Abstract
Early development of the lens and retina depends upon reciprocal inductive interactions between the embryonic surface ectoderm and the underlying neuroepithelium of the optic vesicle. FGF signaling has been implicated in this signal exchange. The docking protein FRS2α is a major mediator of FGF signaling by providing a link between FGF receptors (FGFRs) and a variety of intracellular signaling pathways. After FGF stimulation, tyrosine-phosphorylated FRS2α recruits four molecules of the adaptor protein Grb2 and two molecules of the protein tyrosine phosphatase Shp2, resulting in activation of the Ras/extracellular signal-regulated kinase (ERK) and phosphatidylinositol-3 kinase/Akt signaling pathways. In this report, we explore the role of signaling pathways downstream of FRS2α in eye development by analyzing the phenotypes of mice that carry point mutations in either the Grb2-(Frs2α4F) or the Shp2-binding sites (Frs2α2F) of FRS2α. Although Frs2α4F/4F mice exhibited normal early eye development, all Frs2α2F/2F embryos were defective in eye development and showed anophthalmia or microphthalmia. Consistent with the critical role of FRS2α in FGF signaling, the level of activated extracellular signal-regulated kinase in Frs2α2F/2F embryos was significantly lower than that observed in wild-type embryos. Furthermore, expression of Pax6 and Six3, molecular markers for lens induction, were decreased in the Frs2α2F/2F presumptive lens ectoderm. Similarly, the expression of Chx10 and Bmp4, genes required for retinal precursor proliferation and for lens development, respectively, was also decreased in the optic vesicles of Frs2α2F/2F mice. These experiments demonstrate that intracellular signals that depend on specific tyrosine residues in FRS2α lie upstream of gene products critical for induction of lens and retina.
Keywords: FGF receptor, mouse eye development, signal transduction
In vertebrates, development of the ocular lens begins with a reciprocal inductive interaction between the presumptive lens region of the embryonic head surface ectoderm and the underlying presumptive retina of the optic vesicle. Inductive signaling initiates a series of events that include lens pit invagination, formation of the lens vesicle, and differentiation of the lens fiber cells that confer the properties of transparency and refractility on the mature lens. FGF signaling pathways have been implicated in the development of the lens and neural retina (1, 2).
Cellular responses to FGFs are mediated by four receptor tyrosine kinases, designated FGF receptor (FGFR)1–FGFR4 (3, 4). FGFRs are activated by binding of FGF and heparin to the extracellular ligand-binding domain, thus stimulating FGFR dimerization and transautophosphorylation (5, 6). The activated FGFR phosphorylates a variety of signaling proteins (i.e., Shc, phospholipase Cγ, and STAT1), including the docking protein FRS2α and its close homologue, FRS2β, resulting in a coordinated assembly of a signaling complex that transmits multiple signaling pathways (3, 7–13). Disruption of the Frs2α gene results in early embryonic lethality due to multiple defects in FGF signaling, indicating a critical role of FRS2α in early embryogenesis (ref. 10 and unpublished data). Using mouse embryonic fibroblasts (MEFs) isolated from Frs2α–/– mice, we have demonstrated that FRS2α functions as a focus for assembly of a multiprotein complex that controls the Ras/extracellular signal-regulated kinase (ERK) signaling cascade and the phosphatidylinositol (PI)-3 kinase/Akt antiapoptic signaling pathway (10). The two members of the FRS2 family, FRS2α and FRS2β (9, 13), contain myristyl anchors and phosphotyrosine-binding domains at their N termini followed by a long region containing multiple tyrosine phosphorylation sites. We have shown that four tyrosine residues on FRS2α serve as binding sites for the adaptor protein Grb2, and two tyrosines serve as a binding site for the protein tyrosine phosphatase Shp2 (7–9). Complex formation between tyrosine-phosphorylated FRS2α and Shp2 leads to tyrosine phosphorylation of Shp2 by FGFR, resulting in recruitment of an additional Grb2 molecule by the tyrosine-phosphorylated protein tyrosine phosphatase. Experiments with transfected Frs2α–/– MEFs expressing tyrosine phosphorylation site mutants of FRS2α have shown that Shp2-binding sites play a primary role in FGF stimulation of the Ras/ERK signaling cascade, whereas Grb2-binding sites on FRS2α are primarily responsible for recruitment of Gab1, phosphatidylinositol (PI-3) kinase stimulation, and recruitment of the ubiquitin ligase Cbl (8, 10, 12).
When the FGF-dependent signaling pathway is inhibited within the cells of the lens placode, lens morphogenesis and development are defective, and expression of the lens induction marker Pax6 is diminished (14). Because loss- and gain-of-function analyses indicate that Pax6 is critical for lens development (15–19), regulation of Pax6 by FGFR activity suggests this pathway has a central role in lens induction (2). Available data also suggest that the FGF-dependent signaling pathway has an important role in retinal development. When the lens placode is surgically removed, development of the retina ceases unless recombinant FGF ligands are provided (20). In this setting, the transcription factor Mitf appears to be a critical downstream mediator (21). Combined, these observations raise the possibility that the FGF pathway is involved in both directions of the reciprocal inductive exchange that initiates development of lens and retina. Furthermore, it was proposed that FGF signaling cooperates with the activity of bone-morphogenetic proteins (Bmps) in regulating early eye development (2).
