ABSTRACT
Bacillus Calmette–Guerin (BCG) therapy for non-muscle invasive bladder cancer (NMIBC) can significantly reduce the risk of recurrence and progression. However, BCG therapy may fail in up to a half of treated patients and may also cause toxicities. Biomarkers to predict the effectiveness of BCG therapy are desired to pre-select patients for BCG therapy to maximize efficacy while avoid unnecessary toxicity. Twelve cytokines were measured in 100 blood and 112 urine samples using cytokine antibody array and correlated with recurrence-free survival in overall and BCG-treated NMIBC patients. Of the 12 cytokines, interleukin (IL) -2, IL-8, IL-10, tumor necrosis factor (TNF)-α, granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon (IFN)-γ were measurable in more than 30% of peripheral blood leukocyte (PBL) samples. Only IL-8 in PBL was found to be significantly associated with tumor recurrence, especially in those who receiving BCG therapy (hazard ratio [HR] = 4.24; 95% confidence interval [95%CI] = 1.65–10.88; p = 0.003). The median recurrence-free survival time for BCG-treated patients with high baseline IL-8 levels were much shorter than those with low IL-8 levels (7.9 vs. >78.4 mo, p = 0.004). Furthermore, consistent associations between urinary IL-8 levels and tumor recurrence in patients receiving BCG therapy were observed in 58 pre-BCG and 54 long-term post-BCG-treated urine samples (both p ≤ 0.005). High urinary baseline IL-8 level also predicted shorter time to tumor recurrence in NMIBC patients (both p ≤ 0.004). By using antibody array-based technology in two separate cohorts of NMIBC patients, we found that PBL and urinary baseline IL-8 levels were significantly associated with tumor recurrence after BCG therapy.
KEYWORDS: Antibody array, BCG therapy, bladder cancer, IL-8, tumor recurrence
Abbreviations
- BCG
Bacillus Calmette-Guerin
- CIs
confidence intervals
- ELISA
enzyme-linked immunoadsorbent assay
- FBS
fetal bovine serum
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HR
hazard ratio
- IFNγ
interferon-γ
- IL-2
interleukin-2
- IL-8
interleukin-8
- IL-10
interleukin-10
- MST
median recurrence-free survival time
- NMIBC
non-muscle invasive bladder cancer
- PBL
peripheral blood leukocyte
- PBS
phosphate-buffered saline
- RFU
relative fluorescence unit
- SD
standard deviation
- TNF-α
tumor necrosis factor-α
- TUR
transurethral resection
Introduction
Bladder cancer is the fourth most prevalent cancer in men and accounts for approximately 5% of all newly diagnosed malignancies in the United States, with an estimated 74,000 new cases and 16,000 deaths in 2015.1 Non-muscle-invasive bladder cancer (NMIBC) comprises the great majority (70–80%) of bladder cancer.2 Emerging at the 1970s, intravesical bacillus Calmette–Guerin (BCG) therapy has proven to be the only successful adjuvant therapy for preventing or delaying recurrence and progression after transurethral resection (TUR).3 However, intravesical BCG therapy has certain disadvantages, including potential toxicity and a high non-response rate of 30–50% in the treated cases.4 In addition, BCG therapy may cause significant side effects in approximately 20% patients, such as hematuria and dysuria, even life-threatening sepsis, which affect the course of therapy and lead to worse outcome.5,6 Identifying patients before therapy who are more likely to fail or benefit from this cytostatic therapy is essential to improving survival outcome. Therefore, reliable markers to predict BCG effectiveness at pre-treatment or an early phase after starting therapy are highly demanded.
For decades, people have observed that fever after BCG therapy might serve as an important predicting marker of recurrence for NMIBC patients.7 It is well known that the antitumor activity of intravesical BCG therapy mainly depend on the BCG-induced inflammatory response,8 which is impaired in bladder cancer as reflected by an imbalanced production of immuno-modulating cytokines.9 Many cytokines such as interleukin (IL) -2, IL-8, IL-10 and tumor necrosis factor (TNF)-α regulate or assist in the active immune response.10 Recently, based on a BCG clinical trial of NMIBC patients conducted in our center (NCT01007058), we observed that increases in urinary cytokines after BCG instillation correlated with recurrence and we further developed a nomogram using changes of nine inducible urinary cytokines that showed excellent accuracy in predicting the likelihood of recurrence.11
Conversely, several above-mentioned cytokines, such as IL-8 and IL-10, were also reported to promote tumor progression. Experiments have demonstrated that these cytokines may facilitate bladder cancer development, and blocking these cytokines might inhibit tumor metastasis.12,13 These opposite results suggest that cancer recurrence might be either stimulated or restrained by inflammatory and immune processes depending on different cellular context. Although elevated cytokine levels after BCG instillation in a short-time frame can predict a better response in NMIBC patients, a long-term inflammation status with high baseline levels of cytokines might negatively affect BCG response.
