Abstract
Background
The influence of childbearing in the development of obesity is situated within two different but related contexts: pregnancy-related weight gain and weight gain prevention and control in young adult women. Pregnancy related weight gain contributes to long-term weight retention in childbearing women.
Objective
To present the study design, data collection procedures, recruitment challenges, and the baseline characteristics for the eMoms of Rochester study, a randomized clinical trial testing the effect of electronically-mediated behavioral interventions to prevent excessive gestational weight gain (GWG) and postpartum weight retention among women aged 18–35 years of diverse income and racial/ethnic backgrounds in an urban setting.
Design
Randomized double blind clinical trial. A total of 1,722 women at or below 20 weeks gestation were recruited primarily from obstetrics practices and randomized to 3 treatment groups: control arm; intervention arm with access to intervention during pregnancy and control at postpartum (e-intervention 1); and intervention arm with access to intervention during pregnancy and postpartum (e-intervention 2). Enrollment and consent were completed via study staff or online. Data were collected via online surveys, medical charts, and measurement of postpartum weights. The primary endpoints are gaining more weight than recommended by the Institution of Medicine guidelines and weight retained at 12 months postpartum.
Conclusion
This study will provide evidence on the efficacy of behavioral interventions in the prevention of excessive GWG and postpartum weight retention with potential dissemination to obstetrics practices and/or health insurances.
Keywords: gestational weight gain, maternal weight, pregnancy weight, postpartum weight retention, internet, randomized clinical trial
INTRODUCTION
The influence of pregnancy in the development of obesity is at the intersection of two research areas: pregnancy-related weight gain and weight gain prevention and control in young adult women. Approximately 53% of pregnant women gain more weight during pregnancy[1] than recommended by the Institute of Medicine (IOM) (Table I).[2] Excessive gestational weight gain (GWG) is associated with maternal and offspring morbidity, including postpartum weight retention and childhood obesity.[3–10] Longitudinal data indicate that women gain a considerable amount of weight during their twenties and thirties[11] and that parity is associated with weight and waist circumference increases.[12]
Table 1.
Recommended Gestational Weight Gain (IOMa)
| Pre-pregnancy BMI | Total Weight Gain Range in kg (pounds) |
|---|---|
| Underweight (< 18.5 kg/m2) | 12.5 – 18 (28–40) |
| Healthy weight (18.5 – 24.9 kg/m2) | 11.5 – 16 (25–35) |
| Overweight (25.0 – 29.9 kg/m2) | 7 – 11.5 (15–25) |
| Obese (≥30.0 kg/m2) | 5 – 9 (11–20) |
Weight Gain During Pregnancy: Reexamining the Guidelines.
Reprinted with permission from the National Academies Press, Copyright 2009, National Academy of Sciences.
Institute of Medicine
Several trials have tested the effect of lifestyle interventions during pregnancy on weight accumulation. Randomized and non-randomized trials suggest that educational interventions comprised of physical activity (PA) and dietary counseling during pregnancy, commonly combined with weight monitoring, may lower the risk of excessive GWG[13–15]. Similarly, postpartum trials of both diet and PA or diet alone were effective in minimizing weight retention after childbirth[16]. To our knowledge, few studies have examined the effect of a combined pregnancy and postpartum intervention on GWG and postpartum weight retention[17] adapting the interventions to the lifestyle changes involved in transitioning from pregnancy to postpartum life [18]. eMoms of Rochester trial (eMoms) was designed to fill this gap.
The objective of the eMoms’ study was to slow the accumulation of weight in childbearing women during pregnancy and the postpartum period by decreasing the prevalence of excessive GWG and minimizing postpartum weight retention in a socio-economically and racially/ethnically diverse sample of women. eMoms is part of the National Heart, Lung, and Blood Institute Early Adult Reduction of weight through LifestYle interventions (EARLY) consortium of weight control studies that tested innovative intervention approaches that incorporated digital technologies among young adults aged 18–35 to facilitate recruitment and retention. [19] In addition to using digital technologies for most of the study procedures (e.g., enrollment, intervention delivery, adverse event reporting), eMoms has other unique features such as a three arm design, a large sample size to examine differential effects by subgroup, and a long follow-up period. The purpose of this report is to present the eMoms study design, data collection procedures, recruitment challenges, and the baseline characteristics of the study sample.
MATERAIL AND METHODS
Study Design
The goals of the eMoms interventions were to decrease the prevalence of excessive pregnancy weight gain and to decrease the amount of postpartum weight retention. The efficacy of the interventions was evaluated in a double blind, randomized, controlled effectiveness trial with a parallel group design in which the individual is the unit of randomization and analysis. The study was conducted in a metropolitan county in upstate NY, and started in 2009 with a projected duration of 5 years and consisting of two phases. In phase I (February, 2010 to May, 2011), two electronic behavioral change interventions for weight management and control in pregnancy and postpartum were developed based on formative research that investigated access and use of electronic communication media, women’s preferences for intervention features (e.g.; weight gain tracker), barriers, strategies, and topics of interest and their potential differences between income groups.[20–22] In phase II, 1722 pregnant women aged 18–35 in the first 20 weeks of pregnancy were randomized to 3 arms: e-intervention group 1 receiving the electronic intervention during pregnancy only; e-intervention group 2 receiving the electronic intervention during pregnancy and postpartum; and a control group who was exposed to electronic material unrelated to weight management. Recruitment for Phase II started in May 2011 and lasted 14 months.
The primary hypotheses are: 1) the proportion of women who experience excessive GWG will be 10 percentage points lower in the intervention compared to the control conditions; 2) the control group will have an average weight retention of at least 4 pounds greater at 12 months postpartum compared to e-intervention group 1 and e-intervention group 2. By having two intervention groups, we will test whether interventions during pregnancy have continued effects on postpartum weight retention or whether interventions adapted to women’s new life demands after pregnancy are needed to minimize weight retention after delivery. Secondary research questions include other weight outcomes (e.g. rate of gain by trimester, returning to early pregnancy weight, and retaining 5 pounds or more) and behavioral mediators of intervention effects. The protocol was approved by the University of Rochester Research Subject Review Board and the Cornell University Institutional Review Board.
Source Population
The county has a population of 75,468 females aged 20–34 (Census 2010). [23] Based on the number of total births in the county in 2007, there were a prorated 10,851 births during 15 months (the study accrual time) of which 5,765 were term singleton births to women aged 18 to 35 with BMI 18.5 to <35.0 kg/m2 without weight-affecting medical conditions according to data from a local population-based perinatal registry.[24]
Eligibility criteria
Inclusion and exclusion criteria are listed in Table 2 and were self-reported by participants at enrollment. Since eMoms is part of an NHLBI EARLY Trials cooperative agreement, some of the exclusion criteria were common to all trials.[19]
Table 2.