Here, we show that an FRS2α mutant mouse in which the two tyrosine residues serving as the Shp2-binding sites are mutated (Frs2α2F) has a variably penetrant defect in early eye development that can result in anything from microphthalmia to anophthalmia. In severe cases, expression of Pax6 and Six3 in the lens placode and of Chx10 in the presumptive retina is greatly diminished or absent, indicating a failure of inductive signaling in both tissues. This conclusion is reinforced by the observation that severely affected embryos do not develop eyes. These data contrast with the absence of early eye development defects in mice in which the four Grb2-binding sites of FRS2α were mutated (Frs2α4F). These results show that FGFR-dependent signaling pathways that lie downstream of phosphotyrosine residues that function as Shp2-binding sites are critical for inductive signaling. Finally, we show that in the Frs2α2F/2F mutant, expression of Bmp4 is all but lost from the optic primordium, suggesting that FGF signaling is upstream of Bmp4 function in a pathway regulating eye development.
Materials and Methods
Gene Targeting. The 129sv/EV mouse genomic fragments encoding the Grb2- or Shp2-binding sites were replaced with the mutated cDNA fragments (7, 8). Standard techniques were used for gene targeting. The floxed neo cassette was deleted by crossing with transgenic mice (22) that express cre recombinase in the germline. PCR genotyping was performed by using the primers: 5′-AGAATGGTGGCACAAACCAATAATCC-3′ and 5′-CAATTCTTAACACCCACAAGGCCG-3′ (for details, see Supporting Text, which is published as supporting information on the PNAS web site).
Immunoprecipitation and Immunoblot Analysis. The embryonic day 14.5 embryos (E14.5) were homogenized and solubilized in lysis buffer, and the lysates were subjected to immunoprecipitation and immunoblotting with indicated antibodies as described (13).
In Situ Hybridization. The Frs2α, Frs2β, Six3, Rx, and Bmp4 RNA probes are as described (13, 25, 32, 33). Whole-mount and section RNA in situ experiments were performed as described (13).
Immunofluorescence Experiments and Paraffin Histology. The following antibodies have been used for immunostaining of frozen sections: rabbit polyclonal anti-Pax6 antibodies (1:2,000) (Covance, Princeton, NJ; PRB-228P): rabbit polyclonal anti-Chx10 antibodies (1:750) (Exalpha, Maynard, MA; X1180P), anti-ERK2 antibodies (1:300) (Cell Signaling Technology, Beverly, MA), and Alexa Fluor secondary antibodies (Molecular Probes). Immunostaining with anti-pERK antibodies (antibodies that recognize specifically the activated form of ERK2) (Cell Signaling Technology) was performed by using the TSA biotin system following the manufacturer's protocol (Perkin–Elmer).
Paraffin sections were prepared for hematoxylin/eosin staining by using standard methods (23).
Treatment with FGFR Tyrosine Kinase Inhibitor SU5402. Embryos were treated with 40 μM SU5402 (Calbiochem) in 2 ml of RPMI medium 1640 with 0.1% DMSO and 1% BSA, preequilibrated at 37°C/5% CO2 for 1 h before fixation, as described (24). Control embryos were incubated in 2 ml of RPMI medium 1640 with 0.1% DMSO and 1% BSA and preequilibrated at 37°C, 5% CO2 for 1 h.
Results
Biochemical analyses have shown that FRS2α represents a bifurcation point for activation of the PI-3 kinase and ERK signaling pathways in response to FGF stimulation (10). To understand how these pathways might contribute to the biological effects of FGFs, we generated mutant mice expressing forms of FRS2α that lacked either the Shp2-binding sites (Y436 and Y471, the 2F mutant) or the Grb2-binding sites (Y196, Y306, Y349, and Y392, the 4F mutant) by gene targeting (see Fig. 6, which is published as supporting information on the PNAS web site).
Frs2α2F/2F Mutant Mice Have an Embryonic Lethal Phenotype. Initial analysis indicated that very few Frs2α2F/2F embryos of 129sv background reached advanced stages of development. To minimize this problem, the Frs2α+/2F and Frs2α+/4F mice were backcrossed onto the outbred Swiss–Webster strain (Taconic Farms), and agouti heterozygotes were selected for breeding. We analyzed them in the sixth to ninth generations. Although the Swiss–Webster strain from Taconic Farms carries the mutant allele Pdebrd1 that leads to retinal degeneration postnatally, it is not likely to interfere with the developmental stages analyzed. No gross abnormality was detected in heterozygous mice. Screening of >150 offspring older than 3 weeks and 56 newborn (postnatal day 0) pups derived from Frs2α+/2F intercrosses failed to identify viable homozygotes (see Table 1, which is published as supporting information on the PNAS web site). Furthermore, at E9.5 and E17.5, the frequency of viable Frs2α2F/2F embryos was subMendelian at 16.9% (12/71) and 9.1% (5/55), respectively. This suggested that Frs2α2F/2F embryos had a recessive lethal phenotype, and that they were lost over a range of embryonic stages. Additional investigation of these mice has shown that Frs2α2F/2F embryos exhibit a variety of developmental defects, such as branchial arch, limb, and heart defects (N.G., I.L., and J.S., unpublished results), some of which are likely to be lethal. The details of these defects will be described elsewhere. In contrast to Frs2α2F/2F mice, Frs2α4F/4F mice could survive as adults albeit at the reduced frequency of 8.8% (7/80) from heterozygous intercrosses. The frequency of Frs2α4F/4F embryos surviving at E17.5 was Mendelian at 27.1% (13/48). Some newborn Frs2α4F/4F pups did not suckle and died by postnatal day 1 (21/110, 19.1%), but those that did survive were healthy and fertile. There were no obvious morphological defects except an eyelid development defect that arose with low penetrance.
