Abstract
It is becoming clear that steroids can be synthesized de novo by the brain and other nervous systems. Such steroids are called neurosteroids, and de novo neurosteroidogenesis from cholesterol is a conserved property of vertebrate brains. In this study, we show that the newt brain actively produces 7α-hydroxypregnenolone, a previously undescribed amphibian neurosteroid that stimulates locomotor activity. 7α-hydroxypregnenolone was identified as a most abundant amphibian neurosteroid in the newt brain by using biochemical techniques combined with HPLC, TLC, and GC-MS analyses. The production of 7α-hydroxypregnenolone in the diencephalon and rhombencephalon was higher than that in the telencephalon and peripheral steroidogenic glands. In addition, 7α-hydroxypregnenolone synthesis in the brain showed marked changes during the annual breeding cycle, with a maximal level in the spring breeding period when locomotor activity of the newt increases. Behavioral analysis of newts in the nonbreeding period demonstrated that administration of this previously undescribed amphibian neurosteroid acutely increased locomotor activity. In vitro analysis further revealed that 7α-hydroxypregnenolone treatment resulted in a dose-dependent increase in the release of dopamine from cultured brain tissue of nonbreeding newts. The effect of this neurosteroid on locomotion also was abolished by dopamine D2-like receptor antagonists. These results indicate that 7α-hydroxypregnenolone acts as a neuronal activator to stimulate locomotor activity of breeding newts through the dopaminergic system. This study demonstrates a physiological function of 7α-hydroxypregnenolone that has not been described previously in any vertebrate class. This study also provides findings on the regulatory mechanism of locomotor activity from a unique standpoint.
Keywords: neurosteroids, dopamine release, newt brain, seasonal changes
The brain traditionally has been considered to be a target site of peripheral steroid hormones. In contrast to this classical concept, findings over the past decade have shown that the brain itself also has the capability of forming steroids de novo, the so-called “neurosteroids.” Studies on mammals (for reviews, see refs. 1–3) and nonmammals (for reviews, see refs. 3–6) indicate that de novo neurosteroidogenesis in the brain from cholesterol is a conserved property of vertebrates.
When understanding the function of neurosteroids, physiological changes in neurosteroid levels must be taken into account. Seasonal changes in neurosteroid levels in the brain have been demonstrated in seasonally breeding wild animals, such as amphibians and birds (7–9). Thus, seasonally breeding vertebrates are excellent animal models to investigate physiological functions of neurosteroids. Previous studies with the seasonally breeding frog and newt have demonstrated that their brains possess the key steroidogenic enzyme, cytochrome P450 side-chain cleavage enzyme (P450scc), and produce pregnenolone, a precursor of steroid hormones (7, 8). Other enzymes, such as 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase (3β-HSD) and 17β-HSD, also are expressed in the amphibian brain and are involved in the conversion of pregnenolone to progesterone, androstenedione, and testosterone (8, 10, 11).
Preliminary studies in our laboratory suggested that amphibians actively produce an unknown neurosteroid from pregnenolone in the brain (M.M., K.U., and K.T., unpublished data). Because its production has been suggested to be greater than those of any neurosteroids identified previously in amphibians, this unknown amphibian neurosteroid may be involved in key aspects of brain function. Therefore, the present study was conducted to identify this unknown amphibian neurosteroid and demonstrate its physiological function by using the newt, a seasonally breeding amphibian. First, we identified this unknown neurosteroid from the brain by using biochemical techniques combined with HPLC, TLC, and GC-MS analyses. Subsequently, we examined seasonal changes in the formation of this neurosteroid in the brain. Finally, we investigated the function and mode of action of this neurosteroid. Here, we show that, in the seasonally breeding newt, the brain abundantly produces 7α-hydroxypregnenolone, which stimulates locomotor activity of newts by means of the dopaminergic system in the breeding period.
Materials and Methods
Subjects. Adult male Japanese red-bellied newts (Cynops pyrrhogaster) were collected and used in the present study. This species is a seasonal breeder and shows breeding seasons in spring (March–May) and occasionally in autumn (October and November). Newts were kept in ordinary water aquariums maintained at 18 ± 2°C under a daily photoperiod cycle of 12-h light/12-h dark (lights on at 7:00 a.m.). The experimental protocols were approved in accordance with the Guide for the Care and Use of Laboratory Animals of Hiroshima University.
Biochemical Analyses Combined with HPLC, TLC, and GC-MS of 7α-Hydroxypregnenolone Production. To identify an unknown neurosteroid produced from pregnenolone in the newt brain, the radioactive metabolite of [7-3H]pregnenolone [specific activity, 14 Ci/mmol (1 Ci = 37 GBq); distribution of 3H, 7α, 47.6%; 7β, 31.5%; 4(α+β), 8.7%; 2α, 10%; PerkinElmer] was analyzed by HPLC using brain homogenates as described in refs. 12–14. In brief, brain homogenates containing 20 mg of tissue from the newt were incubated in 500 μl of PBS (10 mM phosphate buffer/140 mM NaCl, pH 7.5) containing 1 million cpm [7-3H]pregnenolone, 0.24 mM NADPH, and 4% propylene glycol for 30 min at 25°C. After incubation, steroids were extracted by ethyl acetate and subjected to HPLC analysis by using a reversed-phase column, LiChrospher 100 RP-18 (4.0 mm × 250 mm, Kanto, Tokyo). The column was eluted with a 30-min linear gradient of 40–70% acetonitrile at a flow rate of 0.7 ml/min, followed by an isocratic elution of 70% acetonitrile. The eluate was fractionated every minute from 5 to 35 min and counted in a liquid scintillation counter. To confirm the involvement of steroidogenic enzyme in the formation of the unknown neurosteroid, newt brain homogenates and [3H]pregnenolone were incubated with ketoconazole (Sigma), an inhibitor of P450s, at a final concentration of 10–4 M.
