ABSTRACT
The human leukocyte antigen (HLA) class II antigen-processing machinery (APM) presents to cognate CD4+ T-cells antigenic peptides mainly generated from exogeneous proteins in the endocytic compartment. These CD4+ T cells exert helper function, but may also act as effector cells, thereby recognizing HLA class II antigen-expressing tumor cells. Thus, HLA class II antigen expression by tumor cells influences the tumor antigen (TA)-specific immune responses and, depending on the cancer type, the clinical course of the disease. Many types of human cancers express HLA class II antigens, although with marked differences in their frequency. Some types of cancer lack HLA class II antigen expression, which could be due to structural defects or deregulation affecting different components of the complex HLA class II APM and/or from lack of cytokine(s) in the tumor microenvironment. In this review, we have summarized the information about HLA class II antigen distribution in normal tissues, the structural organization of the HLA class II APM, their expression and regulation in malignant cells, the defects, which have been identified in malignant cells, and their functional and clinical relevance.
KEYWORDS: Antigen-processing; HLA class II; immune response, tumor
Abbreviations
- APC
antigen presenting cell
- APM
antigen-processing machinery
- CIITA
class II transactivator
- CLIP
class II associated invariant chain peptide
- CRC
colorectal carcinoma
- CREB
cAMP-responsive element binding protein
- CTL
cytotoxic T lymphocyte
- DAC
5′-aza-2′-deoxycytidine
- DC
dendritic cells
- ER
endoplasmic reticulum
- GILT
gamma interferon inducible lysosomal thiolreductase
- HLA
human leukocyte antigen
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papilloma virus
- IFN
interferon
- Ii
invariant chain
- MHC
major histocompatibility complex
- MIIC
MHC class II compartment
- MSI
microsatellite instability
- MSI-H
high level microsatellite instability
- PADRE
pan-HLA-DR reactive epitope
- Rb
retinoblastoma
- RFX
regulatory factor X
- TAA
tumor-associated antigen
- TCR
T-cell receptor
- TSA
trichostatin A
- USF-1
upstream regulatory factor.
Introduction
Malignant tumors, as a genetic disease, are caused by structural alterations of the genome which can give rise to the expression of tumor-associated antigens (TAA) in the form of either structurally altered molecules or of overexpressed normal molecules. TAA may be recognized by the host's immune system and may induce a T-cell-mediated immune response. Consequently, outgrowing cancers develop different strategies to evade potential destruction by the host's immune system. In particular, immune evasion mechanisms affecting the expression and/or function of human leukocyte antigens (HLA) are of special interest to tumor immunologists, since these molecules play a crucial role in the interaction of malignant cells with immune cells. As summarized in Table 1, the two types of classical HLA antigens, HLA class I and II antigens, share certain similarities, but also substantial differences.
Table 1.
Comparison of the major characteristics of the HLA class I and II APM pathway.
| Steps of APM | HLA class I APM | HLA class II APM |
|---|---|---|
| Antigen presentation | HLA-A, -B, -C | HLA-DR, -DQ, DP |
| Stabilization and loading | Tapasin | HLA-DM |
| Peptide generation | Proteasome subunits | Lysosomal enzymes |
| Transactivation | No | Yes, CIITA |
| Assembly and stabilization | β2-m | Invariant chain |
| Peptide loading compartment | ER | MCII |
| Presentation to | CD8+ T cells | CD4+ T cells |
| Regulation by cytokines | IFNγ | IFNγ |
| Expression by tumor cells | Loss Downregulation |
Loss Down- or upregulation de Novo expression |
Analyses of tumor cell lines and of large numbers of surgically removed tumor lesions of distinct histology have demonstrated defects in HLA class I surface expression in most of the tumors tested. This was associated with a downregulation or loss of components of the HLA class I antigen-processing machinery (APM) as summarized in Table 11,2 in solid and hematopoietic tumors; however, the frequency of these defects strongly varied among the different types of cancer. Multiple molecular mechanisms have been shown to underlie these abnormalities, which cause defective synthesis and/or expression of HLA class I antigen/tumor antigen (TA)-derived peptide complexes. The latter mediate the interactions of tumor cells with antigen-specific cytotoxic T lymphocytes (CTL). The functional significance of these defects is indicated by their negative impact on the CTL-mediated elimination of tumor cells.3 An impaired interaction of immune cells with HLA class I antigen-deficient cancer cells also accounts for the association between defective HLA class I APM component expression in tumors and poor clinical course identified in various types of malignancies. Alterations of HLA class I APM and their significance in malignant tumors have been recently reviewed by various groups and we refer the interested reader to these reviews.3-8
In contrast, HLA class II antigens expressed by malignant cells of solid tumors have been characterized only to a limited extent, although the aberrant expression of HLA class II antigens by melanoma cells was first described more than 30 y ago.9 Growing evidence indicates that HLA class II antigen expression by tumor cells has a significant impact on their immunogenicity.10 In addition, more information is available about the molecular mechanisms leading to aberrant HLA class II antigen expression by tumor cells. In this review, we will provide an update about HLA class II antigens in cancer. We will describe HLA class II antigen expression in normal tissues, the physiological organization of components of the HLA class II APM pathway, expression patterns of HLA class II antigens in tumors related to the so far identified molecular and regulatory defects as well as their functional and clinical relevance.
