing a permissive situation for AML1-ETOtr to promote leukemia (Fig. 5). The observation that a truncated form of AML1-ETO could act as a dominant factor in promoting AML is an example in the understanding of tumorigenesis in which the loss of molecular events associated with the function of the C-terminal NCoR/SMRT-interacting domain of AML1-ETO promotes beneficial conditions for t(8;21) leukemogenesis. Various scenarios could be envisioned for such loss of molecular events by additional mutations leading to (i) the dysfunction of AML1-ETO C-terminal domain by truncations or mutations in AML1-ETO directly, (ii) the reduction of factor(s) physically interacting with the C-terminal domain of AML1-ETO, or (iii) changes of signaling pathways further downstream of AML1-ETO C-terminal domain (Fig. 5). All of these possible changes can reduce the negative effect of AML1-ETO C-terminal region on leukemia development and facilitate t(8;21) associated neoplasm. Therefore, AML1-ETO C-terminal deletion is not the only possible additional mutation related to the dysfunction of the C-terminal domain. The next challenge in understanding t(8;21) associated leukemogenesis would be to characterize the signaling pathways related to multiple functional domains of AML1-ETO.
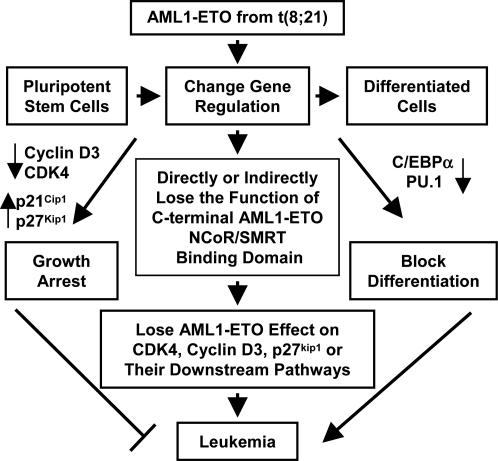
Fig. 5.
Model of secondary mutational event(s) in t(8;21) leukemogenesis. After the acquisition of the t(8;21) chromosomal abnormality, AML1-ETO suppresses both cell proliferation and differentiation by means of the change of the expression of cell-cycle and differentiation regulators. Such dichotomous effect is not sufficient for leukemogenesis. Additional mutations are required to promote leukemia. These mutations could directly or indirectly affect molecular events associated with the function of AML1-ETO C-terminal NCoR/SMRT interacting domain, including the change of the expression of cell-cycle regulators, such as CDK4, cyclin D3, and p27kip1, to release the negative effect of full-length AML1-ETO to cell cycle and promote leukemia development. Such mutations may result in the expression of AML1-ETO without its C-terminal portion (such as alternative RNA splicing or DNA point mutation), disruption of the interaction of AML1-ETO C-terminal region with its binding partners (such as NCoR and SMRT), dysregulated expression of AML1-ETO target-gene expression (such as p21), or changes of signaling pathways further downstream from the primary AML1-ETO targets.
Supplementary Material
Acknowledgments
We thank Drs. Jon Aster (Harvard Medical School, Boston), Paul Burgoyne (National Institute for Medical Research, London), Alan Friedman (The Johns Hopkins University, Baltimore), Scott Hiebert (Vanderbilt University School of Medicine, Nashville, TN), Timothy Ley (Washington University School of Medicine, St. Louis), Misao Ohki, Warren Pear (University of Pennsylvania Medical Center, Philadelphia), Daniel Tenen (Harvard Institutes of Medicine, Boston), Bert Vogelstein (The Johns Hopkins University Medical Institutions, Baltimore), Xiao-Fan Wang (Duke University Medical Center, Durham, NC), Malcolm Wood (The Scripps Research Institute), and members from their laboratories for valuable discussion, DNA constructs, and mice. This work was supported by National Institutes of Health Grants CA72009 (to K.A. and D.-E.Z.) and CA96735 (to D.-E.Z.). L.F.P. is a Skaggs Postdoctoral Fellow at The Scripps Research Institute. The Stein Endowment Fund has partially supported the Molecular and Experimental Medicine departmental molecular biology service laboratory for DNA sequencing and oligonucleotide synthesis. This is manuscript 16043-MEM from The Scripps Research Institute.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AML, acute myeloid leukemia; En, embryonic day n; HA, hemagglutinin.
See Commentary on page 16985.