The radioactive metabolite of pregnenolone also was analyzed by TLC using a silica gel plate (Merck). The HPLC peak corresponding to the unknown neurosteroid was collected, dried, and subjected to TLC analysis by using ethyl acetate/n-hexane/acetic acid (16:8:1 ratio) as the mobile phase. The unknown neurosteroid was further examined in GC-MS analysis. Trimethylsilyl ether derivative of the metabolite obtained from native pregnenolone (Sigma) was prepared before GC-MS by reacting the dried sample with bis(trimethylsilyl)trifluoroacetamide (Wako Pure Chemical, Osaka) for 30 min at 60°C. For the identification of the unknown neurosteroid, a GC-MS system (GCMS-QP5000, Shimadzu) using a CP-Sil 5CB capillary column (0.25 mm × 30 m, Varian) was used as described in ref. 15. The column was maintained at 220°C for 5 min, and then the temperature was raised to 300°C at the rate of 5°C per min. These biochemical analyses were repeated independently at least four times. Both 7α-hydroxypregnenolone and its stereoisomer 7β-hydroxypregnenolone, which were used as reference standards in these analyses, were purchased from Steraloids (Newport, RI).
Comparisons of the Production of 7α-Hydroxypregnenolone Among Different Brain Regions and Different Seasons. To compare 7α-hydroxypregnenolone synthesis among different brain regions, we subdivided the newt brain into the telencephalon, diencephalon, and rhombencephalon. Each homogenate containing 20 mg of tissue was incubated separately with tritiated pregnenolone for 10 min at 25°C. After incubation, extracted steroids were subjected to HPLC analysis. The production of 7α-hydroxypregnenolone in each brain region was calculated as percentage of total radioactivity incubated. To determine seasonal changes in the production of 7α-hydroxypregnenolone in the brain, newts were collected throughout the year, i.e., in February, April, June, August, October, and December. The time lapse between the beginning and the end of tissue sampling at each time of year did not exceed 2 h, and this sampling always was performed between 3:00 and 5:00 p.m. Brains were removed from the skull, weighed, snap-frozen in liquid nitrogen, and stored at –80°C. Each brain homogenate containing 20 mg of tissue was treated as described above for the separate brain regions. Again, the production of 7α-hydroxypregnenolone in each month was calculated as percentage of total radioactivity incubated.
Measurement of Endogenous 7α-Hydroxypregnenolone in the Brain and Plasma. To measure the concentration of endogenous 7α-hydroxypregnenolone in the newt brain and plasma, GC-MS analysis was performed as described in ref. 16. Steroids in brain tissue were extracted by solid phase extraction using C18 columns. The brain tissue (200 mg) was homogenized in an aliquot of methanol/H2O (75:25, vol/vol; 1 ml) on ice. After centrifugation (3,000 × g, 5 min), brain samples (supernatant of homogenate) were diluted to a final concentration of 5% methanol. Brain samples then were extracted with C18 columns previously equilibrated with 5 ml of methanol and 5 ml of methanol/H2O (5:95, vol/vol) successively. The brain sample was passed through the cartridge, and the eluate was discarded. The cartridge then was washed with 5 ml of methanol/H2O (5:95, vol/vol). The steroid fraction was eluted with 5 ml of methanol into a glass vial and evaporated to dryness under a gentle stream of nitrogen. Then, the brain sample was applied to a GC-MS system as described above. The concentration of 7α-hydroxypregnenolone in the brain was calculated as ng/mg tissue. Steroids in plasma (400 μl) also were extracted by solid phase extraction, and plasma samples also were applied to a GC-MS system. In addition, the internal standard [17,21,21,21-2H]pregnenolone was prepared as described in ref. 16 (for details, see Supporting Methods, which is published as supporting information on the PNAS web site).
Surgery, Steroid Treatment, and Behavioral Testing. To determine whether 7α-hydroxypregnenolone exerts any action in the newt brain, an intracerebroventricular (i.c.v.) injection of 7α-hydroxypregnenolone (Steraloids) was given through a 0.4-mm-diameter cannula as described in ref. 17. The cannula was implanted at the junction of the parietal and frontal bones in the cranial midline under anesthesia (0.2% MS-222, Sigma) as described in refs. 18 and 19. One week after surgery, polyethylene tubing was connected to the cannula to allow administration of 7α-hydroxypregnenolone. Neurosteroid administration and behavioral testing were conducted by using free-moving newts. By using a microsyringe attached to the polyethylene tubing, 7α-hydroxypregnenolone dissolved in isotonic saline containing 0.1% DMSO was injected over a period of 30 sec into the third ventricle at different doses (0.1, 0.5, and 1.0 ng in a 3-μl solution). Control treatment consisted of an equal volume of vehicle alone. For behavioral testing, newts were placed individually in a water-filled aquarium maintained at 18 ± 2°C; each testing arena was marked with parallel lines to define four equal sectors. Immediately after administration of 7α-hydroxypregnenolone, locomotor activity was quantified by counting the total number of lines crossed during a 30-min observation as described in refs. 19 and 20. Locomotion consisted of a combination of walking and swimming movements. The effect of 7β-hydroxypregnenolone, a stereoisomer of 7α-hydroxypregnenolone, on locomotion also was examined.
Measurements of Monoamine Concentrations. To determine whether 7α-hydroxypregnenolone influences the concentrations of monoamines in the brain, several monoamines were measured in the newt brain by HPLC electrochemical detector (ECD) (ECD-300, Eicom, Kyoto), as described in Supporting Methods.
