ABSTRACT
Tumor microenvironment (TME) promotes immune suppression through recruiting and expanding suppressive immune cells such as regulatory T cells (Tregs) to facilitate cancer progression. In this study, we identify a novel CD39+ γδTreg in human colorectal cancer (CRC). CD39+ γδTregs are the predominant regulatory T cells and have more potent immunosuppressive activity than CD4+ or CD8+ Tregs via the adenosine-mediated pathway but independent of TGF-β or IL-10. They also secrete cytokines including IL-17A and GM-CSF, which may chemoattract myeloid-derived suppressive cells (MDSCs), thus establishing an immunosuppressive network. We further demonstrate that tumor-derived TGF-β1 induces CD39+ γδT cells from paired normal colon tissues to produce more adenosine and become potent immunosuppressive T cells. Moreover, CD39+ γδTreg infiltration is positively correlated with TNM stage and other unfavorable clinicopathological features, implicating that CD39+ γδTregs are one of the key players in establishment of immunosuppressive TME in human CRC that may be critical for tumor immunotherapy.
KEYWORDS: Adenosine, CD39+ γδTregs, human CRC, tumor-derived TGF-β1
Introduction
Human colorectal cancer (CRC) is one of the most common fatal malignancies worldwide. Accumulating evidence has demonstrated that tumor microenvironment (TME) has an intimate relationship with the initiation, promotion, and progression of CRC via multiple mechanisms such as promoting angiogenesis and immune suppression.1 Immune cells are the core components of the TME.2 However, different profiles of infiltrating immune cells are correlated with differential clinical outcomes in human CRC.3 Within the TME, tumor cells and stromal cells can recruit and differentiate immune cells into an immunosuppressive status, such as regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) through secretion of cytokines, chemokines, and a variety of metabolites.4 Those immunosuppressive cells thus establish an immunosuppressive network,5,6 which inhibits antitumor effector immune cells such as CD8+ T lymphocytes and cytotoxic functions of natural killer (NK) cells, subsequently promoting tumor progression.
Recent studies have demonstrated that increased proportion of tumor-infiltrating Tregs predicts a poor prognosis of patients with cancer.6,7 Thus, the development of therapeutic modalities to relieve immunosuppression from Tregs on effector immune cell populations seems promising. Currently, depletion of Tregs through CD25 such as daclizumab and ONTAK has been used in clinical trials, yielding somewhat promising results.8,9 In addition, other approaches including vaccines that decrease the frequency and function of Tregs have also shown limited clinical benefits for cancer patients.10,11 However, the role of Tregs in human CRC patients is still controversial.12-16 Given the inconsistent results concerning the prognostic value of Tregs in patients with CRC, it is not surprising that there is to date no clinical trial reporting effective immunotherapies targeting Tregs in CRC. Therefore, it is drastically needed to understand the exact role of those regulatory T cells in human CRC. Since regulatory T cells are heterogeneous population, it is tempting to speculate that there may be another immunosuppressive T-cell population that plays a pivotal role in facilitating CRC progression and immune evasion.
In this study, we found that approximately 50% of tumor-infiltrating γδT cells express high levels of CD39 molecule. Surprisingly, tumor-infiltrating CD39+ γδT cells are the predominant regulatory T cells in human CRC and exhibit potent, direct immunosuppressive function on effector T cells via the adenosine-mediated pathway (CD39+ γδTregs). Those CD39+ γδTregs could be induced by tumor-derived TGF-β1 to generate more exogenous adenosine, and exert inhibitory function. In addition, CD39+ γδTreg infiltration in tumor is positively associated with advanced TNM stage and other unfavorable clinicopathological features of CRC. Thus, we have unraveled novel regulatory γδT cells in human CRC that may promote immune suppression and tumor progression.
Results
Tumor-infiltrating CD39+ γδT cells are the potent immunosuppressive T cells in human CRC
In human CRC tissues, we found that approximately 50% of γδT cells expressed high level of CD39 (Fig. 1A). In addition, CD39+ γδT cells were significantly increased in the tumor tissues compared with the paired normal tissues (Fig. 1B). Previous studies have demonstrated that CD39+ immune cells exhibit immunosuppressive phenotype with an immunoregulatory property.17-19 Indeed, CD39+ γδT cells expressed high levels of transcription factor Forkhead box P3 (FOXP3), cytotoxic T-like antigen-4 (CTLA-4), and programmed death-1 (PD-1); of which, the levels of CTLA-4 and PD-1 were significantly higher than those on CD39+ CD4+ and CD39+ CD8+ T cells (Fig. 1C and D), suggesting that CD39+ γδT cells may possess an immunosuppressive function. We also found that CD39+ γδT cells were abundant and their absolute number in CD3+ T cells was significantly more than CD4+ CD25+ CD127low conventional CD4+ Treg cells in the tumor tissues (Fig. 1E). Next, we examined their immunosuppressive function on effector T cells.17 We sorted CD39+ γδT cells and conventional CD4+ Tregs, which expressed markedly high level (approximately 90%) of CD45RO and low level (about 10%) of CD45RA (Fig. S1) from tumor tissues and then co-cultured with allogeneic peripheral blood CD3+ T cells stimulated with CD3 and CD28 mAbs. As shown in Fig. 1F, CD39+ γδT cells and conventional Tregs exhibited potent immunosuppressive function on T cells. In addition, CD39+ γδT cells exerted more potent immunosuppressive activity than conventional CD4+ Tregs (Fig. 1F), suggesting that CD39+ γδT cells are the predominant immunosuppressive T cells in human CRC.
Figure 1.

Tumor-infiltrating CD39+ γδT cells are abundant and express higher immunosuppression-related molecules in human colorectal cancer. (A) Representative flow cytometric analysis of CD39 expression in γδT cells in the tumor tissues. Flow plots were gated on CD45+ CD3+ TCRγδ+ cells. (B) Representative flow cytometric analysis of CD39+ γδT cells in the tumor and paired normal tissues. Plots were gated on CD45+ CD3+ T cells. Bar diagram summarizes the percentages of CD39+ γδT cells in the CD45+ CD3+ cells. N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 109; ***p < 0.001. (C, D) Single-cell suspensions from tumors were stained with a panel of antibodies and analyzed by FCM. Flow plots were gated on CD45+ CD3+ TCRγδ+ CD39+, CD45+ CD3+ CD4+ CD39+, or CD45+ CD3+ CD8+ CD39+ T cells (C). Representative histograms are shown. Bar diagram summarizes the percentages of FOXP3+ cells, CTLA-4+ cells, and PD-1+ cells in CD39+ γδT, CD39+ CD4+ T, and CD39+ CD8+ T cells, respectively (D). Data are shown as mean ± SEM; n = 5; ns: no significance; *p < 0.05; **p < 0.01. (E) Representative flow cytometric analysis of CD39+ γδT and CD4+ Treg cells in tumor tissue. Flow plots of CD39+ γδT cells were gated on CD45+ CD3+ TCRγδ+ cells, and CD4+ Tregs was identified as CD45+ CD3+ CD4+ CD25+ CD127low cells (left panel). Bar diagram summarizes the absolute numbers of CD39+ γδT and CD4+ Treg cells in the CD45+ CD3+ cells (105) (right panel). Data are shown as mean ± SEM; n = 8; **p < 0.01. (F) Sorted CD39+ γδT, CD4+ Treg cells from tumors were in vitro co-cultured with CFSE-labeled allogeneic CD3+ T cells in the presence of CD3 and CD28 mAbs. CD3+ T-cell proliferation was evaluated on day 6 by FCM (left panel). Bar diagram summarizes the percentages of proliferated cells (CFSElow) in CD3+ T cells (right panel). T: tumor tissue. Data are shown as mean ± SEM; n = 5; *p < 0.05; **p < 0.01.