eMoms of Rochester Study Eligibility Criteria
| Inclusion Criteria | |
| Age 18 – 35 at the time of enrollment | |
| Consented at or before 20 weeks gestation | |
| Intending to be available for a 24-month intervention | |
| Plan to deliver in one of the 4 hospitals in Rochester, NY | |
| Plan to carry the pregnancy to term | |
| Plan to keep the baby | |
| Read and understand English | |
| Exclusion criteria | |
| Study Specific | |
| BMI < 18.5 kg/m2 or ≥ 35.0 kg/m2. | |
| Multiple gestation. If multiple gestation was diagnosed after enrollment, participant was terminated from the study | |
| Medical conditions prior to pregnancy which could influence weight loss or gain: cystic fibrosis, hyperthyroidism, renal insufficiency, proteinuria, cerebral palsy, lupus erythematosus; rheumatoid arthritis, Crohn’s disease (severity and other autoimmune diseases evaluated case by case), ulcerative colitis, maternal congenital heart disease (patients are often underweight); hypertension treated with medication | |
| Psychiatric medication associated with major weight gain or loss (e.g. Lithium & Divalproex) Having had three or more consecutive miscarriages (spontaneous abortions), because of the higher probability of another miscarriage. |
|
| Common Exclusion Criteria to EARLY trials | |
| Household member on study staff | |
| Past or planned (within the next 24 months) weight loss surgery (e.g. gastric bypass, lap band, or liposuction); current participation in a commercial weight loss program (e.g. Weight Watcher's, Jenny Craig); currently enrolled or planned to enroll in a weight loss or another weight gain prevention study | |
| Regular use of systemic steroids, prescription weight loss drugs, and/or diabetes medications (oral or injected- insulin, metformin, byetta, TZDs, other). “Regular use” is defined as “taking this medication most days of the week for the previous month” | |
| Current treatment for eating disorder Positive screening for bulimia |
|
| Cardiovascular event (heart attack, stroke, episode of heart failure, or revascularization procedure) within the last 6 months. Revascularization is defined as bypass surgery or stents | |
| Mental or psychiatric condition that precludes giving informed consent and completing questionnaires | |
| Current treatment for malignancy (other than non-melanoma skin cancer and CIN cervix) or in remission for less than 5 years | |
Recruitment and Enrollment
The study had two sources of subject recruitment, obstetric and family private practices and clinics (hereafter, practices) and the community.
Practices
Twenty out of 29 area practices agreed to participate in the study. Additionally, obstetric ultrasound offices were included to capture potential participants who obtained prenatal care in non-participating practices or in small practices where study staff was not regularly assigned. Memoranda of agreement were signed by all participating practices
Community-Based Recruitment
We created ‘brand recognition’ and curiosity among pregnant and non-pregnant women of childbearing age in the area with a community outreach strategy. Newspaper articles, brochures and posters in community settings, participation in community events (e.g. church meetings, fairs), television, radio and transit bus advertising, online ads on area websites, mass mailings coordinated with advertising campaigns, and emails to colleges’ and universities’ alumni networks in the area were utilized.
Participant enrollment
A majority of participants were recruited face-to-face at practices by study or practice staff. The procedures for participants screening and enrollment were adapted to each practice’s needs. In brief, study staff screened for eligibility and consented participants on-site, or practice staff referred potential participants to the study after prescreening for age range, gestational age, and BMI category. Additionally, pregnant women recruited via our community outreach activities had two options for screening and enrollment: contact study staff via telephone, or visit the study website to screen and self-enroll. Participants provided written consent either online or in-person. Medical record release forms were signed by participants in person to ensure access to medical records for chart audits.
Recruitment, recruitment tracking, screening, randomization, and most data entry were accomplished using online tools specifically developed for this study. To decrease the risk of inadvertent entry of inaccurate data by staff or participants the tools included multiple data checks for data quality control purposes. For example, during data collection, the online surveys and online abstraction forms had range checks embedded that flagged out-of-range values for participants to check for accuracy.
Randomization
Once consented, participants were randomized via a computer-generated algorithm. A temporary password and unique, but otherwise non-informative, study ID were immediately created. The email address and temporary password gave the subject access only to web pages corresponding to their assigned treatment. This process ensured blinding of study staff and participants to study arm allocation. Four strata were defined by early pregnancy BMI and income (two BMI groups: BMI 18.5–<25.0 and BMI 25.0–<35.0; and two income groups: above and below Medicaid eligibility). Block randomization (block size 6) within four strata to three study arms was done to obtain balanced treatment allocation within important known predictors of gestational weight gain and postpartum weight retention. Previous work by Olson [25,26] indicated BMI category and Medicaid eligibility were among the strongest predictors.
Intervention
The intervention was designed based on evidence-based behavior change strategies such as goal-setting and self-monitoring which were adapted and informed by formative research that explored media use and potential content and intervention elements. Details of the intervention development, content, and intervention implementation have been published elsewhere.[22] Briefly, the theoretical models for the intervention were Fishbein and Yzer’s Integrative Model of Behavioral Prediction [27]combined with Fogg’s Behavior Model for Persuasive.[28] During pregnancy, the online intervention features had 5 behavioral targets for the prevention of excessive gestational weight gain: entering weight in the project website ‘weight gain tracker’; physical activity and diet goal setting; increasing caloric intake by the recommended amount in the second and third trimester; improving or maintaining the nutritional quality of their diets by consuming 5 servings of fruits and vegetables per day; avoiding excess sugar and fat intake and emotional eating; and engaging in 30 minutes of moderate to vigorous physical activity on at least 5 days per week. These behavioral targets were adapted to the postpartum period. For example, the intervention included a ‘weight loss tracker’ to encourage safe weight loss according to participant’s weight and breast feeding goals. Website content for the control arm of the study included information on having a healthy pregnancy without providing behavior change strategies, self-monitoring tools, or logistical tips, which were similar to what is available on government websites. The information available to control participant was also available to intervention participants. Most of the intervention was delivered via a website with some reminders and informational content pushed out via either text or email messages (e.g.; pregnancy-related tips).
Data collection
Data were obtained from online surveys, medical chart reviews (prenatal, labor and delivery, and 6 week postpartum), and in-person measurements by study staff (postpartum weight and height) (table 3). Once a participant entered a data collection time point for the study the website alerted them as to which surveys, dietary recalls, and/or weights they were being asked to complete.
Table 3.
eMoms of Rochester. Variables, Data Sources and Data Collection Points
| Early Pregnancy (randomization to 28 weeks gestation) |
Late Pregnancy (32 weeks gestation to delivery) |
Postpartum | |||||
|---|---|---|---|---|---|---|---|
| 6 weeks | 6 months | 12 months | 18 months | ||||
| Sociodemographic data | online survey | online survey | online survey and chart audit | online survey | online survey | online survey | |
| Maternal/Infant Health | |||||||
| Infant birthweight and gestational age | chart audit | ||||||
| Parity | chart audit | ||||||
| Prenatal tests (e.g.; glucose screen, urinary tests) | chart audit | ||||||
| Prenatal diagnoses | chart audit | ||||||
| Infant health | online survey and chart audit | online survey | online survey | online survey | |||
| Behavioral variables | |||||||
| Smoking | online survey | online survey | chart audit | online survey | online survey | online survey | |
| Alcohol | online survey | online survey | chart audit | online survey | online survey | online survey | |
| Sleep | online survey | online survey | online survey | online survey | online survey | online survey | |
| Breastfeeding | online survey and chart audit | ||||||
| Physical Activity | online survey | online survey | online survey | online survey | online survey | ||
| Sedentary Behavior | online survey | online survey | online survey | online survey | online survey | ||
| Dietary and Energy Intake | online survey | online survey | online survey | online survey | online survey | ||
| Eating Patterns | online survey | online survey | online survey | online survey | online survey | ||
| Weight management | online survey | online survey | |||||
| Neighborhood Environment | online survey | online survey | online survey | online survey | online survey | ||
| Psychosocial Variables | |||||||
| Depression | online survey | online survey | online survey | online survey | online survey | ||
| Social Support | online survey | online survey | online survey | online survey | online survey | ||
| Perceived Stress | online survey | online survey | online survey | online survey | online survey | ||
| Behavioral intentions, self-efficacy, barriers, beliefs, attitudes | online survey | online survey | online survey | online survey | online survey | ||
| Anthropometry | |||||||
| Early Pregnancy Weight | self-reported at enrollment | chart audit | |||||
| Gestational weights | chart audit | ||||||
| Postpartum Weight | chart audit | measured by study staff | measured by study staffa | measured by study staff | |||
| Height | measured by study staff | ||||||
| Other | |||||||
| Adverse Events | online survey | online survey | online survey | online survey | online survey | ||
| Prescription Medications | online survey | online survey | online survey | online survey | online survey | online survey | |
| Genomic DNA | by study staff at any face-to-face encounter | ||||||
| Process Variables | website log activity and custom reports | ||||||
Postpartum outcome measure tested at 12 months.