In this study, we used a two-phase design with a total of 100 blood and 112 urine samples from NIMBC patients to investigate the prognostic value of baseline levels of cytokines. In the first phase, we compared the baseline levels of 12 cytokines, including IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon (IFN)-γ, in PBL samples from 100 NMIBC patients (50 with recurrence and 50 without) to identify candidate biomarkers for predicting tumor recurrence. In the second phase, we collected urine samples at baseline before (pre-BCG group) or long-term after BCG instillation (long-term post-BCG group) from 112 NMIBC patients receiving BCG therapy, and measured these cytokines in urine samples to validate the predictive values of those cytokines.
Materials and methods
Study population and recruitment
Patients with NMIBC (Tis, Ta or T1 according to European Association of Urology guidelines3) were recruited from The University of Texas MD Anderson Cancer Center. There were no age and sex restrictions on recruitment. All patients in this study were Caucasians. This study was approved by the institutional review board of MD Anderson. Written informed consent to participate in the study was obtained from each participant. All participants were interviewed to collect information about demographics, smoking history and medical history. All participants were followed up with periodic cystoscopic medical examination. The endpoint of interest in each patient was tumor recurrence, defined as a newly identified bladder tumor after a previous negative follow-up cystoscopy. Vital status of each participant was ascertained by MD Anderson Tumor Registry and was further confirmed by the Social Security Death Index.
Purification, culture and cryopreservation of PBL
Blood samples were contained in coded heparinized tubes and sent to the laboratory for PBL purification. PBLs were isolated by Ficoll-Paque™ PLUS density centrifugation (GE Healthcare, Amersham, UK) and washed twice in phosphate buffered saline (PBS, Gibco BRL, Grand Island, NY). PBLs were cultured in 2 mL RPMI-1640 medium containing 10% FBS and 1% penicillin-streptomycin (Sigma-Aldrich, St Louis, MO) in a humidified atmosphere incubator preset to 37°C and 5% CO2 or cryopreserved in liquid nitrogen in heat-inactivated fetal bovine serum (FBS, Gibco BRL, Grand Island, NY) supplemented with 10% DMSO (Sigma-Aldrich, St Louis, MO) at a concentration of 5.0 × 106/mL.
Cytokine release induction in PBL
To detect the cytokine release levels from PBL, we activated PBLs using anti-CD3 and anti-CD28 antibodies as stimuli according to previous reports.14 In brief, PBLs were washed twice with PBS and re-suspended in 1 mL RPMI-1640 medium. Followed by counting cell number, PBLs were added to 96-well plate and co-cultured with anti-CD3 and anti-CD28 monoclonal antibodies (0.1 μg/mL) for 24 h in RPMI-1640 medium. After centrifugation at 1,500 g for 10 min, cell-free supernatant was collected for cytokine assay.
Collection of urine samples
Spontaneous urine samples (volume up to 100 mL) were collected from 112 NMIBC patients receiving BCG therapy. To avoid the interference of BCG reagent on urinary cytokine levels in early stage (0–24 h) after BCG instillation,15 urine samples were split into two subsets, 58 were collected before starting BCG therapy (pre-BCG group), and 54 collected during a prolonged follow-up period after BCG therapy (long-term post-BCG group). In the long-term post-BCG group, the average time of urine sample collection was 160 d from the beginning of BCG instillation. All urine samples were centrifuged at 1,500 g for 10 min. Cell-free urine samples were stored at −80°C and used for cytokine assay.
Cytokine expression measurement
Cytokine levels in cell-free supernatants from blood or urine samples were measured using the Pathscan® cytokine antibody array kit (Cell Signaling, Danvers, MA). Experiments were performed according to the manufacturer recommended protocol. Briefly, 75 μL of fluid sample was added to a nitrocellulose-coated glass slide pre-coated with primary antibodies. The plate was incubated overnight at 4°C with constant rocking. After washing with PBS/0.05% Tween buffer, the plate was then serially exposed to the biotinylated detection antibody cocktail for 1 h (Cell Signaling, Danvers, MA) and Alexa Fluor® 647-conjugated streptavidin (Life Technologies, Gaithersburg, MD) for 30 min at room temperature. After further washing, the slides were dried and imaged using IN Cell Analyzer 2200 (GE Healthcare, Waukesha, WI). High-content images were acquired at 20× magnification with 705/72 m (for Cy5) emission filters, and images were processed and analyzed using IN Cell Developer Toolbox 1.9.2. The spots whose intensity lies above the threshold are considered positive and the overall intensity of the fluorescence was calculated.