Tumor-infiltrating CD39+ γδT cells express higher levels of immunosuppression-related molecules
Since CD39+ γδT cells have not been discovered previously in cancers, we then extensively examined their phenotype. We first examined their phenotype in the tumor tissues as compared with the paired normal tissues. As shown in Fig. 2A and B, tumor-infiltrating CD39+ γδT cells expressed remarkably higher levels of CTLA-4, PD-1, FOXP3, CD25, and CD161 as well as chemokine receptor CCR6, glucocorticoid-induced tumor necrosis factor receptor related gene (GITR), PD-L1 (the ligand of PD-1), and cytotoxic receptor NKp44, whereas they expressed significantly lower levels of immune effector marker TRAIL, NKp46, and co-stimulation marker CD80. Other surface markers such as NKp30, NKG2D, CD122, CD83, and CD86 were not significantly different (Fig. S2A). They predominately expressed Vδ1 TCR (Fig. S2B). Furthermore, we found that tumor-infiltrating CD39+ γδT cells produced significantly more IL-10, IL-17A, GM-CSF, TGF-β1, TNF-α, S100A9 and less IFNγ and IL-2 (Fig. 2C and D). However, CD39+ γδT cells from tumor and the paired normal tissues secreted similar levels of IL-4, IL-8, IL-9, perforin, and granzyme B (Fig. S2C).
Figure 2.

Phenotype of CD39+ γδT cells in CRC versus paired normal tissue. (A, B) Representative flow cytometric analysis of phenotype of CD39+ γδT cells in tumor and paired normal tissues. Flow plots were gated on CD45+ CD3+ TCRγδ+ CD39+ cells (A). Bar diagram summarizes the percentages of indicated markers in CD39+ γδT cells (B). N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. (C, D) Representative flow cytometric analysis of cytokine production by CD39+ γδT cells in tumor and paired normal tissues. Flow plots were gated on CD45+ CD3+ TCRγδ+ CD39+ cells (C). Bar diagram summarizes the percentages of indicated cytokines in CD39+ γδT cells (D). N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; **p < 0.01; ***p < 0.001; ****p < 0.0001.
We next compared the phenotype of CD39+ γδT cells with CD39− γδT cells in the tumor. It was noted that CD39+ γδT cells expressed higher levels of FOXP3, CD25, CTLA-4, PD-1, PD-L1, CD161, GITR, NKp44, and NKp46 (Fig. S3A and B). There were no significant differences in the expression levels of CCR6, CD80, CD83, CD86, TRAIL, NKG2D, NKp30, and CD122 (Fig. S3E). Furthermore, CD39+ γδT cells produced more IL-10, IL-17A, GM-CSF, TGF-β1, TNF-α and less IFNγ than CD39− γδT cells (Fig. S3C and D), whereas these two subpopulations produced similar levels of IL-2, IL-4, IL-8, IL-9, S100A9, perforin, and granzyme B (Fig. S3E). Collectively, it appears that tumor-infiltrating CD39+ γδT cells exhibit much greater immune regulatory phenotype than CD39+ γδT cells from the paired normal tissues or tumor-infiltrating CD39− γδT cells.
Tumor-infiltrating CD39+ γδT cells have potent immunosuppressive activity compared with other regulatory T cells in human CRC
Our initial study demonstrated that tumor-infiltrating CD39+ γδT cells had potent immunosuppressive activity as compared with conventional CD4+ Tregs (Fig. 1F). We next examined different subsets of regulatory T cells within the CRC. We sorted CD39+ γδT cells from the tumor and paired normal tissues. In addition, CD39− γδT, CD4+ Treg, and CD39+ CD8+ T cells were also sorted from the tumor tissues. As shown in Fig. 3A and B, tumor-infiltrating CD39+ γδT cells significantly inhibited T-cell proliferation. Furthermore, tumor-infiltrating CD39+ γδT cells exhibited more potent inhibitory effect than all other T-cell subsets (Fig. 3A and B).
Figure 3.
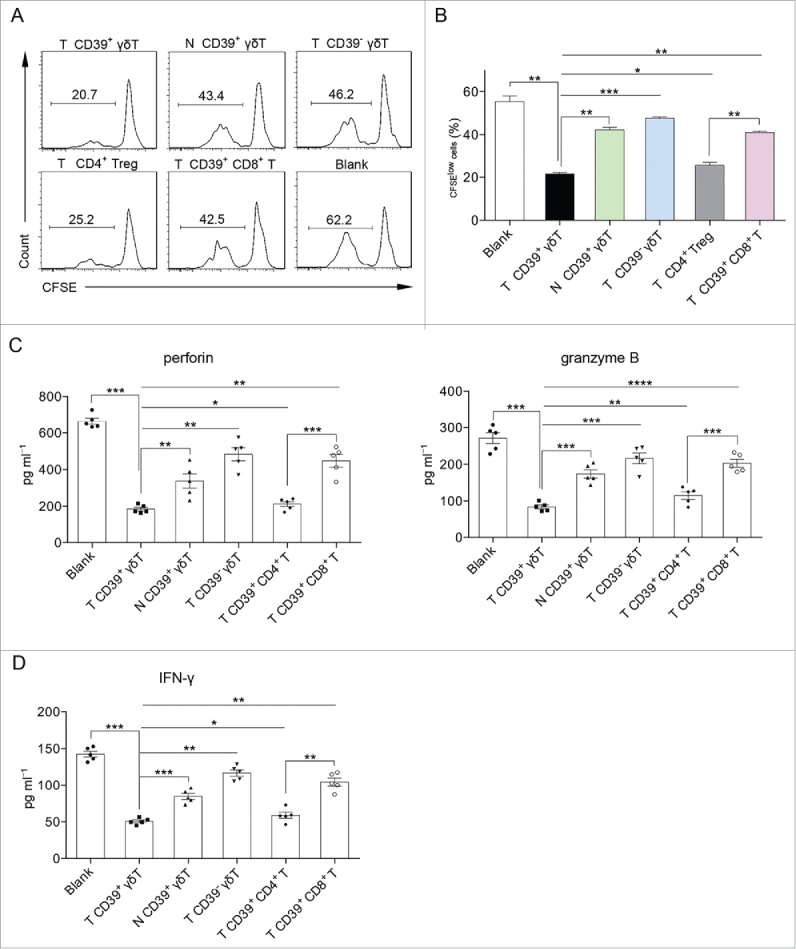
CD39+ γδT cells are the predominant immunosuppressive T cells in CRC. (A, B) Sorted CD39+ γδT, CD39− γδT, CD4+ Treg, CD39+ CD8+ T cells from tumor tissue and CD39+ γδT cells from paired normal tissue were in vitro co-cultured with CFSE-labeled allogeneic CD3+ T cells in the presence of CD3 and CD28 mAbs, respectively. CD3+ T cell proliferation was evaluated on day 6 by FCM (A). Bar diagram summarizes the percentages of proliferated cells (CFSElow) in CD3+ T cells (B). N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; *p < 0.05; **p < 0.01; ***p < 0.001. (C) Sorted CD39+ γδT, CD39− γδT, CD39+ CD4+ T, CD39+ CD8+ T cells from tumor tissue and CD39+ γδT cells from paired normal tissue were in vitro co-cultured with allogeneic CD8+ T cells in the presence of CD3 and CD28 mAbs. Concentrations of perforin (left panel) and granzyme B (right panel) in the supernatants were detected on day 6 by ELISA. N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. (D) Sorted CD39+ γδT, CD39− γδT, CD39+ CD4+ T, CD39+ CD8+ T cells from tumor tissue and CD39+ γδT cells from paired normal tissue were in vitro co-cultured with allogeneic CD4+ T cells in the presence of CD3 and CD28 mAbs. IFNγ levels in the supernatants were detected on day 6 by ELISA. N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; *p < 0.05; **p < 0.01; ***p < 0.001.
