Abstract
Background
Hypertension is a risk factor for peripheral arterial disease (PAD). Subjects with PAD are at increased risk of future cardiovascular (CV) events. Resistin is involved in the pathological processes of CV diseases. The aim of this study is to investigate whether resistin level is correlated with PAD in hypertensive patients.
Methods
One hundred and twenty-four hypertensive patients were enrolled in this study. Ankle-brachial index (ABI) values were measured using the automated oscillometric method. An ABI value < 0.9 defined the low ABI group. Anthropometric analysis with waist circumference and body mass index, and fasting serum levels of blood urea nitrogen, creatinine, glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total calcium, phosphorus, and high-sensitivity C-reactive protein (hs-CRP) were measured using standard enzymatic automated methods. Serum levels of human resistin were determined using a commercially available enzyme immunoassay.
Results
Eighteen hypertensive patients (14.5%) were included in the low ABI group. Hypertensive patients in the low ABI group were older (p = 0.043) and had higher serum creatinine (p < 0.001), high-sensitivity C-reactive protein (hs-CRP; p = 0.013), and resistin (p < 0.001) levels but a lower estimated glomerular filtration rate (p = 0.002) than patients in the normal ABI group. After the adjustment for factors that were significantly associated with PAD on multivariate logistic regression analysis, serum resistin (odds ratio [OR], 1.176; 95% confidence interval [CI], 1.028–1.345; p = 0.018) was also an independent predictor of PAD in hypertensive patients.
Conclusions
A high serum resistin level is an independent predictor of PAD in hypertensive patients.
Keywords: Peripheral arterial disease, Ankle-brachial index, Hypertension, Resistin
Background
Peripheral arterial disease (PAD) is a reflection of systemic atherosclerotic disease, which affected 202 million people worldwide in 2010 [1]. Although PAD can be asymptomatic and subclinical, symptomatic PAD is associated with reduced functional capacity and decreased quality of life [2]. PAD is also associated with an increased risk of incident coronary and cerebrovascular disease morbidity and mortality [2, 3]. The ankle-brachial index (ABI), the ratio of the systolic blood pressure (SBP) at the ankle to that in the arm, is non-invasive and inexpensive and currently used to assess PAD [3]. In a systematic review study, ABI had a sensitivity of 97% and specificity of 89% for detecting significant arterial disease on angiography [4].
Resistin is an adipocyte-derived signaling cysteine-rich molecule consisting of 114 amino acids [5]. Resistin is involved in the pathological processes of cardiovascular (CV) diseases including inflammation, endothelial dysfunction, thrombosis, angiogenesis, and smooth muscle cell dysfunction [6]. Serum resistin levels were higher in PAD patients than in healthy controls and an independent risk factor of PAD in a Chinese study [7]. PAD has been known to increase the risk of CV morbidity and mortality, resistin is involved in the pathological processes of CV diseases, and hypertension is one of the risk factors of PAD [2, 3]. The aim of this study is to examine the relationship between serum resistin levels and PAD in hypertensive patients.
Methods
Patients
From January to December 2012, 124 hypertensive patients enrolled at a medical center in Hualien, Taiwan. The blood pressure (BP) of each patient was measured in the morning by trained staff using standard mercury sphygmomanometers with appropriate cuff sizes after sitting for at least 10 min. Systolic BP and diastolic BP were taken three times at 5-min intervals and averaged for analysis. Patients were regarded as having hypertension if they had a systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, or received any anti-hypertensive medication in the past 2 weeks. Patients were diagnosed with diabetes mellitus if their fasting plasma glucose level was ≥126 mg/dL or they had used oral hypoglycemic medications or insulin [8]. The Protection of the Human Subjects Institutional Review Board of Tzu-Chi University and Hospital approved this study. All patients provided informed consent prior to participating in this study. The patients were excluded if they had an acute infection, acute myocardial infarction, or pulmonary edema, had a history of carotid artery stenosis or stroke; used protease-activated receptor-1 antagonists or warfarin at the time of blood sampling; or declined to provide informed consent.
Anthropometric analysis
Waist circumference was measured using a tape measure at the point between the lowest ribs and the hip bones with the hands on the hips. Participant weight was measured in light clothing and without shoes to the nearest 0.5 kg, while height was measured to the nearest 0.5 cm. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared [9–11].