Statistical analysis
All statistical analyses were performed using the Stata 10.1 statistical software program (version 10.1; Stata Corp). The cytokine expression levels between groups with different clinical outcomes were analyzed using Wilcoxon rank-sum test. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between cytokine levels and the risk of recurrence while adjusting for age, gender, smoking status and tumor stage. Cytokine concentrations were analyzed as a categorical variable by setting cutoff points according to ROC curve in either overall participants or stratified subgroups. The Kaplan–Meier method was used to estimate the median recurrence-free survival. Survival estimates between groups were compared using the log-rank test statistic. All statistical tests were two-sided, and associations were considered statistically significant at p levels less than 0.05.
Results and discussion
In the present study, a total of 100 NMIBC patients with PBL samples (50 with recurrence and 50 without recurrence) and 112 NMIBC patients with urine samples were included in the study. In the patient group with urine samples, all patients were treated with BCG since we intended to investigate the prognostic value of baseline levels of urinary cytokine in BCG therapy. To avoid the short-term influence of BCG therapy on urinary cytokine levels, we separated these patients into two sub-groups based on whether the urine samples were collected before (pre-BCG) or during a prolonged follow-up period after BCG therapy (long-term post-BCG). Descriptive characteristics of patients were shown in Table 1. The mean ages of the PBL and urine groups were 66.43 and 64.35 y, respectively. The proportion of female patients in the urine group was higher than the PBL group (25.89% vs. 10.00%). The distributions in smoking status, tumor stage and clinical outcome were similar between two groups.
Table 1.
Baseline characteristics of patients with bladder cancer.
| Characteristics | PBL group (n = 100) | Urine group (n = 112) |
|---|---|---|
| Mean of age ± SD | 66.43 ± 9.47 | 64.35 ± 10.58 |
| Gender | ||
| Male | 90 (90.00) | 83 (74.11) |
| Female | 10 (10.00) | 29 (25.89) |
| Smoking status | ||
| Never-smoker | 25 (25.00) | 30 (26.79) |
| Former-smoker | 53 (53.00) | 50 (44.64) |
| Current-smoker | 22 (22.00) | 32 (28.57) |
| Tumor stage | ||
| Stage Tis or Ta | 46 (46.00) | 49 (43.75) |
| Stage T1 | 54 (54.00) | 63 (56.25) |
| BCG therapy | 51 (51.00) | 112 (100.00) |
| Clinical outcome | ||
| With recurrence | 50 (50.00) | 48 (42.86) |
| Without recurrence | 50 (50.00) | 64 (57.14) |
Abbreviations: PBL, peripheral blood leukocyte; BCG, bacillus Calmette–Guerin; SD, standard deviation.
The association between high baseline IL-8 level in PBL and tumor recurrence
The levels of IL-4, IL-5, IL-6, IL-12, IL-13 and IL-17 in activated PBL were not measureable in more than 30% of patients, and these six cytokines were not included in further analysis. The intra-assay coefficient of variation for the levels of IL-2, IL-8, IL-10, TNF-α, GM-CSF and IFNγ in PBLs ranged from 3.24% to 9.57%. Higher IL-8 levels in PBL were observed in recurrent patients (p = 0.002), especially in BCG-treated groups (p = 0.005) (Table 2).
Table 2.
Cytokine concentrations in PBL of patients with or without recurrence.
| Overall patients |
BCG-treated patients |
|||||
|---|---|---|---|---|---|---|
| Cytokine | Recurrence (n = 50) | No recurrence (n = 50) | p valuea | Recurrence (n = 28) | No recurrence (n = 23) | p valuea |
| IL-8 (RFU/109) | 2.4 (0.7) | 2.1 (1.0) | 0.002 | 2.4 (0.8) | 1.9 (1.0) | 0.005 |
| IL-2 (RFU/108) | 3.3 (1.8) | 3.5 (2.3) | 0.874 | 3.3 (1.7) | 3.5 (2.3) | 0.947 |
| IL-10 (RFU/107) | 4.4 (3.3) | 4.7 (4.2) | 0.833 | 4.1 (3.1) | 3.9 (3.3) | 0.661 |
| TNF-α (RFU/108) | 8.2 (4.1) | 8.2 (5.2) | 0.574 | 7.8 (3.9) | 8.7 (5.3) | 0.629 |
| IFNγ (RFU/108) | 5.9 (6.5) | 6.8 (5.2) | 0.134 | 5.9 (7.0) | 7.9 (5.3) | 0.087 |
| GM-CSF (RFU/105) | 7.9 (6.8) | 8.1 (8.6) | 0.853 | 8.6 (7.8) | 7.5 (9.8) | 0.632 |
Abbreviations: PBL, peripheral blood leukocyte; BCG, bacillus Calmette–Guerin; RFU, relative fluorescence unit.
p-value was computed using Wilcoxon rank-sum test.
