Abstract
Background
In the general population, at least 50% of time awake is spent in sedentary behaviours. Sedentary behaviours are activities that expend less energy than 1.5 metabolic equivalents, such as sitting. The amount of time spent in sedentary behaviours is a risk factor for diseases such as type 2 diabetes, cardiovascular disease, and death from all causes. Even individuals meeting physical activity guidelines are at a higher risk of premature death and adverse metabolic outcomes if they sit for extended intervals. The associations between sedentary behaviour with type 2 diabetes and with impaired glucose tolerance are stronger for women than for men. It is not known whether sedentary behaviour in pregnancy influences pregnancy outcomes, but if those negative outcomes observed in general adult population also occur in pregnancy, this could have implications for adverse outcomes for mothers and offspring.
We aimed to determine the proportion of time spent in sedentary behaviours among pregnant women, and the association of sedentary behaviour with pregnancy outcomes in mothers and offspring.
Methods
Two researchers independently performed the literature search using 5 different electronic bibliographic databases. Studies were included if sedentary behaviours were assessed during pregnancy. Two reviewers independently assessed the articles for quality and bias, and extracted the relevant information.
Results
We identified 26 studies meeting the inclusion criteria. Pregnant women spent more than 50% of their time in sedentary behaviours. Increased time in sedentary behaviour was significantly associated with higher levels of C Reactive Protein and LDL Cholesterol, and a larger newborn abdominal circumference. Sedentary behaviours were significantly higher among women who delivered macrosomic infants. Discrepancies were found in associations of sedentary behaviour with gestational weight gain, hypertensive disorders, and birth weight. No consistent associations were found between sedentary behaviour and other variables such as gestational diabetes. There was considerable variability in study design and methods of assessing sedentary behaviour.
Conclusions
Our review highlights the significant time spent in sedentary behaviour during pregnancy, and that sedentary behaviour may impact on pregnancy outcomes for both mother and child. The considerable heterogeneity in the literature suggests future studies should use robust methodology for quantifying sedentary behaviour.
Electronic supplementary material
The online version of this article (doi:10.1186/s12966-017-0485-z) contains supplementary material, which is available to authorized users.
Keywords: Sedentary behaviours, Sedentarism, Pregnancy
Background
Sedentary behaviours are activities that expend very low energy, close to the basal metabolic rate, without significantly increasing energy expenditure. This equates to activities such as sitting or lying, that utilise less than 1.5 metabolic equivalent units, or times the basal metabolic rate [1, 2]. Sedentary behaviours are thus distinct from lack of physical activity, although the latter is sometimes mistakenly used as a marker of sedentary behaviour in the literature [3].
Epidemiological studies have shown that in the general adult population, around 55 to 60% of time awake is spent in sedentary behaviours [4, 5]. In the UK, children, young people, adults and older adults, spend on average at least half of their waking hours being sedentary [6, 7]. In pregnant women the situation appears to be similar or even worse [8–12], although the literature has not been systematically reviewed.
The quantity of time spent in sedentary behaviours is a key risk factor for diseases such as type 2 diabetes [13], cardiovascular disease [14], metabolic syndrome [15] and death from all causes [14, 16, 17]. New evidence also suggests that sedentary behaviour has an adverse effect on mental wellbeing, including depression [3]. Importantly some studies have exposed that even when individuals meet physical activity recommendations, they are still at a higher risk of premature death and adverse metabolic health if they sit for extended intervals [2, 18–20]. Sedentary behaviours, mostly television watching, are also linked to high risk of obesity and type 2 diabetes in the general population, independent of physical activity levels [1, 20], and in some studies the associations between sedentary behaviours with type 2 diabetes and with impaired glucose tolerance were stronger for women than for men [18, 21, 22].
If the negative health outcomes associated with sedentary behaviour in the general population, also occur in pregnancy, this could have implications for development of cardiometabolic complications such as gestational weight gain, gestational diabetes mellitus and hypertension, as well as mental wellbeing. It is not known whether sedentary behaviour in pregnancy influences outcomes for the baby such as birthweight or gestation at delivery.
We aimed to carry out a systematic review of the literature investigating sedentary behaviours during pregnancy to determine:
the time spent in sedentary behaviours and the prevalence of sedentary behaviours among pregnant women, and
whether sedentary behaviours are associated with pregnancy outcomes in mothers and offspring.
Methods
Data sources and searches
The Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines were followed for the conduct [23], and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for the reporting of this systematic review [24]. The systematic review was registered in PROSPERO with the number CRD42015023611.
Two researchers (CF, KL) independently performed the literature search using 5 different electronic bibliographic databases: MEDLINE, EMBASE, Web of Science, CINAHL and SPORTDiscus. The strategy (Fig. 1) was developed using Boolean. In MEDLINE medical subject headings (MeSH) used were: pregnant women (used also for pregnant woman), pregnancy (used also for pregnancies and gestation), prenatal care and sedentary lifestyle (used also for sedentary lifestyles). In EMBASE, main terms used were: pregnant woman (used also for pregnant women), pregnancy (used also for child bearing, childbearing, gestation, gravidity, intrauterine pregnancy, labour presentation, pregnancy maintenance and pregnancy trimesters), prenatal care (used also for ante natal care, antenatal care and antenatal control), prenatal period (used also for antenatal period) and sedentary lifestyle (used also for sedentary life style). The following keywords were also used for plain text searching in all databases: pregnan*, gestation*, gravid*, antenatal, prenatal, sedentar*, sitting, television, screen-based, TV, watching and viewing. Recursive searching of reference lists of retrieved articles was performed to identify any additional studies (Additional file 1).
Fig. 1.
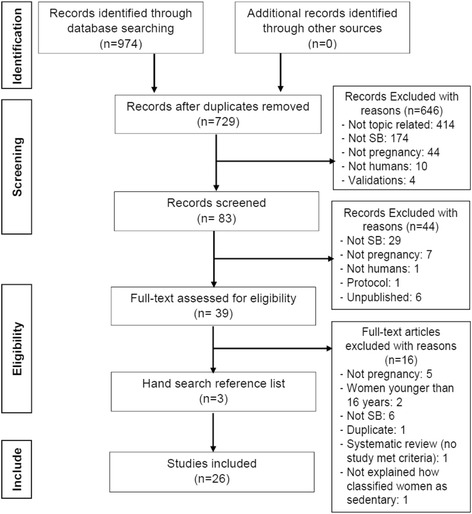
Search strategy flow diagram
Studies were included if the sample considered pregnant women over 16 years old, and if sedentary behaviours (specified as watching TV, sitting or lying, low energy expenditure activities, etc.) were assessed at any point during gestation. Only published studies were included. There were no exclusions related to study design, language, ethnicity, socioeconomic status, parity or physical condition.