Measurement of Dopamine Release. To determine whether 7α-hydroxypregnenolone influences the release of dopamine, the newt brain was preincubated with physiological saline (111 mM NaCl/1.9 mM KCl/1.1 mM CaCl2/2.4 mM NaHCO3) and incubated with the saline containing 7α-hydroxypregnenolone (10–8, 10–7, and 10–6 M) for 10 min at 25°C. The dopamine concentration in the saline was measured by HPLC-ECD (ECD-300, Eicom).
Treatment with Dopamine Antagonist. Finally, to characterize the mode of action of 7α-hydroxypregnenolone on locomotion, newts received an i.p. injection of mammalian dopamine D1-like receptor antagonist (SCH23390) or dopamine D2-like receptor antagonist (haloperidol or sulpiride) 60 min before an i.c.v. injection of 7α-hydroxypregnenolone. Locomotor activity was measured as described above.
Results
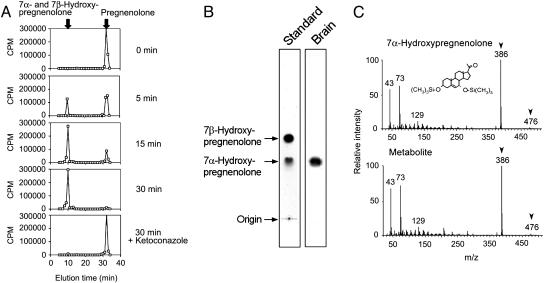
Identification of 7α-Hydroxypregnenolone in the Brain. In this study, we first found that an unknown neurosteroid was actively metabolized from pregnenolone in the newt brain. The production of the unknown neurosteroid in the newt brain was 93.1 ± 3.5% of total radioactivity incubated (n = 4 samples; one sample consisted of 20 mg of brain tissue from one newt). Because the production of the unknown neurosteroid was greater than that of any known neurosteroid, we propose that this neurosteroid might well be involved in important aspects of brain function in the newt. We identified this neurosteroid by using the following biochemical techniques combined with HPLC, TLC, and GC-MS analyses. Newt brain homogenates were incubated with tritiated pregnenolone as a precursor, and radioactive metabolites were analyzed by reversed-phase HPLC. A major radioactive peak of the metabolite was detected ∼20 min before the elution of pregnenolone, a precursor (Fig. 1A). Several nonradioactive steroids were used as reference standards for HPLC analysis, and 7α- and 7β-hydroxypregnenolone exhibited the same retention time of the radioactive peak under a similar chromatographic condition (Fig. 1A). The detection of 3H-7α- and 7β-hydroxypregnenolone was feasible by HPLC, because the radioactive pregnenolone used in this study was labeled with 3H at multiple positions, including the 7α- or 7β-position (see Materials and Methods). The radioactive metabolite corresponding to 7α- and 7β-hydroxypregnenolone increased in a time-dependent manner (Fig. 1A), and the inhibitor of cytochrome P450s, ketoconazole (10–4 M), reduced the metabolite (Fig. 1A). This HPLC peak was collected and subjected to TLC. By using nonradioactive 7α- and 7β-hydroxypregnenolone as reference standards, standard steroids were visualized by iodine atmosphere, and the metabolite of tritiated pregnenolone was detected by autoradiography. As shown in Fig. 1B, only 7α-hydroxypregnenolone had the same retention position as the radioactive metabolite of pregnenolone under the identical chromatographic condition. The metabolite of pregnenolone was further analyzed by GC-MS. Trimethylsilyl ether derivatives of the authentic 7α- and 7β-hydroxypregnenolone and the metabolite obtained from nonradioactive pregnenolone were prepared and subsequently applied to GC-MS analysis. Although 7α- and 7β-hydroxypregnenolone had the same mass spectrum, their retention times were different in GC-MS (7α-hydroxypregnenolone, 18.3 min; 7β-hydroxypregnenolone, 19.7 min). Based on GC-MS, 7α-hydroxypregnenolone and the metabolite had the identical retention time (18.3 min) and the same diagnostically important ions (Fig. 1C). Thus, we can identify the unknown neurosteroid converted from pregnenolone in the newt brain as 7α-hydroxypregnenolone.
Fig. 1.
Identification of 7α-hydroxypregnenolone in the brain. Newts in the spring breeding period (April and May) were used. (A) HPLC profile of an unknown metabolite of pregnenolone extracted from the newt brain homogenates after different incubation times with [3H]pregnenolone at 25°C by using a reversed-phase column. The ordinate indicates the radioactivity measured in each HPLC fraction, and the arrows indicate elution positions of standard steroids, pregnenolone and 7α- and 7β-hydroxypregnenolone. (B) Autoradiography of the unknown pregnenolone metabolite and standard steroids 7α- and 7β-hydroxypregnenolone on TLC under the same condition as in A. Standard steroids 7α- and 7β-hydroxypregnenolone were visualized by iodine atmosphere. (C) GC-MS of trimethylsilyl ether derivatives of an unknown pregnenolone metabolite and the authentic 7α-hydroxypregnenolone. The arrowheads indicate diagnostically important ions of 7α-hydroxypregnenolone (m/z 386 and 476).
