The article by Boles et al. (1) in a recent issue of PNAS is profoundly important because it addresses a major change in the etiology of human bacterial diseases that has passed unnoticed during the last half of the past century. Acute diseases caused by mobile cells of specialized pathogens have largely disappeared because we have identified the pathogens and countered with vaccines and antibiotics. Diphtheria, typhoid fever, and posttraumatic gangrene have ceased to threaten us. However, these acute epidemic diseases have been largely replaced (2) by environmental organisms that gain a foothold in the human body, especially in compromised organs (like the lung in cystic fibrosis), and initiate the invidious twin processes of inflammation and chronic disease. The new microbial enemies are common and ubiquitous in nature, they live in protected biofilms where they resist antibiotics and host defenses, and they can mount small or large acute attacks on the host that may eventually succeed when his or her defenses are depleted.
The “Insurance Hypothesis” in Biofilms in Bacterial Disease
In their article, Boles et al. (1) relate modern biofilm diseases to the basic ecological principles that govern all forms of life on the planet, and they wisely invoke the insurance hypothesis that is well established in general ecology (3). Microbial ecology has not yet embraced either the concepts or the methods developed to study the ecology of higher plants and animals, so microbial ecologists stand to learn every bit as much from this masterful review as do the medical ecologists to whom it may seem to be directed. When a human is attacked by bacteria, our elaborate innate and acquired defense mechanisms swing into action, and the attackers soon become defenders, as cytokines immobilize phagocytes, and defensins and antibodies flood the area and kill the invaders. If the threatening agents are homogeneous, and if they operate as individuals, the fight is won or lost in a matter of hours, and a successful defense leaves the human better prepared for another attack from the same source. If the threatening agents operate from a defended foothold in the host's tissues, and if the host's counterattack is unsuccessful, the aggressors will regroup for a series of attacks that will continue until the host's defenses are weakened sufficiently for them to succeed. Bacterial aggressors become beleaguered defenders when the human body counterattacks, and bacteria living in biofilms are subjected to relentless attacks by the host defenses, and by the antibiotics we have increasingly coopted to assist us in these life and death struggles.
The Boles et al. (1) article addresses a set of issues of pivotal importance in the etiology and persistence of the chronic biofilm infections that now comprise 65–80% (2) of the bacterial infections treated by physicians in the developed world. However, its much broader central theme is the extension of the well established insurance hypothesis from science-based ecology into the fields of microbial ecology and infectious diseases. They succeed because they restate the hypothesis very clearly and because they ask and answer critical questions in the experiments they present as evidence. The central question is: “Is genomic diversity helpful to biological communities under attack?” The answer is that biological communities generate genomic diversity when they are threatened, and that this diversity assures much higher rates of survival when the community is challenged by specific lethal antagonists. The question and the answer are equally unequivocal, and neither needs expansion in this Commentary.
Broader Implications of the Insurance Hypothesis in Biofilms in the Environment
What does elicit comment is the significance of this concept in science and medicine. When our concept of bacteria in natural and pathogenic ecosystems consisted of seeing them as individual cells swimming or floating in bulk fluid, and occasionally adhering temporarily to available surfaces, we assigned a single phenotype to a limited number of clones. Because successful clones would outgrow less successful clones in a particular environment, the environment would elicit a suitable phenotype from a limited number of clones, and all of the bacterial cells of a given species would resemble each other very closely. An effective antibacterial agent would wreak havoc in such a homogeneous population, killing bacterial cells in the open and in all of their hiding places. This was the mindset that guided the thinking of both experts and dilettantes, from the mid-1850s until recent times, and it persists in some circles even today.