Two reviewers (CF, KL) independently assessed articles for inclusion according to the inclusion/exclusion criteria. After screening the titles and abstracts, the reviewers selected potentially relevant studies. If it was not possible to determine relevance from titles and abstracts, full texts were retrieved. Any disagreements that could not be resolved by consensus were discussed with a third reviewer.
Two reviewers (CF, KL) independently extracted relevant information on study characteristics, methodology, and study results using a data extraction form in order to determine whether the study reported the time that pregnant women spent in sedentary behaviours, the prevalence of sedentarism among pregnant women, and whether the sedentary behaviours were linked to pregnancy outcomes.
For presentation in the tables reporting time and proportion of time in sedentary behaviours, we standardised the outcomes (converted to the same units) in order to make them comparable. Due to the heterogeneity of outcome data, a narrative synthesis was developed.
Quality and risks of bias were assessed using objective criteria relating to sample population and recruitment, reliability of instruments, use of validated outcome measures, follow-up, risk of bias and data analysis, using a quality assessment instrument that was modified from the Grading of Recommendations Assessment Development and Evaluation (GRADE) Guidelines used in assessment of clinical trials [25–28]. A paper could attain a maximum score of 8, a score of 1–3 indicating poor quality, 4–6 intermediate, and 7–8 good quality.
Results
From 974 abstracts, 39 full text articles were assessed and 26 studies met the inclusion critera for the systematic review (Fig. 1).
Characteristics of included studies
Characteristics of the 26 included studies are displayed in Table 1. Seventeen were cohort studies [8, 12, 29–43], 7 were cross-sectional studies [9–11, 44–47], and 2 were randomised controlled trials [48, 49].
Table 1.
Description of included studies (arranged alphabetically)
| Author | Country | Number of participants | Study design | Criteria for inclusion | Assessment method | Definition of sedentary | Quality | |
|---|---|---|---|---|---|---|---|---|
| Both, et al. (2010) [31] | UK | 11759 | Cohort | Pregnant women due to deliver between April 1st 1991 and December 31st 1992. | Self-reported questionnaire. | Non-objective. | Who declared being mostly sitting. | Intermediate |
| Chasan-Taber, et al. (2014) [40] | USA | 1276 | Cohort | Women of Puerto Rican or Dominican Republic heritage. | Modified version of the Pregnancy Physical Activity Questionnaire (PPAQ). | Non-objective. | Activities expending <1.5 METs. | Intermediate |
| Chasan-Taber, et al. (2015) [34] | USA | 1240 | Cohort | Women of Puerto Rico or Dominican Republic heritage. | PPAQ. | Non-objective. | The sum of the MET-h/day spent watching TV/videos or sitting/standing at home, work, or during transportation. | Intermediate |
| Di Fabio, et al. (2015) [8] | USA | 46 | Cohort | Healthy pregnant women, including women between 18 and 45 years of age and singleton pregnancy. | - 7 day record diary. | - Non-objective. | Activities expending ≤1.5 METs (independent of nighttime sleep). | Intermediate |
| - SenseWear® Mini armband accelerometer. | - Objective. | |||||||
| - ActivPAL™ Multi-sensor accelerometer. | - Non-objective. | |||||||
| Evenson, et al. (2010) [37] | USA | 1280 | Cohort | Pregnant women ≥16 years of age. | The Behavioral Risk Factor Surveillance System (BRFSS). | Non-objective. | Two questions on TV watching and computer usage outside of work hours were used as SB indicators. Women were also asked if they were ‘mostly sitting’ during their usual daily activities. | Poor |
| Evenson, et al. (2011) [11] | USA | 359 | Cross-sectional | Pregnant women ≥16 years. | ActiGraph accelerometer. | Objective. | Activities expending <100 counts per minute. | Intermediate |
| Gollenberg, et al. (2010) [36] | USA | 1006 | Cohort | Latina ethnicity, age 16–40 years old, singleton pregnancy, and no prior participation in the study. | Modified version of the Kaiser Physical Activity Survey (KPAS). | Non-objective. | Hours spent TV watching per day and frequency of sitting at work. | Intermediate |
| Gradmark, et al. (2011) [47] | Sweden | 101 | Cross-sectional | Normal weight and overweight women without diabetes were studied. | Actiheart monitor. | Objective. | Epochs with valid heart rate data and zero accelerometry counts/min. | Intermediate |
| Hawkins, et al. (2014 Im.) [48] | USA | 260 | Randomized controlled trial | Women in their first trimester of pregnancy, between 16 and 40 years old, and at high risk for GDM. | Pregnancy Physical Activity Questionnaire (PPAQ). | Non-objective. | The amount of time spent watching TV or videos, or sitting or standing at home, work, or during transportation. | Good |
| Hawkins, et al. (2014 PA) [10] | USA | 294 | Cross-sectional | Women in the 2003–2006 NHANES study cycles who self-reported currently being pregnant, were 16 year or older, and who had available data on C reactive protein, physical activity, and SB. | ActiGraph accelerometer. | Objective. | Activities expending <100 counts per minute. | Intermediate |
| Hayes, et al. (2014) [49] | UK | 183 | Randomized controlled trial | All obese (BMI ≥ 30 K/m2) pregnant women. | - Acti-Graph accelerometer. | - Objective. | - Accelerometry: any minute with ≤100 counts/min. | Good |
| - Recent Physical Activity Questionnaire (RPAQ). | - Non-objective. | - RPAQ, minutes spent on activities <1.5 MET. | ||||||
| Hegaard, et al. (2010) [32] | Denmark | 4558 | Cohort | Danish-speaking pregnant women. | Self-reported questionnaires. | Non-objective. | Those who chose “mostly sitting” to describe most correctly her level of leisuretime activity. | Intermediate |
| Hegaard, et al. (2011) [35] | Denmark | 4718 | Cohort | Age ≥ 18 years, Danish speaking, singleton pregnancy, and intended spontaneous vaginal delivery. | Self-administered questionnaire. | Non-objective. | Those who answered: “Reading, watching television, or pursuing some other sedentary occupation”, as the most appropriate description of her activities. | Intermediate |
| Hjorth, et al. (2012) [9] | Ethiopia | 304 | Cross-sectional | All pregnant women who attended routine visits at the antenatal care clinic. | - Actiheart (heart rate and movement device). | - Objective. | Energy expenditure ≤1.5 METs. | Intermediate |
| - 24 h physical activity recall. | - Non-objective. | |||||||
| Jiang, et al. (2012) [30] | China | 862 | Cohort | Pregnant women over 20 years old in a singleton pregnancy, and had no disease including gestational diabetes (GD), hypertension, heart disease, chronic renal disease, and other diseases restricting physical activity. | Pedometer. | Objective. | Less than 5000 steps per day. | Intermediate |
| Kamareswaran, et al. (2013) [41] | UK | 10 | Cohort | Type 1 diabetes, current insulin pump therapy, and a viable singleton pregnancy. | Actiheart (heart rate and movement device). | Objective. | Activities expending ≤1 MET. | Intermediate |