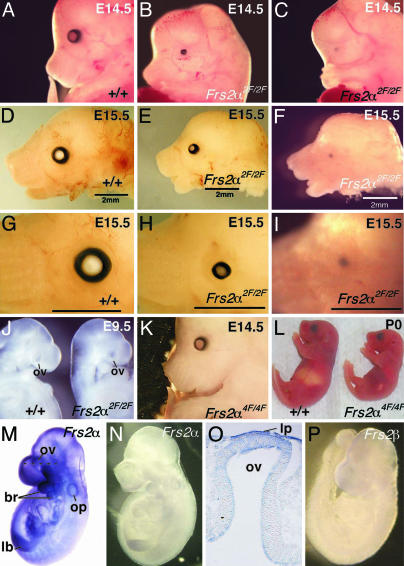
Frs2α2F/2F Mutant Mice Exhibit a Variably Penetrant Eye Defect. Surveying the gross morphology of mutant embryos revealed that all Frs2α2F/2F embryos had severe defects in eye development. Approximately 70% of the Frs2α2F/2F embryos had bilateral microphthalmia, and of the rest, ≈30% had bilateral anophthalmia or microphthalmia on one side and anophthalmia on the other (Fig. 1A). The severity of microphthalmia was variable (compare Fig. 1 B and E). Frs2α2F/2F embryos clearly had normal evagination of the optic vesicle (the future neural retina and retinal pigmented epithelium) from the diencephalon at E9.5 (Fig. 1J). This phenotype is very similar to that of Pax6Sey/Sey embryos and contrasts with that of the Rx (25) and Lhx2 (26) mutants, where an optic vesicle is never formed. The Frs2α4F/4F embryos, on the other hand, showed no abnormalities in the major structures of the eye at E14.5 (Fig. 1K) or the day of birth (Fig. 1L), with the exception of the aforementioned eyelid development defect.
Fig. 1.
Frs2α2F/2F mice have a variably penetrant eye phenotype. (A–I) Whole-mount embryos that are wild type (A, D, and G) or Frs2α2F/2F homozygotes where eye development is either mildly (B, E, and H) or severely (C, F, and I) affected. (A–C) E14.5. (D–I) E15.5. At higher magnification (G–I), microphthalmia (H) or anophthalmia (I) is clearly apparent. (J) E9.5 wild-type (Left) and Frs2α2F/2F embryos showing formation of the optic vesicle. (K and L) Frs2α4F4F mutant mice have no microphthalmia or anophthalmia apparent at E14.5 (K) and are smaller than wild type at the day of birth (L). In situ hybridizations using Frs2α antisense (M) and control sense (N) probes on wild-type E9.5 embryos. (O) Cryosection of the eye region of the embryo shown in (M). (P) In situ hybridization on E9.5 embryo using Frs2β antisense probe. ov, optic vesicle; br, branchial arch; op, otic pit; lb, limb bud; and lp, lens placode.
We examined the expression patterns of the two members of the FRS2 family Frs2α and Frs2β during the early phase of eye development using in situ hybridization analyses. We previously reported (13) that Frs2α is expressed ubiquitously as early as E8.5. Indeed, at E9.5 also, Frs2α is expressed ubiquitously (Fig. 1M), mainly in the forebrain, eye primordia, first and second branchial arches, otic vesicle, and limb bud (Fig. 1M). A horizontal section of a labeled embryo at the level of the eye primordium showed high Frs2α expression in the surface ectoderm, including the presumptive lens and the distal part of the optic vesicle that is the presumptive retina (Fig. 1O). By contrast, the expression of Frs2β is very low in eye tissues (Fig. 1P). The sense probes of Frs2α showed no staining, confirming the specificity of the in situ hybridization analyses (Fig. 1N).
The early stages of eye development are well defined morphologically. After the optic vesicle makes contact, the overlying surface ectoderm thickens to form the lens placode. The lens placode first invaginates to form the lens pit and then separates from the surface ectoderm to form the lens vesicle. Coordinately, the distal optic vesicle invaginates to form the optic cup, with the inner layer, the presumptive retina, developing into the neuroretina and the outer layer forming the retinal pigment epithelium.