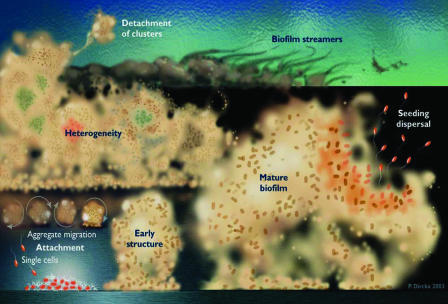
This simple image and the position of bacteria in the grand scheme of life were both irrevocably changed when direct observations of bacteria growing in natural and pathogenic ecosystems (4) showed that they grow predominantly in matrix-enclosed biofilms. Homogeneous legions of planktonic cells are rare and are virtually confined to the nutrient-poor ecosystems of the deep oceans (5) and the deep subsurface, and all bacteria that live in the biosphere and in contact with plants and animals grow in sessile mixed-species communities. Our concept of these biofilms has evolved from one of cells randomly immobilized throughout an exopolysaccharide matrix (6) to one of highly structured communities of amazing structural and functional sophistication (7). Biofilms can be visualized (Fig. 1) as arrays of microcolonies that arise from the colonization of a surface by planktonic cells that then adopt a radically different biofilm phenotype (8) and produce matrix material until this “slime” comprises as much as 85% of the volume of the biofilm. These sessile communities are intersected by water channels (9) that constitute primitive circulatory systems, and individual cells communicate with cells of the same and of other species by chemical signals (10) that regulate cell distribution and community architecture. Cells with peculiar metabolic capabilities cooperate with cells with reciprocal capabilities (Fig. 1, the middle portion) to form highly efficient physiological consortia, whereas other cells merely persist in the anaerobic depths of the community (11) and await opportunities for growth in different environmental circumstances. Each cell in a biofilm exists in its own environmental “niche” (12), and the whole community contains cells of a huge variety of clonal variants of a large number of bacterial species, expressing a spectrum of phenotypes that range from fast-growing to virtually dormant. Microelectrode studies of living biofilms have shown that aerobic cells live in immediate juxtaposition with fastidious anaerobes (11), whereas cells in adjacent areas of biofilms “see” a very wide variety of concentrations of both protons (pH) and metal ions (13). This amazing diversity of bacterial phenotypes in biofilms is further increased by horizontal gene transfer between sessile cells immobilized in matrices, which is actually seen to be as much as 1,000 times more frequent (14) than that seen between planktonic cells dancing to the Brownian music in fluids.
Fig. 1.
Artist's conception of formation, maturation, and dynamics of microbial biofilms. In the foreground, a single-species biofilm formation is initiated by the adhesion of planktonic cells to a surface, and their subsequent production of matrix material to form a mature community, from which planktonic cells can emerge by simple reversion from the biofilm to the planktonic phenotype is shown. In the middle portion, in the majority of natural biofilms, bacterial cells with complementary metabolic capabilities develop into mixed-species microcolonies that can carry out very complex substrate conversions with remarkable speed and efficiency. At the top, the material properties of biofilms are similar to those of viscous solids, and these sessile communities respond to shear forces by elastic deformation and oscillation on the surfaces to which they are attached by various hold-fast structures. [Reproduced with permission from ref. 16 (Copyright 2003, Future Drugs, Ltd.)]. Image courtesy of Peg Dirckx (Center for Biofilm Engineering, Montana State University, Bozeman).
If we then join Boles et al. (1) in the exploration of the application of the insurance hypothesis, we can start with the predominance of biofilms in the primitive earth, where the stable juxtaposition of cells provided by this mode of growth presented the first bacterial antagonists with targets of infinite complexity. Because bacteriophage and free-living amoebae joined metals and toxic chemicals among bacterial antagonists, the inherent functional diversity of biofilms protected them from complete killing, just as it protects biofilm bacteria in chronic infections from host defenses and modern antibiotics (15). Each time we kill 99% of the bacteria in a pathogenic biofilm, only to have the populations rebound by the growth of the survivors in the resultant puree of the dead bodies of their compatriots, we repeat the phylogeny of bacteria. The insurance hypothesis elucidated by Boles et al. provides a useful rationale that explains the role of biofilms in the evolution of natural and pathogenic bacterial communities as we know them today. They predominate in ecosystems ranging from the most sybaritic (the human mouth) to the most severe (the dry valleys of Antarctica), and their numbers are cogent evidence of their success. Boles et al. are students of the etiology of modern bacterial diseases, and this extension of the insurance hypothesis also provides a particularly useful rationale for our understanding of this important process. From the moment they are sensed by the host, bacteria growing in biofilms come under attack by innate host defenses, and later by acquired host defenses and by antibiotics, and their inherent diversity ensures that some individuals survive each attack. If the defenses succeed, the war is quickly finished, and the host is better prepared for another attack. However, the number of people who suffer from chronic infections (e.g., otitis media and prostatitis) attests to the frequent failure of these molecular and cellular defenses, and to the importance of ecological theory in the etiology of chronic diseases caused by bacteria growing in biofilms.