| Li & Zhao (2007) [46] | China | 405 | Cross-sectional | Pregnant women working in a sewing factory. | Self-reported questionnaire. | Non-Objective. | According to the job, women were assigned to the study group (persistent sedentary) or control group. | Poor |
| Loprinzi, et al. (2013) [44] | USA | 206 | Cross-sectional | All women who answered the 2003–2006 National Health and Examination Survey. | ActiGraph accelerometer. | Objective. | Activity counts between 0 and 99 counts/min. | Intermediate |
| Lynch, et al. (2012) [43] | USA | 1355 | Cohort | Women from the ambulatory obstetric practices at ≤20 weeks of gestation. | PPAQ. | Non-objective. | The amount of time spent watching TV or videos, or sitting or standing at home, work, or during transportation. | Intermediate |
| Oken, et al. (2006) [29] | USA | 1581 | Cohort | Women attending initial prenatal visit, who delivered live infants. | Modified version of the leisure time activity section of the Physical Activity Scale for the Elderly (PASE). | Non-objective. | Hours per week spent watching TV or videos. | Intermediate |
| Padmapriya, et al. (2015) [42] | Singapore | 1171 | Cohort | Pregnant women aged 18 years and above attending first trimester antenatal dating ultrasound scan clinics. | Interview questionnaire. | Non-objective. | Hours spent on sitting plus hours spent on watching TV per day. | Intermediate |
| Reid, et al. (2014) [39] | Northern Ireland | 100 | Cohort | Healthy women, ≥16 years old, with singleton pregnancies, between 26 and 37 week gestation. | Body-media SenseWear Pro3 armband. | Objective. | Activities expending ≤1 MET. | Intermediate |
| Rhodes, et al. (2014) [33] | Canada | 157 | Cohort | The cohorts were couples without children, first-time parents during the first year of their parenthood experience, and second time parents during the first year of this parenting experience between the ages of 25 and 40 years of age. | GT1M Activity Monitor (accelerometer and stepcounter). | Objective. | Activities expending 0–100 average acceleration counts/min. | Intermediate |
| Ruifrok, et al. (2014) [12] | Netherlands | 111 | Cohort | Healthy pregnant women. Trial 1: nulliparous pregnant women without BMI restrictions, able to read, write and speak Dutch, and within their first 14 weeks of pregnancy; Trial 2: overweight and obese pregnant women at risk for gestational diabetes. | ActiTrainer accelerometer (Acti-Graph). | Objective. | Activities expending <100 counts/min. | Intermediate |
| Van Raaij, et al. (1990) [38] | Netherlands | 18 | Cohort | Healthy women judged by medical histories, blood pressure, hemoglobin concentration, and urine analysis. | - Open-circuit indirect calorimetry. | - Objective. | Lying, sitting quietly or very light sitting activity, or light-to-moderate sitting activity. | Intermediate |
| - Physical activity diaries. | - Non-objective. | |||||||
| Watts, et al. (2013) [45] | Australia | 81 | Cross-sectional | Pregnant women regardless of their pregnancy trimester. | The Australian Women’s Activity Survey (AWAS). | Non-objective. | Frequency and duration of sitting behavior. | Poor |
Most studies were carried out in the USA (n = 11) and Europe (n = 9), and the remaining were in China (n = 2), Africa (n = 1), Canada (n = 1), Australia (n = 1) and Singapore (n = 1). One study included couples (for the purpose of this review we only considered data from the women, not the men) [33]; 2 other studies included both pregnant and non-pregnant women (non-pregnant women were considered in this review when comparisons between the two groups were made) [33, 47]. Three studies were conducted in Hispanic pregnant women [34, 40, 43], and 1 in Latina pregnant women [36]. One study was conducted in nulliparous pregnant women, 1 in obese pregnant women [49], 1 in pregnant women with type 1 diabetes mellitus [41], and 1 in pregnant women with sedentary lifestyles [38]. Thirteen studies utilised objective methods to assess sedentary behaviours (accelerometers, pedometers, combined heart rate and accelerometer device, and indirect calorimetry), and 13 studies employed non-objective measures including 4 administrating the Pregnancy Physical Activity Questionnaire (PPAQ), 9 using another kind of survey or questionnaire (The Australian Women’s Activity Survey, Modified version of the Kaiser Physical Activity Survey, Behavioral Risk Factor Surveillance System, modified version of the leisure time activity section of the Physical Activity Scale for the Elderly, and other type of non-objective appraisal methods) (Table 2). The PPAQ has been validated among pregnant women, similarly 2 of the administered surveys were also validated among pregnant women, meanwhile 3 studies used validated questionnaires, but not validated among pregnant women. Finally, 4 of the questionnaires were not validated.
Table 2.
Characteristics of included studies
| Number of studies | Participants (N) | |
|---|---|---|
| Assessment tool | ||
| Accelerometer | 7 | 1356 |
| Accelerometer and HR sensor | 3 | 415 |
| Pedometer | 1 | 862 |
| Other objective | 2 | 118 |
| Pregnancy Physical Activity Questionnaire (PPAQ) | 4 | 4131 |
| Other self-reported | 9 | 26559 |
Amount and proportion of time spent in sedentary behaviours (Table 3)
Table 3.
Time and proportion of time spent in sedentary behaviours
| Studies | N | Mean or median (SD or SE or IQR) | |
|---|---|---|---|
| Time spent in SB (objective) | |||
| Time spent in SB (h/day) | Ruifrok 2014 [12] | 111 | 8.6 (SD 2.86) |
| Hawkins 2014 [10] | 294 | 9.2 (SE 16.2)a | |
| Loprinzi 2013 [44] | 206 | 7.7 (SE 0.2)a | |
| Hjorth 2012 [9] | 304 | 18.3a (IQR16.65–19.6) | |
| Evenson 2011 [11] | 359 | 7.07 (SE 0.165)a | |
| Di Fabio 2015 [8] | 46 | 12.65 (SD 1.95)a | |
| Sitting quietly or very light sitting activities (h/day) | Van Raaij 1990 [38] | 18 | 6.7 (SD1.6)a |
| Light to moderate sitting activities (h/day) | Van Raaij 1990 [38] | 18 | 1.6 (SD1.1)a |
| Sit/lie time (h/day) | Di Fabio 2015 [8] | 46 | 18.2 (IQR17.1–19) w18; 18.3 (IQR17.6–19.4) w35 |
| Time spent in SB (non-objective) | |||
| Television time (h/day) | Padmapriya 2015 [42] | 1171 | 2.4 (SD1.5)a |
| Total sitting time (h/day) | Padmapriya 2015 [42) | 1171 | 8.6 (SD3.3)a |
| Proportion of time spent in SB (objective) | |||
| % of day spent in SB | Hjorth 2012 [9] | 304 | 76.4% (IQR 69.37–81.6a) |
| % of wear time spent in SB | Ruifrok 2014 [12] | 111 | 65% |
| Evenson 2011 [11] | 359 | 57.1% (SE 0.77) | |
| Hawkins 2014 [10] | 294 | 64.4% (SE 0.02)a | |
| % of time awake in SB | Di Fabio 2015 [8] | 46 | 76% (SD11) w18–78% (SD13) w35 |
| % of day time in sit/lie | Di Fabio 2015 [8] | 46 | 76% (IQR71–79) w18; 76% (IQR73–81) w35 |
a Numbers were calculated as means and converted to the same units
The amount of time spent in sedentary behaviours was estimated in 8 studies using either objective [8–12, 30, 38, 44] or non-objective methods [42] (Table 3).
