ABSTRACT
PD-L1/2 expression in solid tumors inhibits chimeric antigen receptor (CAR) T-cell efficacy. A PD-1 dominant negative receptor expressed in CAR T cells provides cell-intrinsic checkpoint blockade and augments antitumor efficacy. A combinatorial immunotherapeutic strategy of combining CAR T cells with checkpoint blockade is a promising treatment approach for solid tumors.
KEYWORDS: Adoptive T-cell therapy, breast cancer, immunosuppression, lung cancer, mesothelioma, PD-1 blockade, tumor microenvironment
Abbreviations
- CAR
chimeric antigen receptor
- CRISPR
clustered regularly interspaced short palindromic repeats
- LAG3
lymphocyte-activation gene 3
- MDSC
myeloid-derived suppressor cell
- MSLN
mesothelin; PD-1, programmed death-1
- PD-L1
programmed death-ligand 1
- PSCA
prostate-specific cell antigen
- TAM
tumor-associated macrophage
- TIM-3
T-cell membrane protein 3
- Treg
regulatory T cells
Chimeric antigen receptors (CARs) are synthetic receptors that redirect T cells to cancer cell surface antigens to eliminate the targeted cancer cells. The components of CARs are an extracellular single chain variable fragment (scFv), a transmembrane domain, and an intracellular signaling domain. First-generation CARs linked scFv with the CD3ζ intracellular signaling domain to induce antigen-specific T-cell activation. Subsequent generations of CARs incorporated additional co-stimulatory signals (e.g., domains of CD28, 4–1BB, and OX40) alone (second generation) or in combination with (third generation) to further augment and sustain T-cell effector functions.
Application of CAR T-cell therapy to solid tumors faces a series of challenges, including efficient trafficking and infiltration to tumor, and overcoming tumor-mediated immunosuppression. We have demonstrated augmented efficacy following regional administration of mesothelin (MSLN)-specific CAR T cells in an orthotopic model of pleural mesothelioma; a single, low dose of CAR T cells was able to suppress tumor growth and establish long-term immunity.1 In this clinically-relevant model, we investigated the effect of repeated antigen stress on CAR T cells that results in CAR T-cell exhaustion and further explored strategies to enhance CAR T-cell functional persistence.2 We used three CARs that incorporated an anti-human, MSLN-specific scFv and CD3ζ, CD28/ CD3ζ, or 4–1BB/ CD3ζ signaling domains (Mz, M28z, and MBBz). We purposefully administered a very low dose of MSLN-targeted CAR T cells (Effector to Target, E:T ratio = 1:7,500). Although second generation CAR T cells (M28z and MBBz) exhibited superior antitumor efficacy compared with first-generation CAR T cells (Mz), mice treated with M28z eventually died from tumor relapse, whereas a relatively higher proportion of MBBz-treated mice remained tumor free. Upon ex vivo stimulation, tumor-harvested M28z cells had decreased cytotoxicity and Th1 cytokine production, which is a phenotype associated with T-cell exhaustion. Notably, although MBBz cells better retained effector functions compared with M28z cells, overall cytotoxicity and cytokine production also decreased with repeated antigen stimulation, thus indicating that MBBz cells are also sensitive to exhaustion, albeit at a relatively lower magnitude. These observations underscore the need to establish CAR T-cell functional persistence within the immunosuppressive solid tumor microenvironment for long-term antitumor efficacy.
Exhausted CAR T cells overexpressed inhibitory receptors, including programmed death-1 (PD-1), with corresponding upregulation of PD-1 ligands (PD-L1 and PD-L2) on the tumor cells. In line with the beneficial effects of PD-1 and PD-L1 blocking antibodies in the clinical treatment of solid tumors,3 treatment with the PD-1 blocking antibody was able to partially rescue effector functions of M28z both in vitro and in vivo, and reduce tumor burden significantly. However, tumor relapse was observed following cessation of antibody administration. While long-term, repeated antibody treatment was able to control tumor progression it did not achieve tumor eradication. Taken together, our observation suggests that adaptive resistance plays an important role in CAR T-cell exhaustion. The PD-1 blocking antibody was able to rescue M28z function; its effect is short-lived and repeated antibody administration is required to suppress tumor progression.