HLA class II antigen expression in normal cells
It has been assumed for a long time that under physiological conditions the constitutive expression of the gene products of HLA class II loci is primarily restricted to professional antigen presenting cells (APCs) and to thymic epithelial cells in man and in other animal species.11 With the availability of HLA class II antigen-specific monoclonal antibodies (mAb), the expression of these antigens could be studied in a variety of normal tissues, revealing that HLA class II antigens have a broader distribution in normal tissues than originally postulated11-13: A weak to moderate expression of HLA class II antigens has been shown in skin, breast, lung and kidney tissues (www.proteinatlas.org 11), indicating that in addition to APC, other tissues are able to constitutively express HLA class II antigens.
Organization of the HLA class II antigen-processing machinery
The increased availability of mAbs recognizing HLA class II APM components during the last few years has greatly facilitated the characterization of the organization and functional properties of this machinery 14 (Fig. 1). HLA class II antigens are heterodimers consisting of a 33 kDa α and a 29 kDa β chain. Both chains are glycosylated and polymorphic, although to a different extent. They are synthesized in the endoplasmic reticulum (ER), where they assemble with the invariant chain (Ii) also known as CD74. The li, a 33 kDa polypeptide, is involved in the stabilization and proper folding of HLA class II antigens as well as in the prevention of binding cellular peptides on HLA class II antigens, while it could also serve as vehicle to load antigenic peptides on MHC class I molecules.15 In addition, the li facilitates the export of HLA class II molecules from the ER and is responsible for directing the HLA class II complexes into specialized endosomal/lysosomal antigen loading compartments, termed MHC class II containing compartment (MIIC). Here, the li is proteolytically degraded by cathepsin into the 14 amino acid long class II-associated invariant chain peptide (CLIP) occupying the HLA class II peptide-binding groove.16
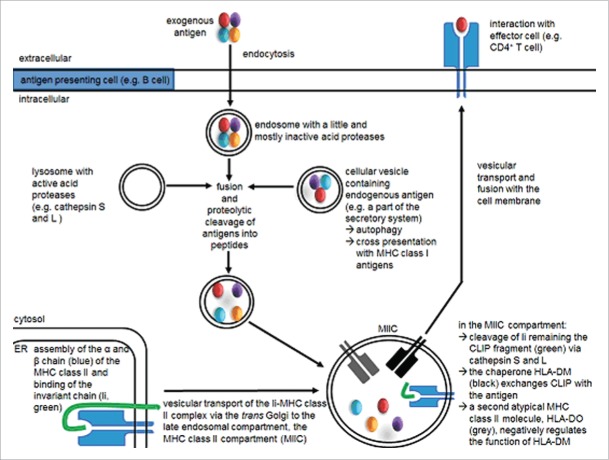
Figure 1.
HLA class II antigen-processing and presentation pathway. In APCs, newly synthesized HLA class II molecules are assembled in the ER and bind the Ii. The Ii direct the transport of HLA class II molecules directly or indirectly into the MIIC, where the li is degraded by different proteases leaving the peptide fragment CLIP still embedded in the HLA class II binding groove. An HLA class II-like molecule, HLA-DM, facilitates the release of CLIP and assists in the exchange of CLIP with relevant exogeneous antigenic fragments. Then the HLA class II peptide complex is transported to cell surface for presentation to CD4+ T cells.