The time spent in sedentary behaviours during pregnancy assessed objectively, varied between 7.07 and 18.3 h per day. Of these studies 1 declared that sleeping was included [9], 2 stated that sleep time was not considered [8, 11], and the rest did not declare anything regarding sleep [10, 12, 44]. Meanwhile the study which assessed using a questionnaire found that women spent 2.4 h per day watching television and the mean of total sitting time was 8.6 h per day [42] (Table 3).
Among the 5 studies assessing the proportion of time spent in sedentary behaviours all used objective devices, finding that pregnant women spent more than 50% of their time (range 57.1 to 78%) in sedentary activities [8–12] (Table 3).
Definitions of sedentary behaviours
The definition of time spent in sedentary behaviours differed according to method of assessment. Studies that used accelerometers defined activities with less than 100 counts per minute as sedentary behaviours, while activities expending 1.5 metabolic equivalents or less was used for combined heart-rate and activity monitors. Meanwhile, non-objective methods focused mostly on television viewing and sitting time.
Prevalence of sedentarism among pregnant women (Table 4)
Table 4.
Prevalence of sedentarism among pregnant women
| Sedentary activity definition | Studies | Assessment method | N | Prevalence |
|---|---|---|---|---|
| Sedentary | Jiang 2012 [30] | Objective | 862 | 18% |
| Hegaard 2011 [35] | Non-objective | 4718 | 29% | |
| Watching TV or videos 5 or more (h/day) | Evenson 2011 [11] | Non-objective | 359 | 15.3% |
| Watching TV 2 or more (h/day) | Oken 2006 [29] | Non-objective | 1581 | 34% |
| Watching TV 3 or more (h/day) | Padmapriya 2015 [42] | Non-objective | 1171 | 31.9% |
| Mostly sitting during day | Evenson 2011 [11] | Non-objective | 359 | 24% |
Five studies determined the prevalence of sedentarism among the pregnant population, all except 1 [30] used non-objective methods to assess activity behaviour, and all used their own cut-offs to classify women as sedentary. Two used the term “sedentary”, defining this as <5000 daily steps [30] or considering women as ‘sedentary’ if they declared “watching television, or pursuing some other sedentary occupation” as the most appropriate description of their activities [35], respectively. One study focused on the second trimester of pregnancy and found that prevalence of sedentarism was 18% [30], the other study assessed women on the third trimester of pregnancy finding that 29% were sedentary [35]. Three studies analysed the prevalence of sedentary women, however these 3 studies did not use the term ‘sedentary’, but used different activity categories defined variously by the authors as: “watching television (for a certain amount of time)”, or being “mostly sitting”. One study found that 15.3% of the studied women watched television or videos for 5 or more hours per day [37], other study found that 34% viewed television 2 h or more per day [29], and the last one found that 31.9% watched television more than 21 h per week, i.e. about 3 h per day [42]. Additionally 1 of the studies found that 24% of women were “mostly sitting” during usual daily activities [37] (Table 4). Comparison of data was difficult due to different cut-offs to define sedentary behaviour and categorisation of sedentarism.
Change in sedentary behaviour during pregnancy
Among the included studies, 5 aimed to determine whether time spent in sedentary behaviours was stable or changed during gestation [8, 10–12, 37]. Four of these studies examined minutes per day or percentage of day spent in sedentary activities based on objective measures [8, 10–12]. Of these, only 1 found that the percentage of time awake spent in sedentary behaviours significantly increased between week 18 and 35 of gestation [8]. Another study found that women spent a mean of 40 min (standard deviation ±75) less in “very light sitting activities” (activities that spend around 1.3 times the basal metabolic rate) in later gestation than in earlier gestation [38]. The 3 studies which objectively assessed time or percentage of time of monitored time spent in sedentary behaviours, did not find significant differences in time spent in sedentary behaviours between trimesters of gestation [10–12]. When focused on the number of sedentary pregnant women across gestation, more women were sedentary during the third trimester than during the second trimester (18%, n = 155; 24.9%, n = 215, respectively) [30]. When the time spent between trimesters in TV watching and computer use was compared, no differences were found [37].
Five studies compared sedentary behaviours between pregnant and non-pregnant women [35, 38, 42, 43, 47]. Four compared from before pregnancy to during pregnancy, and 1 compared pregnant women versus one year postpartum women [38]. Three studies used non-objective methods [35, 42, 43], and 2 objective procedures [38, 47] to assess sedentary behaviours. All found that the time spent in sedentary activities is significantly greater among pregnant than non-pregnant women.
When the number of women that watched television for long periods was compared before and after pregnancy, 1 study observed that the number increased [42], and the other found no change [29].
Additional factors affecting sedentary lifestyles
Some studies considered additional factors which could influence the development of sedentary lifestyles. These factors included: smoking, meeting physical activity recommendations, parity, maternal age, and education level. Time spent in sedentary behaviours was significantly less among women who smoked cigarettes in the past 5 days, compared to those who did not [11]. Time spent in sedentary behaviours at 35 weeks of gestation was significantly less among women meeting physical activity guidelines compared to women who did not [8]. During pregnancy women expecting their first child decreased their sedentary time significantly more than non-pregnant women without children, as well as first time pregnant women also decreased their sedentary time significantly more than those expecting their second baby as pregnancy advanced [33]. When the changes before and during gestation were compared, women aged 16–19 years, significantly decreased their sedentary activity compared to those aged 20–24 years. Women who had completed college, also significantly decreased their sedentary activity during pregnancy, compared with those with less than a high school education [43].
Interruptions during sedentary time
One study focused on the transitions between sit to stand, using an objective device that evaluates postural allocation [8]. No differences were found in sit/lie and upright time between week 18 and 35 of gestation. However, the number of transitions between sedentary (sit/lie) to upright per day and the number of sit/lie bouts increased significantly from week 18 to week 35 of gestation, whilst the length of sit/lie bout in minutes per day significantly decreased across this gestation window.