Online surveys
Surveys covered the following topics: media use, socio-demographic data, attitudes and beliefs about weight, physical activity, and diet, smoking, alcohol intake, sleep, depression, eating patterns, physical activity and sedentary behaviors, supportive relationships, employment status, and neighborhood food and physical activity environment. Participants also entered medication use in an online tracking tool and completed two online 24-hour dietary recalls (one weekday and one weekend) at each data collection time point.[29] Participants were offer the option to complete surveys by telephone when an online survey hadn’t been completed by the end of each data collection period.
Adverse Events
Medical adverse events were self-reported via an online questionnaire at all data collection points except at baseline. Adverse events covered pregnancy, labor and delivery, and infant complications, and non-pregnancy related medical events. Each adverse event, the study time point, and the date it was reported were available by participant ID within the eMoms staff website for review by the blinded study coordinator. Since adverse events were self-reported online, questions were devised to define the event severity in order to minimize study staff contact for determination of a serious adverse event (SAE) (e.g. whether a health care provider was seen, and what, if any, treatment was administered). Further information for the determination of an SAE, if needed, was sought from the participant directly or via the medical record. Potential SAEs were triaged within the online system to the Medical Officer for review.
Safety Alerts
The study generated safety alerts to participants for corrective actions in relation to:
-Inadequate weight gain during pregnancy
Women in the intervention arms were encouraged to enter their weights, measured by the health care providers at prenatal care visits, into an online weight gain grid. We defined two different safety alert levels for inadequate pregnancy weight gain for each of the three BMI groups using the 2009 IOM guidelines [2] to prevent maternal and fetal adverse outcomes. Based on the participants weight entries a first level of alert was sent when a participant’s weight gain fell below the recommended range. The non-blinded interventionists emailed a personalized message with reference to relevant website content and encouraged the participant to discuss the under-gain with their prenatal care provider at the regularly scheduled visit. A second level of alert was sent when participants had serious under-gain that reached the small-for-gestational-age-risk threshold. In this case, the interventionist followed-up with a phone call and a referral letter to the participant indicating the need to discuss inadequate weight gain with her prenatal care provider.
-Rapid weight loss in postpartum
Considerable and rapid weight loss may interfere with exclusive breastfeeding, especially in the first 6 months postpartum. Thus, for women in the intervention arm whose weight loss exceeded 6% of their previous weight reported in the online tracking tool within one month, the interventionist contacted the research participant to investigate potential reasons for weight loss.
-Depression
When participants scored the depression scale in the online survey over a threshold indicating severe depression or if they indicated suicidal ideation, an automatically generated email prompted the study coordinator to send a letter to the respondent within 2 weeks (severe depression) or 1 week (suicidal ideation) encouraging them to consult their provider and including related community and online resources. At a second consecutive score over the threshold, in addition to the letter, the project Safety Officer followed with a phone call to answer any questions and assist her in finding treatment.
Chart abstraction
To minimize practice site burden, we abstracted the bulk of prenatal data from electronic hospital records and collected the data only available in practice records directly from practice sites. Medical records existed in a variety of formats: paper, electronic (scanned and live), inpatient/outpatient, remote access or on site access.
Postpartum weight and height measurement
Using weight collection and pediatric appointment features on the study website, weight measurements were scheduled around the time of well-baby check appointments. To maximize retention, in addition to pediatric practices, locations such as workplaces, the medical center, and participant’s homes were included for weight collection visits. Weight and height were measured with participants in street clothing and no shoes in a private place. Weights were measured to the nearest 0.1 kg at 6, 12, and 18 months postpartum by study staff using autocalibrated ‘EatSmart’ digital scales (EatSmart, NJ). Weights were measured twice and the average of the two weights differing by <0.2 kg rounded to the nearest 0.1 kg was used; otherwise, a third weight was taken. If only one pair of weights differed by <0.2 kg, the average of those two measures was used; if both pairs of measures differed by <0.2 kg, the average of all three measures was used. Height was measured using the pediatric office stadiometer or a Shorr Infant/Child/Adult Height/Length measuring board stadiometer (Weigh and Measure, LLC, Olney, MD) to the nearest 0.1 cm. The average of two measurements differing by <0.5 cm was used; otherwise, a third measurement was taken following the same process as described for weight measurements.
Process measures
Given that the intervention was delivered online, a rich database of process measures was collected to quantify exposure to the intervention both in magnitude (e.g. frequency of website intervention feature use such as time of weight entry) and in quality (e.g. types of website intervention features utilized such as dietary and physical activity assessment results from the goal setting tool;) as well as the timing of utilization (e.g. pregnancy by trimester or postpartum such as weight entry at prenatal visits). Examples of how the data on process measures can be used to provide insight into website utilization can be found in Demment et al.[30]
Incentives
Participants were offered incentives up to $140.00 in cash, check or electronic gift cards to one of five vendors for completion of data collection activities. Incentives ranged from $5.00 (first time login) to $10.00 (online surveys). Participants who completed all questionnaires and weight collections were eligible to earn an additional $150. A cash incentive was used for postpartum weight collection visits to promote retention. The incentive process for non-cash incentives was managed through the “Earnings” section of the website. The total amount earned was shown and a participant could select an e-gift card or check at any point in the study.
Outcomes Measures
Pregnancy Outcome
Gestational weight gain (GWG) is defined as the difference between weight in kilograms at the last prenatal visit and weight at the first prenatal visit (hereafter, anchor weight). Weights were abstracted from the prenatal charts. The estimation of GWG can be influenced by late entry into prenatal care (e.g.; in the second trimester in which women start gaining substantial amounts of weight) or by having their last prenatal visit before term (e.g.; preterm delivery). Consequently, if participants’ anchor weights were recorded after 14 weeks gestation and/or if the last prenatal visit was before 37 weeks gestation, their anchor and last prenatal weights were estimated using multiple imputation. To answer the primary pregnancy hypothesis, GWG is modeled as a categorical variable: “excessive” defined as gaining weight above the upper limit, and “not excessive” defined as gaining within and below the lower limit of the IOM guidelines for GWG (Table 1).[2]
Postpartum outcome
Postpartum weight retention is the difference between the measured weights at each postpartum data collection point minus the anchor weight. Weight postpartum was obtained at 6 weeks from medical records, and measured by study staff at 6, 12 and 18 months, with the 12-month measure as the primary endpoint. Based on previous observational data[31,32], any postpartum weight collected within 3 weeks (± 1.5 weeks) of the 6 weeks postpartum data point and within 3 months (± 1.5 months) of the 6, 12, and 18 months postpartum data points were considered valid measures of the weight at each time point. Weights obtained outside these windows will be used to impute the weight to the appropriate time point. For the primary postpartum hypothesis, postpartum weight retention will be modeled as a continuous variable.
Statistical Considerations
Originally, eMoms of Rochester was designed to evaluate the primary hypotheses within four income and BMI strata separately with a total accrual goal of 3,453 subjects randomized. This sample size was determined by the power to detect a 5 pound difference in weight retention at 18 months postpartum. However, the rate of accrual we initially predicted was an over-estimate given the initial challenges of working with multiple practices and a fixed timeline of 15 months for the study accrual. After 9 months of accrual and 954 subjects randomized, the study was redesigned to allow for an overall intervention assessment with a target sample size of 1641. We intentionally over-accrued and randomized 1722 subjects in response to fraudulent subjects and a few subjects with invalid consents whose data could not legally be included in any analyses. After the accrual was completed, the study was again re-designed due to financial constraints. The primary postpartum endpoint was moved from the 18 to the 12 month postpartum time point The power calculation that follows justifies this primary outcome at 12 months following delivery.
Final Sample Size Justification
The significance level will be 1.67% (2-sided), conservatively using a Bonferroni procedure to reflect the 3 primary comparisons in this study. The mean clinically important difference in weight retention at 12 months postpartum between either of the intervention arms and the control arm was estimated to be 4 pounds with an estimated standard deviation of 14 pounds [16]. The total sample size required to achieve an estimated statistical power of 90% is 999 (333/ arm). We assumed a 15% attrition rate due to miscarriage and withdrawal between randomization and delivery and an additional attrition of 30% and 40% between delivery and 12 and 18 months postpartum, respectively, due to second pregnancies and withdrawals.