Biochemical investigations
Fasting blood samples (approximately 5 mL) were immediately centrifuged at 3000 × g for 10 min. Serum levels of blood urea nitrogen (BUN), creatinine (Cre), fasting glucose, total cholesterol (TCH), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total calcium, phosphorus, and high-sensitivity C-reactive protein (hs-CRP) were measured using an autoanalyzer (COBAS Integra 800; Roche Diagnostics, Basel, Switzerland) [9–11]. Serum levels of human resistin (SPI-BIO; Montigny le Bretonneux, France) [12] and intact parathyroid hormone (iPTH; Diagnostic Systems Laboratories, Webster, TX, USA) [9–11] were determined using a commercially available enzyme immunoassay or enzyme-linked immunosorbent assay, respectively. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
ABI measurements
Using an oscillometric method, ABI values were measured using an ABI-form device (VaSera VS-1000; Fukuda Denshi Co, Ltd, Tokyo, Japan) that automatically and simultaneously measures BP in both arms and ankles [13]. With the participants lying in the supine position, occlusion and monitoring cuffs were placed tightly around the four extremities, and an electrocardiogram was recorded and the heart sounds were measured for at least 10 min. ABI was calculated as the ratio of the ankle SBP divided by the arm SBP, and the lower value of the ankle SBP was used for the calculation. We repeatedly measured these parameters for both legs of each participant and expressed the mean values. PAD was diagnosed based on an ABI < 0.9 [14]. In this study, left or right ABI values < 0.9 were used to define the low ABI group.
Statistical analysis
Data were tested for normal distribution using the Kolmogorov–Smirnov test. Normally distributed data are expressed as mean ± standard deviation and comparisons between patients were performed using the Student’s independent t-test (two-tailed). Non-normally distributed data were expressed as medians and interquartile ranges and comparisons between patients were performed using the Mann–Whitney U test (TG, fasting glucose, iPTH, hs-CRP, and resistin). Data expressed as the number of patients were analyzed by the χ 2 test. Because TG, fasting glucose, iPTH, hs-CRP, and resistin was not normally distributed and underwent base 10 logarithmic transformations to achieve normality. Clinical variables that correlated with serum resistin levels in hypertensive patients were evaluated using univariate linear regression analysis. Variables that were significantly associated with resistin levels in hypertensive patients were tested for independence on multivariate forward stepwise regression analysis. Variables that were significantly associated with PAD were tested for independence on multivariate logistic regression analysis (adapted factors: smoking, age, Cre, eGFR, hs-CRP, and resistin). The receiver operating curve (ROC) was used to calculate the area under the curve (AUC) to identify the cutoff value of resistin predicting PAD in hypertensive patients. Data were analyzed using SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL, USA). P values < 0.05 were considered statistically significant.
Results
The clinical and laboratory characteristics of the 124 hypertensive patients are shown in Table 1. Sixty-three patients (50.8%) had diabetes mellitus and 99 patients (79.8%) had dyslipidemia. Eighteen hypertensive patients (14.5%) were included in the low ABI group. Patients in the low ABI group were older (p = 0.043) and had higher serum Cre (p < 0.001), hs-CRP (p = 0.013), and resistin (p < 0.001) levels but had lower eGFR (p = 0.002) levels than those in the normal ABI group. Current smokers among low ABI group were significantly higher than the normal ABI group (p = 0.031). The use of drugs included angiotensin-converting enzyme inhibitors (ACEi; n = 44; 35.5%), angiotensin receptor blockers (ARB; n = 67; 54.0%), β-blockers (n = 72; 58.1%), calcium channel blockers (CCB; n = 56; 45.2%), statins (n = 69; 55.4%), fibrate (n = 30; 24.2%), aspirin (n = 72; 58.1%), and clopidogrel (n = 30; 24.2%). There was no statistically significant difference based on gender, co-existing diabetes or dyslipidemia, or use of ACEi, ARB, β-blockers, CCB, statins, fibrate, aspirin, or clopidogrel between the two groups. The resistin levels also did not differ statistically based on gender, co-existing diabetes or dyslipidemia, or ACEi, ARB, β-blocker, CCB, statin, fibrate, aspirin, or clopidogrel use (Table 2).
Table 1.
