ABSTRACT
The use of high-dose interleukin-2 (IL-2) has fallen out of favor due to severe life-threatening side effects. We have recently described a unique way of directly targeting IL-2 to cytotoxic lymphocytes using a virally encoded immune evasion protein and an IL-2 mutant that avoids off-target side effects such as activation of regulatory T cells and vascular endothelium.
KEYWORDS: Cytokine, cytotoxic lymphocytes, immunotargeting, immunotherapy, Interleukin-2 (IL-2), natural killer cell (NK), NKG2D
Systemic high-dose IL-2 as a curative form of immunotherapy
High-dose interleukin-2 (IL-2) cytokine therapy was first approved by the FDA for treatment of advanced renal cell carcinoma (RCC) in 1992 and for advanced melanoma in 1998. High-dose IL-2 activates and induces proliferation of natural killer (NK) cells and CD8+ cytotoxic T cells, leading to potent anticancer activity, which results in a durable complete remission in up to 9% of RCC patients with a median durability of 70 mo. Many patients experience remission lasting more than 10 y.1 This rate is in contrast to the <1% complete remission rate of the anti-PD1 therapy nivolumab, which received Food and Drug Administration (FDA) fast-track approval. Despite highly promising results, high-dose IL-2 therapy is rarely used today because many patients experience severe adverse effects, such as fever, malaise, and systemic capillary leak. As a result, close to 50% of patients discontinue therapy, and a further 2–5% of patients will die as a result of therapy-induced pulmonary edema and cardiovascular instability.
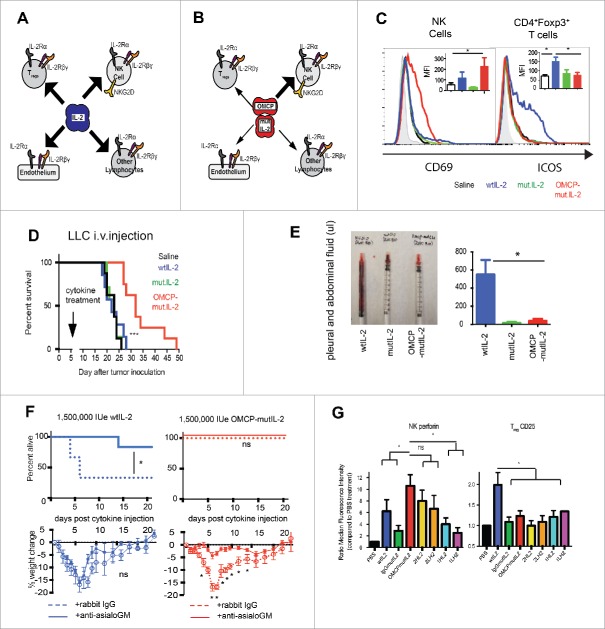
These side effects are the result of off-target signaling via the IL-2 receptor (IL-2R). Cytotoxic lymphocytes (CTLs) express the low-affinity IL-2Rβγ form of the receptor at baseline but after initial activation display the high-affinity IL-2Rαβγ receptor that is ultimately required for peak effector function. In contrast, vascular endothelium expresses the high-affinity IL-2Rαβγ receptor at baseline and thus high-dose IL-2 results in inflammation, vascular leakage, and the associated adverse events due to direct vascular toxicity. Furthermore, CD4+ Foxp3+ Tregs also express the high-affinity IL-2Rαβγ receptor at baseline and are preferentially activated by IL-2. Tregs normally function to downregulate immune responses, and in the context of cancer immunotherapy limit the therapeutic potential of the CTLs (Fig. 1A).
Figure 1.