Additionally, we found that tumor-infiltrating CD39+ γδT cells remarkably inhibited perforin and granzyme B secretion by CD8+ T cells (Fig. 3C) and IFNγ production by CD4+ T cells (Fig. 3D). Interestingly, tumor-infiltrating CD39+ γδT cells rendered much greater immunosuppressive property than other immunosuppressive T cells, including CD39+ CD4+20,21 and CD39+ CD8+ T cells18,22,23 (Fig. 3C and D). Taken together, these data suggest that CD39+ γδT cells are the predominant regulatory T cells in human CRC not only in quantity but also in quality.
CD39+ γδT regulatory cells (CD39+ γδTregs) function directly via the adenosine-mediated pathway
Previous studies have shown that Tregs inhibit effector T cells via IL-1024,25 and/or transforming growth factor (TGF)-β24,26 However, we found that IL-10 level was very low under all co-culture conditions (Fig. S4A). In vitro blocking assay indicated that CD39+ γδTreg-mediated T-cell suppression was not affected by IL-10 (Fig. S4B) or TGF-β neutralizing mAbs (Fig. S5). Next, we investigated whether co-inhibitory markers such as CTLA-4 and PD-1 on CD39+ γδTregs play a role in the immunosuppressive function. Surprisingly, CTLA-4 and PD-1 blocking mAbs only showed marginal effect on their suppressive function (Fig. S6A and B), suggesting that neither CTLA-4 nor PD-1 is the key mediator in immunosuppression of CD39+ γδTregs.
Previous studies have shown that CD39 and CD73 as ectonucleotidase can collaboratively hydrolyze extracellular ATP/ADP to adenosine.27,28 Adenosine, which is present at increased concentrations in cancer tissue,29,30 has a crucial role in the activity alteration of immune cells including Tregs31 and CD8+ T cells.32 We found that a large fraction of CD39+ γδT cells co-expressed CD73 (Fig. 4A). Strikingly, we found the concentration of extracellular adenosine was more than 10-fold increase in supernatants derived from CRC tissues over that from paired normal tissues (Fig. 4B), and tumor cells expressed high level of CD73 and remarkably low level of CD39 in tumor tissue compared with tumor-infiltrating γδT cells (Fig. S7), suggesting that CD39+ γδTregs might contribute to a considerable extent to such higher level of adenosine in tumor tissue. We also found that the level of extracellular adenosine from co-culture of tumor-infiltrating CD39+ γδT cells was significantly higher than that from co-cultures with other cell subsets (Fig. 4C). To examine whether adenosine is involved in immunosuppression mediated by CD39+ γδTregs, we added two adenosine receptor antagonists A2A (SCH58261) and A2B (PSB603) in the co-culture system. Strikingly, effector T-cell inhibition by CD39+ γδTregs was almost completely blocked by SCH58261 and PSB603 (Fig. 4D). In addition, inhibition of IFNγ production by CD4+ T cells and perforin secretion by CD8+ T cells were also ameliorated by the addition of SCH58261 and PSB603 (Fig. 4E), suggesting that the adenosine-mediated signaling pathway plays a critical role in immunosuppression of CD39+ γδTregs. In addition, we found that CD73 blocking mAb almost completely inhibited the immunosuppression and adenosine generation of CD39+ γδTregs (Fig. S8). It appears that all other regulatory T-cell-mediated suppressive activity was also affected at various degrees in the presence of two adenosine receptor antagonists.
Figure 4.
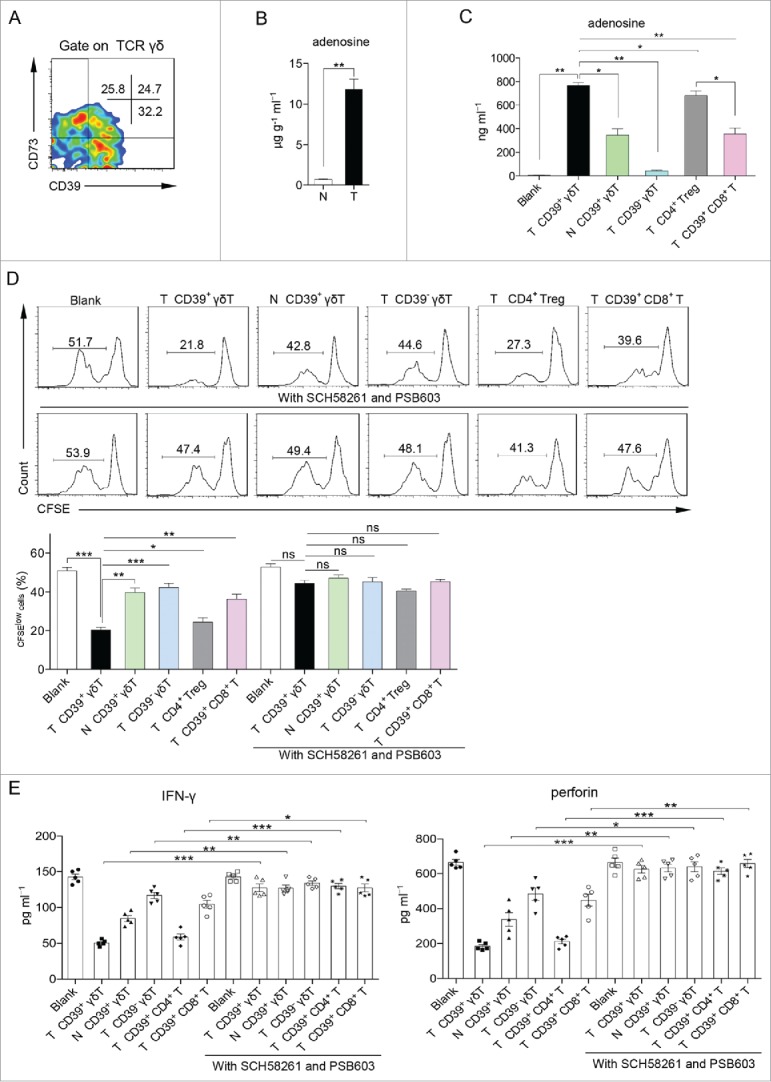
CD39+ γδT regulatory cells (CD39+ γδTreg) exhibit direct immunosuppression via the adenosine-mediated pathway. (A) Representative flow cytometric analysis of CD39 and CD73 expression on γδT cells in tumor tissue. Flow plots were gated on CD45+ CD3+ TCRγδ+ cells. (B) Concentrations of adenosine in the supernatants from 1 g of tumor and paired normal tissues were detected by HPLC. N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; **p < 0.01. (C) HPLC assay to measure adenosine concentrations in the supernatants of Fig. 3A on day 6. N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; *p < 0.05; **p < 0.01. (D) Sorted CD39+ γδT, CD39− γδT, CD4+ Treg, CD39+ CD8+ T cells from tumor tissue and CD39+ γδT cells from paired normal tissue were in vitro co-cultured with CFSE-labeled allogeneic CD3+ T cells in the presence of CD3 and CD28 mAbs, pretreated with or without A2A (SCH58261) and A2B (PSB603) adenosine receptor antagonists. CD3+ T-cell proliferation was evaluated on day 6 by FCM (upper panel). Bar diagram summarizes the percentages of proliferated cells (CFSElow) in CD3+ T cells (lower panel). N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; ns: no significance; *p < 0.05; **p < 0.01; ***p < 0.001. (E) Sorted CD39+ γδT, CD39− γδT, CD39+ CD4+ T, CD39+ CD8+ T cells from tumor tissue and CD39+ γδT cells from paired normal tissue were in vitro co-cultured with allogeneic CD4+ and CD8+ T cells in the presence of CD3 and CD28 mAbs, pretreated with or without A2A (SCH58261) and A2B (PSB603) adenosine receptor antagonists. IFNγ (left panel) or perforin (right panel) levels in the supernatants were detected on day 6 by ELISA. N: normal tissue; T: tumor tissue. Data are shown as mean ± SEM; n = 5; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Tumor-derived TGF-β1 induces CD39+ γδTreg differentiation with excessive adenosine production
TGF-β1 plays a crucial role in the induction of CD4+ Tregs.33,34 Given the much greater immunosuppressive ability of tumor-infiltrating CD39+ γδT cells than those from the paired normal tissues, we reasoned that the TME (e.g., TGF-β1) might be involved in the induction of CD39+ γδTregs. We found that the mRNA and the protein expression levels of TGF-β1 in the CRC tissues were significantly higher than those in the paired normal tissues (Fig. 5A and B). We also found that CD39+ γδT cells from the normal tissues pretreated with tumor supernatants (TS) exhibited potent immunosuppressive activity (Fig. 5C and D). The immunosuppressive effect was abrogated when TGF-β1 neutralizing mAb was added. In addition, CD39+ γδT cells co-cultured with TS produced high levels of exogenous adenosine, which was also significantly inhibited by TGF-β1 neutralizing mAb (Fig. 5E). To further determine the role of TGF-β1 in the induction of CD39+ γδTregs, we directly stimulated CD39+ γδT cells from the normal tissues with varying concentrations of TGF-β1. We found that TGF-β1 induced CD39+ γδTregs with higher adenosine secretion in a dose-dependent manner (Fig. 5F–H). In addition, both TS and TGF-β1 could induce CD39 expression on CD39− γδT cells from the normal tissues (Fig. S9).