Exogeneous engulfed proteins delivered into the endosomal/lysosomal network are also exposed to distinct endosomal/lysosomal aspartyl and cysteine proteases including cathepsin S,16 peptidases and reductases, such as the gamma-interferon inducible lysosomal thiol reductase GILT, yielding peptide ligands for HLA class II molecules.17,18 In the MIIC, the exchange of CLIP for such antigenic high-affinity peptides is facilitated by low pH, endosomal proteases and the assistance of the non-classical MHC class II molecule HLA-DM. The latter serves as a peptide editor.19 Following peptide loading, the HLA class II/peptide complex is then transported via the trans-Golgi to the cell surface and there presented to cognate CD4+ T cells. The CD4+ T cells have a helper function or display an effector function with HLA class II bearing tumor cells. The function of HLA class II proteins has been associated with the regulation of immune responses by presenting antigenic peptides to CD4+ T cells and by controlling B-cell differentiation into antibody-producing blasts. Efficient and long-lasting TAA-specific immunity requires both CD8+ CTL and CD4+ T lymphocytes during priming and effector phases of TAA-specific immune responses.20,21 However, not only exogeneous, but also endogeneous peptides can be loaded onto HLA class II molecules; 22 this process is mediated by an Ii-independent, but proteasome- and peptide transporter-dependent presentation pathway.23
Regulation of HLA class II antigen expression
The expression levels of HLA class II antigens are tightly regulated to ensure an immune response directed against pathogens as well as malignant or virally transformed cells.24 The promoters of HLA class II and related genes share a set of conserved sequence elements, the W/S, X1, X2 and Y boxes, which interact with transcription factors (TF) including members of the regulatory factor X (RFX) family, the nuclear factor Y and the cAMP-responsive element binding protein CREB.25,26 All these TF and co-factors bind to cis regulatory elements of the HLA class II promoters to form a highly stable multimeric complex known as MHC enhanceosome. Due to their ubiquitous expression, the enhanceosome components fail to account for the cell type specificity and/or IFNγ inducibility of HLA class II antigen expression.27 In contrast, the HLA class II transactivator CIITA, the master key transcriptional activator interacting with the DNA-binding proteins of the HLA class II promoters, exhibits a cell type-specific, cytokine-inducible and differentiation-specific expression pattern. This is controlled by the alternative usage of the four distinct CIITA promoters (CIITA-P) I, II, III and IV. CIITA-PIV is involved in the IFNγ-mediated induction of HLA class II surface expression. In addtion, to promote the binding of TF to CIITA-PIV, IFNγ also includes the acetylation of histones leading to an accessibility of CIITA-PIV.28 Furthermore, a variety of signal transduction pathways, such as PKA, upregulates CIITA expression, which, in turn, enhances HLA class II antigen cell surface expression.29
Expression of HLA class II antigens in malignant tumors
Malignant transformation of cells is not only associated with changes in HLA class I, but also in HLA class II antigen expression.12 The frequency of these changes varies among the different types of hematopoietic and solid tumors depending on their origin and/or molecular phenotype 23,30,31: for example, more than 80% of ductal breast carcinoma lesions analyzed lack HLA class II antigen expression.29,30 In contrast, about 50% of papillary thyroid carcinoma and 60% of primary melanoma express HLA class II antigens 31,32 suggesting a gain of HLA class II antigen expression in these tumor types. In addition, a heterogeneous intra-tumoral HLA class II antigen expression pattern was detected in HLA class II antigen-positive tumor lesions.33 While there is concordant information in the literature about the frequency of HLA class II antigen expression in some tumor types, in others the information is conflicting. For example, the frequency of HLA class II positivity in colorectal carcinoma (CRC) reported in the literature varied between 21 and 55% depending on the study.34-38 These different results may reflect differences in the methodology and antibodies used, in the characteristics of the patients' population included in the studies and in the molecular pathogenesis of the disease. In this context, it is noteworthy that HLA class II antigen expression in CRC is closely related to the high-level microsatellite instability (MSI-H) phenotype 33,39 underlining that a precise molecular classification is required to obtain reliable information about the frequency of HLA class II antigen expression in defined tumor subtypes.37 This conclusion is further supported by the observation in head and neck cancers, where HLA class II antigen expression is related to human papillomavirus infection of the lesions.40
Altered constitutive and IFNγ inducible HLA class II antigen expression in cancer cells