Associations between sedentary behaviours and maternal and infant outcomes
Birth and gestation outcomes associated with sedentary behaviours were studied in 14 of the included studies [10, 12, 30–32, 34, 36, 39, 40, 44–47, 49]. Of these, 7 were focused on pregnancy outcomes including gestational weight gain (GWG) and maternal depression [12, 30, 34, 40, 44–46], 5 on metabolic outcomes [10, 36, 44, 47, 49], and 5 on infant outcomes [12, 31, 32, 39, 49].
Associations between sedentary behaviours and pregnancy outcomes (Table 5)
Table 5.
Associations between sedentary behaviours and maternal health outcomes
| Author | Participants | Association (Yes/No) | |
|---|---|---|---|
| Pregnancy Outcomes | |||
| GWG | Ruifrok 2014 [12] | 111 | No |
| Chasan-Taber 2014 [40] | 1276 | No | |
| Jiang 2012 [30] | 862 | Yesa (p < 0.001) | |
| Hypertensive disorders | Chasan-Taber 2015 [34] | 1240 | No |
| Loprinzi 2013 [42] | 206 | No | |
| Li 2007 [46] | 405 | Yesb (p < 0.05) | |
| Depression | Watts 2013 [45] | 81 | No |
| Metabolic Outcomes | |||
| Glucose levels | Loprinzi 2013 [44] | 206 | Trend (p = 0.06) |
| Hayes 2014 [49] | 183 | No | |
| Insulin sensitivity | Gradmark 2011 [47] | 101 | No |
| GDM | Hayes 2014 [49] | 183 | No |
| AGT | Gollenberg 2010 [36] | 1006 | No |
| CRP | Loprinzi 2013 [44] | 206 | Yesc (p < 0.05) |
| Hawkins 2014 [10] | 294 | Yesc (p < 0.05) | |
| Blood lipids levels (Total Cholesterol, HDL-cholesterol and triglycerides) | Loprinzi 2013 [44] | 206 | Yesd (LDL p < 0.05) |
| Infant outcomes | |||
| Birth Weight | Ruifrok 2014 [12] | 111 | No |
| Hegaard 2010 [32] | 4558 | No | |
| Both 2010 [31] | 11759 | Yese (p < 0.05) | |
| Macrosomia | Reid 2014 [39] | 100 | Yesf (p < 0.05) |
| New-born abdominal circumference | Hayes 2014 [49] | 183 | Yesg (p < 0.05) |
| Gestational length | Ruifrok 2014 [12] | 111 | No |
| Both 2010 [31] | 11759 | No | |
| Risk of preterm delivery | Both 2010 [31] | 11759 | No |
a GWG was higher in the sedentary group compared with the active group, b the sedentary group developed more hypertension, c Increased time in sedentary behaviours is associated with higher levels of CRP, d increased time in sedentary behaviour is associated with higher LDL cholesterol, e Increased time in sedentary behaviour is associated with lower birth weight, f women delivering macrosomic infants had higher levels of SB, g the association between SB and new-born abdominal circumference was inverse at baseline, and positive at 36 weeks
Three studies investigated whether there is an association between sedentary behaviours and gestational weight gain [12, 30, 40]. One study found no association between percentage of time spent in sedentary behaviours with gestational weight gain at 15 weeks of gestation, between 15 and 32–35 weeks of gestation, or with gestational weight gain per week [12]. Likewise, change in percentage of time in sedentary behaviours during 15 to 32–35 weeks of gestation was not associated with total gestational weight gain or with gestational weight gain per week. Another study also observed no significant associations between sedentary activity and inadequate or excessive gestational weight gain, at each stage of pregnancy [40]. However, in another study the ‘Active’ group (named according to author´s categorisation) gained significantly lower maternal weight during the second and third trimesters than the ‘sedentary’ group (named according to author´s categorisation) [30].
Three studies explored the association between pregnancy sedentary behaviours and hypertensive disorders during gestation. Two studies found no association [34, 44], but 1 study found that women who had persistent sedentary work (and were not authorised to move from their work place during working hours), such as sewing operators, developed significantly more gestational hypertension than women in the control group, whose work was also mostly sedentary, but whom were allowed to move during working time, such as secretaries [46].
No association was found between pregnancy sedentary behaviours and depression [45].
Associations between sedentary behaviours and metabolic outcomes (Table 5)
The relationship between time spent in sedentary behaviours and fasting glucose levels was analysed in 1 study, finding a positive association [44]. On the other hand, sedentary behaviours were not associated with altered insulin sensitivity [47], gestational diabetes mellitus [49], or abnormal glucose tolerance [36]. Two studies found associations between sedentary behaviours and C-reactive protein (CRP) [10, 44]. In 1 study sedentary time and proportion of wear time spent sedentary were positively associated with CRP among women in the second trimester, but this finding was no longer statistically significant in analyses adjusting for confounders [10]. In the other study the positive association between sedentary behaviours and CRP levels remained after adjustment for confounders [44]. A significantly positive association between time spent in sedentary behaviours and higher LDL cholesterol was found in 1 study, but no association was found with any other blood lipid marker [44].
Associations between sedentary behaviours and infant outcomes (Table 5)
Two studies found no association between birth weight and mother’s sedentary behaviours during pregnancy [12, 32]. One study found a significant association between lower birthweight with time spent in sedentary lifestyle in each trimester of gestation [31], whilst another found that women who delivered macrosomic infants (birthweight ≥4000 g) spent significantly more time sedentary than women delivering offspring weighing less than 4000 g [39]. The 1 study exploring the correlation between the new born abdominal circumference (as an indicator for abdominal adiposity) with mothers’ time spent sedentary found differing results according to gestation. At 16–18 weeks of gestation a significantly inverse association was found between infant abdominal circumference and time spent sedentary, however at 36 weeks of gestation, the relationship became significantly positive [49]. No associations were found between sedentary behaviours and gestational length [12, 31], or risk of preterm delivery [31].
Quality assessment results
Both reviewers agreed that 2 (7.7%) of the studies were of good quality [48, 49], 3 (11.5%) were classified as of poor quality [37, 45, 46], and the rest 21 as intermediate (80.8%).
The 2 studies that were classified as good quality were randomised controlled trials.
Of those classified as poor quality the main reasons were small sample size [45, 46], use of a non-objective appraisal tool to classify women as sedentary [37, 45, 46] and lack of detail about the outcome measures [37, 46].
Discussion
Main findings
There is increasing interest in research in the general population about whether reducing time spent in sedentary behaviours has a beneficial effect on health [50, 51]. Here we systematically reviewed the literature in this field among pregnant women. Our key findings were that pregnant women spend at least half of their time in sedentary activities, which is similar to time reported in children, young people, adults and older adults in the UK [6]. Whether sedentary behaviours impact on pregnancy outcomes was less clear-cut with inconsistencies in the literature.