Figure 5.
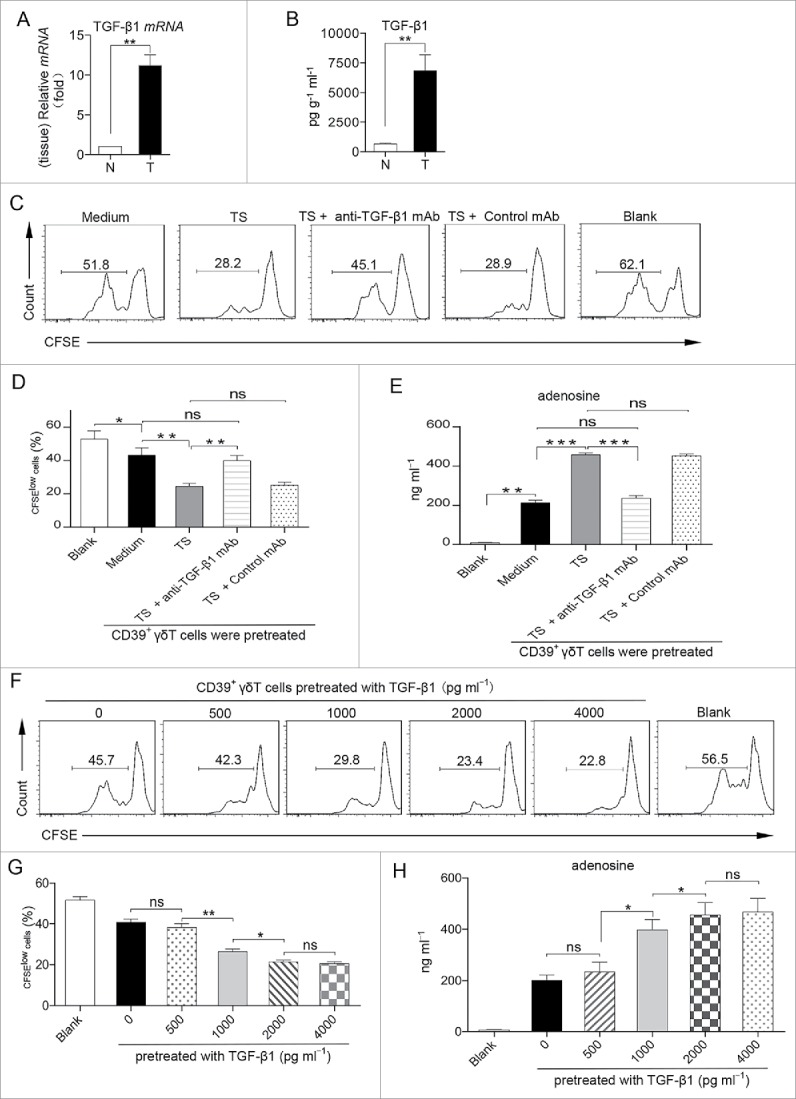
TGF-β1 in the tumor microenvironment induces CD39+ γδTregs. (A, B) The relative mRNA level of TGF-β1 in the tumor and paired normal tissues was determined by RT-PCR and normalized to GAPDH (A). Concentrations of TGF-β1 in the tumor and paired normal tissue-derived supernatants were detected by ELISA (B). Data are shown as mean ± SEM; n = 5; N: normal tissue; T: tumor tissue; **p < 0.01. (C–E) Sorted CD39+ γδT cells from paired normal tissue were pretreated with medium, TS, TS with anti-TGF-β1 mAb, or TS with control mAb in the presence of CD3 and CD28 mAbs. These CD39+ γδT cells were washed and harvested on day 6, and then co-cultured with CFSE-labeled allogeneic CD3+ T cells in the presence of CD3 and CD28 mAbs for 6 d; CD3+ T-cell proliferation was evaluated by FCM (C). Bar diagram summarizes the percentages of proliferated cells (CFSElow) in CD3+ T cells (D). Meanwhile, the concentrations of adenosine in the supernatants of co-culture system were detected by HPLC (E). Data are shown as mean ± SEM; n = 4; TS: tumor supernatant; ns: no significance; *p < 0.05; **p < 0.01; ***p < 0.001. (F–H) Sorted CD39+ γδT cells from paired normal tissue were pretreated with varying concentrations of TGF-β1 in the presence of CD3 and CD28 mAbs. These CD39+ γδT cells were washed and harvested on day 6, and then co-cultured with CFSE-labeled allogeneic CD3+ T cells in the presence of CD3 and CD28 mAbs for additional 6 d; CD3+ T-cell proliferation was evaluated by FCM (F). Bar diagram summarizes the percentages of proliferated cells (CFSElow) in CD3+ T cells (G). Meanwhile, the concentrations of adenosine in the supernatants of co-culture system were detected by HPLC (H). Data are shown as mean ± SEM; n = 4; ns: no significance; *p < 0.05; **p < 0.01.
TGF-β1 can be secreted by different cell subsets including tumor cells. Indeed, human CRC SW480 cell line expressed remarkably high mRNA and protein levels of TGF-β1, but not TGF-β2 or TGF-β3 (Fig. 6A and B). Knockdown of TGF-β1 by siRNA significantly reduced both the mRNA and protein expression levels of TGF-β1 in SW480 cells (Fig. 6C and D). Similarly, CD39+ γδT cells from normal tissues co-cultured with SW480 remarkably inhibited CD3+ T-cell proliferation with significant more adenosine secretion, whereas TGF-β1 neutralizing mAb and TGF-β1 siRNA knockdown significantly decreased the property of SW480 in the induction of CD39+ γδTregs (Fig. 6E–G). Taken together, these data suggest that tumor-derived TGF-β1 induces CD39+ γδTregs with increased exogenous adenosine production, thus exhibiting more potent immunosuppressive activity.
Figure 6.