The association between six measurable cytokines and the risk of recurrence was shown in Table 3. Patients with higher PBL IL-8 level had more than 2.5-fold increased risk of recurrence in overall analysis (HR: 2.62, 95%CI: 1.36–5.06; p = 0.004) and stratified analysis for BCG-treated group (HR: 4.24, 95%CI: 1.65–10.88; p = 0.003). Kaplan–Meier analysis demonstrated that the median recurrence-free survival time for those patients with high IL-8 levels were much shorter than those with low IL-8 levels (14.0 mo vs. >78.4 mo; p = 0.003) (Table 3; Fig. 1A). Similarly, for BCG-treated patients only, high IL-8 level also predicted a shorter median recurrence-free survival time (7.9 mo vs. >78.4 mo; p = 0.004) (Table 3; Fig. 1B). In contrast, there was no significant association between IL-8 and recurrence in patients receiving TUR only (data not shown). None of the other cytokines were significantly associated with tumor recurrence either in overall or in stratified analysis.
Table 3.
Recurrence risk of patients with bladder cancer as estimated by cytokine levels in PBL.
| Cytokine levels in PBL | Recurrence (high/low) | No recurrence (high/low) | HR (95% CI)a | p value | MST (high/low) | Log-rank p value |
|---|---|---|---|---|---|---|
| IL-8 | ||||||
| Overall | 38/12 | 21/29 | 2.62 (1.36–5.06) | 0.004 | 14.0/>78.4 | 0.003 |
| BCG | 22/6 | 8/15 | 4.24 (1.65–10.88) | 0.003 | 7.9/>78.4 | 0.004 |
| IL-2 | ||||||
| Overall | 28/22 | 24/26 | 1.20 (0.68–2.12) | 0.520 | 31.6/>78.4 | 0.557 |
| BCG | 16/12 | 11/12 | 1.42 (0.65–3.12) | 0.382 | 21.0/33.3 | 0.514 |
| IL-10 | ||||||
| Overall | 15/35 | 9/41 | 1.57 (0.85–2.93) | 0.151 | 20.6/50.2 | 0.170 |
| BCG | 7/21 | 4/19 | 1.27 (0.51–3.15) | 0.601 | 31.6/21.0 | 0.775 |
| TNF-α | ||||||
| Overall | 25/25 | 19/31 | 1.57 (0.88–2.81) | 0.127 | 16.6/50.2 | 0.174 |
| BCG | 13/15 | 10/13 | 1.85 (0.79–4.34) | 0.155 | 20.6/33.3 | 0.399 |
| IFNγ | ||||||
| Overall | 7/43 | 10/40 | 0.90 (0.40–2.06) | 0.814 | >78.4/31.6 | 0.730 |
| BCG | 3/25 | 8/15 | 0.45 (0.13–1.58) | 0.214 | >78.4/16.7 | 0.197 |
| GM-CSF | ||||||
| Overall | 18/32 | 14/36 | 1.51 (0.84–2.72) | 0.172 | 10.3/50.2 | 0.163 |
| BCG | 11/17 | 5/18 | 1.82 (0.83–4.02) | 0.138 | 6.0/38.9 | 0.120 |
Abbreviations: PBL, peripheral blood leukocyte; BCG, bacillus Calmette–Guerin; HR, hazard ratio; 95%CI, 95% confidence interval; MST, median recurrence-free survival time.
Adjusted by age, sex, smoking status and tumor stage.
Figure 1.
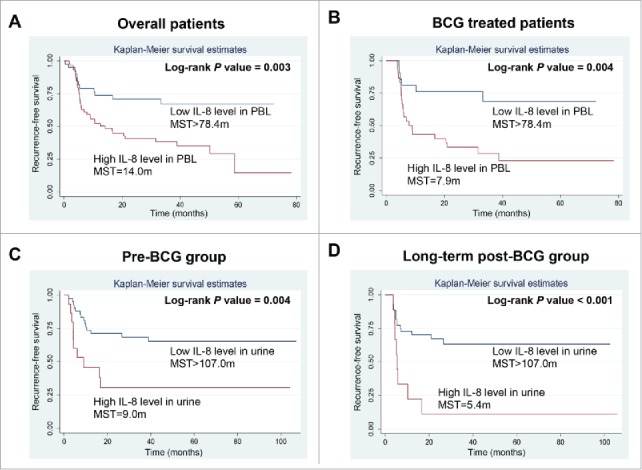
Kaplan–Meier curves for patients with different IL-8 levels in PBL and urine samples. Baseline IL-8 levels in PBL were associated with recurrence-free time in overall analysis (A) and stratified analysis in BCG-treated group (B). In urine samples, high baseline IL-8 levels predicted shorter recurrence-free time either in pre-BCG group (C) or in long-term post-BCG group (D). Abbreviations: PBL, peripheral blood leukocyte; BCG, bacillus Calmette–Guerin; MST, median recurrence-free survival time.