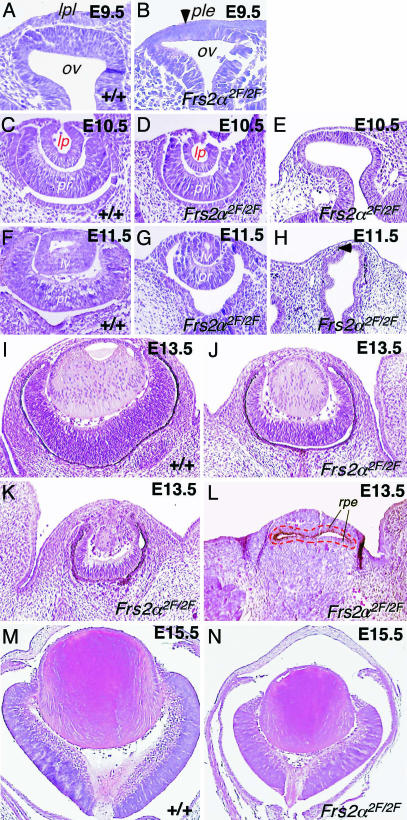
Histological analysis (Fig. 2) revealed that the Frs2α2F/2F embryos had a variably penetrant defect in these early stages of eye development. At E9.5, morphological defects in the Frs2α2F/2F eye are subtle, but the placodal thickening in the wild-type presumptive lens region (Fig. 2 A) is absent from mutant embryos (Fig. 2B, arrowhead). At E10.5, mildly affected mutant eyes were slightly smaller than those of wild type (Fig. 2 C and D). In severely affected mutants, eye development had not advanced beyond E9.5, and the optic vesicle and presumptive lens ectoderm remained (Fig. 2E). At E11.5, the mildly affected mutant optic cup was smaller than that of wild type (Fig. 2 F and G), whereas in severely affected eyes, the optic vesicle was distorted and became separated from the surface ectoderm by the intervening mesenchyme (Fig. 2H, arrowhead). In mildly affected mutant eyes at E13.5, differentiation of lens fiber cells had occurred, but eyes were smaller than those of wild types or heterozygotes (Fig. 2 I and J). More severely affected eyes were in the process of degeneration at E13.5 (Fig. 2K). Those that were most severely affected had no morphological indication of lens or retina development and, according to pigmentation, showed a transformation of presumptive retina into retinal pigment epithelium (Fig. 2L). The major feature of the E15.5 Frs2α2F/2F eye was reduced in size, and this was apparent in both the lens and the retina (Fig. 2 M and N).
Fig. 2.
Frs2α2F/2F mice exhibit defective early and late eye development. Hematoxylin/eosin-stained sections through the eye region of embryos of the indicated ages and genotypes. lpl, lens placode; ple, presumptive lens ectoderm; ov, optic vesicle; lp, lens pit; pr, presumptive retina; lv, lens vesicle; rpe, retinal pigment epithelium. In L the dashed red line indicates the boundary of pigment epithelial tissue.
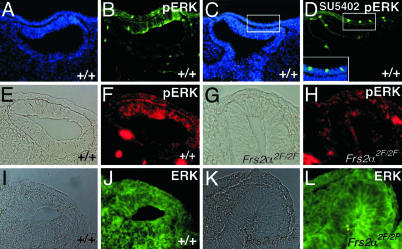
Reduced ERK Activation in Primordial Eye of Frs2α2F/2F Mice. Because Shp2 recruitment has been shown to be critical for FGF stimulation of ERK (8, 10, 27, 28), we have explored ERK activation in the eye primordium during the interaction between the presumptive lens and retina at E9.5 by staining eye tissue with antibodies that recognize specifically the activated form of ERK2. As might be expected given previous demonstrations of a requirement for FGFR activity in lens (29) and retina induction (21), activated ERK2 could be detected in the lens placode and the distal part of the optic vesicle (Fig. 3 A and B). Importantly, labeling for activated ERK2 was greatly diminished when embryos were treated with FGFR inhibitor SU5402 (Fig. 3 C and D) (24). Thus, ERK activation in the lens placode and distal optic vesicle depends upon the tyrosine kinase activity of FGFR, as might be expected given previous analysis (24). Interestingly, in the Frs2α2F/2F mutant eye primordia, activation of ERK2 was very low in both the optic vesicle and overlying surface ectoderm (compare Fig. 3 E and F with G and H). This was true for all four mutant embryos assessed and indicated that, as might be expected, ERK2 activation depends on the integrity of FRS2α. The amount of ERK2 in these tissues was similar regardless of embryo genotype, as revealed by staining with anti-ERK2 antibodies (Fig. 3 J and L).
Fig. 3.
ERK activation is diminished in the eye region of Frs2α2F/2F mice. Cryosections from E9.5 embryos of the indicated genotypes. A and B, C and D, E and F, G and H, I and J, and K and L are the same sections. E, G, I, and K are bright-field images. Immunofluorescence labeling with antibodies that recognize specifically the activated form of ERK2 (pERK) (B, D, F, and H) and ERK (J and L). (A and C) Nuclei are labeled blue with Hoechst 33258. (C and D) The embryo was treated for 1 hr with the FGFR kinase inhibitor SU5402. (D Inset) A magnified and merged image of the two images indicated in squares in (C and D) showed nuclear localization of pERK in a few cells. Secondary antibodies were green for B and D and red for F and H.
Impaired Expression of Pax6, Chx10, Six3, and Bmp4 in Frs2α2F/2F Mice. To understand the developmental pathways in which FRS2α functions, we next examined the expression of several genes that are important in early eye development and thus serve as useful markers of inductive signaling.