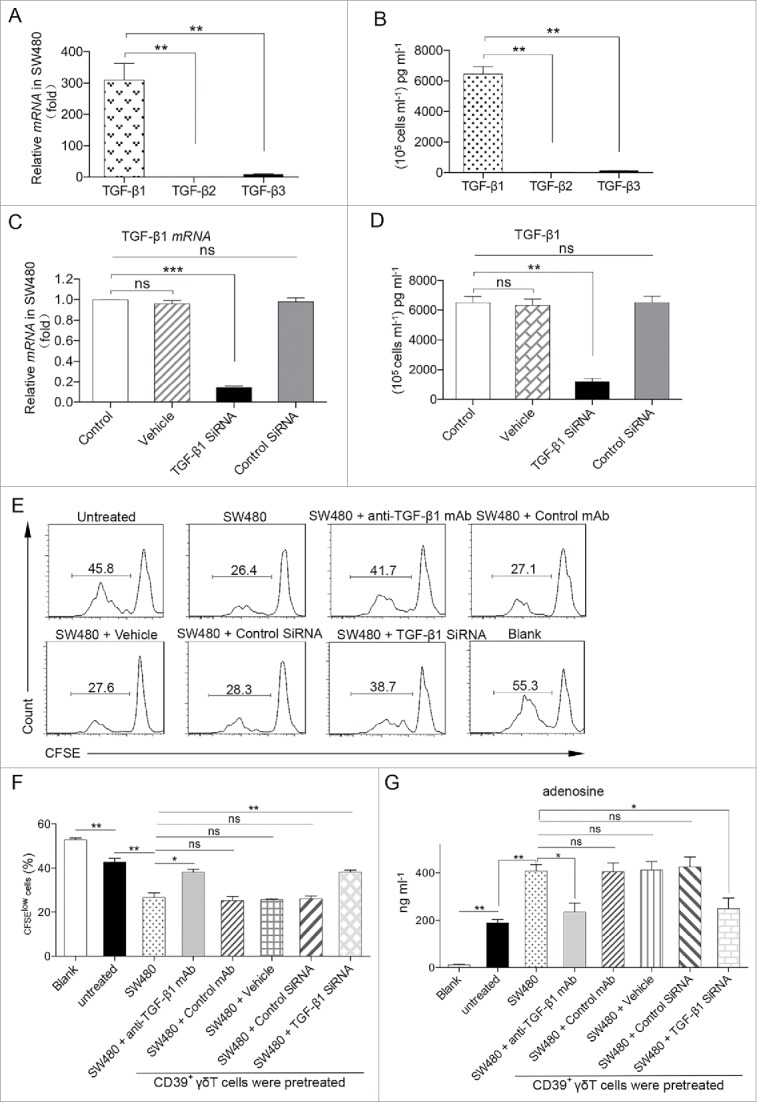
Tumor-derived TGF-β1 plays a pivotal role in the induction of CD39+ γδTregs. (A, B) The relative mRNA levels of TGF-β1, TGF-β2, and TGF-β3 in SW480 cells were determined by RT-PCR and normalized to GAPDH (A). Concentrations of TGF-β1, TGF-β2, and TGF-β3 in SW480 cells-derived supernatant were detected by ELISA (B). Data are shown as mean ± SEM; n = 5; **p < 0.01. (C, D) The relative mRNA levels of TGF-β1 in SW480, vehicle pretreated SW480, control siRNA-transfected SW480, and TGF-β1-siRNA-transfected SW480 cells were determined by RT-PCR and normalized to GAPDH (C). Concentrations of TGF-β1 in supernatants derived from different treated SW480 cells were detected by ELISA respectively (D). Data are shown as mean ± SEM; n = 5; ns: no significance; RT-PCR: real-time PCR; **p < 0.01; ***p < 0.001. (E–G) Sorted CD39+ γδT cells from paired normal tissue were co-cultured with SW480 cells in the presence of TGF-β1 mAb or control mAb, vehicle pretreated SW480, and control siRNA or TGF-β1-siRNA-transfected SW480 cells in the presence of CD3 and CD28 mAbs. These CD39+ γδT cells were washed and harvested on day 4, and then co-cultured with CFSE-labeled allogeneic CD3+ T cells in the presence of CD3 and CD28 mAbs for additional 6 d; CD3+ T-cell proliferation was evaluated by FCM (E). Bar diagram summarizes the percentages of proliferated cells (CFSElow) in CD3+ T cells (F). Meanwhile, the concentrations of adenosine in the supernatants of co-culture system were detected by HPLC (G). Data are shown as mean ± SEM; n = 4; ns: no significance; *p < 0.05; **p < 0.01.
Tumor-infiltrating CD39+ γδTregs correlate positively with malignant clinicopathological features in CRC
Since tumor-infiltrating CD39+ γδTregs are predominant regulatory T cells with more potent immunosuppressive activity than other regulatory T cells in human CRC, we next investigated the implication of CD39+ γδTregs to clinical features of human CRC. We collected and analyzed clinical data from 109 CRC patients and found that the frequency of tumor-infiltrating CD39+ γδTregs was substantially and positively correlated with advanced TNM stage. Similar results were observed when the percentage of tumor-infiltrating CD39+ γδTregs in CD3+ cells was analyzed (Fig. 7A). Moreover, the frequency and the percentage of tumor-infiltrating CD39+ γδTregs were positively correlated with other clinicopathological features, including tumor size, tumor invasion, lymph node metastasis, lymphatic/or vascular invasion, and serum CEA levels (Fig. 7B–F) but not with tumor differentiation, perineuronal invasion, or mismatch repair (MMR) status (Fig. S10). These findings suggest that tumor-infiltrating CD39+ γδTregs are associated with tumor invasiveness and progression. Thus, the frequency of tumor-infiltrating CD39+ γδTregs may be a prognostic factor in human CRC.
Figure 7.

Tumor-infiltrating CD39+ γδTregs correlate with malignant clinicopathological features in CRC. (A) The CD39+ γδT cells in tumor were analyzed by FCM. The potential correlations between the frequency of CD39+ γδTregs and TNM stage were analyzed. The frequencies of tumor-infiltrating CD39+ γδTregs were positively correlated with TNM stage (left panel). CD39+ γδT cell percentages in CD45+ CD3+ T cells were analyzed by FCM. The potential correlations between the CD39+ γδT cell percentages and TNM stage were analyzed. The CD39+ γδT cells percentages in CD45+ CD3+ T cells were positively correlated with TNM stage (right panel). Data are shown as man ± SEM; n = 109. (B–F) Potential correlations of CD39+ γδTreg frequency and percentages with other clinicopathological features. Data are shown as mean ± SEM; CEA: carcinoembryonic antigen; n = 109.
Discussion
Tregs are highly enriched in the TME and are considered to be a pivotal mediator of immune suppression, therefore facilitating tumor progression. In this study, we identify novel CD39+ γδTregs, which are significantly increased in human CRC. CD39+ γδTregs express high levels of CTLA-4, PD-1, FOXP3, and CD25 and secrete cytokines including IL-10, IL-17A, and GM-CSF. Tumor-infiltrating CD39+ γδTregs are abundant in the TME and exhibit potent immunosuppressive activity than conventional CD4+ Tregs or other regulatory T cells. It appears that tumor-derived TGF-β1 induces CD39+ γδTreg differentiation with more exogenous adenosine production. More importantly, CD39+ γδTregs are positively correlated with malignant clinicopathological features of human CRC. These findings suggest that novel CD39+ γδTregs are the critical regulatory T cells in human CRC that promote tumor progression.