IL-8, an important chemotactic and pro-inflammatory cytokine, has been shown to be associated with tumor progression in bladder cancer.15-22 High expression levels of IL-8 were observed in both tissue and urine samples of patients with bladder cancer,23 and were reported to be associated with tumor recurrence.24 The majority of IL-8 in the bladder is produced by leukocytes such as neutrophils, T cells and macrophages.25,26 However, to date, whether the production levels of IL-8 in leukocytes contribute to tumor progression in patients with bladder cancer still remains largely unknown. In this study, our data showed that high secretion levels of IL-8 in PBL were significantly associated with shorter recurrence-free time in patients with bladder cancer. There was also suggestive evidence that IFNγ in PBL was protective against recurrence, particularly in patients receiving BCG therapy (Table 1), although the difference did not reach statistical significance. These findings were consistent with previous reports. Elsasser-Beile et al. 27 reported that IFNγ level was significantly lower in bladder cancer patients as compare with the healthy controls. Kaempfer et al. 28 also observed a lower inducibility of IFNγ mRNA in PBL from NMIBC patients with tumor relapse. Interestingly, a molecular mechanism reported by Gusella et al. 29 indicated that IFNγ suppresses IL-8 expression in monocytes, which might partially explain the contrasting roles of IL-8 and IFNγ in PBLs for predicting tumor recurrence from bladder cancer.
The association between high urinary IL-8 level and tumor recurrence
Considering that the significant association between IL-8 levels in PBL and tumor recurrence in BCG-treated patients, we wondered whether baseline levels of urinary IL-8, which are mainly secreted from activated PBLs,30 could also predict BCG therapy effectiveness. We therefore conducted the analysis in 112 urine samples from NMIBC patients receiving BCG therapy. Because literature showed that BCG therapy may shortly affect urinary cytokine levels,31 we conducted two sub-groups, pre-BCG (n = 58) and long-term post-BCG groups (n = 54), to ensure the cytokine levels are at baseline. We found that patients with high urinary IL-8 levels had approximately 4-fold increased risk of tumor recurrence either in pre-BCG group (HR: 3.72, 95%CI: 1.49–9.28; p = 0.005) or in long-term post-BCG group (HR: 4.25, 95%CI: 1.63–11.05; p = 0.003), compare with those with low IL-8 levels (Table 4). In addition, the median recurrence-free survival time for those patients with high urinary IL-8 level were also significantly shorter than those with low IL-8 levels in both the pre-BCG and long-term post-BCG groups (9.0 mo vs. >107.0 mo, p = 0.004 and 5.4 mo vs. >107.0 mo, p <0.001, respectively) (Table 4; Fig. 1C and D).
Table 4.
Recurrence risk of BCG-treated patients as estimated by urinary IL-8 level.
| Urinary IL-8 level | Clinical outcomea | HR (95% CI) b | p value | MST (months) | Log-rank p value |
|---|---|---|---|---|---|
| Pre-BCG | |||||
| Low | 14/28 | 1 (reference) | — | >107.0 | — |
| High | 11/5 | 3.72 (1.49–9.28) | 0.005 | 9.0 | 0.004 |
| Long-term post-BCG | |||||
| Low | 15/30 | 1 (reference) | — | >107.0 | — |
| High | 8/1 | 4.25 (1.63–11.05) | 0.003 | 5.4 | <0.001 |
Abbreviations: NMIBC, non-muscle invasive cancer; BCG, bacillus Calmette–Guerin; HR, hazard ratio; 95%CI, 95% confidence interval; MST, median recurrence-free survival time.
The results of clinical outcome are presented as patient number of recurrence vs. no recurrence.
Adjusted by age, sex, smoking status and tumor stage.
Urquidi et al. reported high urinary IL-8 level in patients with bladder cancer.32 Similarly, Sheryka et al. also found patients with tumor recurrence had higher baseline IL-8 level, and urinary IL-8 level was associated with tumor stage.23 Although these studies have suggested that increased urinary IL-8 expression might play a critical role in the development of bladder cancer, whether elevated levels of IL-8 in urine are associated with patients' survival still remains unclear. In the present study, our data clearly showed that patients with high baseline IL-8 levels in urine had approximately 4-fold increased risk of tumor recurrence after BCG treatment. This result strongly suggests screening baseline urinary IL-8 level is helpful for selecting suitable therapy for bladder cancer patients.