Targeted delivery of IL-2. (A) Proposed model of competition between NK cells and stromal cells for IL-2. (B) NKG2D targeted OMCP-mutIL-2 preferentially delivers cytokine to NK cells. Width of arrow indicates proposed strength of IL-2 signaling. (C) In vitro flow cytometrically evaluated activation of A/J spleen-derived lymphocyte subsets after 36 h of culture in 100 IUe/mL of cytokines or OMCP-mutIL-2 construct. Graph demonstrating one representative histogram plot and median fluorescent intensity (MFI) ± S.E.M. across 5–7 separate experiments. (D) Survival of C57BL/6 mice treated with 750,000 IUe of cytokine 5 d after injection of 1×105 LLC intravenously (E) ascites (representative syringe—left; average from all mice in the group—right) in A/J mice treated with 200,000 IUe of either wild-type IL-2 (blue) or OMCP-mutIL-2 (red). (F) Animal mortality and morbidity, as assessed by survival (top) and weight loss (bottom) of A/J mice treated with supratherapeutic doses of wild-type IL-2 or OMCP-mutIL-2. NK cells were either depleted with anti-asialo GM (solid line) or not (dashed line). (G) In order to compare antibody-mediated delivery of mutIL-2 we engineered four anti-human NKG2D single chain variable fragment domains based on the described sequence of the KYK1 and KYK2 antibodies.11 1HL2 and 1LH2 are derived from the KYK1 antibody and 2HL2 and 2LH2 from the KYK2 antibody. The binding coefficients of OMCP, KYK1, and KYK2 are 0.1 nM, 27 nM, and 6 nM, respectively. Similar to what is described for in vitro human studies NK activation was measured by expression of intracellular perforin, whereas Treg activation by surface CD25 levels, both expressed as relative ration to saline control. After 48 h in 100 U/mL NK cells treated with 2HL2 and 2LH2 antibodies synthesized as much perforin as OMCP-mutIL-2 treated cells but lower levels of perforin were evident in 1HL2 and 1LH2 treated NK cells. Higher levels of CD25 were evident in wild-type IL-2 treated cultures over all constructs, *p <0.05; ***p <0.001; ns = p > 0.05. Data from Figure A-F modified from Ghasemi et. al., Nature Communications 2016; 7:12878; http://dx.doi.org/10.1038/ ncomms12878.
Alternative forms of IL-2 and IL-2 delivery
Previous investigators have hypothesized that a form of IL-2 that prevents Treg activation could greatly enhance therapeutic efficacy.2 There are multiple described mutations that decrease IL-2 affinity for IL-2Rα, such as the substitution of alanine for arginine (R38A) and lysine for phenylalanine (F42K).3 Alternately, antibody-bound forms of IL-2 have been developed to reduce IL-2Rα binding.4 Unfortunately, IL-2Rα-inhibited therapies have reduced NK-cell activation and a correspondingly reduced therapeutic index due to the requirement of the high-affinity receptor for peak cytotoxicity. Another strategy has been to create mutants with increased affinity for IL-2Rβ in an effort to remove the preference for IL-2Rα.5 The IL-2Rβ-enhanced IL-2 mutants still bind and activate Tregs and vascular endothelium and thus still have “off target” side effects. Several groups have attempted to direct cytokine delivery to the tumor bed or vasculature via a targeted antibody-IL-2 conjugate but these therapies may be limited due to the ability of the tumor to escape by shedding, mutating, or downregulating the targeted antigen. Furthermore, the tumor-associated microenvironment actively excludes lymphocytes, thus preventing CTLs from encountering IL-2. Others have extended the half-life of IL-2 by conjugating it with anti-IL-2 antibodies or stabilizing the cytokine in the blood stream through the addition of polyethyline glycol.6 None of these approaches, however, significantly ameliorates Treg activation with unproven results in human trials.7
Unique method for IL-2 delivery to cytotoxic lymphocytes as a superior form of immunotherapy
We have recently described a new form of IL-2 immunotherapy through targeted delivery of an IL-2 mutant directly to CTLs. We utilized a high-affinity, virally encoded NKG2D ligand known as orthopoxvirus major histocompatibility complex class I like protein or OMCP8 (Fig. 1B). Since NKG2D is expressed constitutively on murine NK cells as well as activated murine CD8+ T cells, the new OMCP-(R38A/F42K)IL-2 protein resulted in preferential binding to and activation of NK cells over Tregs (Fig. 1B and C), improved immunotherapy against both solid and hematopoietic tumors (Fig. 1D), and no systemic capillary leak or animal mortality (Fig. 1E and F). Limited animal morbidity, manifest as weight loss, was completely dependent on NK activation (Fig. 1F). OMCP offers the highest known NKG2D binding affinity of any known ligand9,10 and has broad applications in both human and veterinary immuno-oncology due to potential reactivity with multiple species. Nevertheless, the use of anti-NKG2D antibodies to target IL-2 to NK cells is also a possibility (Fig. 1G). Taken together our work demonstrates the utility of targeted cytokine delivery for the treatment of cancer immunotherapy and possibly other forms of immune suppression.