TME promotes tumor progression and metastasis through multiple mechanisms such as the induction of regulatory T cells and immune evasion.35,36 Although conventional Tregs are thought to be the major immunosuppressive T cells in many types of cancer, the role of Tregs in human CRC is not conclusive. In addition, it is unknown whether other regulatory T cells exist in human CRC. Previous studies have shown that γδ1 T cells are the major immunosuppressive T cells in human breast cancer.37,38 In this study, we unexpectedly discover that CD39+ γδTregs are the major regulatory T cells within the human CRC. CD39+ γδTregs not only are more in numbers compared with CD4+ Tregs but also exhibit more potent immunosuppressive activity than CD4+ Tregs. CD39+ γδTregs are expanded in the tumor tissues and are induced and differentiated by tumor-derived TGF-β1. This notion is supported by three lines of evidence. First, we show that freshly isolated human CRC tissues contain high levels of TGF-β1 which can induce CD39+ γδT cells of paired normal colon tissues differentiation into CD39+ γδTregs and this induction can be blocked by anti-TGF-β1 neutralizing mAb. Second, we show that co-cultures of CD39+ γδT cells from normal colon tissues with TGF-β1-producing human colon cancer SW480 cell line, but not TGF-β1-knockdown SW480 cells, induce CD39+ γδTregs. Finally, CD39+ γδT cells of normal colon tissues can be differentiated into CD39+ γδTregs by directly stimulation with TGF-β1. Thus, the CRC microenvironment may favor the induction of CD39+ γδTregs by secretion of TGF-β1. This is different from previous study on mouse showing that CD39+ γδT cells derived from axillary and inguinal lymph nodes are induced by IL-2,17 further highlighting the differences between humans and mice. However, the molecular mechanisms underlying TGF-β1-mediated acquisition of immunosuppressive properties by CD39+ γδTregs need further investigation.
Although TGF-β1 is critical in the induction of CD39+ γδTregs, immunosuppressive activity mediated by CD39+ γδTregs is independent of TGF-β. Recent studies have demonstrated that adenosine not only plays a crucial role in establishing immunosuppressive environment but also promotes tumor cell migration and metastasis directly through activation of its receptor.39,40 In this study, we demonstrate that CD39+ γδTregs function via the adenosine-mediated pathway and are independent of TGF-β or IL-10. This inhibitory effect appears to function via CD73 and A2A or A2B receptor.41,42 This is different from the conventional view that Tregs suppress effector T cells in an IL-10-/or TGF-β-dependent manner.25,43 Interestingly, a recent study reported that a subset of CRC-infiltrating CD4+ Tregs also express high CD39.44 However, it is unknown whether this population is correlated with clinicopathological characteristics in human CRC. CD39 expression on different immune cells exhibits diverse functions. A recent study shows that CD39 is only a surface marker and does not have function17, whereas other study indicates that co-expression of CD39 and CD161 on human Th17 cells can boost sphingomyelinase bioactivity to mediate downstream signals including STAT3 and mTOR, suggesting that CD39 is a functional marker on human Th17 cells.45 In addition, a recent study suggests that CD39 is a marker for human exhausted CD8+ T cells possibly due to ectonucleotidase.46 In this study, we show that CD39 is highly expressed on tumor-infiltrating γδT cells. More importantly, it functions as a ectonucleotidase,47 hydrolyzing extracellular ATP/ADP in collaboration with CD73 to adenosine that provides γδTregs with the capacity to exhibit local inhibitory functionality. Our data are consistent with the current findings that CD39+ Tregs suppress antitumor immunity,31,48,49 and CD39+ CD8+ T cells substantially inhibit IFNγ production by CD39− CD8+ T in Crohn's disease in mice via the adenosine-mediated pathway.23 Moreover, we show that CD39+ γδTregs not only have direct immunosuppressive function on effector T cells but also secrete large amounts of IL-17A, TNF-α, and GM-CSF, which may mobilize and recruit PMN-MDSCs into the TME as we previously reported,50 thus establishing an immunosuppressive network in CRC to promote tumor progression and metastasis.
In summary, our study has unraveled tumor-infiltrating CD39+ γδTregs as the predominant regulatory T cells and a key mediator in human CRC. CD39+ γδTregs are induced by tumor-derived TGF-β1, exhibit potent immunosuppressive effect via the adenosine-mediated pathway, and positively correlate with unfavorable clinicopathological features of human CRC. Although CD39+ γδTregs did not show significant correlation with tumors with MMR status, tumors with MMR deficiency show trending lower CD39+ γδTregs frequency. This may be related to better immunotherapeutic efficacy shown in CRC patients bearing MMR deficiency.51 Nevertheless, our findings suggest that CD39+ γδTregs may be a prognostic factor of human CRC and eradication of these cells may have a potential for effective human CRC treatment.
Materials and methods
Clinical specimens
Tumor (T, homogeneous cellularity, without foci of necrosis) and paired normal tissues (N) were obtained from 109 patients with colorectal cancer who underwent surgical resection at the Second Affiliated Hospital, Zhejiang University School of Medicine. Normal autologous tissue was obtained from a macroscopically normal part of the excised intestine, at least 5–10 cm away from the tumor. None of the patients had received radiotherapy or chemotherapy before operation. Peripheral blood samples were obtained from healthy donors from the Zhejiang Blood Center, all of whom were negative for antibodies against hepatitis C virus, hepatitis B virus, HIV, and syphilis. All samples were anonymously coded in accordance with local ethical guidelines (as stipulated by the Declaration of Helsinki), and written informed consent was obtained and the protocol was approved by the Review Board of the Second Affiliated Hospital, Zhejiang University School of Medicine.
Cell isolation and culture
Freshly excised tissues were cut into small pieces and then digested in RPMI 1640 medium containing 2% FBS, type IV collagenase (1 mg/mL), and hyaluronidase (10 ng/mL) for 2–2.5 h at 37 ºC. Total γδT, CD39+ γδT, CD4+ Treg (CD4+ CD25+ CD127low T cells), CD39+ CD4+ T, and CD39+ CD8+ T cells in single-cell suspensions were sorted by an Aria II cell sorter (BD Biosciences). For CD3+, CD4+, and CD8+ T-cell isolation, peripheral blood mononuclear cells (PBMCs) were labeled with relative microbeads (StemCell Technologies) and separated by magnetic activated cell sorting (MACS) following the manufacturer's instructions. The purity of all sorted cells was greater than 90%.
Cancer cell line
Human CRC SW480 cell line was obtained from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China and maintained in RPMI-1640 medium containing 10% fetal calf serum.
Flow cytometry (FCM)
For extracellular staining of immune markers, we prepared single cell suspensions by mechanic dispersion and enzymatic digestion of normal and tumor tissues. We preincubated fresh tissue cells (1 × 106/mL) in a mixture of PBS, 2% fetal calf serum, and 0.1% (w/v) sodium azide with FcgIII/IIR-specific antibody to block nonspecific binding and stained with different combinations of fluorochrome-coupled antibodies (Table S2). For intracellular staining, we followed the manufacturer's protocol after 6-h incubation in the presence of Leukocyte Activation Cocktail (BD PharMingen). Fluorescence data were collected on a FACS Canto II system (BD Biosciences) and analyzed with FlowJo software (Tree Star).
RNA extraction and gene expression quantitative Real-time PCR
Tissue and cell RNAs were extracted using RNeasy Mini Kit (Qiagen) and reverse transcribed into cDNA by PrimeScript RT reagent kit with gDNA Eraser (Takara). Real-time PCR was performed with an ABI 7500 Fast real-time PCR system (Applied Biosystems) according to the manufacturer's instructions for the target genes (Table S3). Data were calculated by the comparative ΔΔCT method with the GAPDH gene as endogenous control.
siRNA silencing
siRNA-mediated gene silencing was performed with validated control or TGF-β1-specific siRNAs according to the manufacturer's instructions (GenePharma).