In the present study, we collected urine samples both before and at a more prolonged time (average 160 d) after BCG instillation. For decades, clinicians have observed that urinary IL-8 levels could be influenced by BCG instillation. Several prior studies found that higher inducible urinary IL-8 levels in the early stage (usually in first 24 h) after BCG instillation were associated with better clinical outcomes. de Boer et al.15 noted that the urinary IL-8 levels in BCG responders were sharply increased in first 4 h after BCG instillation and then gradually decreased and became stable in 24 h. Thalmann et al.17 also reported the acute post-BCG levels (6 h after BCG instillation) of IL-8 were 25-fold increased and significantly associated with immune response to BCG therapy. However, contradicted results were also reported by other groups. Sagnak et al.21 observed higher levels of urinary IL-8 (2 and 4 h after BCG instillation) in patients who developed tumor recurrence, compare with those in remission. Almost all previous studies focused on the acute changes of urinary cytokine levels after BCG instillation,4 which exhibited unstable cytokine levels and might also contribute to the contradictory conclusions.15 However, the association between long-term urinary cytokine levels after BCG treatment and patients' survival has hardly been investigated. Only Sheryka et al. measured urinary IL-8 level 1 mo after BCG therapy in 21 BCG-treated patients.23 They found the 1 mo post-BCG instillation levels of urinary IL-8 were greater in patients with tumor recurrence compared with those whose tumor was in remission. In the present study, our data further confirmed that long-term post-BCG levels of IL-8 in urine was a predictor of tumor recurrence for NMIBC patients. Mounting evidence from basic science research suggested IL-8 as an important pro-inflammatory and pro-angiogenesis cytokine playing a critical role in tumorigenesis, 33 and targeting IL-8 could reduce tumor metastasis in a variety of human cancers, including bladder cancer.34-36 Taken together, it is reasonable to hypothesize that persistent high baseline IL-8 levels might lead to inflammation and angiogenesis, resulting in increased risk for tumor recurrence or metastasis.
Compared with numerous previous reports which investigated the prognostic values of cytokines in bladder cancer, our study has several unique findings. First, we, for the first time, comprehensively evaluated the predicting values of 12 cytokine levels both in PBL and urine samples in bladder cancer, and identified high levels IL-8 either in PBL or in urine were potential prognostic biomarkers in patients receiving BCG therapy. Second, in contrast to conventional enzyme-linked immunoadsorbent assays (ELISAs) used in previous studies, the cytokine antibody array implemented in this study has several advantages: it is a high-throughput system measuring 12 cytokines in a single sample, which reduced the operator error and saved time; 37 the sample volume required for this assay (only 75 μL) is generally much smaller than that required for ELISA assay. There are also a few limitations in this study. First, we did not measure cytokine concentrations in PBL and urinary samples collected from the same patient and therefore were unable to analyze the correlation of IL-8 levels between the two types of samples. Certainly, a significant correlation of IL-8 levels between PBL and urine would strengthen our analysis. Nevertheless, this does not repudiate the significant association observed between IL-8 and recurrence. Second, this is a single point analysis of cytokines in PBL and urine and did not consider the cytokine level fluctuations influenced by varying host and environment factors. Third, the moderate sample size in our study limits the statistical power to conduct analysis in stratified subgroups. Future large-scale studies with repeated collection of samples are warranted to validate our findings.
In conclusion, by using an antibody array-based technology, we observed that elevated baseline IL-8 levels in PBL and urine were significantly associated with tumor recurrence after BCG therapy. To our knowledge, this is the first epidemiological study combining PBL and urine samples to investigate predictive value of baseline IL-8 level in bladder cancer. If validated, baseline IL-8 level may potentially serve as a simple non-invasive predictive biomarker for bladder cancer patients waiting for BCG therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The work was supported by National Institutes of Health grants P50 CA91846 and U01 CA127615.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5-29; PMID:25559415; http://dx.doi.org/23827737 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J, Rouprêt M. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 2011; 59:997-1008; PMID:21458150; http://dx.doi.org/23827737 10.1016/j.eururo.