To license CAR T cells with checkpoint blockade without being reliant on repeated antibody administration, we genetically engineered CAR T cells to overexpress a PD-1 dominant negative receptor (PD-1 DNR) that lacks the PD-1 transmembrane and intracellular signaling domains (see Fig. 1). We hypothesized that PD-1 DNR expressed on the CAR T-cell surface can act as a “decoy receptor” to bind and block the PD-L1/2 inhibitory signal. Indeed, M28z CAR T cells co-transduced with PD-1 DNR cells had increased proliferation, enhanced cytotoxicity, and augmented cytokine secretion upon repeated antigen stimulation compared with M28z cells. Mice injected with M28z PD-1 DNR cells better controlled tumor burden and prolonged median survival. Other strategies used to intrinsically block the PD-1 inhibitory signal in CAR T cells include co-expression of a switch receptor that contains the PD-1 extracellular domain and CD28 transmembrane, and intracellular domains (PD-1 CD28) in anti-MSLN or anti-prostate-specific cell antigen (PSCA) CAR T cells expressing the 4–1BB co-stimulatory signal,4 as well as the generation of PD-1 deficient anti-CD19 CAR T cells using CRISPR/Cas9 gene editing technology.5
Figure 1.
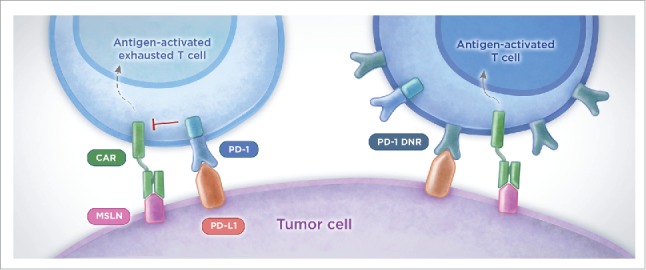
PD-1 cell-intrinsic strategy counteracts PD-L1-mediated immunosuppression and enhances efficacy of CAR T-cell therapy. To overcome PD-L1 mediated immunosuppression, MSLN-specific CAR T cells were genetically engineered to overexpress a PD-1 dominant negative receptor (PD-1 DNR) that lacks the intracellular inhibitory signaling domain. PD-1 DNR competes with the endogenous PD-1 and saturates the PD-L1 ligand expressed on tumor cells, thereby limiting activation of PD-1 inhibitory signaling. PD-1 DNR CAR T cells rescue effector functions in vitro and enhance control of tumor burden in vivo. PD, programmed-death; MSLN, mesothelin; CAR, chimeric antigen receptor; DNR, dominant negative receptor.
In our study, PD-1 blockade by both anti-PD-1 antibody and PD-1 DNR augmented CAR T-cell efficacy. Compared with combined CAR T-cell and antibody treatment, there are several advantages of using genetically engineered cells. First, blockade of the PD-1 signal through genetic engineering provides a sustainable effect. Second, genetically engineered CAR T cells provide tumor-limited PD-1 blockade. Anti-PD-1 antibody efficacy can be limited by inefficient tumor penetrance, short half-life, and non-specific toxicity. Nevertheless, MSLN-targeted CARs, in combination with checkpoint blockade either by antibody administration or by PD-1 DNR, provides opportunity to treat a wide array of solid tumors.6 We are planning to initiate a combination immunotherapy clinical trial for patients with metastatic lung cancer, breast cancer, and mesothelioma at Memorial Sloan Kettering Cancer Center.
Some mice treated with a single dose of M28z PD-1 DNR CAR T cells demonstrated long-term tumor relapse, which suggests that there are redundant mechanisms of immunoinhibition that must be overcome within the tumor microenvironment. In addition to PD-1, multiple co-inhibitory receptors (e.g., TIM-3, LAG3, and TIGIT) are also expressed on exhausted T cells.7 Simultaneously targeting multiple inhibitory pathways may further enhance CAR T-cell potency. A better understanding of inhibitory mechanisms influencing tumor-infiltrating T cells, both endogenous and CAR T cells can help design next-generation immunotherapy. Adoptive T-cell therapy by T-cell engineering provides opportunities to design rational combinatory strategies to improve CAR T-cell therapy that may lead to successful treatment of solid tumors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Institutes of Health (P30 CA008748), the U.S. Department of Defense (BC132124), Memorial Sloan Kettering's Office of Technology Development, and the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center.
References
- 1.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014; 6:261ra151; PMID:25378643; http://dx.doi.org/ 10.1126/scitranslmed.3010162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016; 126:3130-44; PMID:27454297; http://dx.doi.org/ 10.1172/JCI83092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8:328rv4; PMID:26936508; http://dx.doi.org/26979791 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, Newick K, Lo A, June CH, Zhao Y et al.. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016; 76:1578-90; PMID:26979791; http://dx.doi.org/ 10.1158/0008-5472.CAN-15-2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rupp L, Schumann K, Roybal KT, Marson A, Lim WA. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. The J Immunol. 2016; 196:214.24; http://www.jimmunol.org/content/196/1_Supplement/214.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 2016; 6:133-46; PMID:26503962; http://dx.doi.org/ 10.1158/2159-8290.CD-15-0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016; 13:143-58; PMID:26598942; http://dx.doi.org/ 10.1038/nrclinonc.2015.209 [DOI] [PubMed] [Google Scholar]