For SW480 cells transfection, the siRNA was designed to target the sequences of human TGF-β1 (sense: 5′-CACUGCAAGUGGACAUCAATT-3′ antisense: 5′-UUGAUGUCCACUUGCAGUGTT-3′). Scrambled siRNA (sense: 5′-UUCUUCGAAGGUGUCACGUTT-3′ antisense: 5′-ACGUGACACGUUCGGAGAATT-3′) was included as negative control.
Transfection of siRNA was conducted on six-well plates with Lipofectamine 3000 (Invitrogen) as following the manufacturer's instructions. After 48 h of transfection, the cells were collected to perform real-time PCR analysis to confirm the knockdown efficiency.
CD3+ T-cell proliferation assay
For CD39+ γδT cells mediated CD3+ T-cell suppression experiment, sorted tumor-infiltrating CD39+ γδT cells were co-cultured with allogeneic peripheral blood CFSE (Invitrogen)-labeled CD3+ T cells in the presence of anti-CD3 (Clone HIT3a, 10 µg/mL) and anti-CD28 antibodies (Clone CD28.2, 10 µg/mL). At day 6, cells were harvested, and CFSElow CD3+ T cells were detected by FCM.
ELISA assay
For CD39+ γδT cells mediated CD4+ and CD8+ T-cell suppression experiments, sorted tumor-infiltrating CD39+ γδT cells were co-cultured with allogeneic peripheral blood CD4+ or CD8+ T cells in presence of anti-CD3 (Clone HIT3a, 10 µg/mL) and anti-CD28 antibodies (Clone CD28.2, 10 µg/mL). At day 6, supernatants were collected for IFNγ (eBioscience), perforin (Abcam), or granzyme B (eBioscience) detection by ELISA kits. IL-10 levels were determined by ELISA kits in the supernatants of CD3+ T-cell proliferation assay. TGF-β1 (eBioscience), TGF-β2 (eBioscience), and TGF-β3 (Lifespan) in the supernatants derived from tumor, normal tissues, or SW480 were also measured by ELISA kits.
Blocking assay
Anti-IL-10 (Clone JES3-19F1, 0.1 µg/mL), anti-TGF-β neutralizing antibody (ab50716, 0.1 µg/mL), anti-CD73 (Clone 4G4, 20 µg/mL), anti-CTLA-4 (Clone L3D10, 0.5 µg/mL), anti-PD-1 blocking antibody (Clone EH12.2H7, 0.5 µg/mL) or A2A (SCH58261, CAS No. 160098-96-4, 0.1 mM) and A2B (PSB603, CAS No. 1092351-10-4, 0.05 mM) adenosine receptor antagonists were used to block the inhibitory function of CD39+ γδT cells in CD3+ T-cell proliferation or cytokines secretions by CD4+ and CD8+ T cells.
Extracellular adenosine detection
Adenosine concentrations were measured using a high-performance liquid chromatography (HPLC) system equipped with an Inertsil ODS-SP C18 chromatogram column (4.6 mm × 250 mm, 5 µm, Japan) using a mobile phase consisting of acetonitrile and 0.04 M potassium dihydrogen phosphate at the volume ratio of 5: 95. Identification and quantification of adenosine peaks were done by comparison to retention times of known standards and peak integration and normalization.
In vitro CD39+ γδT cells induction
CD39+ γδT cells isolated from normal colon tissues were cultured in medium, supernatants derived from tumor tissues (TS), or recombinant human TGF-β1 (concentration gradient as 500, 1,000, 2,000, 4,000 pg/mL, PeproTech), or co-cultured with SW480 or TGF-β1-siRNA pretreating SW480 cells in the presence of anti-CD3 (Clone HIT3a, 10 µg/mL) and anti-CD28 antibodies (Clone CD28.2, 10 µg/mL). Anti-TGF-β1 neutralizing antibody (Clone 19D8, 0.1 µg/mL) was added when needed. At day 6, CD39+ γδT cells were washed, harvested, and then co-cultured with CFSE-labeled allogeneic peripheral blood CD3+ T cells for 6 d. Supernatants were collected for adenosine assay; meanwhile, the CFSElow CD3+ T cells were detected by FCM.
Statistical analysis
Results were exhibited as means ± SEM. Statistical analysis was performed using GraphPad Prism software version 6. The statistical significance of differences between tumor and paired normal tissue groups was determined by paired Student's t-test; otherwise unpaired Student's t-test was applied. All data were analyzed using two-tailed tests unless otherwise specified, and a p value < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the major international cooperation projects of the National Natural Science Foundation of China (Grant No.81520108024 to JH) and National Natural Science Foundation of China (Grant No.81472640, 81672802 to FMQ; 81572800 to PW). This work was partly granted by Natural Science Foundation of Zhejiang Province (Grant No. LY15H160036 to XY.Y. and No. LY15H160041 to P.W.). This work was also funded by the Zhejiang Province Key Discipline of Traditional Chinese Medicine (Grant No. 2012-XK-A27 to J.H.) and Zhejiang Province Key Innovation Discipline of Medicine (Grant No. 2011-CX11 to J.H.).
Author contributions
GM.H. participated in the design and coordination of the research project, collected and processed the specimen, performed flow cytometry, HPLC, cell sorting, some in vitro experiments, analyzed the data, and contributed to the manuscript writing. P.W. participated in the design and coordination of the research project, performed some ELISA tests and analyzed the data. P.C. performed gene silencing and detections, and participated in collection and analysis of clinicopathological data of patients. ZG.Z., Z.W., XY.Y., and X.S. participated in the collection of the specimen. D.W. performed some of the flow cytometry. J.Y. and T.Z. performed some ELISA tests. XC.W. provided some of the specimen. FM.Q. provided helpful edits. J.Y. and. J.H. participated in the design and coordination of the research project, supervised the research project, analyzed the data, revised the manuscript, and approved the final version of the manuscript.