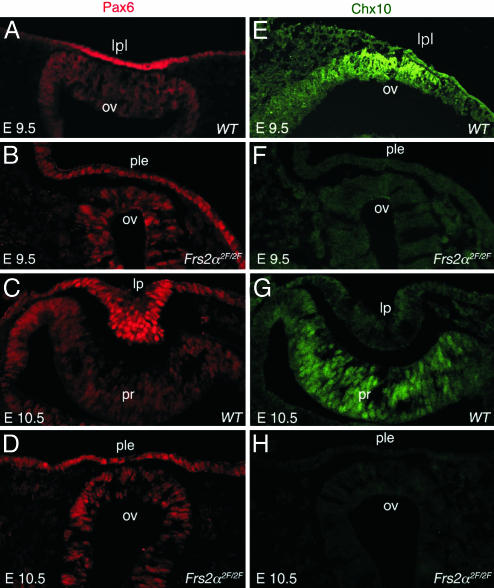
The transcription factor Pax6 is critical for eye development and is known to have an essential autonomous function in lens development (15, 18). An intense region of Pax6 immunoreactivity can be detected in the wild-type lens placode (Fig. 4A) and is a response to lens induction signals (29). In Frs2α2F/2F embryos at E9.5 (Fig. 4B), Pax6 is not up-regulated in the presumptive lens ectoderm. Similarly, at E10.5, the wild-type pattern of Pax6 labeling is strong in the lens pit and weaker in the optic cup (Fig. 4C). In severely affected E10.5 embryos where lens pit and optic cup invagination have failed, Pax6 is detectable, although at much reduced levels (Fig. 4D). In particular, in Frs2α2F/2F embryos at both E9.5 (Fig. 4B) and E10.5 (Fig. 4D), the Pax6 level and distribution closely resemble the preinduction pattern observed at E8.75 (30).
Fig. 4.
Frs2α2F/2F mice exhibit defective lens and retina induction. Immunofluorescence labeling for Pax6 (A–D) and Chx10 (E–H) of eye-region cryosections from mice of the indicated ages and genotypes. lpl, lens placode; ov, optic vesicle; ple, presumptive lens ectoderm; lp, lens pit; pr, presumptive retina.
Chx10 is a homeodomain transcription factor with an important role in retinal progenitor cell proliferation (31). It is first expressed in the central presumptive retina at E9.5 (Fig. 4E) and depends upon inductive signals from presumptive lens (21). In wild-type embryos, Chx10 continues to be detectable at E10.5 in the invaginating optic cup (Fig. 4G). In Frs2α2F/2F embryos, Chx10 immunoreactivity is absent (Fig. 4 F and H). This indicates that inductive signaling from the presumptive lens to the presumptive retina is disrupted.
Six3 is one of the Six/sine oculis family of homeobox genes (32). At E9.5, the Six3 transcript is present in the optic vesicle and overlying surface ectoderm in wild-type embryos (Fig. 5A). However, in the Frs2α2F/2F mutant, expression of Six3 was reduced in both the optic vesicle and surface ectoderm; the reduction was more pronounced in the surface ectoderm (Fig. 5B). Epistatic analysis suggests that, in the lens lineage, Six3 lies downstream of Pax6 (18). This observation provides further evidence that the integrity of FRS2α is required for normal lens induction.
Fig. 5.
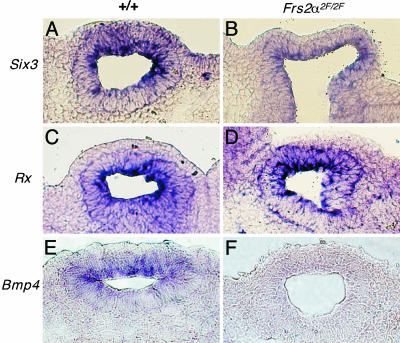
Modifications in the expression patterns of lens and retina induction markers in Frs2α2F/2F mice. Eye region section in situ hybridizations for Six3 (A and B), Rx (C and D), and Bmp4 (E and F) for wild type (Left) and Frs2α2F/2F (Right) embryos at E9.5.
Rx is a homeobox-containing gene expressed in the optic vesicle in wild-type embryos at E9.5 (Fig. 5C) and is required for the formation of the optic cup (25). Rx expression levels in the optic vesicle were unchanged in the Frs2α2F/2F mutant embryos (Fig. 5D). By contrast, expression of Bmp4 was greatly reduced in Frs2α2F/2F mutant embryos. Bmp4 is a member of the transforming growth factor β family of cytokines and has been implicated in eye development through its regulation of Sox2 expression (33). Bmp4 is normally expressed in the optic vesicle and the presumptive lens during the early phases of eye development, and its expression is up-regulated in the distal optic vesicle and lens placode, probably as a response to inductive signaling (33). In four E9.5 Frs2α2F/2F embryos assessed, Bmp4 expression was reproducibly low (Fig. 5 E and F). This suggests that FRS2α lies upstream of Bmp4, and by inference, that FRS2α activation of ERK may be required for stimulation of Bmp4 transcription.