Analysis Plan Summary
Treatment groups are defined according to randomization (intent-to-treat, ITT). For the pregnancy outcome, a multiple logistic regression model will be used to assess the effect of the pregnancy intervention on the odds of excessive GWG. The covariates included in the primary analysis besides randomized treatment assignment and the stratification factors (early pregnancy BMI and income strata) will be early pregnancy BMI (continuous), ultrasound adjusted gestational age of the baby to account for differing pregnancy durations, and the weeks between first and last pregnancy weight, and between the last pregnancy weight and delivery to adjust for pregnancy duration and measurement time. Although the randomization was done at the individual level, it is possible that the standard of care and other unmeasured factors may influence the effect of the intervention on the outcome. We expect that these ‘clinic factors’ will be balanced across arms. However, we will test for the presence of potential clinic effects, using a linear mixed model with clinic as random effects. Clinic effects will be dropped if not significant. For the postpartum outcome, in addition to randomized treatment, the model will include the factors time, income strata at randomization and early pregnancy BMI. Since weight retention was measured at 6 weeks, and 6 and 12 months and will be likely correlated across time in the same participant, we will handle the correlation structure using a generalized estimating equation approach unless significant clinic effects indicate the mixed model approach. The test for the primary postpartum hypotheses will be a treatment construct of weight retention at 12 months. For all outcomes, sensitivity analysis will be conducted after the ITT analysis is completed by testing the intervention effect on the outcomes once removing the participants that were ineligible randomized (protocol violations). Missing data will be addressed using multiple imputation within randomization strata (BMI and income). Since we do not expect to observe a monotone or nearly monotone missing data pattern, we will use a Markov Chain Monte Carlo procedure that handles arbitrary missing data patterns. The pregnancy and postpartum analysis data sets will have separate imputation procedures.
Descriptive Statistics
In this report, we are presenting selected baseline characteristics of participants for the entire sample by the best estimate of early pregnancy BMI. We define the best estimate of BMI as obtained by the most accurate height and weight based on the various sources of data we have for those measurements. For height, the sources in order of accuracy are: height measured by study staff at a postpartum weight collection visit; height recorded in the prenatal chart; height self-reported at screening. For weight, the sources in order of accuracy are: measured by health care provider before 14 weeks gestation obtained from prenatal chart; pre-pregnancy weight reported in the pre-natal chart; pre-pregnancy/early pregnancy weight self-reported at screening. So for example, if we have height recorded from all sources, we used height measured by study staff in the BMI calculation. Otherwise, we used the second best source. Baseline characteristics reported are:
-
-
Demographics, gestational age at enrollment, earliest pregnancy BMI and the gestational age at measurement.
-
-
Smoking: Non-smoker, ex-smoker, and current smoker based on answers to the following questions: "have you smoked at least 100 cigarettes in your entire life?; do you now smoke cigarettes?; how long has it been since you last smoked cigarettes regularly?”[33]
-
-
Alcohol intake: Percentage of participants who never drink.[]
-
-
Depression: Center for Epidemiologic Studies Short Depression Scale (CES-D), a previously validated 10-item screening questionnaire for depression with a score that ranges from 0–30.[34,35]]
-
-
Physical Activity (PA): Pregnancy Physical Activity Questionnaire (PPAQ)[36] to estimate the percentage of participants meeting the American College of Obstetrician and Gynecologist (ACOG) PA recommendations (accumulation of 30 minutes or more a day of moderate intensity most days of the week).[37] The PPAQ includes an open-ended section to add activities not listed that were scored.by two investigators (IDF, SG) matching the reported activities with those of the Compendium of Physical Activities.[38] The duration of time spent in each activity was multiplied by its intensity to obtain the average weekly energy expenditure (MET-h·week−1) attributable to each activity. A participant met the ACOG recommendation if the scores was >7.5 MET hrs/week of any activity of moderate intensity or greater (this is the equivalent of 30 minutes/day of activity at >3 METS multiplied by 5 days).
-
-
Perceived Availability of Healthy Foods Scale: Availability of healthy food in a neighborhood (quality and variety of fruits and vegetables and availability of low fat products) on a five-point Likert scale.[39] We dichotomized the responses into the proportion that strongly agree and agree versus all other responses.
-
-
Proportion of participants who live in a high crime neighborhood from the Physical Activity Neighborhood Environment Survey [40]: This item is considered to hinder physical activity.
RESULTS
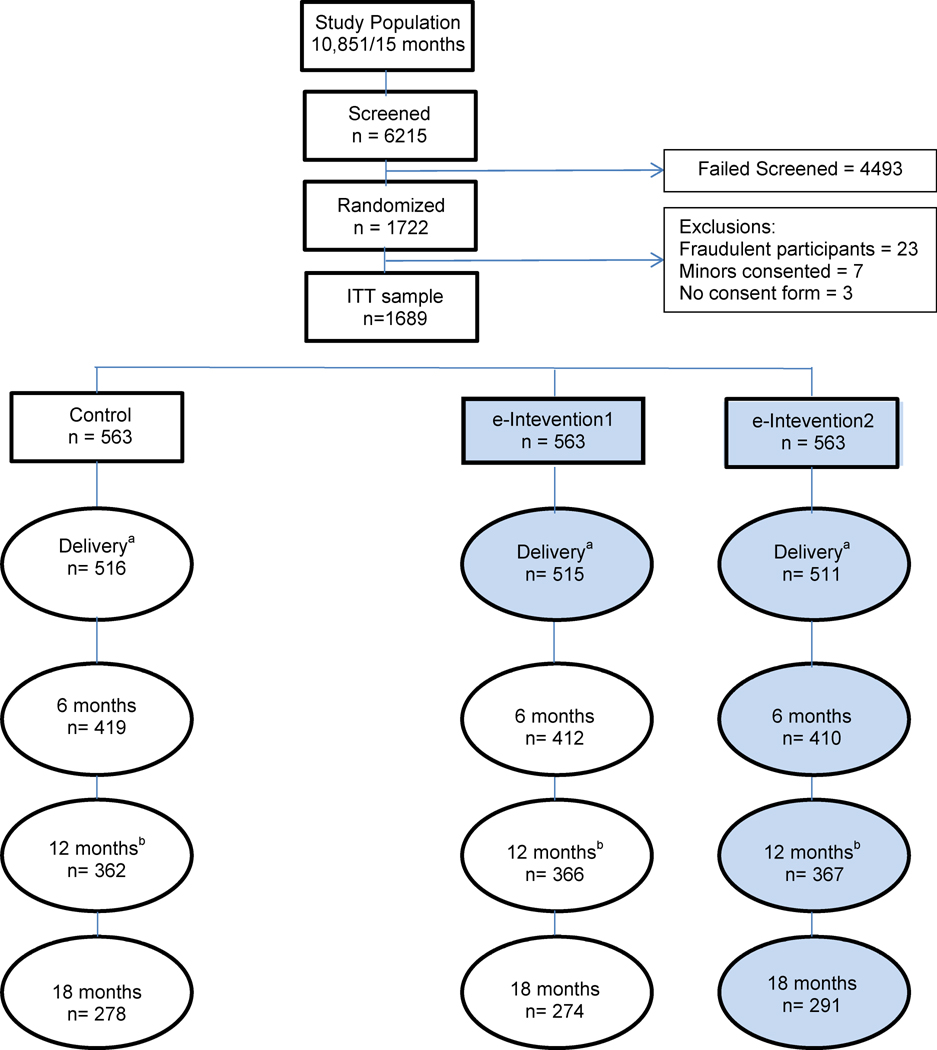
We recruited 1722 pregnant women in 14 months (28% of the 6215 approached) who were randomized into 3 equally sized arms (Figure 1). Approximately 70% of participants agreed to provide a saliva sample and 65% accepted to be contacted for further research. Reasons for ineligibility are listed in Table 4. If multiple reasons were applicable to a subject, one reason was selected for reporting. Attrition was approximately as originally estimated: 15% during pregnancy and 30% of the delivery sample at 12 months postpartum. The study had a total of 135 protocol violations due to ineligible persons randomized (n=104), lack of consent form on file (n=3), randomization problems (n=5), and fraudulent participants (n=23).