Clinical variables of the 124 hypertensive patients in the normal or low ankle brachial index group
| Characteristic | All patients (n = 124) | Normal ABI group (n = 106) | Low ABI group (n = 18) | p value |
|---|---|---|---|---|
| Age (years) | 64.40 ± 9.81 | 63.67 ± 9.00 | 68.72 ± 13.15 | 0.043* |
| Height (cm) | 161.33 ± 8.84 | 161.75 ± 8.62 | 158.83 ± 9.92 | 0.197 |
| Body weight (kg) | 69.96 ± 12.49 | 70.51 ± 12.78 | 66.78 ± 10.39 | 0.243 |
| Waist circumference (cm) | 91.87 ± 9.00 | 91.63 ± 8.52 | 93.28 ± 11.65 | 0.476 |
| Body mass index (kg/m2) | 26.80 ± 3.72 | 26.84 ± 3.69 | 26.53 ± 3.96 | 0.740 |
| Left ankle-brachial index | 1.06 ± 0.11 | 1.09 ± 0.08 | 0.87 ± 0.10 | <0.001* |
| Right ankle-brachial index | 1.05 ± 0.12 | 1.08 ± 0.08 | 0.84 ± 0.10 | <0.001* |
| Systolic blood pressure (mmHg) | 134.63 ± 16.97 | 133.62 ± 16.11 | 140.56 ± 20.92 | 0.109 |
| Diastolic blood pressure (mmHg) | 74.10 ± 10.18 | 74.30 ± 10.46 | 72.94 ± 8.47 | 0.603 |
| Total cholesterol (mg/dL) | 174.18 ± 38.59 | 174.38 ± 40.54 | 173.00 ± 24.90 | 0.889 |
| Triglyceride (mg/dL) | 128.00 (93.25–177.25) | 122.00 (90.75–178.25) | 137.00 (114.00–172.50) | 0.383 |
| HDL-C (mg/dL) | 45.19 ± 12.81 | 45.38 ± 12.85 | 44.11 ± 12.88 | 0.700 |
| LDL-C (mg/dL) | 103.19 ± 30.46 | 103.03 ± 32.11 | 104.11 ± 18.47 | 0.890 |
| Fasting glucose (mg/dL) | 111.00 (96.25–137.75) | 111.00 (96.75–137.25) | 110.00 (94.50–154.00) | 0.980 |
| Blood urea nitrogen (mg/dL) | 17.36 ± 6.11 | 16.99 ± 5.75 | 19.56 ± 7.76 | 0.100 |
| Creatinine (mg/dL) | 1.13 ± 0.33 | 1.09 ± 0.28 | 1.38 ± 0.47 | <0.001* |
| eGFR (mL/min) | 67.81 ± 20.37 | 70.10 ± 18.81 | 54.33 ± 24.35 | 0.002* |
| Total calcium (mg/dL) | 9.16 ± 0.37 | 9.15 ± 0.38 | 9.20 ± 0.33 | 0.560 |
| Phosphorus (mg/dL) | 3.55 ± 0.51 | 3.54 ± 0.53 | 3.57 ± 0.38 | 0.854 |
| Intact parathyroid hormone (pg/mL) | 46.65 (33.53–61.95) | 46.30 (32.40–59.85) | 51.20 (36.45–77.78) | 0.272 |
| hs-CRP (mg/dL) | 0.21 (0.15–0.29) | 0.20 (0.14–0.27) | 0.28 (0.20–0.55) | 0.013* |
| Resistin (ng/mL) | 7.19 (5.21–10.33) | 6.67 (4.77–8.59) | 11.79 (9.94–16.72) | <0.001* |
| Male, n (%) | 82 (66.1) | 71 (67.0) | 11 (61.1) | 0.627 |
| Diabetes, n (%) | 63 (50.8) | 52 (49.1) | 11 (61.1) | 0.344 |
| Dyslipidemia, n (%) | 99 (79.8) | 85 (80.2) | 14 (77.8) | 0.814 |
| Smoking, n (%) | 11 (8.9) | 7 (6.6) | 4 (22.2) | 0.031* |
| ACE inhibitor use, n (%) | 44 (35.5) | 39 (36.8) | 5 (27.8) | 0.460 |
| ARB use, n (%) | 67 (54.0) | 57 (53.8) | 10 (55.6) | 0.888 |
| β-blocker use, n (%) | 72 (58.1) | 60 (56.6) | 12 (66.7) | 0.424 |
| CCB use, n (%) | 56 (45.2) | 49 (46.2) | 7 (38.9) | 0.563 |
| Statin use, n (%) | 69 (55.4) | 58 (54.7) | 11 (61.1) | 0.614 |
| Fibrate use, n (%) | 30 (24.2) | 28 (26.4) | 2 (11.1) | 0.161 |
| Aspirin, n (%) | 72 (58.1) | 64 (60.4) | 8 (44.4) | 0.205 |
| Clopidogrel, n (%) | 30 (24.2) | 27 (25.5) | 3 (16.7) | 0.420 |
Values for continuous variables are shown as mean ± standard deviation after analysis by Student’s t-test; variables not normally distributed are shown as median and interquartile range after analysis by the Mann–Whitney U test; values are presented as number (%) and analysis after analysis by the chi-square test
ABI ankle brachial index, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, hs-CRP high-sensitivity C-reactive protein, ACE angiotensin-converting enzyme, ARB angiotensin-receptor blocker, CCB calcium-channel blocker
*Values of p < 0.05 were considered statistically significant
Table 2.