Disclosures of potential conflict of interest
E.L. and A.S.K. have filed a patent on data described in this manuscript with technology licensed to Courier Therapeutics (http://www.couriertherapeutics.com). Targeted Cytokine Delivery to Cytotoxic Lymphocytes—Inventors: Alexander S. Krupnick, Daved H. Fremont, Eric Lazear, U.S. Provisional Application number 62/091,898, filed December 15, 2014. EL, ASK, DW, and JW are major shareholders and SH is an employee of Courier Therapeutics.
Funding
This work is supported by the Biostatistics Shared Resource and Siteman Flow Cytometry Core (P30CA091842), NIH/NIAID R01 AI019687, U19AI109948, NIAID contracts HHSN272200700058C & HHSN272201200026C, as well as the Rheumatic Diseases Core Center (P30 AR48335). ASK is supported by NIH R01HL094601, PO1 AI116501, the Barnes Jewish Hospital Research Foundation, the Doug and Ann Brown Foundation, Daniel, Jordan and Paula Bergstein, and Courier Therapeutics.
References
- 1.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg 1998; 228:307-19; PMID:9742914; http://dx.doi.org/25271022 10.1097/00000658-199809000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+CD25hi Foxp3+ regulatory T cells in cancer patients. Blood 2006; 107:2409-14; PMID:16304057; http://dx.doi.org/25271022 10.1182/blood-2005-06-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton KM, Ju G, Grimm EA. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res 1993; 53:2597-602; PMID:849542225271022 [PubMed] [Google Scholar]
- 4.Rosalia RA, Arenas-Ramirez N, Bouchaud G, Raeber ME, Boyman O. Use of enhanced interleukin-2 formulations for improved immunotherapy against cancer. Curr Opin Chem Biol 2014; 23:39-46; PMID:25271022; http://dx.doi.org/ 10.1016/j.cbpa.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, Moraga I, Raeber ME, Bowman GR, Novick P et al.. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 2012; 484:529-33; PMID:22446627; http://dx.doi.org/ 10.1038/nature10975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006; 311:1924-7; PMID:16484453; http://dx.doi.org/ 10.1126/science.1122927 [DOI] [PubMed] [Google Scholar]
- 7.Yang JC, Topalian SL, Schwartzentruber DJ, Parkinson DR, Marincola FM, Weber JS, Seipp CA, White DE, Rosenberg SA. The use of polyethylene glycol-modified interleukin-2 (PEG-IL-2) in the treatment of patients with metastatic renal cell carcinoma and melanoma. A phase I study and a randomized prospective study comparing IL-2 alone versus IL-2 combined with PEG-IL-2. Cancer 1995; 76:687-94; PMID:8625167; http://dx.doi.org/ 10.1002/1097-0142(19950815)76:4%3c687::AID-CNCR2820760424%3e3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- 8.Ghasemi R, Lazear E, Wang X, Arefanian S, Zheleznyak A, Carreno BM, Higashikubo R, Gelman AE, Kreisel D, Fremont DH et al.. Selective targeting of IL-2 to NKG2D bearing cells for improved immunotherapy. Nat Commun 2016; 7:12878; PMID:27650575; http://dx.doi.org/17548517 10.1038/ncomms12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JA, Trossman DS, Yokoyama WM, Carayannopoulos LN. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J Exp Med 2007; 204:1311-7; PMID:17548517; http://dx.doi.org/ 10.1084/jem.20062026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazear E, Peterson LW, Nelson CA, Fremont DH. Crystal structure of the cowpox virus-encoded NKG2D ligand OMCP. J Virol. 2013 Jan; 87(2):840-50; PMID:23115291; http://dx.doi.org/18809410 10.1128/JVI.01948-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong KY, Baskar S, Zhang H, Mackall CL, Rader C. Generation, affinity maturation, and characterization of a human anti-human NKG2D monoclonal antibody with dual antagonistic and agonistic activity. J Mol Biol 2008; 384:1143-56; PMID:18809410; http://dx.doi.org/ 10.1016/j.jmb.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]