References
- 1.Rowley, J. D. (2001) Nat. Rev. Cancer 1, 245–250. [DOI] [PubMed] [Google Scholar]
- 2.Downing, J. R. (2001) Leukemia 15, 664–665. [DOI] [PubMed] [Google Scholar]
- 3.Kundu, M. & Liu, P. P. (2001) Curr. Opin. Hematol. 8, 201–205. [DOI] [PubMed] [Google Scholar]
- 4.Nucifora, G. & Rowley, J. D. (1995) Blood 86, 1–14. [PubMed] [Google Scholar]
- 5.Langabeer, S. E., Walker, H., Rogers, J. R., Burnett, A. K., Wheatley, K., Swirsky, D., Goldstone, A. H. & Linch, D. C. (1997) Br. J. Haematol. 99, 925–928. [DOI] [PubMed] [Google Scholar]
- 6.Rege, K., Swansbury, G. J., Atra, A. A., Horton, C., Min, T., Dainton, M. G., Matutes, E., Durosinmi, M., Treleaven, J. G., Powles, R. L., et al. (2000) Leuk. Lymphoma 40, 67–77. [DOI] [PubMed] [Google Scholar]
- 7.Rowe, D., Cotterill, S. J., Ross, F. M., Bunyan, D. J., Vickers, S. J., Bryon, J., McMullan, D. J., Griffiths, M. J., Reilly, J. T., Vandenberghe, E. A., et al. (2000) Br. J. Haematol. 111, 1051–1056. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi, H., Shimizu, K., Kozu, T., Maseki, N., Kaneko, Y. & Ohki, M. (1991) Proc. Natl. Acad. Sci. USA 88, 10431–10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson, P., Gao, J., Chang, K. S., Look, T., Whisenant, E., Raimondi, S., Lasher, R., Trujillo, J., Rowley, J. & Drabkin, H. (1992) Blood 80, 1825–1831. [PubMed] [Google Scholar]
- 10.Nisson, P. E., Watkins, P. C. & Sacchi, N. (1992) Cancer Genet. Cytogenet. 63, 81–88. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi, H., Kozu, T., Shimizu, K., Enomoto, K., Maseki, N., Kaneko, Y., Kamada, N. & Ohki, M. (1993) EMBO J. 12, 2715–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yergeau, D. A., Hetherington, C. J., Wang, Q., Zhang, P., Sharpe, A. H., Binder, M., Marin-Padilla, M., Tenen, D. G., Speck, N. A. & Zhang, D. E. (1997) Nat. Genet. 15, 303–306. [DOI] [PubMed] [Google Scholar]
- 13.Okuda, T., Cai, Z., Yang, S., Lenny, N., Lyu, C., van Deursen, J. M. A., Harada, H. & Downing, J. R. (1998) Blood 91, 3134–3143. [PubMed] [Google Scholar]
- 14.Rhoades, K. L., Hetherington, C. J., Harakawa, N., Yergeau, D. A., Zhou, L., Liu, L. Q., Little, M. T., Tenen, D. G. & Zhang, D. E. (2000) Blood 96, 2108–2115. [PubMed] [Google Scholar]
- 15.Yuan, Y., Zhou, L., Miyamoto, T., Iwasaki, H., Harakawa, N., Hetherington, C. J., Burel, S. A., Lagasse, E., Weissman, I. L., Akashi, K., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 10398–10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higuchi, M., O'Brien, D., Kumaravelu, P., Lenny, N., Yeoh, E. J. & Downing, J. R. (2002) Cancer Cells 1, 63–74. [DOI] [PubMed] [Google Scholar]
- 17.de Guzman, C. G., Warren, A. J., Zhang, Z., Gartland, L., Erickson, P., Drabkin, H., Hiebert, S. W. & Klug, C. A. (2002) Mol. Cell. Biol. 22, 5506–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwieger, M., Lohler, J., Friel, J., Scheller, M., Horak, I. & Stocking, C. (2002) J. Exp. Med. 196, 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulloy, J. C., Cammenga, J., Berguido, F. J., Wu, K., Zhou, P., Comenzo, R. L., Jhanwar, S., Moore, M. A. & Nimer, S. D. (2003) Blood 102, 4369–4376. [DOI] [PubMed] [Google Scholar]
- 20.Grisolano, J. L., O'Neal, J., Cain, J. & Tomasson, M. H. (2003) Proc. Natl. Acad. Sci. USA 100, 9506–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutterbach, B., Westendorf, J. J., Linggi, B., Patten, A., Moniwa, M., Davie, J. R., Huynh, K. D., Bardwell, V. J., Lavinsky, R. M., Rosenfeld, M. G., et al. (1998) Mol. Cell. Biol. 18, 7176–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelmetti, V., Zhang, J., Fanelli, M., Minucci, S., Pelicci, P. G. & Lazar, M. A. (1998) Mol. Cell. Biol. 18, 7185–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, J., Hoshino, T., Redner, R. L., Kajigaya, S. & Liu, J. M. (1998) Proc. Natl. Acad. Sci. USA 95, 10860–10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pear, W. S., Miller, J. P., Xu, L., Pui, J. C., Soffer, B., Quackenbush, R. C., Pendergast, A. M., Bronson, R., Aster, J. C., Scott, M. L., et al. (1998) Blood 92, 3780–3792. [PubMed] [Google Scholar]