Discussion
Biochemical and genetic experiments have shown that the docking protein FRS2α and its close homologue FRS2β are major mediators of signaling via FGFRs. In FGF-stimulated cells, tyrosine-phosphorylated FRS2α recruits four Grb2 molecules and two molecules of protein tyrosine phosphatase Shp2. Experiments performed with PC12 cells or with Frs2α–/– mouse embryonic fibroblasts expressing phosphorylation site mutants of FRS2α have shown that the four tyrosine residues (Y196, Y306, Y349, and Y392) that function as Grb2-binding sites have a primary role in recruitment of Gab1, Cbl, and PI-3 kinase activation and a secondary role in activation of the Ras/ERK signaling cascade. The other two phosphorylation sites (Y436 and Y471) that function as a binding site for Shp2 play an important role in the activation of the Ras/ERK signaling pathway (10). The most mildly affected Frs2α2F/2F embryos survived up to E18.5, whereas Fgf4, Fgfr1, Fgfr2, Frs2α, or Shp2 mutant embryos show earlier embryonic lethality (10, 28, 34–37). This indicates that the signaling pathways independent of the Shp2-binding sites of FRS2α contribute significantly to FGF signaling during development. It is also possible that Shp2 activation still occurs at low levels in Frs2α2F/2F embryos via alternative Shp2-binding sites. For example, Shp2 could be indirectly recruited by FRS2α by means of Grb2-mediated recruitment of Gab1.
Using early eye development as a model system for exploring the biological roles of FGF-dependent signaling pathways, we demonstrate that, whereas eye development is severely impaired in Frs2α2F/2F mice, the eyes of Frs2α4F/4F mice develop normally. Because Y436 and Y471 serve as a binding site for Shp2, the simplest interpretation of these results is that recruitment of Shp2 by tyrosine-phosphorylated FRS2α is responsible for ERK-dependent control of eye development. It also follows that recruitment of Grb2, Gab1, and PI-3 kinase stimulation plays a less important role in FGF stimulation of eye development. However, it is important to remember that the tyrosine residues that function as a binding site for Shp2 may also be responsible for binding of a yet-to-be-identified SH2 domain-containing protein that is responsible for eye development in response to FGF stimulation. Furthermore, it is possible that the progression of eye development in some Frs2α2F/2F embryos may depend on the secondary role of the FRS2α Grb2-binding sites in activating Ras/ERK or on Ras/ERK activation mediated by Shc.
The paired and homeodomain transcription factor Pax6 is known to have a critical autonomous function in development of the lens. This is indicated by the inability of Pax6Sey/Sey cells to contribute to the lens in chimeric mice (19) and by the failure of lens development when the presumptive lens is derived from Pax6Sey/Sey embryos in tissue recombination experiments (15) or when Pax6 is conditionally deleted in the presumptive lens (18). Pax6 is thought to be at the apex of a genetic hierarchy regulating lens developed based on its ability to induced ectopic lenses when misexpressed in Xenopus (16, 17). It has been shown that the presumptive lens ectoderm requires FGFR activity if Pax6 is to be up-regulated in the normal way in the lens placode; in mice expressing a dominant interfering FGFR mutant in the presumptive lens ectoderm, Pax6 expression and lens development are both inhibited (29). Pax6 up-regulation in the lens placode at E9.0-E9.5 therefore represents a response to inductive signals. Based on its diminished expression in the Pax6 conditional null (18), the transcription factor gene Six3 is believed to be genetically downstream of Pax6 in the lens placode.
The lens phenotype observed in the Frs2α2F/2F mouse is consistent with these data. In this mutant, the most severe morphological change is the absence of formation of the lens placode and lens pit. Furthermore, Pax6 expression is never up-regulated in the presumptive lens and remains as the broad ectodermal distribution that is most similar to the preinduction pattern observed at E8.5 (30). Similarly, the abnormally low level of Six3 expression in the presumptive lens also suggests that inductive signaling is defective.
It has also been shown that induction of the retina can be a consequence of response to FGF in the distal optic vesicle (21). Specifically, expression of the homeodomain transcription factor Chx10 and down-regulation of the transcription factor Mitf (38) in the central presumptive retina both depend on FGF signals from the presumptive lens ectoderm (21). Similarly, Pax6 is normally down-regulated in the central region of the presumptive retina at E10.5 and becomes emphasized in the anterior rim of the optic cup. The retinal phenotype observed in severely affected Frs2α2F/2F embryos can also be ascribed to a failure of inductive signaling. Evidence for this includes the absence of any morphological sign of retinal development, the absence of Chx10, and low levels of Six3. In addition, Pax6 remains in the central presumptive retina.
These data provide evidence that FRS2α is required for expression of these gene products and implies that the FGF signaling pathway is critical for induction of both lens and retina. Given the nature of the Frs2α2F/2F mutation, these data also allow us to go further and suggest that the Shp2-ERK component of the FGF signaling pathway may play a role in early inductive interactions in the eye. When combined with evidence that the ERK response is also involved in lens fiber cell differentiation (39), we can suggest that the FGFR–FRS2α–Shp2–ERK axis is active throughout lens development. However, at this time, alternative mechanisms downstream of FRS2α cannot be ruled out.
Of great interest is the observation that in the Frs2α2F/2F mutant mouse, Bmp4 expression is absent from the optic vesicle at E9.5. The Bmp4 mutant has a failure of lens development (33), and this has implied that Bmp4 may be involved in lens induction. Interestingly, Bmp4 is required for up-regulation of the Sry family transcription factor Sox2 in both presumptive lens and retina (33). Although Sox2 may have other functions, it is known to have an important role in lens lineage differentiation through its regulation of crystallin gene expression (40). Based upon explant analysis, it has been suggested that Bmp4 is one of several optic vesicle signals required for lens induction (33).