Clinical characteristics and serum resistin levels of 124 hypertensive patients
| Characteristic | Number (%) | Resistin (ng/mL) | p value | |
|---|---|---|---|---|
| Sex | Male | 82 (66.1) | 7.26 (5.10–10.11) | 0.631 |
| Female | 42 (33.9) | 6.95 (5.60–11.98) | ||
| Diabetes | No | 61 (49.2) | 6.86 (5.12–10.01) | 0.472 |
| Yes | 63 (50.8) | 7.41 (5.24–10.89) | ||
| Dyslipidemia | No | 25 (20.2) | 7.23 (5.14–9.55) | 0.818 |
| Yes | 99 (79.8) | 7.18 (5.20–10.89) | ||
| ACE inhibitor | No | 80 (64.5) | 7.17 (5.14–10.81) | 0.938 |
| Yes | 44 (35.5) | 7.22 (5.26–10.16) | ||
| ARB | No | 57 (46.0) | 7.20 (4.91–10.44) | 0.833 |
| Yes | 67 (54.0) | 7.18 (5.24–10.19) | ||
| β-blocker | No | 52 (41.9) | 6.95 (5.34–9.22) | 0.491 |
| Yes | 72 (58.1) | 7.39 (4.94–11.74) | ||
| CCB | No | 68 (54.8) | 7.04 (4.67–10.16) | 0.498 |
| Yes | 56 (45.2) | 7.42 (5.40–10.79) | ||
| Statin | No | 55 (44.6) | 7.41 (5.68–10.55) | 0.260 |
| Yes | 69 (55.4) | 6.88 (4.77–10.02) | ||
| Fibrate | No | 94 (75.8) | 7.33 (5.36–10.51) | 0.253 |
| Yes | 30 (24.2) | 6.76 (4.56–8.56) | ||
| Aspirin | No | 52 (41.9) | 8.90 (5.78–10.24) | 0.525 |
| Yes | 72 (58.1) | 7.88 (4.92–10.16) | ||
| Clopidogrel | No | 94 (75.8) | 8.53 (5.36–10.17) | 0.253 |
| Yes | 30 (24.2) | 7.61 (4.56–8.56) | ||
Data are expressed as median and interquartile range after analysis by the Mann–Whitney U test
ACE angiotensin-converting enzyme, ARB angiotensin-receptor blocker, CCB calcium-channel blocker
*Values of p < 0.05 were considered statistically significant after analysis by the Mann–Whitney U test
Uni- and multivariate linear analyses of the clinical variables associated with serum resistin levels in hypertensive patients are shown in Table 3. Waist circumference (r = 0.261; p = 0.003), TCH (r = 0.182; p = 0.043), LDL-C (r = 0.190; p = 0.035), BUN (r = 0.237; p = 0.008), Cre (r = 0.298; p = 0.001), and logarithmically transformed hs-CRP (log-hs-CRP, r = 0.294; p = 0.001) were positively correlated, while eGFR (r = −0.278; p = 0.002) was negatively correlated with serum resistin levels in hypertensive patients. Multivariate forward stepwise linear regression analysis of the variables significantly associated with fasting serum resistin levels revealed that waist circumference (β = 0.204; p = 0.014), LDL-C (β = 0.165; p = 0.044), Cre (β = 0.243; p = 0.004), and log-hs-CRP (β = 0.236; p = 0.005) were independent predictors of resistin values for hypertensive patients.
Table 3.