References
- 1.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol 2011; 11:702-11; PMID:21941296; http://dx.doi.org/ 10.1038/nri3064 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A et al.. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782-95; PMID:24138885; http://dx.doi.org/ 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.org/ 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 5.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 2007; 117:1155-66; PMID:17476345; http://dx.doi.org/ 10.1172/JCI31422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M et al.. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:15322536; http://dx.doi.org/ 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- 7.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006; 24:5373-80; PMID:17135638; http://dx.doi.org/ 10.1200/JCO.2006.05.9584 [DOI] [PubMed] [Google Scholar]
- 8.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann NY Acad Sci 2009; 1174:99-106; PMID:19769742; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04939.x [DOI] [PubMed] [Google Scholar]
- 9.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E et al.. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 2005; 115:3623-33; PMID:16308572; http://dx.doi.org/ 10.1172/JCI25947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prins RM, Wang X, Soto H, Young E, Lisiero DN, Fong B, Everson R, Yong WH, Lai A, Li G et al.. Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients. J Immunother 2013; 36:152-7; PMID:23377664; http://dx.doi.org/ 10.1097/CJI.0b013e3182811ae4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW et al.. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010; 59:663-74; PMID:19890632; http://dx.doi.org/ 10.1007/s00262-009-0782-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjornbeth BA, Taskén K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother 2008; 57:813-21; PMID:17962941; http://dx.doi.org/ 10.1007/s00262-007-0417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuo C, Li Z, Xu Y, Wang Y, Li Q, Peng J, Zheng H, Wu P, Li B, Cai S. Higher FOXP3-TSDR demethylation rates in adjacent normal tissues in patients with colon cancer were associated with worse survival. Mol Cancer 2014; 13:153; PMID:24938080; http://dx.doi.org/ 10.1186/1476-4598-13-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 2009; 27:186-92; PMID:19064967; http://dx.doi.org/ 10.1200/JCO.2008.18.7229 [DOI] [PubMed] [Google Scholar]
- 15.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L et al.. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 2010; 33:435-41; PMID:20386463; http://dx.doi.org/ 10.1097/CJI.0b013e3181d32f01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer 2010; 126:2635-43; PMID:19856313. [DOI] [PubMed] [Google Scholar]
- 17.Otsuka A, Hanakawa S, Miyachi Y, Kabashima K. CD39: a new surface marker of mouse regulatory gammadelta T cells. J Allergy Clin Immunol 2013; 132:1448-51; PMID:23870672; http://dx.doi.org/ 10.1016/j.jaci.2013.05.037 [DOI] [PubMed] [Google Scholar]
- 18.Parodi A, Battaglia F, Kalli F, Ferrera F, Conteduca G, Tardito S, Stringara S, Ivaldi F, Negrini S, Borgonovo G et al.. CD39 is highly involved in mediating the suppression activity of tumor-infiltrating CD8+ T regulatory lymphocytes. Cancer Immunol Immunother 2013; 62:851-62; PMID:23359087; http://dx.doi.org/ 10.1007/s00262-013-1392-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Telesford KM, Ochoa-Reparaz J, Haque-Begum S, Christy M, Kasper EJ, Wang L, Wu Y, Robson SC, Kasper DL et al.. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun 2014; 5:4432; PMID:25043484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, Kassam N, Enjyoji K, Robson SC et al.. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant 2009; 9:2303-11; PMID:19656134; http://dx.doi.org/ 10.1111/j.1600-6143.2009.02777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Begum-Haque S, Telesford KM, Ochoa-Reparaz J, Christy M, Kasper EJ, Kasper DL, Robson SC, Kasper LH. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes 2014; 5:552-61; PMID:25006655; http://dx.doi.org/ 10.4161/gmic.29797 [DOI] [PubMed] [Google Scholar]
- 22.Boer MC, van Meijgaarden KE, Bastid J, Ottenhoff TH, Joosten SA. CD39 is involved in mediating suppression by Mycobacterium bovis BCG-activated human CD8(+) CD39(+) regulatory T cells. Eur J Immunol 2013; 43:1925-32; PMID:23606272; http://dx.doi.org/ 10.1002/eji.201243286 [DOI] [PubMed] [Google Scholar]
- 23.Bai A, Moss A, Rothweiler S, Serena Longhi M, Wu Y, Junger WG, Robson SC. NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nat Commun 2015; 6:8819; PMID:26549640; http://dx.doi.org/ 10.1038/ncomms9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8:523-32; PMID:18566595; http://dx.doi.org/ 10.1038/nri2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res 2007; 13:4345-54; PMID:17671115; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-0472 [DOI] [PubMed] [Google Scholar]
- 26.Gigante M, Ranieri E. TGF-beta promotes immune suppression by inhibiting Treg cell apoptosis. Immunotherapy 2010; 2:608; PMID:20919442; http://dx.doi.org/ 10.2217/imt.10.54 [DOI] [PubMed] [Google Scholar]
- 27.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2006; 2:409-30; PMID:18404480; http://dx.doi.org/ 10.1007/s11302-006-9003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013; 19:355-67; PMID:23601906; http://dx.doi.org/ 10.1016/j.molmed.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res 1997; 57:2602-5; PMID:9205063 [PubMed] [Google Scholar]
- 30.Hausler SF, Del Barrio IM, Diessner J, Stein RG, Strohschein J, Honig A, Dietl J, Wischhusen J. Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion. Am J Transl Res 2014; 6:129-39; PMID:24489992. [PMC free article] [PubMed] [Google Scholar]
- 31.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M et al.. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204:1257-65; PMID:17502665; http://dx.doi.org/ 10.1084/jem.20062512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linnemann C, Schildberg FA, Schurich A, Diehl L, Hegenbarth SI, Endl E, Lacher S, Müller CE, Frey J, Simeoni L et al.. Adenosine regulates CD8 T-cell priming by inhibition of membrane-proximal T-cell receptor signalling. Immunology 2009; 128:e728-37; PMID:19740334; http://dx.doi.org/ 10.1111/j.1365-2567.2009.03075.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 2005; 201:1061-7; PMID:15809351; http://dx.doi.org/ 10.1084/jem.20042276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding R, Steinman RM, Suthanthiran M. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25- T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci USA 2007; 104:2821-6; PMID:17307871; http://dx.doi.org/ 10.1073/pnas.0611646104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 2005; 202:919-29; PMID:16186184; http://dx.doi.org/ 10.1084/jem.20050463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol 2007; 178:2883-92; http://dx.doi.org/ 10.4049/jimmunol.178.5.2883 [DOI] [PubMed] [Google Scholar]
- 37.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007; 27:334-48; PMID:17656116; http://dx.doi.org/ 10.1016/j.immuni.2007.05.020 [DOI] [PubMed] [Google Scholar]
- 38.Ye J, Ma C, Wang F, Hsueh EC, Toth K, Huang Y, Mo W, Liu S, Han B, Varvares MA et al.. Specific recruitment of gammadelta regulatory T cells in human breast cancer. Cancer Res 2013; 73:6137-48; PMID:23959855; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA 2010; 107:1547-52; PMID:20080644; http://dx.doi.org/ 10.1073/pnas.0908801107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desmet CJ, Gallenne T, Prieur A, Reyal F, Visser NL, Wittner BS, Smit MA, Geiger TR, Laoukili J, Iskit S et al.. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc Natl Acad Sci USA 2013; 110:5139-44; PMID:23483055; http://dx.doi.org/ 10.1073/pnas.1222085110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cekic C, Linden J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res 2014; 74:7239-49; PMID:25341542; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 2008; 111:251-9; PMID:17909080; http://dx.doi.org/ 10.1182/blood-2007-03-081646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E et al.. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005; 202:1075-85; PMID:16230475; http://dx.doi.org/ 10.1084/jem.20051511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scurr M, Ladell K, Besneux M, Christian A, Hockey T, Smart K, Bridgeman H, Hargest R, Phillips S, Davies M et al.. Highly prevalent colorectal cancer-infiltrating LAP(+) Foxp3(-) T cells exhibit more potent immunosuppressive activity than Foxp3(+) regulatory T cells. Mucosal Immunol 2014; 7:428-39; PMID:24064667; http://dx.doi.org/ 10.1038/mi.2013.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai A, Robson S. Beyond ecto-nucleotidase: CD39 defines human Th17 cells with CD161. Purinergic Signal 2015; 11:317-9; PMID:26059452; http://dx.doi.org/ 10.1007/s11302-015-9457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta PK, Godec J, Wolski D, Adland E, Yates K, Pauken KE, Cosgrove C, Ledderose C, Junger WG, Robson SC et al.. CD39 expression identifies terminally exhausted CD8+ T Cells. PLoS Pathogens 2015; 11:e1005177; PMID:26485519; http://dx.doi.org/ 10.1371/journal.ppat.1005177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal 2007; 3:171-80; PMID:18404431; http://dx.doi.org/ 10.1007/s11302-006-9050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 2010; 139:1030-40; PMID:20546740; http://dx.doi.org/ 10.1053/j.gastro.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell'Acqua ML et al.. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007; 110:1225-32; PMID:17449799; http://dx.doi.org/ 10.1182/blood-2006-12-064527 [DOI] [PubMed] [Google Scholar]
- 50.Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W et al.. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014; 40:785-800; PMID:24816404; http://dx.doi.org/ 10.1016/j.immuni.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D et al.. PD-1 blockade in tumors with mismatch-repair deficiency. N Eng J Med 2015; 372:2509-20; PMID:26028255; http://dx.doi.org/ 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