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Böhle A, Palou Redorta J et al.. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013; 64:639-53; PMID:23827737; http://dx.doi.org/ 10.1016/j.eururo.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 4.Zuiverloon TC, Nieuweboer AJ, Vekony H, Kirkels WJ, Bangma CH, Zwarthoff EC. Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol 2012; 61:128-45; PMID:22000498; http://dx.doi.org/ 10.1016/j.eururo.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 5.Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, van Andel G, Gontero P, Turkeri L, Marreaud S, Collette S et al.. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol 2014; 65:69-76; PMID:23910233; http://dx.doi.org/ 10.1016/j.eururo.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 6.van der Meijden AP, Sylvester RJ, Oosterlinck W, Hoeltl W, Bono AV, Group EG-UTC. Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur Urol 2003; 44:429-34; PMID:14499676; http://dx.doi.org/ 10.1016/S0302-2838(03)00357-9 [DOI] [PubMed] [Google Scholar]
- 7.Luftenegger W, Ackermann DK, Futterlieb A, Kraft R, Minder CE, Nadelhaft P, Studer UE. Intravesical versus intravesical plus intradermal bacillus Calmette-Guerin: a prospective randomized study in patients with recurrent superficial bladder tumors. J Urol 1996; 155:483-7; PMID:8558641; http://dx.doi.org/ 10.1016/S0022-5347(01)66427-9 [DOI] [PubMed] [Google Scholar]
- 8.Bohle A, Brandau S. Immune mechanisms in bacillus Calmette-Guerin immunotherapy for superficial bladder cancer. J Urol 2003; 170:964-9; PMID:12913751; http://dx.doi.org/ 10.1097/01.ju.0000073852.24341.4a [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi A, Dejima T, Yamada H, Shibata K, Nakamura R, Eto M, Nakatani T, Naito S, Yoshikai Y. IL-17 production by gammadelta T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guerin treatment against bladder cancer. Eur J Immunol 2011; 41:246-51; PMID:21182095; http://dx.doi.org/ 10.1002/eji.201040773 [DOI] [PubMed] [Google Scholar]
- 10.Hirahara K, Vahedi G, Ghoreschi K, Yang XP, Nakayamada S, Kanno Y, O'Shea JJ, Laurence A. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology 2011; 134:235-45; PMID:21977994; http://dx.doi.org/ 10.1111/j.1365-2567.2011.03483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamat AM, Briggman J, Urbauer DL, Svatek R, Nogueras Gonzalez GM, Anderson R, Grossman HB, Prat F, Dinney CP. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): Nomogram of Changes in Urinary Cytokine Levels Predicts Patient Response to Bacillus Calmette-Guerin. Eur Urol 2016; 69:197-200; PMID:26119560; http://dx.doi.org/ 10.1016/j.eururo.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton MR, Askeland EJ, Andresen ED, Chehval VA, Wang X, Askeland RW, O'Donnell MA, Luo Y. Anti-interleukin-10R1 monoclonal antibody in combination with bacillus Calmette–Guerin is protective against bladder cancer metastasis in a murine orthotopic tumour model and demonstrates systemic specific anti-tumour immunity. Clin Exp Immunol 2014; 177:261-8; PMID:24593764; http://dx.doi.org/ 10.1111/cei.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou Z, Wang Y, Liu L, Li L, Yeh S, Qi L, Chang C. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget 2015; 6:26065-78; PMID:26305549; http://dx.doi.org/ 10.18632/oncotarget.4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods 2003; 275:251-5; PMID:12667688; http://dx.doi.org/ 10.1016/S0022-1759(03)00010-3 [DOI] [PubMed] [Google Scholar]
- 15.de Boer EC, Somogyi L, de Ruiter GJ, de Reijke TM, Kurth KH, Schamhart DH. Role of interleukin-8 in onset of the immune response in intravesical BCG therapy for superficial bladder cancer. Urol Res 1997; 25:31-4; PMID:9079743; http://dx.doi.org/ 10.1007/BF00941903 [DOI] [PubMed] [Google Scholar]
- 16.Rabinowitz R, Smith DS, Tiemann DD, Hudson MA. Urinary interleukin-8/creatinine level as a predictor of response to intravesical bacillus Calmette-Guerin therapy in bladder tumor patients. J Urol 1997; 158:1728-31; discussion 31-2; PMID:9334588; http://dx.doi.org/9302115 10.1016/S0022-5347(01)64111-9 [DOI] [PubMed] [Google Scholar]
- 17.Thalmann GN, Dewald B, Baggiolini M, Studer UE. Interleukin-8 expression in the urine after bacillus Calmette-Guerin therapy: a potential prognostic factor of tumor recurrence and progression. J Urol 1997; 158:1340-4; PMID:9302115; http://dx.doi.org/ 10.1016/S0022-5347(01)64210-1 [DOI] [PubMed] [Google Scholar]
- 18.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol 2000; 164:2129-33; PMID:11061941; http://dx.doi.org/ 10.1016/S0022-5347(05)66983-2 [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Dubey D, Bansal P, Mandhani A, Naik S. Urinary interleukin-8 predicts the response of standard and low dose intravesical bacillus Calmette-Guerin (modified Danish 1331 strain) for superficial bladder cancer. J Urol 2002; 168:2232-5; PMID:12394765; http://dx.doi.org/ 10.1016/S0022-5347(05)64361-3 [DOI] [PubMed] [Google Scholar]