So far, five distinct HLA class II phenotypes have been described in human tumors, which are characterized by (i) a strong cytokine-independent homogeneous overexpression, (ii) total lack of expression, (iii) downregulation, (iv) altered IFNγ inducibility of basal HLA class II surface antigens in terms of level and kinetics quantity and time and (v) lack of IFNγ inducibility. In addition, a novel, cytokine-independent mechanism of HLA class II antigen upregulation has recently been detected in Schwann cells, which express high levels of HLA class II antigens upon inactivation of the tumor suppressor gene neurofibromin 1.41 The molecular mechanisms mediating the selective lack or downregulation of constitutive as well as IFNγ-mediated upregulation of HLA class II antigens by malignant cells have been characterized to a limited extent. They appear to be mainly mediated by structural abnormalities, by epigenetic, transcriptional and post-transcriptional regulation of HLA class II molecules and/or APM components (Table 2) 42 and by genomic instability.33 Furthermore, the function of genes encoding IFNγ as well as components of the IFNγ signal pathway, which regulate HLA class II antigen expression, may be impaired by multiple mechanisms. They include either gene-inactivating mutations, silencing through promoter methylation, transcriptional downregulation or post-translational alterations, such as an altered phosphorylation pattern.42-45 In the following section, we will focus on structural alterations and de-regulation of HLA class II antigen expression since they are the best characterized abnormalities affecting HLA class II antigen expression in tumors.
Table 2.
Molecular mechanisms causing HLA class II abnormalities in malignant tumors.
| Molecular mechanism | Tumor type |
|---|---|
| Mutations in CIITA and RFX5 | Colorectal cancer (MSI-H subtype)33, 47 |
| CIITA promoter polymorphism A to G substitution | Melanoma 99 |
| Transcriptional downregulation of the CIITA promoter due to HASH-1, L-myc and c-myc overexpression | SCLC, neuroblastoma 50 B cell tumors 49 |
 Hypermethylation of CIITA Hypermethylation of CIITA |
Teratocarcinoma 108 Embryonal carcinoma 108 Lymphoblastic leukemia 98,109 Ocular melanoma 100 Head and neck squamous cell carcinoma 102 B-cell lymphoma 95,96 |
Histone acetylation of CIITA
|
Squamous cell carcinoma 58 Leukemia60,109 |
| Post-transcriptional regulation of HLA class II antigens | Head and neck squamous cell carcinoma, ocular melanoma 101,102 |
| Absence of AIRE | Thymoma 106 |
| STAT1 defects associated with impaired IFNγ induction | Thymoma 106 |
| Low expression of GILT | Melanoma, large B cell lymphoma 66 |
| Decreased expression of HLA-DM | B-cell lymphoma 49 |
| Hypermethylation of HLA class II gene promoters | Esophageal squamous cell carcinoma 62 |
| Low CLIP expression | Acute myeloid leukemia 107 |
| Lack of HLA-DM | Head and neck squamous cell carcinoma 101 |
| CLIP occupation | B-cell lymphoma 103 |
| CIITA gene fusion | B-cell lymphoma 46 |
Structural alterations of HLA class II apm components in malignant cells
Sequence abnormalities, such as mutations or rearrangements in CIITA, RFX5, RFXAP and/or HLA class II alleles leading to impaired constitutive or IFNγ-inducible HLA class II expression have been described in particular in diffuse large B-cell lymphoma, CRC and melanoma.46,47 These structural alterations appear to occur at a low frequency, although this might be underestimated due to the heterogeneity of clinical samples. Mutations in the HLA class II-regulatory gene RFX5 were found at frequency of about 30% of HLA class II antigen-negative microsatellite-unstable (MSI-H) CRC (28.9%), while CIITA mutations were only found in about 5% of these lesions.33
Also in tumor types distinct from CRC, structural alterations of HLA class II antigen-regulatory genes have been detected. In melanoma A to G substitutions in the 5′ flanking region of the CIITA-PIII were found, which were associated with higher levels of constitutive HLA-DR expression.48 Recently, CIITA has been described as a recurrent gene fusion partner in B-cell lymphoma and Hodgkin's disease.46 The presence of the CIITA rearrangement was associated with HLA class II antigen downregulation and significantly correlated with a shorter disease-specific survival, which is compatible with a reduced immunogenicity of the affected tumor cells.46
Deregulation of HLA class II APM components in malignant cells
Impaired HLA class II antigen cell surface expression may be also caused by the deregulation of APM components at each step of this pathway. Suppression of CIITA transcription mediated by the overexpression of HASH-1, c-Myc and N-Myc, which competitively bind to the E-box in the CIITA-PIV has been reported in neuroblastoma, in small cell lung carcinoma (SCLC) and in B-cell tumors.49,50 In contrast, RET/PTC expression is correlated with increased CIITA expression associated with an increased HLA class II antigen expression.51 The lack of STAT1α and the retinoblastoma tumor suppressor gene affects CIITA expression and consequently leads to an impaired constitutive and IFNγ-inducible HLA class II antigen expression as demonstrated in breast carcinoma and NSCLC cells.50,52 Furthermore, a low frequency of mutations or downregulated expression of the interferon regulatory factor (IRF)-2 have been found in tumors, which is associated with impaired CIITA expression and IFNγ response.53 These data demonstrate an important role of the IFNγ signal pathway for both the basal and IFNγ-induced HLA class II expression.