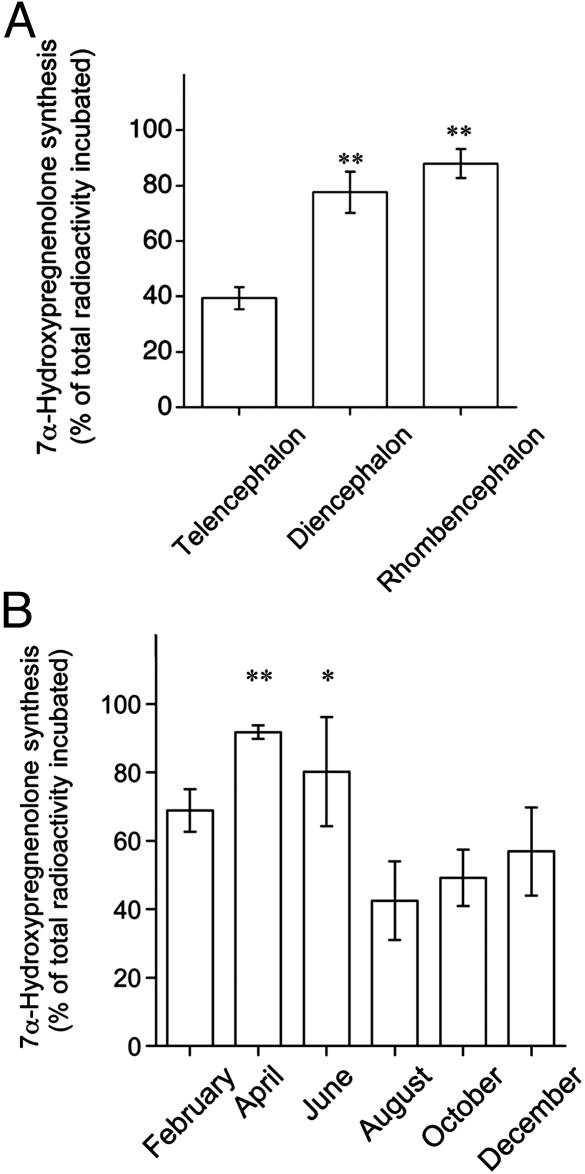
Comparison of 7α-Hydroxypregnenolone Synthesis Among Different Brain Regions and Seasonal Changes in 7α-Hydroxypregnenolone Synthesis in the Brain. This study compared the production of 7α-hydroxypregnenolone among different brain regions. Selected brain regions were incubated with tritiated pregnenolone, and 7α-hydroxypregnenolone synthesis was calculated as percentage of total radioactivity incubated. The production of 7α-hydroxypregnenolone was detected in various brain regions, but there was a clear regional difference (Fig. 2A). 7α-hydroxypregnenolone synthesis in the diencephalon and rhombencephalon was greater than that in the telencephalon (Fig. 2A). In contrast to the brain, 7α-hydroxypregnenolone synthesis was very low in peripheral steroidogenic glands, such as the testis, liver, and kidney/adrenal gland (see Fig. 5, which is published as supporting information on the PNAS web site). In particular, 7α-hydroxypregnenolone synthesis was negligible in the testis and kidney/adrenal gland.
Fig. 2.
Comparisons of 7α-hydroxypregnenolone synthesis among different brain regions and seasons. (A)7α-Hydroxypregnenolone synthesis in the newt telencephalon, diencephalon, and rhombencephalon. Newts in the spring breeding period (April and May) were used. Each column and vertical line represent the mean ± SEM percent of total radioactivity incubated (five samples, each consisting of 20 mg of tissue of each brain region from five newts). **, P < 0.01 vs. telencephalon by one-way ANOVA, followed by Duncan's multiple range test. (B) Seasonal changes in 7α-hydroxypregnenolone synthesis in the brain. Newt brains were obtained in February, April, June, August, October, and December. Each column and vertical line represent the mean ± SEM percent of total radioactivity incubated (four samples, each equivalent to 20 mg of brain tissue from one newt). *, P < 0.05, and **, P < 0.01 vs. August by one-way ANOVA, followed by Duncan's multiple range test.
To understand the function of 7α-hydroxypregnenolone in the brain, physiological changes in 7α-hydroxypregnenolone levels must be taken into account. We therefore examined seasonal changes in 7α-hydroxypregnenolone synthesis in the newt brain. As shown in Fig. 2B, 7α-hydroxypregnenolone synthesis in the brain markedly changed during the annual breeding cycle and was maximal in April and minimal in August, suggesting that 7α-hydroxypregnenolone is actively produced in the newt brain in the spring breeding period. 7α-Hydroxypregnenolone synthesis in the brain was also high in June, but there was large variance in sample values at that time of year (Fig. 2B).
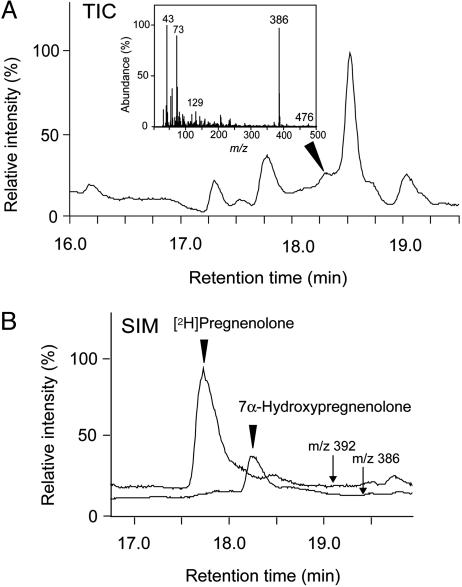
Endogenous Levels of 7α-Hydroxypregnenolone in the Brain and Plasma. To determine the concentration of 7α-hydroxypregnenolone in the newt brain, GC-MS analysis was performed. We measured the concentration of 7α-hydroxypregnenolone in the brain of newts collected in the nonbreeding period (November and December), because our experiment involving administration of 7α-hydroxypregnenolone and behavioral testing were conducted by using nonbreeding newts. Fig. 3A shows the GC-MS total-ion current trace of the extract from newt brain homogenates. As shown in Fig. 3A, 7α-hydroxypregnenolone was detected in the newt brain by GC-MS (see arrowhead). In contrast, 7α-hydroxypregnenolone in the plasma was below the level of detectability (data not shown), suggesting that 7α-hydroxypregnenolone in the plasma is very low. To measure the concentration of 7α-hydroxypregnenolone in the brain, steroids extracted from the brain tissue were further applied to GC-selected ion monitoring (SIM) analysis. Fig. 3B shows the GC-SIM ion chromatograms of m/z 386 and 392 in the extract from newt brain homogenates including the internal standard [2H]pregnenolone. Both ion peaks corresponding to 7α-hydroxypregnenolone (m/z 386) and the internal standard [2H]pregnenolone (m/z 392) could be detected (Fig. 3B). Based on the area of the ion peak corresponding to 7α-hydroxypregnenolone by GC-SIM analysis (Fig. 3B), the concentration and content of 7α-hydroxypregnenolone in the newt brain were calculated as 0.08 ± 0.02 ng/mg tissue and 1.8 ± 0.5 ng per brain, respectively (four samples, each consisting of 200 mg of brain tissue from 10 newts).