- 20.Kocak H, Oner-Iyidogan Y, Kocak T, Oner P. Determination of diagnostic and prognostic values of urinary interleukin-8, tumor necrosis factor-alpha, and leukocyte arylsulfatase-A activity in patients with bladder cancer. Clin Biochem 2004; 37:673-8; PMID:15302609; http://dx.doi.org/ 10.1016/j.clinbiochem.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 21.Sagnak L, Ersoy H, Ozok U, Senturk B, Ercil H, Bahar G, Ozturk E. Predictive value of urinary interleukin-8 cutoff point for recurrences after transurethral resection plus induction bacillus Calmette-Guerin treatment in non-muscle-invasive bladder tumors. Clin Genitourin Cancer 2009; 7:E16-23; PMID:19692317; http://dx.doi.org/ 10.3816/CGC.2009.n.016 [DOI] [PubMed] [Google Scholar]
- 22.Loskog A, Ninalga C, Paul-Wetterberg G, de la Torre M, Malmstrom PU, Totterman TH. Human bladder carcinoma is dominated by T-regulatory cells and Th1 inhibitory cytokines. J Urol 2007; 177:353-8; PMID:17162090; http://dx.doi.org/ 10.1016/j.juro.2006.08.078 [DOI] [PubMed] [Google Scholar]
- 23.Sheryka E, Wheeler MA, Hausladen DA, Weiss RM. Urinary interleukin-8 levels are elevated in subjects with transitional cell carcinoma. Urology 2003; 62:162-6; PMID:12837459; http://dx.doi.org/ 10.1016/S0090-4295(03)00134-1 [DOI] [PubMed] [Google Scholar]
- 24.Reis ST, Leite KR, Piovesan LF, Pontes-Junior J, Viana NI, Abe DK, Crippa A, Moura CM, Adonias SP, Srougi M et al.. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urol 2012; 12:18; PMID:22695075; http://dx.doi.org/ 10.1186/1471-2490-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest 1995; 95:586-92; PMID:7860742; http://dx.doi.org/ 10.1172/JCI117702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassatella MA, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. IL-8 production by human polymorphonuclear leukocytes. The chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol 1992; 148:3216-20; PMID:15781469817975 [PubMed] [Google Scholar]
- 27.Elsasser-Beile U, Kolble N, Grussenmeyer T, Schultze-Seemann W, Wetterauer U, Gallati H, Schulte Mönting J, von Kleist S. Th1 and Th2 cytokine response patterns in leukocyte cultures of patients with urinary bladder, renal cell and prostate carcinomas. Tumour Biol 1998; 19:470-6; PMID:9817975; http://dx.doi.org/ 10.1159/000030039 [DOI] [PubMed] [Google Scholar]
- 28.Kaempfer R, Gerez L, Farbstein H, Madar L, Hirschman O, Nussinovich R, Shapiro A. Prediction of response to treatment in superficial bladder carcinoma through pattern of interleukin-2 gene expression. J Clin Oncol 1996; 14:1778-86; PMID:8656246; http://dx.doi.org/ 10.1200/jco.1996.14.6.1778 [DOI] [PubMed] [Google Scholar]
- 29.Gusella GL, Musso T, Bosco MC, Espinoza-Delgado I, Matsushima K, Varesio L. IL-2 up-regulates but IFN-gamma suppresses IL-8 expression in human monocytes. J Immunol 1993; 151:2725-32; PMID:836048725618112 [PubMed] [Google Scholar]
- 30.Janaszek-Seydlitz W, Prygiel M, Bucholc B, Wiatrzyk A, Czajka U, Gorska P, Soliwoda U. Effect of different bacillus Calmette-Guerin substrains on growth inhibition of T24 bladder cancer cells and cytokines secretion by BCG activated peripheral blood mononuclear cells of PBMCs. Adv Clin Exp Med 2014; 23:877-84; PMID:25618112; http://dx.doi.org/ 10.17219/acem/37330 [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Dowell AC, Patel P, Viney RP, Foster MC, Porfiri E, James ND, Bryan RT. Cytokines as effectors and predictors of responses in the treatment of bladder cancer by bacillus Calmette-Guerin. Future Oncol 2014; 10:1443-56; PMID:25052754; http://dx.doi.org/ 10.2217/fon.14.79 [DOI] [PubMed] [Google Scholar]
- 32.Urquidi V, Chang M, Dai Y, Kim J, Wolfson ED, Goodison S, Rosser CJ. IL-8 as a urinary biomarker for the detection of bladder cancer. BMC urology 2012; 12:12; PMID:22559832; http://dx.doi.org/ 10.1186/1471-2490-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci 2005; 10:853-65; PMID:15569594; http://dx.doi.org/ 10.2741/1579 [DOI] [PubMed] [Google Scholar]
- 34.Ning Y, Lenz HJ. Targeting IL-8 in colorectal cancer. Expert Opin Ther Targets 2012; 16:491-7; PMID:22494524; http://dx.doi.org/ 10.1517/14728222.2012.677440 [DOI] [PubMed] [Google Scholar]
- 35.Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res 2013; 15:210; PMID:24041156; http://dx.doi.org/ 10.1186/bcr3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mian BM, Dinney CP, Bermejo CE, Sweeney P, Tellez C, Yang XD, Gudas JM, McConkey DJ, Bar-Eli M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res 2003; 9:3167-75; PMID:12912969 [PubMed] [Google Scholar]
- 37.Huang RP. An array of possibilities in cancer research using cytokine antibody arrays. Expert Rev Proteomics 2007; 4:299-308; PMID:17425464; http://dx.doi.org/ 10.1586/14789450.4.2.299 [DOI] [PubMed] [Google Scholar]