Correlation between serum resistin levels and clinical variables among the 124 hypertensive patients
| Variable | Log-Resistin (ng/mL) | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| r | p | Beta | p | |
| Age (years) | 0.135 | 0.135 | - | - |
| Height (cm) | 0.006 | 0.949 | - | - |
| Body weight (kg) | −0.117 | 0.197 | - | - |
| Waist circumference (cm) | 0.261 | 0.003* | 0.204 | 0.014* |
| Body mass index (kg/m2) | −0.149 | 0.098 | - | - |
| SBP (mmHg) | −0.102 | 0.261 | - | - |
| DBP (mmHg) | 0.044 | 0.629 | - | - |
| Total cholesterol (mg/dL) | 0.182 | 0.043* | - | - |
| Log-Triglyceride (mg/dL) | 0.015 | 0.872 | - | - |
| HDL-C (mg/dL) | 0.061 | 0.501 | - | - |
| LDL-C (mg/dL) | 0.190 | 0.035* | 0.165 | 0.044* |
| Log-glucose (mg/dL) | −0.059 | 0.517 | - | - |
| Blood urea nitrogen (mg/dL) | 0.237 | 0.008* | - | - |
| Creatinine (mg/dL) | 0.298 | 0.001* | 0.243 | 0.004* |
| eGFR (mL/min) | −0.278 | 0.002* | - | - |
| Total calcium (mg/dL) | −0.094 | 0.302 | - | - |
| Phosphorus (mg/dL) | −0.109 | 0.228 | - | - |
| Log-iPTH (pg/mL) | 0.146 | 0.106 | - | - |
| Log-hs-CRP (mg/dL) | 0.294 | 0.001* | 0.236 | 0.005* |
Data of resistin, triglyceride, glucose, iPTH, and hs-CRP levels showed a skewed distribution and were, therefore, log-transformed before analysis
Analysis of the data was done using the univariate linear regression analyses or multivariate stepwise linear regression analysis (adopted factors: waist circumference, total cholesterol, blood urea nitrogen, creatinine, eGFR, and hs-CRP)
SBP systolic blood pressure, DBP diastolic blood pressure, HDL-C high density lipoprotein-cholesterol LDL-C low-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, iPTH intact parathyroid hormone, hs-CRP high-sensitivity C-reactive protein
*Values of p < 0.05 were considered statistically significant
Adjustment of the factors significantly associated with PAD (smoking, age, Cre, eGFR, hs-CRP, and resistin) on multivariate logistic regression analysis revealed that increased serum resistin level (odds ratio [OR], 1.176; 95% confidence interval [CI], 1.028–1.345; p = 0.018) was an independent predictor of PAD in hypertensive patients (Table 4); plotting of the ROC curve for PAD prediction revealed that the AUC for resistin was 0.870 (95% CI, 0.798–0.923; p < 0.001) (Fig. 1).
Table 4.
Multivariate logistic regression analysis of the factors correlated to peripheral artery disease among the 124 hypertensive patients
| Variable | Odds ratio | 95% Confidence interval | p value |
|---|---|---|---|
| Resistin (ng/mL) (each increase of 1 ng/mL) |
1.176 | 1.028-1.345 | 0.018* |
Analysis of the data was done using multivariate logistic regression analysis (adopted factors: smoking, age, creatinine, estimated glomerular filtration rate, high-sensitivity C-reactive protein, and resistin)
*Values of p < 0.05 were considered statistically significant
Fig. 1.
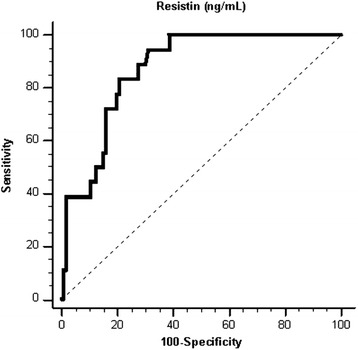
The area under the receiver operating characteristic curve indicates the diagnostic power of resistin for predicting peripheral artery disease of hypertensive patients
Discussion
The results of this study showed that hypertensive patients with PAD were older and had higher serum Cre, hs-CRP, and resistin levels but a lower eGFR. Serum resistin level was an independent clinical predictor of PAD in hypertensive patients after multivariate analysis. Among these patients, waist circumference and LDL-C, Cre, and log-hs-CRP levels were the independent predictors of resistin values.
PAD is a result of the blockage of the arteries supplying blood to the brain, visceral organs, and the limbs and usually occurs secondarily to atherosclerosis [15, 16]. The prevalence of PAD increases sharply with age, and it affects a substantial proportion of the elderly population [1–3]. Moreover, men and women from low- and middle-income countries have modestly lower PAD rates than those in high-income countries [1]. The prevalence in high-income countries at age 45–49 years was 5.28% in women and 5.41% in men; at age 85–89 years, it was 18.38% in women and 18.83% in men. The prevalence in men was lower in low- and middle-income countries than in high-income countries (2.89% at 45–49 years and 14.94% at 85–89 years) [1]. The prevalence in Asian-Americans at age 60–69 years was 2.6% in women and 10.2% in men; at age ≥ 80 years, it was 17.1% in women and 13.8% in men [17].
Most studies found a significant independent association between hypertension and PAD. The odds ratio of hypertension for PAD was 1.32–2.20 [3]. The mean age in this study was 64.4 years and the prevalence was 14.5% in hypertensive patients; of note, hypertensive patients with PAD tended to be older. Male gender, smoking, and comorbid diabetes or dyslipidemia are established risk factors for PAD [2, 3, 16]. Our results also noted current smokers among low ABI group were significantly higher than the normal ABI group. Our study did not find statistically significant differences in gender or co-existing diabetes or dyslipidemia in hypertensive patients, possibly due to an insufficient sample size.