The demonstration that Bmp4 expression is undetectable in the optic vesicle of Frs2α2F/2F mutant mice indicates that, genetically, Bmp4 lies downstream of FGF signaling. In turn, this may suggest that FGF and perhaps other signals are the first step in establishing an exchange of signals between presumptive lens and retina. Although there are other possibilities, these data are consistent with a model in which an FGF response in the presumptive retina stimulates Bmp4 expression and Bmp4 then signals within the eye primordium to induce Sox2 expression. When combined with other analysis (33), this might suggest that lens development depends on both FGF and Bmp4 signals from the presumptive retina. Because FGF signaling up-regulates Pax6 and Bmp4 signaling up-regulates Sox2 in the presumptive lens, this model can explain how Pax6 and Sox2 activities could be combined to initiate crystallin expression (41) in preparation for fiber cell differentiation. Although this is an appealing model, much additional analysis will be required to test this hypothesis. Tissue-specific conditional deletions of FGFRs and BMP receptors will be very valuable tools in this endeavor.
Supplementary Material
Acknowledgments
We thank P. Carmeliet for providing the targeting vector, A. Joyner for providing Cre recombinase mice, and A. Auerbach and staff members in Transgenic Animal Facility of New York University for blastocyst injection. We are thankful to Akihiko Shimono and Nadean Brown for providing the probes. This work was supported by National Institutes of Health Grant 1RO1-AR051448-01 and funds from the Ludwig Institute for Cancer Research (New York and Zurich) (to J.S.); Grant-in-Aid for Scientific Research 12215024 (to M.S.) and 1458069 (to N.G.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Research for the Future program of the Japan Society for the Promotion of Science (to M.S.); National Institutes of Health Grants RO1s EY10559, EY11234, EY14102, and EY115766, and RO3 EY14826, and by funds from the Abrahamson Pediatric Eye Institute Endowment at Children's Hospital Medical Center of Cincinnati (to R.A.L.).
Abbreviations: FGFR, FGF receptor; En, embryonic day n; ERK, extracellular signal-regulated kinase; PI-3, phosphatidylinositol.
References
- 1.Chow, R. L. & Lang, R. A. (2001) Annu. Rev. Cell Dev. Biol. 17, 255–259. [DOI] [PubMed] [Google Scholar]
- 2.Lang, R. A. & McAvoy, J. W. (2004) in Development of the Ocular Lens, eds. Robinson, M. L. & Lovicu, F. J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 261–289.
- 3.Schlessinger, J. (2000) Cell 103, 211–225. [DOI] [PubMed] [Google Scholar]
- 4.Ornitz, D. M. & Itoh, N. (March 9, 2001) Genome Biol. 2, 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed]
- 5.Yayon, A., Klagsburn, M., Esko, J. D., Leder, P. & Ornitz, D. M. (1991) Cell 64, 841–848. [DOI] [PubMed] [Google Scholar]
- 6.Spivak-Kroizman, T., Lemmon, M. A., Dikic, I., Ladbury, J. E., Pinchasi, D., Huang, J., Jaye, M., Crumley, G., Schlessinger, J. & Lax, I. (1994) Cell 79, 1015–1024. [DOI] [PubMed] [Google Scholar]
- 7.Kouhara, H., Hadari, Y. R., Spivak-Kroizman, T., Schilling, J., Bar-Sagi, D., Lax, I. & Schlessinger, J. (1997) Cell 89, 693–702. [DOI] [PubMed] [Google Scholar]
- 8.Hadari, Y. R., Kouhara, H., Lax, I. & Schlessinger, J. (1998) Mol. Cell. Biol. 18, 3966–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong, S. H., Guy, G. R., Hadari, Y. R., Laks, S., Gotoh, N., Schlessinger, J. & Lax, I. (2000) Mol. Cell. Biol. 20, 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadari, Y. R., Gotoh, N., Kouhara, H., Lax, I. & Schlessinger, J. (2001) Proc. Natl. Acad. Sci. USA 98, 8578–8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lax, I., Wong, A., Lamothe, B., Lee, A., Frost, A., Hawes, J. & Schlessinger, J. (2002) Mol. Cell 10, 709–719. [DOI] [PubMed] [Google Scholar]
- 12.Wong, A., Lamothe, B., Lee, A., Schlessinger, J., Lax, I. & Li, A. (2002) Proc. Natl. Acad. Sci. USA 99, 6684–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotoh, N., Laks, S., Nakashima, M., Lax, I. & Schlessinger, J. (2004) FEBS Lett. 564, 14–18. [DOI] [PubMed] [Google Scholar]
- 14.Faber, S. C., Robinson, M. L., Makarenkova, H. P. & Lang, R. A. (2002) Development (Cambridge, U.K.) 129, 3727–3737. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara, M., Uchida, T., Osumi-Yamashita, N. & Eto, K. (1994) Differentiation 57, 31–38. [DOI] [PubMed] [Google Scholar]
- 16.Altmann, C. R., Chow, R. L., Lang, R. A. & Hemmati-Brivanlou, A. (1997) Dev. Biol. 185, 119–123. [DOI] [PubMed] [Google Scholar]
- 17.Chow, R. L., Altmann, C. R., Lang, R. A. & Hemmati-Brivanlou, A. (1999) Development (Cambridge, U.K.) 126, 4213–4222. [DOI] [PubMed] [Google Scholar]
- 18.Ashery-Padan, R., Marquardt, T., Zhou, X. & Gruss, P. (2000) Genes Dev. 14, 2701–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collinson, J. M., Hill, R. E. & West, J. D. (2000) Development (Cambridge, U.K.) 127, 945–956. [DOI] [PubMed] [Google Scholar]
- 20.Hyer, J., Mima, T. & Mikawa, T. (1998) Development (Cambridge, U.K.) 125, 869–877. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen, M. & Arnheiter, H. (2000) Development (Cambridge, U.K.) 127, 3581–3591. [DOI] [PubMed] [Google Scholar]
- 22.White, J. K., Auerbach, W., Duyao, M. P., Vonsattel, J. P., Gusella, J. F., Joyner, A. L. & MacDonald, M. E. (1997) Nat. Genet. 17, 404–410. [DOI] [PubMed] [Google Scholar]
- 23.Culling, C. F. A., Allison, R. T. & Barr, W. T. (1985) Cellular Pathology Technique (Butterworth, London).