Our review highlights the considerable heterogeneity in the definitions of sedentary behaviours and the methods used to assess this. Differences in the reported prevalence of sedentary behaviours between studies could be due to the unclear definition of sedentary behaviours, or classification of sedentary. For example, 1 study used a pedometer, an objective method, to classify women as sedentary, considering less than 5000 steps per day as a sedentary lifestyle [30], meanwhile in another study women were considered sedentary if they answered “Reading, watching television, or pursuing some other sedentary occupation”, as the most appropriate description of their activities during pregnancy [35]. Many of included studies defined sedentary behaviours as activities expending the same or less than one metabolic equivalent [39, 41], however there is no consensus in how many hours per day spent in sedentary behaviours are sufficient to be categorised as sedentary, making it difficult to determine the prevalence of sedentarism. In addition sedentary behaviours were often assessed retrospectively [32, 35], potentially introducing recall bias.
Studies also differed in the assessment measures to calculate sedentary behaviours making comparisons difficult. This corresponds with what has been exposed regarding sedentary behaviours assessment in other populations [6].
Half of the identified studies considered whether sedentary behaviour in pregnancy impacted on maternal or offspring outcomes. This is an important consideration as interventions based on increasing physical activity among obese pregnant women have had limited impact on pregnancy outcomes [49, 52–55]. One study found that reducing time spent in sedentary activity was associated with decreased gestational weight gain [30]. Two other studies, including a large study of >1000 women found no associations with gestational weight gain [12, 40]. Likewise there were discrepancies in studies examining associations of sedentary behaviours with hypertensive disorders [34, 44, 46]. Notably the 1 study which found a significant association was classified as poor quality, which decreases the reliability of the result [46]. Differences in ethnicity between the study populations may partly explain the discrepant findings with gestational weight gain (1 study developed in Denmark, other included only Latin-American pregnant women, and 1 was developed in China) and hypertensive disorders (1 included only Latin-American women, 1 was developed in the USA and 1 in China). No association was found between depression and sedentary behaviours, however the 1 study focusing on that was classified as poor quality [45]. None of the studies reported associations between sedentary behaviour and glucose metabolism, as assessed by fasting glucose levels [44, 49], insulin sensitivity (measured using an oral glucose tolerance test) [47], gestational diabetes mellitus (GDM) [49] and in a large study of >1000 women glucose tolerance measured during a glucose tolerance test [36]. In contrast, 2 studies found associations between higher CRP levels and increased sedentary behaviour [10, 44], and 1 found an association with blood lipids [44] suggesting there may be subtle beneficial effects on maternal metabolism if time spent sedentary is reduced. Overall, there was some suggestion that sedentary behaviours may impact on size at birth [31, 39, 49], but not timing of delivery [12, 31]. However, the largest study including over 11,000 pregnant women and which reported associations of sedentary behaviour with birthweight but not gestational length or risk of preterm birth, assessed sedentary behaviours during pregnancy using a postal questionnaire using the question “Are/were you mostly sitting?” [31].
Strengths and limitations
The strengths of this review include the systematic and comprehensive review process which was followed in line with PRISMA guidelines. Two researchers independently assessed eligibility of the titles, abstracts and full-text studies, extracted the data and assessed the articles for bias.
A further strength of the review is that many of the studies were of considerable sample size. Eleven studies included samples of over 1000 women [29, 34, 36, 37, 40, 42, 43], including 2 assessing more than 4000 women using validated questionnaires [32, 35]. Nevertheless, larger studies using objective assessments of sedentary behaviour in pregnancy would considerably add to the literature in this field.
There are also some potential limitations. Though we used a robust search strategy developed from other systematic reviews of sedentary behaviour in the general population [2, 56, 57], it is possible that some potentially eligible studies may not have been identified. For example, some studies appraise sedentary behaviours when assessing physical activity, but the titles do not mention the key words we chose to identify sedentary behaviours. We included a search of reference lists of all papers that the full text was read, to identify any further additional papers.
A limitation of the data is that only 2 of the identified studies were trials, all the rest were observational. Of the trials, just 1 used an objective method to assess sedentary behaviours, the other employed a questionnaire. Of the 24 observational studies, only 12 used objective instruments, the other 12 utilised self-reported methods to assess sedentary behaviours. Most of these studies were considered of intermediate quality due to the small sample size, or lack of use of a validated questionnaire or objective measurement. Therefore, the use of objective methods, such as accelerometers, or the combination of movement and physiological (e.g. heart rate) devices should be encouraged if we wish to provide a more clear, realistic, and objective estimate of time spent in sedentary behaviours. Also, the cut-offs used for defining sedentary behaviours as to categorise people as sedentary are not clear and differ between studies, and should be standardised.
Although 3 studies (11.5%) were classified as poor quality one of these [37] did not report any maternal or infant outcomes and so will not have influenced our interpretation of the literature. As noted the findings of the other 2 poor quality rated studies [45, 46] should be interpreted with caution. The rest of the studies were classified at least as intermediate quality, mostly because the designs were less reliable (not randomised controlled trials), most of the sample size were small, some utilised non-objective assessment methods, and/or were not validated, but we are confident that they are representative of the available literature.
Conclusions
The observation that pregnant women spend much of their time in sedentary activities opens new approaches aiming to improve pregnant women’s health. However our review has identified important gaps in our understanding in this field. For example only 2 studies considered sleeping time during pregnancy [8, 38] which may be an important consideration when assessing sedentary behaviour due to changing sleep patterns in pregnancy. Further, only 1 study assessed the transitions from sit/lay to stand, or breaks during sedentary time [8], which may be an important area to target in future interventions studies.
Our review highlights a high prevalence of sedentarism and significant time spent in sedentary behaviours, also that changes in sedentary behaviour may impact on pregnancy outcomes for both mother and child, emphasising this as an area for future mechanistic and intervention studies. However, the heterogeneity in the literature suggests future studies should use robust methodology, preferably with objective measures for quantifying sedentary behaviour.
Acknowledgements
We thank Sheila Frisken, librarian from the Royal Infirmary Library, who was very helpful during the research strategy process.
Funding
CF was supported by funds from The National Commission for Scientific and Technological Research (Chile). We also acknowledge the support of Tommy’s and the British Heart Foundation and of the MRC Centre Grant MR/N022556/1
Availability of data and materials
Not applicable.
Authors’ contributions
CF conducted all aspects of the review and wrote the manuscript. KL carried out the literature review and approved the manuscript. DS supervised the conduct of the review and wrote the manuscript. JN supervised the conduct of the review and wrote the manuscript. RR carried out the systematic review and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable as this is a systematic review. We registered our intention to conduct the review with Prospero.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CF
Caterina Fazzi
- CRP
C-reactive protein
- GRADE
Grading of Recommendations Assessment Development and Evaluation
- KL
Kathryn Linton
- MeSH
Medical subject headings
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
Additional file
Database search strategy. (PDF 8 kb)
Contributor Information
Caterina Fazzi, Email: Caterina.fazzi@ed.ac.uk.