A high prevalence of PAD is noted in the US population > 40 years old with renal insufficiency, and even after the adjustment for important confounding factors, persons with renal insufficiency are still more than twice as likely to have an ABI < 0.9 according to the National Health and Nutrition Examination Survey 1999–2000 [18]. Renal insufficiency was also independently associated with future PAD events among postmenopausal women with coronary heart disease. The hazard ratio for PAD in women with a Cre clearance 30–59 mL/min/1.73 m2, < 30 mL/min/1.73 m2 is 1.63, 3.24 compared with persons with a Cre clearance ≥ 60 mL/min/1.73 m2 [19]. Our results also noted that hypertensive patients in the low ABI group had higher serum Cre levels and a lower eGFR. Inflammatory markers such as CRP are associated with PAD in many studies [2, 3]. Wildman et al. noted that the adjusted OR of PAD associated with the highest versus the lowest quartile of CRP was 2.14 in a sample of 4,787 participants aged > 40 years in the National Health and Nutrition Examination Survey 1999–2002 [20]. CRP was associated with fatal and nonfatal cardiovascular disease events as well as nonfatal PAD events in 18,450 apparently healthy participants in the European Prospective Investigation into Cancer and Nutrition-Norfolk cohort [21]. The hs-CRP level was also statistically significantly more correlated in PAD patients, who were at higher risk for CV morbidity and mortality in the presence of an elevated hs-CRP level [22]. Similar to these studies, our study revealed that hypertensive patients with a low ABI had higher serum hs-CRP levels.
Resistin is an adipokine involved in glucose homeostasis, lipid metabolism, and insulin action [23–25]. Circulating resistin levels have been positively associated with central obesity as well as insulin resistance in rodents; however, their significance remained controversial in human studies [25]. Resistin was positively associated with waist circumference and insulin resistance and inversely associated with TCH, HDL-C, and LDL-C after adjustment for age, gender, and BMI in a study with 1,508 Finnish subjects aged 45–74 years [26]. In an Indian study, plasma resistin levels were also positively associated with waist circumference and insulin resistance. Moreover, plasma resistin levels were strongly positively correlated with TCH, HDL-C, and LDL-C levels [27]. Our results also noted that serum resistin levels were positively associated with waist circumference and LDL-C level after multivariate linear regression analysis. Human resistin was shown to be predominantly expressed in peripheral blood mononuclear cells, macrophages, and bone marrow cells by analyses of resistin gene expression across a wide array of human tissues [5, 23].
Human resistin is an inflammatory biomarker that functions through the nuclear factor-κB and other signaling pathways that induce pro-inflammatory processes [6, 25]. Plasma resistin levels are positively associated with CRP and predictive of coronary atherosclerosis in humans [28]. A high serum resistin level is associated with a low eGFR in patients with type 2 diabetes and those with chronic kidney disease [29, 30]. High plasma resistin levels were independently associated with a greater risk of kidney function decline in middle-aged and elderly Chinese patients [31]. Our results showed that serum Cre and log-hs-CRP levels were positively correlated with serum resistin levels in hypertensive patients after multivariate analysis.
Resistin has a pathogenic role in the development and progression of atherosclerosis [6, 23]. Atherosclerosis is the major cause of PAD by arterial stenosis or occlusion, particularly of the lower extremities [15, 16]. Our study revealed that hypertensive patients in the low ABI group had higher serum resistin levels compared to those in the normal ABI group. Adjustment of the confounding factors on the multivariate logistic regression analysis revealed that an increased serum resistin level was an independent predictor of PAD in hypertensive patients.
Use of the ABI test to diagnose PAD has several limitations. Occlusive disease distal to the ankle is not detected by the ABI test, which is also sensitive to the patient’s height [3]. The other limitation of this study is that it was an observational, single-center study with a small sample of hypertensive participants. The use of anti-hypertensive drugs such as ACEi or ARB or of statins is beneficial for reducing major adverse cardiovascular events in patients with PAD and hypertension [32]. However, evidence of the use of various anti-hypertensive drugs in people with PAD is poor [33]. Another limitation is that pharmacological interventions have been shown to influence resistin levels in humans. The use of amlodipine, bisoprolol, or indapamide resulted in significantly lower plasma resistin concentrations after 6 weeks of treatment [34]. Statin therapy did not result in any significant changes in plasma resistin concentrations in a meta-analysis [35]. The current results did not show a correlation between anti-hypertensive drugs, statins, fibrates, aspirin, or clopidogrel and serum resistin levels. Further studies are required to elucidate the relationship between medication use and resistin levels in hypertensive patients.