Good things happen when medical scientists like Pradeep Singh (1) have the imagination to mobilize the time-tested concepts of sciences like ecology to help them understand severe challenges that their patients experience every day. Also, an ancillary benefit occurs when perceptive scientists come to see the evolving patterns of bacterial disease in humans as a valuable experiment that spans the past two centuries. Initially, we gained control of acute epidemic diseases, by the use of vaccines, and then of antibiotics, and our counterattack succeeded against legions of planktonic cells that lacked insurance because they lacked diversity. Ubiquitous environmental organisms then colonized the tissues of compromised individuals and formed biofilms in which the high level of diversity of both phenotypes and genotypes provided insurance for bacterial survival, and the rest is history. This elegant extension of the insurance hypothesis to microbial ecology provides insight into the interactions of bacteria with other organisms in virtually all ecosystems, ranging from simple soils to the complex life webs of the Great Barrier Reef.
See companion article on page 16630 in issue 47 of volume 101.
References
- 1.Boles, B. R., Thoendel, M. & Singh, P. K. (2004) Proc. Natl. Acad. Sci. USA 101, 16630–16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton, J. W., Stewart, P. S. & Greenberg, E. P. (1999) Science 284, 1318–1322. [DOI] [PubMed] [Google Scholar]
- 3.McCann, K. S. (2000) Nature 406, 228–233. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence, J. R., Korber, D. R., Hoyle, B. D. & Costerton, J. W. (1991) J. Bacteriol. 173, 6558–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjelleberg, S. (1993) Starvation in Bacteria (Plenum, New York).
- 6.Costerton, J. W., Geesey, G. G. & Cheng, K.-J. (1978) Sci. Am. 238, 86–95. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W. & Stewart, P. S. (2001) Sci. Am. 285, 75–81. [DOI] [PubMed] [Google Scholar]
- 8.Sauer, K. & Camper, A. K. (2001) J. Bacteriol. 183, 6579–6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoodley, P., deBeer, D. & Lewandowski, Z. (1994) Appl. Environ. Microbiol. 60, 2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, D. G., Parsek, M. R., Pearson, J. P., Iglewski, B. H., Costerton, J. W. & Greenberg, E. P. (1998) Science 280, 295–298. [DOI] [PubMed] [Google Scholar]
- 11.Lewandowski, Z., Lee, W., Characklis, W. G. & Little, B. (1989) Corrosion 45, 92–98. [Google Scholar]
- 12.Costerton, J. W., Lewandowski, Z., deBeer, D., Caldwell, D., Korber, D. & James, G. (1994) J. Bacteriol. 176, 2137–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costerton, J. W. (2000) in Nonculturable Microorganisms in the Environment, eds. Colwell, R. R. & Grimes, D. J. (Am. Soc. Microbiol., Washington, DC), pp. 131–145.
- 14.Ghigo, J.-M. (2001) Nature 412, 442–445. [DOI] [PubMed] [Google Scholar]
- 15.Costerton, J. W., Veeh, R., Shirtliff, M., Pasmore, M., Post, C. & Ehrlich, G. (2003). J. Clin. Invest. 112, 1466–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fux, C. A., Stoodley, P., Hall-Stoodley, L. & Costerton, J. W. (2003) Expert. Rev. Anti. Infect. Ther. 1, 667–683. [DOI] [PubMed] [Google Scholar]