Figure 1.
eMoms of Rochester. Flowchart
aMain pregnancy outcome: gestational weight gain.
bMain postpartum outcome: 12 months postpartum weight retention.
Table 4.
eMoms of Rochester. Reasons for Ineligibility (6,215 screened participants)
| Criteria | N (% of ineligible) | % of screened |
|---|---|---|
| Not interested in participating in study | 831 (19) | 13 |
| Tacit refusal of consent form onlinea | 16 (<1) | <1 |
| Refused to sign consent form | 98 (2) | 2 |
| Age | ||
| <18 years old | 156 (3) | 3 |
| >35 years old | 544 (12) | 9 |
| Out of range, age unspecified | 10 (<1) | <1 |
| Not expected to be available for 24 month intervention | 29 (<1) | <1 |
| Body mass index (BMI) | ||
| BMI<18.5 kg/m2 | 68 (2) | 1 |
| BMI ≥ 35 kg/m2 and BMI <40 kg/m kg/m2 | 687 (15) | 11 |
| BMI out of range, unspecified amount | 44 (1) | <1 |
| Pregnancy related | ||
| Gestational age at screening > 20 weeks | 664 (15) | 11 |
| Multiple gestation | 117 (3) | 2 |
| >=3 consecutive fetal losses prior to this pregnancy | 25 (<1) | <1 |
| Delivering baby outside the study area | 108 (2) | 2 |
| Plans to interrupt pregnancy / fetal loss during screening | 8 (<1) | <1 |
| Does not plan to keep the baby | 4 (<1) | <1 |
| Household member on study staff | 0 | 0 |
| Weight loss surgery, program, or intervention study (Past or planned) | ||
| Past or planned (within 24 months) weight loss surgery | 10 (<1) | <1 |
| Currently/Plan to be enrolled in another diet/PA/weight loss intervention study | 14 (<1) | <1 |
| Medications | ||
| Regularb use of systemic steroids | 3 (<1) | <1 |
| Regularb use of prescription weight loss drugs | 0 | 0 |
| Regularb use of diabetes medications (oral or injected) | 31 (<1) | <1 |
| Cardiovascular eventc within 6 months | 2 (<1) | <1 |
| Currently being treated for cancer (other than non-melanoma skin cancer) | 5 (<1) | <1 |
| Currently being treated for an eating disorder/ has eating disorder | 6 (<1) | <1 |
| Investigator discretion | 3 (<1) | <1 |
| Does not read or understand English | 170 (4) | 3 |
| Has been previously screened for eMoms project | 716 (16) | 12 |
| Currently has no valid email account and not willing to obtain an email account | 42 (<1) | <1 |
| Medical conditions prior to pregnancy which could influence weight loss or gain including kidney disorders | 37 (<1) | <1 |
| Hypertension treated with medication (e-Moms blood pressure criterion) | 18 (<1) | <1 |
| Psychotropic medication (2nd generation anti-psychotics, mood stabilizers) | 4 (<1) | <1 |
| Mental or psychiatric conditions (Schizophrenia, Psychotic disorder NOS, Schizoaffective disorders) | 2 (<1) | <1 |
| Mentally incompetentd | 8 (<1) | <1 |
| Bulimia | 13 (<1) | <1 |
| Total | 4493 (100) | 72 |
Participants did not return a mailed consent form.
Regular use is defined as “taking this medication most days of the week for the previous month”.
Heart attack, stroke, episode of heart failure or revascularization procedure.
Recruiters’ discretion to evaluate the competence of a potential participant to effectively take part in the study.
Protocol Violations
Ineligible persons randomized
Eligibility was determined via self-report at recruitment. However, in the course of reviewing the medical records reasons for ineligibility, not reported at screening, were discovered. Ninety-six participants were found ineligible via chart review (e.g.; autoimmune diseases such as lupus, past weight loss surgery) and one participant contacted the study staff a day after randomization to inform she was not pregnant. Among the 97 ineligible participants randomized, 18 underweight women over-reported and 45 obese type II women under-reported their weight (3.7% of the ITT sample). All 97 participants remained in the study and were analyzed following the ITT principle. Seven participants were, in fact, minors at enrollment and were excluded from the study and analysis because participation as minors is illegal without parental consent.
Lack of consent on file
Three participants were excluded from analysis because they did not have a consent form on file.
Randomization problems
Two participants were randomized in the wrong stratum because one’s weight and another’s height were incorrectly entered, and thus, they were classified into the wrong BMI category. Additionally, three participants received the intervention different from the arm to which they were randomized due to a programming glitch at randomization.
Fraudulent participants
We had a total of 21 observations based on fictitious data and 2 participants who enrolled twice using their actual data. Nineteen of the fraudulent observations were through online enrollment, 18 of which occurred within a 48 hour period (August 19–21, 2011). Project coordinators observed unusual enrollment activity (e.g. same IP address, same estimated delivery date). Further investigation confirmed that information from one participant who had self-enrolled online was used to subsequently enroll 18 additional times. To prevent more fraudulent activity a protocol was developed to monitor online self-screening and consent. Once a subject was deemed eligible based on online self-screening, consent was blocked until after the study coordinator reviewed the following variables within 24 hours: e-mail address, telephone number, mailing address, practice providing prenatal care, IP address, date of birth, estimated delivery date, height, weight, insurance provider, and time and date of completion of online screening. If validity of the potential subject’s responses was in doubt (i.e.; similarity to other subjects enrolled), the subject was contacted via phone to verify her identity and the information provided. Entry into the study was denied if: a) discrepancies existed between information provided in the call and the online screening tool; or b) phone number provided was not valid or a call was not returned within 7 days. After the procedures were established a total of 298 participants were contacted and 1 person was declined. Two additional participants enrolled via study staff providing data that were later discovered to be fraudulent. Another two participants enrolled twice in the study although providing truthful data. All fraudulent observations were excluded from the study.
Baseline Characteristics of Study Participants (Table 5)
Table 5.