In addition, an epigenetic control of different HLA class II APM components including HLA-DR and CIITA due to alterations in their chromatin accessibility and DNA methylation status has been described; these abnormalities lead to a lack of HLA class II antigen expression.42,54 These abnormalities could be reverted by treatment of tumor cells with pharmacologic substances like 5-aza-2-deoxycytidine (DAC) and trichostatin A (TSA), which induce DNA demethylation and block histone deacetylation, respectively.55 The hypermethylation of the CIITA promoter, in particular of CIITA-PIV was found at a high frequency in gastric cancers, HNSCC and ocular melanoma, but not in CRC suggesting tumor type-specific CIITA methylation.33,55,56 Promoters of the HLA class II antigen-encoding genes could also be directly silenced by methylation. In RFX5-negative B-lymphoma cells, the HLA-DO, HLA-DR and HLA-DQ promoters are methylated.57 Furthermore, modification of histone deacetylation could be also associated with CIITA silencing in some tumor types, like squamous cell carcinoma and hematopoietic tumors.58-61 The clinical relevance of the epigenetic control of HLA class II antigen expression was shown for esophageal squamous cell carcinoma and adrenocortical tumors promoting their recurrence and progression.62,63
Impaired HLA class II-restricted TAA presentation due to abnormalities in the processing pathway could limit CD4+ T-cell help for the induction of CD8+ T-cell responses.64 Intracellular li expression and occupancy of HLA class II antigen with CLIP as well as low to basal levels of GILT negatively interfere with the activation of CD4+ T cells reactive against acute myeloid leukemia (AML) and lymphoma cells. This caused immune tolerance or unresponsiveness to leukemia antigens and lead to a significantly shortened disease-free survival of patients with AML or diffuse large B-cell lymphoma.23,65,66 In contrast, some CLIP− tumor cells were able to process li-independent endogeneous antigens generated by the proteasome for HLA class II-restricted presentation thereby activating TAA-specific CD4+ T cells.23,67
Furthermore, it has recently been suggested that the different HLA class II phenotypes in tumors can be controlled at the post-transcriptional level, in particular by microRNAs (miRs).42 Bioinformatic analyses have identified a number of miRs binding to the 3′-untranslated region (3′-UTR) of CIITA. As an example, miR-150 was shown to abrogate CIITA expression in macrophages upon infection with pathogenic mycobacteria.68 However, a miR-mediated altered expression of HLA class II pathway components in tumors has not yet been identified. Furthermore, HLA class II molecules could be regulated by the interaction with ubiquitin ligases leading to their intracellular sequestration and degradation.69,70
Clinical significance of HLA class II antigens in cancer
A functional role of HLA class II antigens on the tumor cell surface is suggested by the observation that HLA class II antigen expression is related to prognosis in several types of cancers. However, the available information about the prognostic significance of HLA class II antigen expression in cancers is conflicting (Table 3). While constitutive HLA class II antigen expression was reported to be associated with a favorable prognosis in some tumor types, e. g. CRC and larynx squamous cell carcinoma,71-74 it was associated with higher metastatic dissemination, increased tumor stage and reduced patients' survival in other malignancies, such as melanoma and cervical carcinoma.75-77 Conflicting is the information about the clinical relevance of HLA class II antigen expression in osteosarcoma; however, the number of tumors analyzed is too small to draw conclusions.78,79
Table 3.