- 24.Corson, L. B., Yamanaka, Y., Lai, K.-M. V. & Rossant, J. (2003) Development (Cambridge, U.K.) 130, 4527–4537. [DOI] [PubMed] [Google Scholar]
- 25.Mathers, P. H., Grinberg, A., Mahon, K. A. & Jamrich, M. (1997) Nature 387, 603–607. [DOI] [PubMed] [Google Scholar]
- 26.Porter, F. D., Drago, J., Xu, Y., Cheema, S. S., Wassif, C., Huang, S. P., Lee, E., Grinberg, A., Massalas, J. S., Bodine, D., et al. (1997) Development (Cambridge, U.K.) 124, 2935–2944. [DOI] [PubMed] [Google Scholar]
- 27.Tang, T. L., Freeman, R. M. Jr., O'reilly, A. M., Neel, B. G. & Sokol, S. Y. (1995) Cell 80, 473–483. [DOI] [PubMed] [Google Scholar]
- 28.Saxton, T. M., Henkemeyer, M., Gasca, S., Shen, R., Rossi, D. J., Shalaby, F., Feng, G. S. & Pawson, T. (1997) EMBO J. 16, 2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faber, S. C., Dimanlig, P., Makarenkova, H. P., Shirke, S., Ko, K. & Lang, R. A. (2001) Development (Cambridge, U.K.) 128, 4425–4438. [DOI] [PubMed] [Google Scholar]
- 30.Grindley, J. C., Davidson, D. R. & Hill, R. E. (1995) Development (Cambridge, U.K.) 121, 1433–1442. [DOI] [PubMed] [Google Scholar]
- 31.Burmeister, M., Novak, J., Liang, M. Y., Basu, S., Ploder, L., Hawes, N. L., Vidgen, D., Hoover, F., Goldman, D., Kalnins, V. I., et al. (1996) Nat. Genet. 12, 376–384. [DOI] [PubMed] [Google Scholar]
- 32.Oliver, G., Mailhos, A., Wehr, R., Copeland, N. G., Jenkins, N. A. & Gruss, P. (1995) Development (Cambridge, U.K.) 121, 4045–4055. [DOI] [PubMed] [Google Scholar]
- 33.Furuta, Y. & Hogan, B. L. M. (1998) Genes Dev. 12, 3764–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldman, B., Poueymirou, W., Papaioannou, V. E., DeChiara, T. M. & Goldfarb, M. (1995) Science 267, 246–249. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi, T. P., Harpal, K., Henkemeyer, M. & Rossant, J. (1994) Genes Dev. 8, 3032–3044. [DOI] [PubMed] [Google Scholar]
- 36.Xu, X., Weinstein, M., Li, C., Naski, M., Cohen, R. I., Ornitz, D. M., Leder, P. & Deng, C. (1998) Development (Cambridge, U.K.) 125, 753–765. [DOI] [PubMed] [Google Scholar]
- 37.Arman, E., Hafener-Krausz, R., Chen, Y., Heath, J. K. & Lonai, P. (1998) Proc. Natl. Acad. Sci. USA 95, 5082–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tachibana, M., Perez-Jurado, L. A., Nakayama, A., Hodgkinson, C. A., Li, X., Schneider, M., Miki, T., Fex, J., Francke, U. & Arnheiter, H. (1994) Hum. Mol. Genet. 3, 553–557. [DOI] [PubMed] [Google Scholar]
- 39.Lovicu, F. J. & McAvoy, J. W. (2001) Development (Cambridge, U.K.) 128, 5075–5084. [DOI] [PubMed] [Google Scholar]
- 40.Kamachi, Y., Sockanathan, S., Liu, Q., Breitman, M., Lovell-Badge, R. & Kondoh, H. (1995) EMBO J. 14, 3510–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamachi, Y., Uchikawa, M., Tanouchi, A., Sekido, R. & Kondoh, H. (2001) Genes Dev. 15, 1272–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.