Fig. 3.
Measurement of endogenous 7α-hydroxypregnenolone in the brain. Newts in the nonbreeding period (November and December) were used. (A) GC-MS total-ion current (TIC) trace of the extract from newt brain homogenates. The arrowhead shows the peak corresponding to 7α-hydroxypregnenolone. (Inset) The mass spectrum of the peak corresponding to 7α-hydroxypregnenolone. (B) GC-SIM mass traces of m/z 386 and 392 in the extract from newt brain homogenates. The arrows show GC-SIM ion chromatograms of m/z 386 and 392, and the arrowheads indicate the retention times of 7α-hydroxypregnenolone and [2H]pregnenolone. The m/z 386 ion is a diagnostic ion of 7α-hydroxypregnenolone (see Fig. 1C), and the m/z 392 ion is a diagnostic ion of [2H]pregnenolone (parent ion).
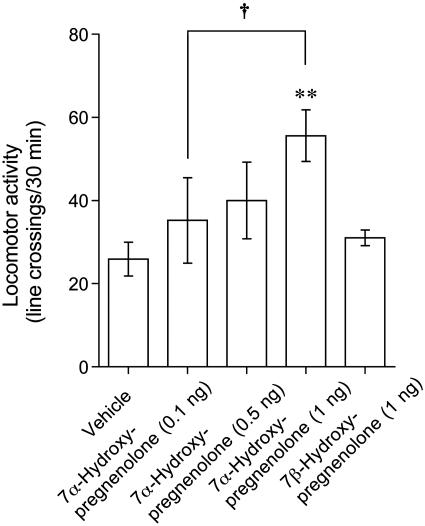
Effects of 7α-Hydroxypregnenolone on Locomotor Activity. To demonstrate whether 7α-hydroxypregnenolone exerts any action in the newt brain, this study further analyzed locomotor behavior after an i.c.v. injection to nonbreeding newts (November and December). As shown in Fig. 4, 7α-hydroxypregnenolone significantly increased locomotor activity of newts during a 30-min observation immediately after an i.c.v. injection. This stimulatory effect tended to be dose-dependent; the effective dose ranged between 0.5 and 1 ng i.c.v. injection (Fig. 4). In contrast, 7β-hydroxypregnenolone, a stereoisomer of 7α-hydroxypregnenolone, at the same high dose (1 ng) did not influence locomotor activity (Fig. 4).
Fig. 4.
Effect of 7α-hydroxypregnenolone on locomotor activity. Newts in the nonbreeding period (November and December) received an i.c.v. injection of vehicle (n = 7), 7α-hydroxypregnenolone (0.1 and 0.5 ng, n = 6; 1 ng, n = 7), or 7β-hydroxypregnenolone (1 ng, n = 6). Each column and vertical line represent the mean ± SEM total number of crossings. **, P < 0.01 vs. vehicle; †, P < 0.05 vs. 0.1 ng of 7α-hydroxypregnenolone injection by one-way ANOVA, followed by Duncan's multiple range test.
Mode of Action of 7α-Hydroxypregnenolone on Locomotion. To reveal the mode of action of 7α-hydroxypregnenolone on locomotion, the concentrations of several monoamines (norepinephrine, epinephrine, dopamine, and 5-hydroxytryptamine) were measured by HPLC-ECD 5 min after an i.c.v. injection of 7α-hydroxypregnenolone to nonbreeding newts (November and December). 7α-Hydroxypregnenolone significantly increased the concentration of dopamine in the newt brain, particularly in the rostral brain region (see Fig. 6, which is published as supporting information on the PNAS web site). However, there were no significant differences in the concentrations of other monoamines, such as norepinephrine, epinephrine, and 5-hydroxytryptamine (Fig. 6). In vitro analysis of brain tissue from nonbreeding newts (November and December) further revealed that 7α-hydroxypregnenolone treatment resulted in a dose-dependent increase in the release of dopamine from the cultured brain tissue after a 10-min incubation (Table 1). The threshold concentration ranged between 10–8 and 10–7 M (Table 1). Interestingly, the effect of 7α-hydroxypregnenolone on locomotion was abolished by i.p. administration of haloperidol or sulpiride, mammalian dopamine D2-like receptor antagonists (Table 2). In contrast, the mammalian dopamine D1-like receptor antagonist, SCH23390, did not abolish the effect of 7α-hydroxypregnenolone (Table 2).