David H. Saunders, Email: Dave.saunders@ed.ac.uk
Kathryn Linton, Email: Lintonk319@doctors.org.uk.
Jane E. Norman, Email: Jane.norman@ed.ac.uk
Rebecca M. Reynolds, Email: R.Reynolds@ed.ac.uk
References
- 1.Pate RR, O’Neill JR, Lobelo F. The evolving definition of “sedentary”. Exerc Sport Sci Rev. 2008;36(4):173–8. [DOI] [PubMed]
- 2.Rezende LFMd, Lopes MR, Rey-Lopez JP, Matsudo VKR, Luiz OdC. Sedentary Behavior and Health Outcomes: An Overview of Systematic Reviews. PLoS ONE. 2014;9(8):e105620. doi:10.1371/journal.pone.0105620. [DOI] [PMC free article] [PubMed]
- 3.What is sedentary behaviour? [Internet]. British Heart Foundation National Centre for Physical Activity and Health, Loughborough University. 2012. www.II.dlpa.bru.nihr.ac.uk/bin/-1/whatissedentarybehaviour.pdf.
- 4.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spittaels H, van Cauwenberghe E, Verbestel V, De Meester F, van Dyck D, Verloigne M, et al. Objectively measured sedentary time and physical activity time across the lifespan. A cross-sectional study in four age groups. Int J Behav Nutr Phys Act. 2012;9(149):1–12. doi: 10.1186/1479-5868-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Sedentary Behaviour and Obesity Expert Working Group, Biddle S, Cavill N, Ekelund U, Gorely T, Griffiths M, et al. Sedentary Behaviour and Obesity: Review of the Current Scientific Evidence. The Sedentary Behaviour and Obesity Expert Working Group, 2010. www.gov.uk/government/uploads/system/uploads/attachment_data/file/213745/dh_128225.pdf.
- 7.Obesity Indicators 2014. Monitoring Progress for the Prevention of Obesity Route Map [Internet]. 2014. Available from: http://www.gov.scot/Publications/2014/12/4260.
- 8.Di Fabio DR, Blomme CK, Smith KM, Welk GJ, Campbell CG. Adherence to physical activity guidelines in mid-pregnancy does not reduce sedentary time: an observational study. International Journal of Behavioral Nutrition and Physical Activity. 2015;12(1). [DOI] [PMC free article] [PubMed]
- 9.Hjorth MF, Kloster S, Girma T, Faurholt-Jepsen D, Andersen G, Kaestel P, et al. Level and intensity of objectively assessed physical activity among pregnant women from urban Ethiopia. BMC Pregnancy Childbirth. 2012;12. [DOI] [PMC free article] [PubMed]
- 10.Hawkins M, Pekow P, Chasan-Taber L. Physical activity, sedentary behavior, and C-reactive protein in pregnancy. Med Sci Sports Exerc. 2014;46(2):284–92. doi: 10.1249/MSS.0b013e3182a44767. [DOI] [PubMed] [Google Scholar]
- 11.Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med. 2011;53(1–2):39–43. doi: 10.1016/j.ypmed.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Ruifrok AE, Althuizen E, Oostdam N, van Mechelen W, Mol BW, de Groot CJ, et al. The relationship of objectively measured physical activity and sedentary behaviour with gestational weight gain and birth weight. J Pregnancy. 2014;2014:567379. doi: 10.1155/2014/567379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu F, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviours in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA Intern Med. 2003;289:1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 14.Katzmarzyk PT, Church T, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease and cancer. Med Sci Sports Exerc. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 15.Bertrais S, Beyeme-Ondoua JP, Czernichow S, Galan P, Hercberg S, Oppert JM. Sedentary behaviors, physical activity, and metabolic syndrome in middle-aged French subjects. Obes Res. 2005;13(5):936–44. doi: 10.1038/oby.2005.108. [DOI] [PubMed] [Google Scholar]
- 16.Dunstan DW, Barr EL, Healy GN, Salmon J, Shaw JE, Balkau B, et al. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121(3):384–91. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 17.Rezende LF, Sá TH, Mielke GI, Viscondi JY, Rey-López JP, LM G. All-Cause Mortality Attributable to Sitting Time. Analysis of 54 Countries Worldwide. Am J Prev Med. 2016;51(2):253-63. [DOI] [PubMed]
- 18.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–13. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González-Gross M, Meléndez A. Sedentarism, active lifestyle and sport: impact on health and obesity prevention. Nutr Hosp. 2013;28(Supl. 5):89–98. doi: 10.3305/nh.2013.28.sup5.6923. [DOI] [PubMed] [Google Scholar]
- 20.Clark BK, Sugiyama T, Healy GN, Salmon J, Dunstan DW, Owen N. Validity and reliability of measures of television viewing time and other non-occupational sedentary behaviour of adults: a review. Obes Rev. 2009;10(1):7–16. doi: 10.1111/j.1467-789X.2008.00508.x. [DOI] [PubMed] [Google Scholar]
- 21.Dunstan DW, Salmon J, Owen N, Armstrong T, Zimmet PZ, Welborn TA, et al. Physical activity and television viewing in relation to risk of undiagnosed abnormal glucose metabolism in adults. (Epidemiology/Health Services/Psychosocial Research) Diabetes Care. 2004;27(11):2603. doi: 10.2337/diacare.27.11.2603. [DOI] [PubMed] [Google Scholar]
- 22.Dunstan DW, Salmon J, Healy GN, Shaw JE, Jolley D, Zimmet PZ, et al. Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care. 2007;30(3):516. doi: 10.2337/dc06-1996. [DOI] [PubMed] [Google Scholar]
- 23.Stroup D., Berlin J., Morton S., Olkin I., Williamson G.D., Rennie D., et al. The Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines Journal of American Medical Association. 2000;283(15). [DOI] [PubMed]
- 24.Shamseer L., Moher D., ClarkeM., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2014. [DOI] [PubMed]
- 25.Guyatt G, Oxman A, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt G, Oxman A, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Balshem H, Helfand M, Schünemann H, Oxman A, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt G, Oxman A, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence-study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Oken N, Rifas-Shiman R, Rich-Edwards G. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108(5):1200–7. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang H, Qian X, Li M, Lynn H, Fan Y, Jiang H, et al. Can physical activity reduce excessive gestational weight gain? Findings from a Chinese urban pregnant women cohort study. Int J Behav Nutr Phys Act. 2012;9:12. doi: 10.1186/1479-5868-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Both MI, Wildhagen MF, Wildschut HI, Overvest MA, Golding J. The association of daily physical activity and birth outcome. A population-based cohort study. Eur J Epidemiol. 2010;25:421–9. doi: 10.1007/s10654-010-9458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegaard HK, Petersson K, Hedegaard M, Ottesen B, Dykes AK, Henriksen TB, et al. Sports and leisure-time physical activity in pregnancy and birth weight: a population-based study. Scand J Med Sci Sports. 2010;20(1):e96–102. doi: 10.1111/j.1600-0838.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes RE, Blanchard CM, Benoit C, Levy-Milne R, Naylor PJ, Symons Downs D, et al. Physical activity and sedentary behavior across 12 months in cohort samples of couples without children, expecting their first child, and expecting their second child. J Behav Med. 2014;37(3):533–42. doi: 10.1007/s10865-013-9508-7. [DOI] [PubMed] [Google Scholar]