Clinical relevance of HLA class II expression.
| Tumor type | HLA class II level | Prognosis | Citation |
|---|---|---|---|
| CRC | High | Favorable | 72-74,80 |
| Larynx squamous cell carcinoma | High | Favorable | 71 |
| Oropharyngeal squamous arcinoma | High | Favorable | 40 |
| Melanoma | High | Poor | 76, 77 |
| Cervical cancer | High | Poor | 75 |
| Osteosarcoma | High | Controverse | 78, 79 |
| Pediatric adrenocortical tumors | Low | Poor | 63 |
In addition, even within defined cancer types, the prognostic value of HLA class II antigens is conflicting depending on the studies. Immunohistochemically detectable HLA class II antigen expression was associated with a better prognostic outcome of CRC patients in some studies,36 but not associated with tumor grade, stage and survival in other studies.37,80 A positive prognostic effect of HLA class II antigen expression in CRC may reflect the increased T-cell infiltration which is one of the strongest favorable prognostic biomarkers in CRC.81 Consequently, high levels of IFNγ might induce HLA class II antigen expression in densely T cell-infiltrated tumors, which has a good prognosis.
Immune selection of HLA class II-deficient tumors
The role of immune selection in the generation of tumors with defects in HLA class I antigen expression has been extensively investigated. In vitro experiments, studies in animal models and clinical investigations have convincingly shown that the selective pressure imposed by HLA class I antigen-restricted, TAA-specific T cells on a tumor cell population can facilitate the outgrowth of tumor cells, which are not recognized by cognate CTL due to defective synthesis and/or expression of HLA class I antigen-TAA-derived peptide complexes. The latter mediate the interactions of tumor cells with host's immune system.6,82 In contrast, limited information is available about the impact of immune selection on HLA class II antigen expression by tumor cells. We are aware of only one example suggesting that immune selection facilitates the outgrowth of HLA class II antigen-negative tumor cells: MSI-H CRCs are characterized by a pronounced antitumoral immune response, which is triggered by a high amount of mutation-induced neoantigens.83 In MSI-H CRCs, pronounced CD4+ T-cell infiltration was associated with HLA class II antigen loss induced by RFX5 or CIITA mutations.47 The association between CD4+ T-cell infiltration and lack of HLA class II antigen expression by tumor cells may reflect the role of immune selection in the generation of tumors without detectable HLA class II antigen expression. The selective pressure imposed by CD4+ cells on the tumor cell population may lead to the elimination of MSI-H CRC cells expressing HLA class II antigens by cognate CD4+ T cells and may facilitate the outgrowth of tumor cells, which have lost the ability to express HLA class II antigens. This possibility is supported by the detection of mutations in HLA class II-regulatory genes in the tumor cells, which do not express HLA class II antigens.33,47 If our interpretation is correct, our results imply that human TAA recognized by cognate CD4+ T cells are effective in inducing a TAA-specific immune response and in mediating tumor rejection through an antigen-specific CD4+ T-cell response. This hypothesis is supported by recent studies in mouse models reporting that the presence of MHC class II molecules on the tumor cell surface can mediate efficient elimination of tumor cells through direct recognition by CD4+ T cells.84-86
Beyond conventional recognition of HLA class II molecules by CD4+ T cells, the expression of HLA class II antigens may have consequences for tumor/immune cell interactions and tumor cell survival. For example, the interaction between LAG3, a surface molecule expressed on exhausted lymphocytes, and HLA class II antigens can enhance survival and apoptosis resistance of melanoma cells.87
Concluding remarks and perspectives
Although there exist major differences in the HLA class I and II APM pathways, interactions between both pathways have been identified, which allow the cross-presentation of antigens as a key feature for the induction of TAA- and viral antigen-specific CD8+ T cell responses.88 However, the mechanisms underlying antigen cross-presentation have not yet been defined, but an escape of internalized proteins from the endosomes via an unknown transporter is currently being discussed. Other possibilities involve the TAP-mediated transport of cytosolic peptides back to the phagosome or endosome 89,90 and the escort of the HLA class I antigens by the Ii to the endosomal compartment.91,92
The role of HLA class II antigen expression by tumors and its clinical relevance remains controversial and appears to depend on the tumor type including its genetic determinants. There is increasing evidence that HLA class II antigens on tumor cells shape the TAA-specific antitumoral immune response suggesting that HLA class II antigens may represent a novel therapeutic target.93 Thus, there are still many open questions, which have to be addressed in order to fully characterize the function of HLA class I and class II antigens in tumors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant from the German Cancer Aid 110703 (BS).