Table 1. Effect of 7α-hydroxypregnenolone on dopamine release.
| 7α-Hydroxypregnenolone concentration, M | Dopamine release, % of baseline |
|---|---|
| 0 (control) | 100.0 ± 24.2 |
| 10-8 | 118.5 ± 28.8 |
| 10-7 | 244.5 ± 57.0* |
| 10-6 | 220.8 ± 14.7* |
Newts in the nonbreeding period (November and December) were used. The brain was incubated with physiological saline containing 7α-hydroxypregnenolone (10-8, 10-7, and 10-6 M; n = 4 samples at each concentration, respectively, with one sample per newt) for 10 min at 25°C, and the dopamine concentration in the saline was measured by HPLC-ECD. Results were expressed as the mean ± SEM percent of baseline dopamine release (805.6 ± 194.5 pg/ml). *, P < 0.01 vs. control by one-way ANOVA, followed by Duncan's multiple range test.
Table 2. Effect of dopamine receptor antagonists on the increase in locomotor activity induced by 7α-hydroxypregnenolone.
| Treatment | Locomotor activity, line crossings per 30 min |
|---|---|
| Vehicle | 25.9 ± 4.1 |
| 7α-Hydroxypregnenolone | 55.6 ± 6.2* |
| 7α-Hydroxypregnenolone + haloperidol | 20.8 ± 2.2† |
| 7α-Hydroxypregnenolone + sulpiride | 25.0 ± 4.5† |
| 7α-Hydroxypregnenolone + SCH23390 | 51.3 ± 11.8* |
Newts (n = 7 per each treatment) in the nonbreeding period (November and December) were used. The dopamine D1-like receptor antagonist, SCH23390 (1 μg, n = 7), or dopamine D2-like receptor antagonist, haloperidol (1 μg, n = 7) or sulpiride (1 μg, n = 7) was injected i.p. 60 min prior to an i.c.v. injection of 7α-hydroxypregnenolone (1 ng). Results were expressed as the mean ± SEM total number of crossings. *, P < 0.01 vs. vehicle. †, P < 0.01 vs. 1 ng of 7α-hydroxypregnenolone alone by one-way ANOVA, followed by Duncan's multiple range test.
Discussion
First, we identified an unknown amphibian neurosteroid as 7α-hydroxypregnenolone in the brain that was abundantly produced in the newt, a seasonally breeding amphibian. The formation of 7α-hydroxylated neurosteroids, such as 7α-hydroxypregnenolone and 7α-hydroxydehydroepiandrosterone, has been observed only in the brain of mammals, such as laboratory rodents and humans (21–24). In mammals, 7α-hydroxylation of dehydroepiandrosterone may be part of a metabolic pathway to more potent derivatives (25), and 7α-hydroxydehydroepiandrosterone is more active than dehydroepiandrosterone in preventing hypoxic cell death of neurons in vitro (26). In contrast to these mammalian studies, no investigation has been published on the formation of 7α-hydroxypregnenolone in nonmammalian vertebrates, to our knowledge. Furthermore, the function of 7α-hydroxypregnenolone is still unclear in the brain of any vertebrates. Because 7α-hydroxypregnenolone is actively produced in the brain of the newt, this seasonally breeding amphibian may serve as an excellent animal model to demonstrate the function of 7α-hydroxypregnenolone. The production of 7α-hydroxypregnenolone in the newt diencephalon and rhombencephalon was higher than that in the telencephalon and peripheral steroidogenic glands. In addition, 7α-hydroxypregnenolone synthesis in the brain showed marked changes during the annual breeding cycle, with a maximal level in the spring breeding period when locomotor activity of wild populations of the same species increases (27). We therefore analyzed the effect of 7α-hydroxypregnenolone on locomotor activity. Interestingly, exogenous 7α-hydroxypregnenolone acutely activated locomotor activity of newts in the nonbreeding period when endogenous 7α-hydroxypregnenolone synthesis in the brain was low. This stimulatory effect tended to occur in a dose-dependent manner with a threshold dose ranging between 0.5- and 1-ng i.c.v. injection (Fig. 4), indicating that the effect of 7α-hydroxypregnenolone may be within the physiological range observed in the brain of normal newts (see Results). Therefore, 7α-hydroxypregnenolone may act as a neuronal activator to stimulate locomotor activity of newts. The increase in locomotor activity of newts in the spring breeding period may be due to an increase in the production of 7α-hydroxypregnenolone (see Fig. 2B). Thus, this study shows a previously undescribed physiological function of 7α-hydroxypregnenolone in the brain of a vertebrate.
In the present study, we used only male newts to demonstrate a physiological function of 7α-hydroxypregnenolone in the brain, because our preliminary studies suggested that 7α-hydroxypregnenolone is produced in the brain of both sexes, but the concentration of 7α-hydroxypregnenolone in the male brain is higher than that in the female brain (M.M., K.U., and K.T., unpublished data). It is well known that sexually mature male newts in the breeding period move around much more than the females, searching for sexually mature female partners or courting the females before the sperm transfer (28, 29). It is therefore possible that 7α-hydroxypregnenolone may actually function in the male brain rather than the female brain.