eMoms of Rochester. Selected baseline characteristics of study ITT sample (n= 1689)
| Early Pregnancy Weight Category | |||||
|---|---|---|---|---|---|
|
| |||||
| Total Sample N = 1689 |
Healthy Weighta n = 871 |
Overweight n = 509 |
Obese Type Ib n = 309 |
P- valuec |
|
| Age at enrollment (years), mean ±sd (n) | 27.5± 4.7(1689) | 27.4±4.7(871) | 27.5± 4.7(509) | 27.7± 4.5(309) | 0.77 |
|
| |||||
| Gestational age at enrollment(weeks), mean ±sd (n) | 11.2 ± 4.3(1689) | 11.3± 4.3, 871 | 11.0 ± 4.2(509) | 11.3 ± 4.2(309) | 0.45 |
|
| |||||
| Early pregnancy weight (kg), mean ±sd (n) | 68.3±12.9(1599) | 59.0 ± 6.3, 826 | 72.8 ± 7.2(481) | 86.9± 9.7(292) | <.0001 |
|
| |||||
| Gestational age at earliest pregnancy weight (weeks), mean ±sd (n) | 8.4 ± 2.1(1599) | 8.5,± 2.1, 826 | 8.4 ± 2.1(481) | 8.4 ± 2.2(292) | 0.30 |
|
| |||||
| Early pregnancy BMI, mean, sd,(n) | 25.4 ± 4.3(1252) | 22.1,± 1.7(670) | 27.2± 1.5,(368) | 32.7± 2.1(214) | <.0001 |
|
| |||||
| Race (n non-missing) | 1573 | 825 | 464 | 284 | <.0001 |
| Black or African American, n (%) | 388 (24.7) | 170 (20.6) | 114 (24.6) | 104 (36.6) | |
| American Indian/Native Hawaiian Pacific Islander, n (%) | 10 (0.6) | 4 (0.5) | 4 (0.9) | 2 (0.7) | |
| Asian, n (%) | 38 (2.4) | 28 (3.4) | 4 (0.9) | 6 (2.1) | |
| White or Caucasian, n (%) | 1069 (68.0) | 594 (72.0) | 316 (68.1) | 159 (56.0) | |
| Multiple, n (%) | 68 (4.3) | 29 (3.5) | 26 (5.6) | 13 (4.6) | |
|
| |||||
| Ethnicity (n non-missing) | 1687 | 869 | 509 | 309 | 0.015 |
| Hispanic/Latina, n (%) | 213 (12.6) | 90 (10.4) | 78 (15.3) | 45 (14.6) | |
|
| |||||
| Education (n non-missing) | 1340 | 720 | 403 | 217 | <.0001 |
| High School or GED and below, n (%) | 291 (21.7) | 143 (19.9) | 96 (23.8) | 52 (24.0) | |
| Some College or Associate Degree or Vocational School, n (%) | 391 (29.2) | 176 (24.4) | 127 (31.5) | 88 (40.6) | |
| College Graduate, n (%) | 326 (24.3) | 188 (26.1) | 96 (23.8) | 42 (19.4) | |
| Master or Doctoral Degree, n (%) | 332 (24.8) | 213 (29.6) | 84 (20.8) | 35 (16.1) | |
|
| |||||
| Personal Income in the past 12 month (n non-missing) | 1195 | 637 | 360 | 198 | 0.04 |
| ≤ $11,999, n (%) | 365 (30.6) | 193 (30.3) | 116 (32.2) | 56 (28.3) | |
| $12,000-24,999, n (%) | 145 (12.1) | 62 (9.7) | 49 (13.6) | 34 (17.2) | |
| $25,000–$34,999, n (%) | 143 (12.0) | 80 (12.6) | 38 (10.6) | 25 (12.6) | |
| $35,000- $49,999, n (%) | 213 (17.8) | 116 (18.2) | 56 (15.6) | 41 (20.7) | |
| ≥$50,000, n (%) | 250 (20.9) | 144 (22.6) | 78 (21.7) | 28 (14.1) | |
| Don't, n (%) | 79 (6.6) | 42 (6.6) | 23 (6.4) | 14 (7.1) | |
|
| |||||
| Relationship status (n non-missing) | 1336 | 717 | 404 | 215 | 0.0004 |
| Single, n (%) | 140 (10.5) | 59 (8.2) | 42 (10.4) | 39 (18.1) | |
| Married / Co-habitating, n (%) | 1183 (88.6) | 654 (91.2) | 356 (88.1) | 173 (80.5) | |
| Separated / Divorced, n (%) | 13 (1.0) | 4 (0.6) | 6 (1.5) | 3 (1.4) | |
|
| |||||
| Parity (n non-missing) | 1686 | 869 | 508 | 309 | <.0001 |
| Nulliparous, n (%) | 775 (46.0) | 439 (50.5) | 215 (42.3) | 212 (39.2) | |
| Primaparous, n (%) | 550(32.6) | 281 (32.3) | 166 (32.7) | 103 (33.3) | |
| Multiparous [n (%)] | 361 (21.4) | 149 (17.2) | 127 (25.0) | 85 (27.5) | |
|
| |||||
| Smoking (n non-missing) | 1393 | 738 | 425 | 230 | 0.01 |
| Non-smoker, n (%) | 941 (67.6) | 522 (70.7) | 280 (65.9) | 139 (60.4) | |
| Exsmoker, n (%) | 364 (26.1) | 177 (24.0) | 120 (28.2) | 67 (29.1) | |
| Current smoker, n (%) | 88 (6.3) | 39 (5.3) | 25 (5.9) | 24 (10.4) | |
|
| |||||
| Alcohol (in the past 30 days) (n non-missing) | 1395 | 740 | 425 | 230 | |
| None, n (%) | 1245 (89.3) | 658 (88.9) | 382 (89.9) | 205 (89.1) | 0.88 |
|
| |||||
| CES-D score, mean ±sd (n) | 8.2±4.9(1414) | 7.8±4.8(749) | 8.6±4.9(430) | 8.8±5.0(235) | 0.003 |
|
| |||||
| ACOG criteria using PPAQ (n non-missing) | 1359 | 729 | 409 | 221 | .001 |
| Met recommendation, n (%) | 599 (44.1) | 349 (47.9) | 175 (42.8) | 75 (33.9) | |
|
| |||||
| Employment for Pay (n non-missing) | 1342 | 719 | 404 | 219 | 0.42 |
| Yes, n (%) | 959 (71.5) | 522 (72.6) | 288 (71.3) | 149 (68.0) | |
|
| |||||
| Perceived Availability of Healthy Foods Scale (n non-missing) | 1338 | 718 | 401 | 219 | |
| Strongly agree, n (%) | 469 (35.0) | 275 (38.3) | 143 (35.7) | 51 (32.3) | 0.0002 |
|
| |||||
| PA neighborhood environment (n non-missing) | 1256 | 677 | 377 | 202 | |
| High Crime, n (%) | 249 (19.8) | 118 (17.4) | 83 (22.0) | 48 (23.8) | 0.06 |
Includes 18 underweight women who over-reported their weight at screening
Includes 45 obese class II women who under-reported their weight at screening
Differences across BMI categories assessed by 1-way ANOVA (F-test; 2df) for continuous variables and by Chi-Square test for categorical variables. Differences in non-normal continuous factors across BMI categories was assessed using the Kruskal-Wallis test.
Overall, the average gestational age at enrollment was at the end of the first trimester (11.2 ±4.2 weeks), but participants had a first recorded weight in the prenatal chart earlier in pregnancy (8.4 ±2.1 weeks) that did not differ across weight categories. Approximately 52% of our sample started pregnancy in a healthy weight category. The early pregnancy mean BMI based on measured weight and height from the prenatal charts for the entire sample is slightly over the lower end of the overweight category (BMI of 25.4 ±4.3). The sample has a high proportion of African American (24.7%) and Hispanic/Latina (12.6%) women. Our participants are highly educated (almost 80% had some college degree or more), the majority are married (88.6%), employed (71.5%), non-smokers (67.6%) and non-drinkers during pregnancy (89.3%), and slightly over half of them have had a prior delivery (54%). These characteristics differ significantly across BMI category except for the alcohol intake measures. Although the mean depression score is in the lower half of the range (mean score 8.2 ±4.9, range 0–30), the mean score increases with increasing weight category. Across BMI categories, the most notable pattern is observed in the physical activity measure with 47.9%, 42.8%, and 33.9% of the healthy weight, overweight, and obese participants, respectively, meeting recommendations. The obese category has the lowest percentage of women strongly agreeing with the high quality of the food environment and the higher proportion of those who live in high crime areas are in the obese category.
DISCUSSION
This report describes the study design, methods, and sample baseline characteristics of the eMoms study, a double blind randomized clinical trial with a parallel group design that tested electronically-mediated behavioral interventions to prevent excessive gestational weight gain and minimize postpartum weight retention in women 18–35 years of age of diverse racial/ethnic and income backgrounds. Our sample has a larger representation of racial/ethnic minorities compared to the county percentages based on 2010 census data (African American 25% vs 16.7%, Hispanic 12.3% vs. 7.3%, study sample and county, respectively)[23] possibly due to a high participation of clinics primarily serving minorities. Our participants are also more educated, almost 50% of our sample has a college degree or more versus 35% in the county, a typical phenomenon of clinical trials volunteers. Interestingly, the proportion of women in the higher income range ($50,000) is lower than observed in the county (study sample 23% vs. county 57%)[23] despite a large representation of women with higher education. Although we have no data to explain the discrepancy between income and education, several reasons might explain it. For example, participants may still be graduate students, may have left the workforce to raise a family, and/or the lower income of educated women may reflect the current economic situation or gender salary inequalities. This discrepancy, however, should be interpreted with caution since a large proportion of education and income data were missing (21% and 29%, respectively).