Conclusions
This study showed that a higher resistin level was an independent predictor of the development of PAD in hypertensive patients. In addition, waist circumference, LDL-C, Cre, and log-hs-CRP were positively associated with resistin values in these patients.
Acknowledgments
Funding
This work was supported by grants from Tzu Chi Hospital (TCRD 101–03) in Taiwan.
Authors’ contributions
B-GH and J-HW conceived and designed the experiments. C-FY, Y-CC, and J-HW performed the experiments. C-JL and B-GH contributed reagents and analyzed the data. B-GH and J-HW wrote the manuscript. All of the authors reviewed and approved the final version of this paper.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study was approved by an independent ethics committee from the Protection of the Human Subjects Institutional Review Board of Tzu-Chi University and Hospital (IRB099-97), and all participants gave written informed consent according to the general recommendations of the Helsinki Declaration.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ABI
Ankle-brachial index
- ACEi
Angiotensin-converting enzyme inhibitors
- ARB
Angiotensin receptor blockers
- AUC
Area under the curve
- BMI
Body mass index
- BP
Blood pressure
- BUN
Blood urea nitrogen
- CCB
Calcium channel blockers
- CI
Confidence interval
- Cre
Creatinine
- CV
Cardiovascular
- eGFR
Estimated glomerular filtration rate
- HDL-C
High-density lipoprotein cholesterol
- hs-CRP
High-sensitivity C-reactive protein
- iPTH
Intact parathyroid hormone
- LDL-C
Low-density lipoprotein cholesterol
- OR
Odds ratio
- PAD
Peripheral arterial disease
- ROC
Receiver operating curve
- TCH
Total cholesterol
- TG
Triglyceride
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol. 2016;19(2):91–5. doi: 10.1053/j.tvir.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–26. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 4.Crawford F, Welch K, Andras A, Chappell FM. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev. 2016;9:CD010680. doi: 10.1002/14651858.CD010680.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Suhaimi EA, Shehzad A. Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur J Med Res. 2013;18:12. doi: 10.1186/2047-783X-18-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165(3):622–32. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, Xu H, Xie N, Huang J, Fang H, Luo M. Association of serum resistin with peripheral arterial disease. Pol Arch Med Wewn. 2013;123(12):680–5. doi: 10.20452/pamw.2011. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. I. Diagnosis and classification of diabetes mellitus: provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Lee CJ, Wang JH, Chen YC, Chen ML, Yang CF, Hsu BG. Serum osteopontin level correlates with carotid-femoral pulse wave velocity in geriatric persons. Biomed Res Int. 2014;2014:570698. doi: 10.1155/2014/570698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai JP, Wang JH, Lee CJ, Chen YC, Hsu BG. Positive correlation of serum adipocyte fatty acid binding protein levels with carotid-femoral pulse wave velocity in geriatric population. BMC Geriatr. 2015;15:88. doi: 10.1186/s12877-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai JP, Wang JH, Chen ML, Yang CF, Chen YC, Hsu BG. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016;16:80. doi: 10.1186/s12872-016-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi PJ, Liou HH, Hsu BG, Tasi JP. Relationship between resistin and mortality in maintenance hemodialysis patients. Clin Nephrol. 2016;86(9):125–31. doi: 10.5414/CN108720. [DOI] [PubMed] [Google Scholar]
- 13.Ho GJ, Chen YC, Yin WY, Chang YJ, Lee MC, Hsu BG. Fasting serum long-acting natriuretic peptide correlates with ankle brachial index in renal transplant recipients. Exp Clin Transplant. 2013;11(4):303–9. doi: 10.6002/ect.2012.0224. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira AC, Macedo FY. A review of simple, non-invasive means of assessing peripheral arterial disease and implications for medical management. Ann Med. 2010;42(2):139–50. doi: 10.3109/07853890903521070. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 16.Krishna SM, Moxon JV, Golledge J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int J Mol Sci. 2015;16(5):11294–322. doi: 10.3390/ijms160511294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32(4):328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 18.O’Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004;109(3):320–3. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 19.O’Hare AM, Vittinghoff E, Hsia J, Shlipak MG. Renal insufficiency and the risk of lower extremity peripheral arterial disease: results from the Heart and Estrogen/Progestin Replacement Study (HERS) J Am Soc Nephrol. 2004;15(4):1046–51. doi: 10.1097/01.ASN.0000119574.27772.FD. [DOI] [PubMed] [Google Scholar]