This study also provides findings on the regulatory mechanism of locomotor activity that depends on 7α-hydroxypregnenolone action. 7α-Hydroxypregnenolone increased the concentration of dopamine in the rostral brain region including the striatum (Fig. 6), which is known to be involved in the regulation of locomotor behavior (30, 31). In addition, 7α-hydroxypregnenolone dose-dependently increased dopamine release from cultured brain tissue within the physiological range of 10–8 to 10–7 M (Table 1). Behavioral analysis further indicated that the effect of 7α-hydroxypregnenolone on locomotor activity was abolished by mammalian dopamine D2-like receptor antagonists haloperidol and sulpiride. It has been reported that in amphibians, dopamine neurons are localized in the medial brain region, such as the posterior tuberal nucleus and ventral tegmental area, and that they project to the rostral brain region, such as the striatum (32, 33). It also has been reported that, as in mammal brain, the amphibian brain possesses dopamine D1- and D2-like receptors (34) and that both haloperidol and sulpiride abolish dopamine actions in amphibians (35). The present and previous studies with amphibians suggest that the stimulatory effect of 7α-hydroxypregnenolone on locomotor activity is mediated by the dopaminergic system. 7α-Hydroxypregnenolone synthesized actively in the diencephalon and rhombencephalon may, by acting on dopamine neurons localized in the posterior tuberal nucleus and ventral tegmental area, induce dopamine release from their termini in the rostral brain region, such as the striatum, and consequently increase locomotor activity of newts. However, it is still not entirely clear whether the effect of 7α-hydroxypregnenolone on locomotion is solely mediated by dopamine D2-like receptors, because haloperidol also has been shown to bind with high affinity to σ receptors in mammals (36).
Because 7α-hydroxypregnenolone acutely increased both locomotor activity and dopamine release, these actions may be nongenomic rather than genomic. Similar effects of the progesterone metabolite allopregnanolone on locomotion (37) and dopamine release (38, 39) have been reported in rats. These effects of allopregnanolone may be through the modulation of GABA type A receptors, because allopregnanolone is considered to be a potent allosteric modulator of GABA type A receptors (40, 41), and dopamine neurons are regulated by GABAergic transmission (42) in mammals. To understand acute actions of 7α-hydroxypregnenolone on dopamine release and locomotor activity in amphibians, we need to analyze the action of 7α-hydroxypregnenolone on GABA type A receptors. Conversely, we have found that the concentration of progesterone in the newt brain increases in the spring breeding period (8). Not only 7α-hydroxypregnenolone but also progesterone or its metabolite allopregnanolone may regulate dopamine release and modulate locomotor activity of newts in the spring breeding period. Because 7-oxo-pregnenolone is produced from pregnenolone and transformed into 7α-hydroxypregnenolone, 7-oxo-pregnenolone also might be involved in regulation of locomotor activity of newts. To draw a firm conclusion, we need to establish steroid specificity with regard to regulation of locomotor behavior.
Interestingly, 7α-hydroxypregnenolone synthesis in the newt brain showed marked changes during the annual breeding cycle and a maximal level in the spring breeding period. Ketoconazole, an inhibitor of P450s, reduced 7α-hydroxypregnenolone synthesis in the newt brain. Cytochrome P4507α (Cyp7b) catalyzes the conversion of pregnenolone to 7α-hydroxypregnenolone in the brain of mammals (43, 44). Therefore, 7α-hydroxypregnenolone synthesis in the newt brain may be a Cyp7b-dependent mechanism, and some factors may induce the expression of Cyp7b in the spring breeding period.
Supplementary Material
Acknowledgments
We thank Dr. G. E. Bentley (University of Washington, Seattle) for his valuable discussion and reading of this manuscript and Drs. H. Hasegawa, H. Takeda, and N. Sakurai (Hiroshima University) for their technical assistance. This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology (Tokyo).
Author contributions: M.M., K.U., E.-E.B., and K.T. designed research; M.M. performed research and analyzed data; and M.M. and K.T. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SIM, selected ion monitoring; ECD, electrochemical detector.
References
- 1.Baulieu, E.-E. (1997) Recent Prog. Horm. Res. 52, 1–32. [PubMed] [Google Scholar]
- 2.Compagnone, N. A. & Mellon, S. H. (2000) Front. Neuroendocrinol. 21, 1–56. [DOI] [PubMed] [Google Scholar]
- 3.Mellon, S. H. & Vaudry, H. (2001) Int. Rev. Neurobiol. 46, 33–78. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsui, K., Ukena, K., Takase, M., Kohchi, C. & Lea, R. W. (1999) Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 124, 121–129. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui, K., Ukena, K., Usui, M., Sakamoto, H. & Takase, M. (2000) Neurosci. Res. 36, 261–273. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsui, K., Matsunaga, M. & Ukena, K. (2003) Avian Poultry Biol. Rev. 14, 63–78. [Google Scholar]