- 34.Chasan-Taber L, Silveira M, Pekow P, Braun B, Manson JE, Solomon CG, et al. Physical activity, sedentary behavior and risk of hypertensive disorders of pregnancy in Hispanic women. Hypertens Pregnancy. 2015;34(1):1–16. doi: 10.3109/10641955.2014.946616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegaard HK, Damm P, Hedegaard M, Henriksen TB, Ottesen B, Dykes AK, et al. Sports and leisure time physical activity during pregnancy in nulliparous women. Matern Child Health J. 2011;15(6):806–13. doi: 10.1007/s10995-010-0647-y. [DOI] [PubMed] [Google Scholar]
- 36.Gollenberg AL, Pekow P, Bertone-Johnson ER, Freedson PS, Markenson G, Chasan-Taber L. Sedentary behaviors and abnormal glucose tolerance among pregnant Latina women. Med Sci Sports Exerc. 2010;42(6):1079–85. doi: 10.1249/MSS.0b013e3181c6dec8. [DOI] [PubMed] [Google Scholar]
- 37.Evenson KR, Wen F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Prev Med. 2010;50(3):123–8. doi: 10.1016/j.ypmed.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Van Raaij JMA, Schonk CM, Vermaat-Miedema SH, Peek MEM, Hautvast JGAJ. Energy cost of physical activity throughout pregnancy and the first year postpartum in Dutch women with sedentary lifestyles. Am J Clin Nutr. 1990;52(2):234–9. doi: 10.1093/ajcn/52.2.234. [DOI] [PubMed] [Google Scholar]
- 39.Reid EW, McNeill JA, Alderdice FA, Tully MA, Holmes VA. Physical activity, sedentary behaviour and fetal macrosomia in uncomplicated pregnancies: a prospective cohort study. Midwifery. 2014;30(12):1202–9. doi: 10.1016/j.midw.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Chasan-Taber L, Silveira M, Lynch KE, Pekow P, Solomon CG, Markenson G. Physical activity and gestational weight gain in Hispanic women. Obesity. 2014;22(3):909–18. doi: 10.1002/oby.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumareswaran K, Elleri D, Allen JM, Caldwell K, Westgate K, Brage S, et al. Physical activity energy expenditure and glucose control in pregnant women with type 1 diabetes: is 30 min of daily exercise enough? Diabetes Care. 2013;36:1095–101. doi: 10.2337/dc12-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padmapriya N, Shen L, Soh SE, Shen Z, Kwek K, Godfrey KM, et al. Physical activity and sedentary behavior patterns before and during pregnancy in a multi-ethnic sample of Asian women in Singapore. Matern Child Health J. 2015;19(11):2523–35. doi: 10.1007/s10995-015-1773-3. [DOI] [PubMed] [Google Scholar]
- 43.Lynch KE, Landsbaugh JR, Whitcomb BW, Pekow P, Markenson G, Chasan-Taber L. Physical activity of pregnant Hispanic women. Am J Prev Med. 2012;43(4):434–9. doi: 10.1016/j.amepre.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loprinzi PD, Fitzgerald EM, Woekel E, Cardinal BJ. Association of physical activity and sedentary behavior with biological markers among U.S. pregnant women. J Womens Health. 2013;22(11):953–8. doi: 10.1089/jwh.2013.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watts JN, Miller YD, Marshall AL. Depressive symptoms during pregnancy: exploring the role of sitting. Ment Health and Phys Act. 2013;6(1):36–42. doi: 10.1016/j.mhpa.2012.08.001. [DOI] [Google Scholar]
- 46.Li CR, Zhao SX. The impact of persistent sedentary work on outcome pregnancy. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi/Zhonghua Laodong Weisheng Zhiyebing Zazhi/Chinese Journal of Industrial Hygiene & Occupational Diseases. 2007;25(8):506–7. [PubMed] [Google Scholar]
- 47.Gradmark A, Pomeroy J, Renstrom F, Steiginga S, Persson M, Wright A, et al. Physical activity, sedentary behaviors, and estimated insulin sensitivity and secretion in pregnant and non-pregnant women. BMC Pregnancy Childbirth. 2011;11:44. [DOI] [PMC free article] [PubMed]
- 48.Hawkins M, Chasan-Taber L, Marcus B, Stanek E, Braun B, Ciccolo J, et al. Impact of an exercise intervention on physical activity during pregnancy: the behaviors affecting baby and you study. Am J Public Health. 2014;104(10):e74–81. doi: 10.2105/AJPH.2014.302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes L, Bell R, Robson S, Poston L. Association between physical activity in obese pregnant women and pregnancy outcomes: the UPBEAT pilot study. Ann Nutr Metab. 2014;64:239–46. doi: 10.1159/000365027. [DOI] [PubMed] [Google Scholar]
- 50.Henson J, Yates T, Biddle SJ, Edwardson CL, Khunti K, Wilmot EG, et al. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. 2013;56(5):1012–20. doi: 10.1007/s00125-013-2845-9. [DOI] [PubMed] [Google Scholar]
- 51.Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010;35:725–40. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 52.Van der Pligt P, Willcox J, Hesketh KD, Ball K, Wilkinson S, Crawford D, et al. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev. 2013;14(10):792–805. doi: 10.1111/obr.12053. [DOI] [PubMed] [Google Scholar]
- 53.Poston L, Bell R, Croker H, Flynn A, Godfrey K, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767–77. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 54.Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ. 2014;348. doi:10.1136/bmj.g1285. [DOI] [PMC free article] [PubMed]
- 55.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. Br Med J. 2012;344. doi:10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed]
- 56.Martin A, Fitzsimons C, Jepson R, Saunders D, Van de Ploeg H, Teixeira P, et al. Interventions with potential to reduce sedentary time in adults: systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1056–63. doi: 10.1136/bjsports-2014-094524. [DOI] [PubMed] [Google Scholar]
- 57.DeMattia L, Lemont L, Meurer L. Do interventions to limit sedentary behaviours change behaviour and reduce childhood obesity? A critical review of the literature. Obes Rev. 2007;8(1):69–81. doi: 10.1111/j.1467-789X.2006.00259.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