- 20.Wildman RP, Muntner P, Chen J, Sutton-Tyrrell K, He J. Relation of inflammation to peripheral arterial disease in the national health and nutrition examination survey, 1999–2002. Am J Cardiol. 2005;96(11):1579–83. doi: 10.1016/j.amjcard.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 21.van Wijk DF, Boekholdt SM, Wareham NJ, Ahmadi-Abhari S, Kastelein JJ, Stroes ES, Khaw KT. C-reactive protein, fatal and nonfatal coronary artery disease, stroke, and peripheral artery disease in the prospective EPIC-Norfolk cohort study. Arterioscler Thromb Vasc Biol. 2013;33(12):2888–94. doi: 10.1161/ATVBAHA.113.301736. [DOI] [PubMed] [Google Scholar]
- 22.Stone PA, Yacoub M. Inflammatory biomarkers in peripheral arterial disease. Semin Vasc Surg. 2014;27(3–4):148–51. doi: 10.1053/j.semvascsurg.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrinol Metab. 2011;22(7):259–65. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Codoñer-Franch P, Alonso-Iglesias E. Resistin: insulin resistance to malignancy. Clin Chim Acta. 2015;438:46–54. doi: 10.1016/j.cca.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Yang Z. Resistin’s, obesity and insulin resistance: the continuing disconnect between rodents and humans. J Endocrinol Invest. 2016;39(6):607–15. doi: 10.1007/s40618-015-0408-2. [DOI] [PubMed] [Google Scholar]
- 26.Malo E, Ukkola O, Jokela M, Moilanen L, Kähönen M, Nieminen MS, Salomaa V, Jula A, Kesäniemi YA. Resistin is an indicator of the metabolic syndrome according to five different definitions in the Finnish Health 2000 survey. Metab Syndr Relat Disord. 2011;9(3):203–10. doi: 10.1089/met.2010.0106. [DOI] [PubMed] [Google Scholar]
- 27.Singh AK, Tiwari S, Gupta A, Shukla KK, Chhabra KG, Pandey A, Pant AB. Association of resistin with insulin resistance and factors of metabolic syndrome in north Indians. Indian J Clin Biochem. 2015;30(3):255–62. doi: 10.1007/s12291-014-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111(7):932–9. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 29.Moreno LO, Salvemini L, Mendonca C, Copetti M, De Bonis C, De Cosmo S, Doria A, Trischitta V, Menzaghi C. Serum resistin and glomerular filtration rate in patients with type 2 diabetes. PLoS One. 2015;10(3):e0119529. doi: 10.1371/journal.pone.0119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawamura R, Doi Y, Osawa H, Ninomiya T, Hata J, Yonemoto K, Tanizaki Y, Iida M, Makino H, Kiyohara Y. Circulating resistin is increased with decreasing renal function in a general Japanese population: the Hisayama Study. Nephrol Dial Transplant. 2010;25(10):3236–40. doi: 10.1093/ndt/gfq155. [DOI] [PubMed] [Google Scholar]
- 31.Liu G, Deng Y, Sun L, Ye X, Yao P, Hu Y, Wang F, Ma Y, Li H, Liu Y, Sun Q, Lin X. Elevated plasma tumor necrosis factor-α receptor 2 and resistin are associated with increased incidence of kidney function decline in Chinese adults. Endocrine. 2016;52(3):541–9. doi: 10.1007/s12020-015-0807-3. [DOI] [PubMed] [Google Scholar]
- 32.Bonaca MP, Creager MA. Pharmacological treatment and current management of peripheral artery disease. Circ Res. 2015;116(9):1579–98. doi: 10.1161/CIRCRESAHA.114.303505. [DOI] [PubMed] [Google Scholar]
- 33.Lane DA, Lip GY. Treatment of hypertension in peripheral arterial disease. Cochrane Database Syst Rev. 2013;12:CD003075. doi: 10.1002/14651858.CD003075.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Skoczylas A, Piecha G, Więcek A. Effects of antihypertensive treatment on plasma apelin, resistin, and visfatin concentrations. Pol Arch Med Wewn. 2016;126(4):243–53. doi: 10.20452/pamw.3390. [DOI] [PubMed] [Google Scholar]
- 35.Sahebkar A, Giorgini P, Ludovici V, Pedone C, Ferretti G, Bacchetti T, Grassi D, Di Giosia P, Ferri C. Impact of statin therapy on plasma resistin and visfatin concentrations: a systematic review and meta-analysis of controlled clinical trials. Pharmacol Res. 2016;111:827–37. doi: 10.1016/j.phrs.2016.07.031. [DOI] [PubMed] [Google Scholar]