- 7.Takase, M., Ukena, K., Yamazaki, T., Kominami, S. & Tsutsui, K. (1999) Endocrinology 140, 1936–1944. [DOI] [PubMed] [Google Scholar]
- 8.Inai, Y., Nagai, K., Ukena, K., Oishi, T. & Tsutsui, K. (2003) Brain Res. 959, 214–225. [DOI] [PubMed] [Google Scholar]
- 9.Lea, R. W., Clark, J. A. & Tsutsui, K. (2001) Microsc. Res. Tech. 55, 12–26. [DOI] [PubMed] [Google Scholar]
- 10.Mensah-Nyagan, A. G., Feuilloley, M., Dupont, E., Do-Rego, J. L., Leboulenger, F., Pelletier, G. & Vaudry, H. (1994) J. Neurosci. 14, 7306–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mensah-Nyagan, A. G., Feuilloley, M., Do-Rego, J. L., Marcual, A., Lange, C., Tonon, M. C., Pelletier, G. & Vaudry, H. (1996) Proc. Natl. Acad. Sci. USA 93, 1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ukena, K., Kohchi, C. & Tsutsui, K. (1999) Endocrinology 140, 805–813. [DOI] [PubMed] [Google Scholar]
- 13.Matsunaga, M., Ukena, K. & Tsutsui, K. (2001) Brain Res. 899, 112–122. [DOI] [PubMed] [Google Scholar]
- 14.Matsunaga, M., Ukena, K. & Tsutsui, K. (2002) Brain Res. 948, 180–185. [DOI] [PubMed] [Google Scholar]
- 15.Takeda, H., Kotake, T., Nakagawa, N., Sakurai, N. & Nevins, D. J. (2003) Plant Physiol. 131, 1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallée, M., Rivera, J. D., Koob, G. F., Purdy, R. H. & Fitzgerald, R. L. (2000) Anal. Biochem. 287, 153–166. [DOI] [PubMed] [Google Scholar]
- 17.Kozaka, T., Fujii, Y. & Ando, M. (2003) J. Exp. Biol. 206, 687–692. [DOI] [PubMed] [Google Scholar]
- 18.Moore, F. L. & Miller, L. J. (1983) Peptides 4, 97–102. [DOI] [PubMed] [Google Scholar]
- 19.Lowry, C. A., Burke, K. A., Renner, K. J., Moore, F. L. & Orchinik, M. (2001) Horm. Behav. 39, 195–205. [DOI] [PubMed] [Google Scholar]
- 20.Moore, F. L., Roberts, J. & Bevers, J. (1984) J. Exp. Zool. 231, 331–333. [DOI] [PubMed] [Google Scholar]
- 21.Akwa, Y., Morfin, R. F., Robel, P. & Baulieu, E.-E. (1992) Biochem. J. 288, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doostzadeh, J. & Morfin, R. (1997) J. Endocrinol. 155, 343–350. [DOI] [PubMed] [Google Scholar]
- 23.Weill-Engerer, S., David, J. P., Sazdovitch, V., Liere, P., Schumacher, M., Delacourte, A., Baulieu, E.-E. & Akwa, Y. (2003) Brain Res. 969, 117–125. [DOI] [PubMed] [Google Scholar]
- 24.Yau, J. L., Rasmuson, S., Andrew, R., Graham, M., Noble, J., Olsson, T., Fuchs, E., Lathe, R. & Seckl, J. R. (2003) Neuroscience 121, 307–314. [DOI] [PubMed] [Google Scholar]
- 25.Lardy, H., Partridge, B., Kneer, N. & Wei, Y. (1995) Proc. Natl. Acad. Sci. USA 92, 6617–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lathe, R. (2002) Steroids 67, 967–977. [DOI] [PubMed] [Google Scholar]
- 27.Uchiyama, R., Maeda, N., Numata, K. & Seki, S. (2002) A Photographic Guide: Amphibians and Reptiles in Japan (Heibonsha, Tokyo).
- 28.Iwata, T., Toyoda, F., Yamamoto, K. & Kikuyama, S. (2000) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 126, 221–229. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsui, Y. (1931) Memoirs Coll. Sci., Kyoto Imperial Univ., Ser. B 7, 159–178. [Google Scholar]
- 30.Sharp, T., Zetterstrom, T., Ljungberg, T. & Ungerstedt, U. (1987) Brain Res. 401, 322–330. [DOI] [PubMed] [Google Scholar]
- 31.Bardo, M. T., Bowling, S. L. & Pierce, R. C. (1990) Psychopharmacology 101, 338–343. [DOI] [PubMed] [Google Scholar]
- 32.González, A. & Smeets, W. J. (1991) J. Comp. Neurol. 303, 457–477. [DOI] [PubMed] [Google Scholar]
- 33.Marín, O., Smeets, W. J. & González, A. (1998) Trends Neurosci. 21, 487–494. [DOI] [PubMed] [Google Scholar]
- 34.Chu, J., Wilczynski, W. & Wilcox, R. E. (2001) Brain Behav. Evol. 57, 328–342. [DOI] [PubMed] [Google Scholar]
- 35.Ryan, G. P., Hackman, J. C., Wohlberg, C. J. & Davidoff, R. A. (1985) Brain Res. 328, 283–290. [DOI] [PubMed] [Google Scholar]
- 36.Moison, D., De Deurwaerdere, P., Cagnotto, A., Marrazzo, A., Prezzavento, O., Ronsisvalle, G., Mennini, T. & Spampinato, U. (2003) Neuropharmacology 45, 945–953. [DOI] [PubMed] [Google Scholar]
- 37.Wieland, S., Belluzzi, J. D., Stein, L., Lan, N. C. (1995) Psychopharmacology 118, 65–71. [DOI] [PubMed] [Google Scholar]
- 38.Bullock, A. E., Clark, A. L., Grady, S. R., Robinson, S. F., Slobe, B. S., Marks, M. J. & Collins, A. C. (1997) J. Neurochem. 68, 2412–2423. [DOI] [PubMed] [Google Scholar]
- 39.Rougé-Pont, F., Mayo, W., Marinelli, M., Gingras, M., Moal, M. L. & Piazza, P. V. (2002) Eur. J. Neurosci. 16, 169–173. [DOI] [PubMed] [Google Scholar]
- 40.Paul, S. M. & Purdy, R. H. (1992) FASEB J. 6, 2311–2322. [PubMed] [Google Scholar]
- 41.Lambert, J. J., Belelli, D., Hill-Venning, C. & Peters, J. A. (1995) Trends Pharmacol. Sci. 16, 295–303. [DOI] [PubMed] [Google Scholar]
- 42.Laviolette, S. R. & van der Kooy, D. (2001) Eur. J. Neurosci. 13, 1009–1015. [DOI] [PubMed] [Google Scholar]
- 43.Rose, K., Stapleton, G., Dott, K., Kieny, M. P., Best, R., Schwarz, M., Russell, D. W., Björkhem, I., Seckl, J. R. & Lathe, R. (1997) Proc. Natl. Acad. Sci. USA 94, 4925–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stapleton, G., Steel, M., Richardson, M., Mason, J. O., Rose, K. A., Morris, R. G. & Lathe, R. (1995) J. Biol. Chem. 270, 29739–29745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.