References
- 1.Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol 2007; 8:1041-8; PMID:17878914; http://dx.doi.org/ 10.1038/ni1516 [DOI] [PubMed] [Google Scholar]
- 2.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol 2008; 8:607-18; PMID:18641646; http://dx.doi.org/ 10.1038/nri2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garrido F, Algarra I, Garcia-Lora AM. The escape of cancer from T lymphocytes: immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol Immunother 2010; 59:1601-6; PMID:20625726; http://dx.doi.org/ 10.1007/s00262-010-0893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukur J, Jasinski S, Seliger B. The role of classical and non-classical HLA class I antigens in human tumors. Semin Cancer Biol 2012; 22:350-8; PMID:22465194; http://dx.doi.org/ 10.1016/j.semcancer.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Campoli M, Ferrone S. HLA antigen and NK cell activating ligand expression in malignant cells: a story of loss or acquisition. Semin Immunopathol 2011; 33:321-34; PMID:21523560; http://dx.doi.org/ 10.1007/s00281-011-0270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol 2014; 27:16-25; PMID:24531241; http://dx.doi.org/ 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbatino F, Schwab JH, Ferrone S, Ferrone CR. Evolution of studies of HLA class I antigen processing machinery (APM) components in malignant cells. Clin Transpl 2013:453-63; PMID:25095541 [PubMed] [Google Scholar]
- 8.Seliger B. The link between MHC class I abnormalities of tumors, oncogenes, tumor suppressor genes, and transcription factors. J Immunotoxicol 2014; 11:308-10; PMID:24479372; http://dx.doi.org/ 10.3109/1547691X.2013.875084 [DOI] [PubMed] [Google Scholar]
- 9.Natali PG, Segatto O, Ferrone S, Tosi R, Corte G. Differential tissue distribution and ontogeny of DC-1 and HLA-DR antigens. Immunogenetics 1984; 19:109-16; PMID:6421723; http://dx.doi.org/ 10.1007/BF00387853 [DOI] [PubMed] [Google Scholar]
- 10.van den Hoorn T, Paul P, Jongsma ML, Neefjes J. Routes to manipulate MHC class II antigen presentation. Curr Opin Immunol 2011; 23:88-95; PMID:21112200; http://dx.doi.org/ 10.1016/j.coi.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 11.Drozina G, Kohoutek J, Jabrane-Ferrat N, Peterlin BM. Expression of MHC II genes. Curr Top Microbiol Immunol 2005; 290:147-70; PMID:16480042 [DOI] [PubMed] [Google Scholar]
- 12.Natali PG, Bigotti A, Nicotra MR, Nardi RM, Delovu A, Segatto O, Ferrone S. Analysis of the antigenic profile of uveal melanoma lesions with anti-cutaneous melanoma-associated antigen and anti-HLA monoclonal antibodies. Cancer Res 1989; 49:1269-74; PMID:2917356 [PubMed] [Google Scholar]
- 13.Real FX, Houghton AN, Albino AP, Cordon-Cardo C, Melamed MR, Oettgen HF, Old LJ. Surface antigens of melanomas and melanocytes defined by mouse monoclonal antibodies: specificity analysis and comparison of antigen expression in cultured cells and tissues. Cancer Res 1985; 45:4401-11; PMID:4028024 [PubMed] [Google Scholar]
- 14.Rocha N, Neefjes J. MHC class II molecules on the move for successful antigen presentation. EMBO J 2008; 27:1-5; PMID:18046453; http://dx.doi.org/ 10.1038/sj.emboj.7601945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walchli S, Kumari S, Fallang LE, Sand KM, Yang W, Landsverk OJ, Bakke O, Olweus J, Gregers TF. Invariant chain as a vehicle to load antigenic peptides on human MHC class I for cytotoxic T-cell activation. Eur J Immunol 2014; 44:774-84; PMID:24293164; http://dx.doi.org/ 10.1002/eji.201343671 [DOI] [PubMed] [Google Scholar]