A major strength of eMoms is that women receive an intervention and are followed from early pregnancy until late postpartum contributing to the literature on both pregnancy-related weight gain and weight management in young adult women. Our data will provide evidence as to whether an intervention delivered only during pregnancy has a long lasting effect on postpartum weight retention once the intervention stops at delivery (e-intervention group 1) or whether there is an additive effect of a postpartum component to a pregnancy intervention by adapting the strategies to their new life stage. Previous studies have examined pregnancy intervention effects on weight measures at 2 months[41,42], 6[43], and 12 months[44] postpartum, and two others with a 12 month follow-up are underway,[45,46] but very few have tested interventions for both the pregnancy and postpartum periods. Huang TT, et al.[17] tested a lifestyle intervention using a three-arm design until 6 months postpartum with positive results, and Rauh et al.[47] have an ongoing cluster randomized pregnancy and postpartum intervention implemented until 6–8 weeks. Thus, eMoms will contribute a longer length intervention and follow-up of postpartum women and the ability to compare three conditions.
Other strengths of our study include: online trial processes and intervention delivery, the recruitment of a racially/ethnically and socioeconomically diverse sample; online randomization minimizing the possibility of bias in intervention allocation; the online intervention delivery that guarantees fidelity and prevents contamination, and the programmed data checks included in the online surveys, chart audit, and weight collection, thus reducing the probability of data entry errors. Finally, a key characteristic of our methods is the use of multiple and flexible modes for recruitment, enrollment, and retention.
The study encountered challenges related to the use of self-reported data. In addition to uncovering ineligible participants randomized during chart abstraction of prenatal medical records, participants also reported inaccurate information of the type and timing of adverse events. In this study, the prenatal record was considered the gold standard compared to self-reported data as it has been done previously in the perinatal literature.[48–50]
Technology support for lifestyle interventions and the use of online methodologies to conduct all aspects of randomized clinical trials have many advantages such as the stigma reduction due to responders’ anonymity, timeliness, fidelity of intervention delivery, lower costs, and larger reach since geographical, time and mobility barriers are minimized.[51–55]. At the same time, online methodologies open the doors for other concerns such as the opportunity for participants to re-enroll in the study using different identities facilitated by the anonymity provided by the internet.[56 In our study, 21 online enrollments, approximately 5% of those who enrolled online, were repeat enrollments, which may have come from either a single participant and/or a participant who shared eligibility information with others. Other studies have reported from 1% to 11% of repeated online enrollment.[56,57] As the use of electronic media in research becomes widespread, investigators should be creative in controlling potential pitfalls that were not as common with previous methods. We developed a monitoring system for every online enrollment to prevent fraudulent participants. Other procedural/design, technical/software, and data analysis strategies have been suggested [58] eMoms was not designed to test whether electronically-mediated interventions are more effective in influencing weight outcomes than supervised or tele-health interventions. As a consequence, when analyzing the result of t, the effect of the intervention in and of itself and the effect of the method of intervention delivery (electronic media) will not be disentangled.
Finally, eMoms was originally designed to enroll 3,353 participants with a follow-up until 18 months postpartum. The challenges faced by working with clinical practices whose main concern is the delivery of health care and whose investment in research is secondary, the need to adhere to the proposed recruitment period, and budget constraints given unplanned extra costs led to changes in the target sample size and to a change in the primary outcome to 12 instead of 18 months. Future trials planning a large RCT should consider these challenges for budgeting and study planning
Conclusions
Our study will provide evidence on the efficacy of behavioral interventions in the prevention of excessive GWG and postpartum weight retention, which potentially lead to obesity and associated cardiovascular risk factors. If the intervention strategies are successful in preventing the outcomes of interest and in engaging young adults, dissemination to practices and/or health insurances should be investigated.
Acknowledgments
This study is supported by a grant from the EARLY Trials, National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant No. HL096760. We would like to acknowledge all the Obstetrics and Gynecology, Ultrasound, and Pediatric practices who contributed their time and personnel resources to this study and the study recruiters, Ashley Eagen, Julia Frisk, Rebecca Horn, Madeline Forster, Kayla Bermudez, and Maura Hamilton.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Isabel Diana Fernandez, Department of Public Health Sciences, Division of Epidemiology, University of Rochester School of Medicine and Dentistry, 265 Crittenden Blvd., CU 420644, Rochester, NY, USA 14642-0644, 585-275-9554.
Susan W. Groth, School of Nursing, University of Rochester.
Jennifer E. Reschke, Department of Public Health Sciences, University of Rochester School of Medicine and Dentistry.
Meredith L. Graham, Division of Nutritional Sciences, Cornell University.
Myla Strawderman, Division of Nutritional Sciences, Cornell University.
Christine M. Olson, Division of Nutritional Sciences, Cornell University.
REFERENCES
- 1.Simas TA, Liao X, Garrison A, Sullivan GM, Howard AE, Hardy JR. Impact of updated Institute of Medicine guidelines on prepregnancy body mass index categorization, gestational weight gain recommendations, and needed counseling. Journal of Women's Health. 2011;20(6):837–844. doi: 10.1089/jwh.2010.2429. [DOI] [PubMed] [Google Scholar]
- 2.IOM (Institute of Medicine) and NRC (National Research Council) Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 3.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med. 2003;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 4.Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. Int J Obes Relat Metab Disord. 2000;24(12):1660–1668. doi: 10.1038/sj.ijo.0801456. [DOI] [PubMed] [Google Scholar]
- 5.Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol. 2010;202(6):574, e571–e578. doi: 10.1016/j.ajog.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322, e321–e328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Matern Child Health J. 2009;13(6):839–846. doi: 10.1007/s10995-008-0413-6. [DOI] [PubMed] [Google Scholar]
- 8.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol. 2002;100(2):245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 9.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106(6):1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 10.Witter FR, Caulfield LE, Stoltzfus RJ. Influence of maternal anthropometric status and birth weight on the risk of cesarean delivery. Obstet Gynecol. 1995;85(6):947–951. doi: 10.1016/0029-7844(95)00082-3. [DOI] [PubMed] [Google Scholar]
- 11.Norman JE, Bild D, Lewis CE, Liu K, West DS, Study C. The impact of weight change on cardiovascular disease risk factors in young black and white adults: the CARDIA study. Int J Obes Relat Metab Disord. 2003;27(3):369–376. doi: 10.1038/sj.ijo.0802243. [DOI] [PubMed] [Google Scholar]
- 12.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes Relat Metab Disord. 2004;28(4):525–535. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am J Clin Nutr. 2010;92(4):678–687. doi: 10.3945/ajcn.2010.29363. [DOI] [PubMed] [Google Scholar]
- 14.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG. 2010;117(11):1316–1326. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 15.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e88. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amorim AR, Linne YM. Lourenco PMC. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD005627.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Huang TT, Yeh CY, Tsai YC. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery. 2011;27(2):257–264. doi: 10.1016/j.midw.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Hutchesson MJ, Hulst J, Collins CE. Weight management interventions targeting young women: a systematic review. J Acad Nutr Diet. 2013;113(6):795–702. doi: 10.1016/j.jand.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Lytle LS, Svetkey LP, Patrick K, Belle SH, Fernandez ID, Jakicic J, Johnson KC, et al. The EARLY Trials: A Consortium of Studies Targeting Weight Control in Young Adults. Transl Behav Med. 2014;4(3):304–313. doi: 10.1007/s13142-014-0252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul KH, Graham ML, Olson CM. The web of risk factors for excessive gestational weight gain in low income women. Matern Child Health J. 2013;17(2):344–351. doi: 10.1007/s10995-012-0979-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham M, Uesugi K, Olson C. Barriers to weight-related health behaviours: a qualitative comparison of the socioecological conditions between pregnant and post-partum low-income women. Matern Child Nutr. 2014 Jul 18; doi: 10.1111/mcn.12135. PMID: 25040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham ML, Uesugi KH, Niederdeppe J, Gay GK, Olson CM. The Theory, Development, and Implementation of an e-Intervention to Prevent Excessive Gestational Weight Gain: e-Moms Roc. Telemed J E Health. 2014;20(12):1135–1142. doi: 10.1089/tmj.2013.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American FactFinder. [Accessed July 3, 2013]; factfinder2.census.gov/faces/tableservices/jsf/pages/productview.