- 25.Li, S., Ilaria, R. L., Jr., Million, R. P., Daley, G. Q. & Van Etten, R. A. (1999) J. Exp. Med. 189, 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burel, S. A., Harakawa, N., Zhou, L., Pabst, T., Tenen, D. G. & Zhang, D. E. (2001) Mol. Cell. Biol. 21, 5577–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naviaux, R. K., Costanzi, E., Haas, M. & Verma, I. M. (1996) J. Virol. 70, 5701–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spooncer, E., Heyworth, C. M., Dunn, A. & Dexter, T. M. (1986) Differentiation (Berlin) 31, 111–118. [DOI] [PubMed] [Google Scholar]
- 29.Kitabayashi, I., Ida, K., Morohoshi, F., Yokoyama, A., Mitsuhashi, N., Shimizu, K., Nomura, N., Hayashi, Y. & Ohki, M. (1998) Mol. Cell. Biol. 18, 846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akashi, K., Traver, D., Miyamoto, T. & Weissman, I. L. (2000) Nature 404, 193–197. [DOI] [PubMed] [Google Scholar]
- 31.Kogan, S. C., Ward, J. M., Anver, M. R., Berman, J. J., Brayton, C., Cardiff, R. D., Carter, J. S., de Coronado, S., Downing, J. R., Fredrickson, T. N., et al. (2002) Blood 100, 238–245. [DOI] [PubMed] [Google Scholar]
- 32.Lutterbach, B., Sun, D., Schuetz, J. & Hiebert, S. W. (1998) Mol. Cell. Biol. 18, 3604–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson, L. F. & Zhang, D. E. (2004) Oncogene 23, 4255–4262. [DOI] [PubMed] [Google Scholar]
- 34.Sherr, C. J. & Roberts, J. M. (1999) Genes Dev. 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- 35.Bernardin-Fried, F., Kummalue, T., Leijen, S., Collector, M. I., Ravid, K. & Friedman, A. D. (2004) J. Biol. Chem. 279, 15678–15687. [DOI] [PubMed] [Google Scholar]
- 36.Lutterbach, B., Westendorf, J. J., Linggi, B., Isaac, S., Seto, E. & Hiebert, S. W. (2000) J. Biol. Chem. 275, 651–656. [DOI] [PubMed] [Google Scholar]
- 37.Le, X. F., Claxton, D., Kornblau, S., Fan, Y. H., Mu, Z. M. & Chang, K. S. (1998) Eur. J. Haematol. 60, 217–225. [DOI] [PubMed] [Google Scholar]
- 38.Tonks, A., Pearn, L., Tonks, A. J., Pearce, L., Hoy, T., Phillips, S., Fisher, J., Downing, J. R., Burnett, A. K. & Darley, R. L. (2003) Blood 101, 624–632. [DOI] [PubMed] [Google Scholar]
- 39.Lane, A. A. & Ley, T. J. (2003) Cell 115, 305–318. [DOI] [PubMed] [Google Scholar]
- 40.McGhee, L., Bryan, J., Elliott, L., Grimes, H. L., Kazanjian, A., Davis, J. N. & Meyers, S. (2003) J. Cell Biochem. 89, 1005–1018. [DOI] [PubMed] [Google Scholar]
- 41.Amann, J. M., Nip, J., Strom, D. K., Lutterbach, B., Harada, H., Lenny, N., Downing, J. R., Meyers, S. & Hiebert, S. W. (2001) Mol. Cell. Biol. 21, 6470–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klampfer, L., Zhang, J., Zelenetz, A. O., Uchida, H. & Nimer, S. D. (1996) Proc. Natl. Acad. Sci. USA 93, 14059–14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhoades, K. L., Hetherington, C. J., Rowley, J. D., Hiebert, S. W., Nucifora, G., Tenen, D. G. & Zhang, D. E. (1996) Proc. Natl. Acad. Sci. USA 93, 11895–11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu, K., Kitabayashi, I., Kamada, N., Abe, T., Maseki, N., Suzukawa, K. & Ohki, M. (2000) Blood 96, 288–296. [PubMed] [Google Scholar]
- 45.Melnick, A., Carlile, G. W., McConnell, M. J., Polinger, A., Hiebert, S. W. & Licht, J. D. (2000) Blood 96, 3939–3947. [PubMed] [Google Scholar]
- 46.Zhang, J., Kalkum, M., Yamamura, S., Chait, B. T. & Roeder, R. G. (2004) Science 305, 1286–1289. [DOI] [PubMed] [Google Scholar]
- 47.Tighe, J. E. & Calabi, F. (1994) Blood 84, 2115–2121. [PubMed] [Google Scholar]
- 48.van de Locht, L. T., Smetsers, T. F., Wittebol, S., Raymakers, R. A. & Mensink, E. J. (1994) Leukemia 8, 1780–1784. [PubMed] [Google Scholar]
- 49.Kozu, T., Miyoshi, H., Shimizu, K., Maseki, N., Kaneko, Y., Asou, H., Kamada, N. & Ohki, M. (1993) Blood 82, 1270–1276. [PubMed] [Google Scholar]
- 50.Era, T., Asou, N., Kunisada, T., Yamasaki, H., Asou, H., Kamada, N., Nishikawa, S., Yamaguchi, K. & Takatsuki, K. (1995) Genes Chromosomes. Cancer 13, 25–33. [DOI] [PubMed] [Google Scholar]
- 51.Wolford, J. K. & Prochazka, M. (1998) Gene 212, 103–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.