- 24.Dye TD, Wojtowycz MA, Aubry RH. A cost evaluation of implementing a quality-oriented, regional perinatal data system. J Public Health Manag Pract. 1997;3(2):37–40. doi: 10.1097/00124784-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychsocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc. 2003;103(1):48–54. doi: 10.1053/jada.2003.50001. [DOI] [PubMed] [Google Scholar]
- 26.Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol. 2004;191:530–536. doi: 10.1016/j.ajog.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Fishbein M, Yzer MC. Using theory to design effective health behavior interventions. Communication Theory. 2003;13:164–183. [Google Scholar]
- 28.Fogg B. Persuasive Technology. Using computers to change what we think and do. San Francisco, CA: Morgan Kaufmann; 2002. [Google Scholar]
- 29.National Institutes of Health. National Cancer Institute. Applied Research. [Accessed March 14, 2014];Cancer Control and Population Sciences. Available at: http://appliedresearch.cancer.gov/asa24/.
- 30.Demment MM, Graham ML, Olson CM. How an online intervention to prevent excessive gestational weight gain is used and by whom: a randomized controlled process evaluation. J Med Internet Res. 2014;16(8):e194. doi: 10.2196/jmir.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janney CA, Zhang D, Sowers M. Lactation and weight retention. Am J Clin Nutr. 1997;66(5):1116–1124. doi: 10.1093/ajcn/66.5.1116. [DOI] [PubMed] [Google Scholar]
- 32.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189(5):1423–1432. doi: 10.1067/s0002-9378(03)00596-9. [DOI] [PubMed] [Google Scholar]
- 33.Center for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire 2009. [Accessed March 14, 2014]; Available at: http://www.cdc.gov/brfss/.
- 34.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 35.Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. J Youth Adolesc. 1991;20(2):149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- 36.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004;36(10):1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 37.American College of Obstetrics and Gynelogy Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 38.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 39.Moore LV, Diez Roux AV, Nettleton JA, Jacobs DR., Jr Associations of the local food environment with diet quality--a comparison of assessments based on surveys and geographic information systems: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2008;167(8):917–924. doi: 10.1093/aje/kwm394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallis JF, Bowles HR, Bauman A, et al. Neighborhood environments and physical activity among adults in 11 countries. Am J Prev Med. 2009;36(6):484–490. doi: 10.1016/j.amepre.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 41.Mottola MF, Giroux I, Gratton R, et al. Nutrition and exercise prevent excess weight gain in overweight pregnant women. Med Sci Sports Exerc. 2010;42(2):265–272. doi: 10.1249/MSS.0b013e3181b5419a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruchat SM, Davenport MH, Giroux I, et al. Nutrition and exercise reduce excessive weight gain in normal-weight pregnant women. Med Sci Sports Exerc. 2012;44(8):1419–1426. doi: 10.1249/MSS.0b013e31825365f1. [DOI] [PubMed] [Google Scholar]
- 43.Vinter CA, Jensen DM, Ovesen P, et al. Postpartum weight retention and breastfeeding among obese women from the randomized controlled Lifestyle in Pregnancy (LiP) trial. Acta Obstetricia et Gynecologica Scandinavica. 2014;93(8):794–801. doi: 10.1111/aogs.12429. [DOI] [PubMed] [Google Scholar]
- 44.Phelan S, Phipps MG, Abrams B, et al. Does behavioral intervention in pregnancy reduce postpartum weight retention? Twelve-month outcomes of the Fit for Delivery randomized trial. Am J Clin Nutr. 2014;99(2):302–311. doi: 10.3945/ajcn.113.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skouteris H, McCabe M, Milgrom J, et al. Protocol for a randomized controlled trial of a specialized health coaching intervention to prevent excessive gestational weight gain and postpartum weight retention in women: the HIPP study. BMC Public Health. 2012;12:78. doi: 10.1186/1471-2458-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vesco KK, Karanja N, King JC, et al. Healthy Moms, a randomized trial to promote and evaluate weight maintenance among obese pregnant women: study design and rationale. Contemp Clin Trials. 2012;33(4):777–785. doi: 10.1016/j.cct.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauh K, Kunath J, Rosenfeld E, Kick L, Ulm K, Hauner H. Healthy living in pregnancy: a cluster-randomized controlled trial to prevent excessive gestational weight gain - rationale and design of the GeliS study. BMC Pregnancy & Childbirth. 2014;14:119. doi: 10.1186/1471-2393-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellison GT, de Wet T, Matshidze KP, Cooper P. The reliability and validity of self-reported reproductive history and obstetric morbidity amongst Birth to Ten mothers in Soweto. Curationis. 2000;23(4):76–80. doi: 10.4102/curationis.v23i4.753. [DOI] [PubMed] [Google Scholar]
- 49.McNamara TK, Orav EJ, Wilkins-Haug L, Chang G. Risk during pregnancy--self-report versus medical record. Am J Obstet Gynecol. 2005;193(6):1981–1985. doi: 10.1016/j.ajog.2005.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: a report from the Children's Cancer Group. Am J Epidemiol. 1997;145(1):58–67. doi: 10.1093/oxfordjournals.aje.a009032. [DOI] [PubMed] [Google Scholar]
- 51.Griffiths F, Lindenmeyer A, Powell J, Lowe P, Thorogood M. Why are health care interventions delivered over the internet? A systematic review of the published literature. J Med Internet Res. 2006;8(2):e10. doi: 10.2196/jmir.8.2.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strecher V. Internet methods for delivering behavioral and health-related interventions (eHealth) Annu Rev Clin Psychol. 2007;3:53–76. doi: 10.1146/annurev.clinpsy.3.022806.091428. [DOI] [PubMed] [Google Scholar]
- 53.Bull SS, Vallejos D, Levine D, Ortiz C. Improving recruitment and retention for an online randomized controlled trial: experience from the Youthnet study. AIDS Care. 2008;20(8):887–893. doi: 10.1080/09540120701771697. [DOI] [PubMed] [Google Scholar]
- 54.Eysenbach G. Issues in evaluating health websites in an Internet-based randomized controlled trial. J Med Internet Res. 2002;4(3):E17. doi: 10.2196/jmir.4.3.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todd NJ, Solis-Trapala I, Jones SH, Lobban FA. An online randomised controlled trial to assess the feasibility, acceptability and potential effectiveness of 'Living with Bipolar': a web-based self-management intervention for bipolar disorder: trial design and protocol. Contemp Clin Trials. 2012;33(4):679–688. doi: 10.1016/j.cct.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Murray E, Khadjesari Z, White IR, et al. Methodological challenges in online trials. J Med Internet Res. 2009;11(2):e9. doi: 10.2196/jmir.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konstan JA, Rosser BRS, Ross MW, Stanton J, Edwards WM. The story of subject naught: A cautionary but optimistic tale of Internet survey research. Journal of Computer-Mediated Communication. 2005;10(2) [Google Scholar]
- 58.Kramer J, Rubin A, Coster W, et al. Strategies to address participant misrepresentation for eligibility in Web-based research. Int J Methods Psychiatr Res. 2014;23(1):120–129. doi: 10.1002/mpr.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]