- 16.Blum JS, Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci USA 1988; 85:3975-9; PMID:3287381; http://dx.doi.org/ 10.1073/pnas.85.11.3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, Alfonso C, Karlsson L, Flavell RA, Cresswell P. Defective antigen processing in GILT-free mice. Science 2001; 294:1361-5; PMID:11701933; http://dx.doi.org/ 10.1126/science.1065500 [DOI] [PubMed] [Google Scholar]
- 18.Norton DL, Haque A. Insights into the role of GILT in HLA class II antigen processing and presentation by melanoma. J Oncol 2009; 2009:142959; PMID:20016802; http://dx.doi.org/ 10.1155/2009/142959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellins ED, Stern LJ. HLA-DM and HLA-DO, key regulators of MHC-II processing and presentation. Curr Opin Immunol 2014; 26:115-22; PMID:24463216; http://dx.doi.org/ 10.1016/j.coi.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB et al.. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 2013; 73:19-29; PMID:23087058; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1127 [DOI] [PubMed] [Google Scholar]
- 21.Fischer WH, thor Straten P, Terheyden P, Becker JC. Function and dysfunction of CD4(+) T cells in the immune response to melanoma. Cancer Immunol Immunother 1999; 48:363-70; PMID:10501848; http://dx.doi.org/ 10.1007/s002620050587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma W, Van den Eynde BJ. Endosomal compartment: Also a dock for MHC class I peptide loading. Eur J Immunol 2014; 44:650-3; PMID:24515851; http://dx.doi.org/ 10.1002/eji.201444470 [DOI] [PubMed] [Google Scholar]
- 23.van Luijn MM, Chamuleau ME, Thompson JA, Ostrand-Rosenberg S, Westers TM, Souwer Y, Ossenkoppele GJ, van Ham SM, van de Loosdrecht AA. Class II-associated invariant chain peptide down-modulation enhances the immunogenicity of myeloid leukemic blasts resulting in increased CD4+ T-cell responses. Haematologica 2010; 95:485-93; PMID:19903675; http://dx.doi.org/ 10.3324/haematol.2009.010595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Elsen PJ, Holling TM, Kuipers HF, van der Stoep N. Transcriptional regulation of antigen presentation. Curr Opin Immunol 2004; 16:67-75; PMID:14734112; http://dx.doi.org/ 10.1016/j.coi.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 25.Kern I, Steimle V, Siegrist CA, Mach B. The two novel MHC class II transactivators RFX5 and CIITA both control expression of HLA-DM genes. Int Immunol 1995; 7:1295-9; PMID:7495736; http://dx.doi.org/ 10.1093/intimm/7.8.1295 [DOI] [PubMed] [Google Scholar]
- 26.Lochamy J, Rogers EM, Boss JM. CREB and phospho-CREB interact with RFX5 and CIITA to regulate MHC class II genes. Mol Immunol 2007; 44:837-47; PMID:16730065; http://dx.doi.org/ 10.1016/j.molimm.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 27.Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med 1994; 180:1367-74; PMID:7931070; http://dx.doi.org/ 10.1084/jem.180.4.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris AC, Beresford GW, Mooney MR, Boss JM. Kinetics of a gamma interferon response: expression and assembly of CIITA promoter IV and inhibition by methylation. Mol Cell Biol 2002; 22:4781-91; PMID:12052885; http://dx.doi.org/ 10.1128/MCB.22.13.4781-4791.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazzaro B, Anderson AE, Kajdacsy-Balla A, Hessner MJ. Antigenic characterization of medullary carcinoma of the breast: HLA-DR expression in lymph node positive cases. Appl Immunohistochem Mol Morphol 2001; 9:234-41; PMID:11556751 [DOI] [PubMed] [Google Scholar]
- 30.Concha A, Ruiz-Cabello F, Cabrera T, Nogales F, Collado A, Garrido F. Different patterns of HLA-DR antigen expression in normal epithelium, hyperplastic and neoplastic malignant lesions of the breast. Eur J Immunogenet 1995; 22:299-310; PMID:7495782; http://dx.doi.org/ 10.1111/j.1744-313X.1995.tb00246.x [DOI] [PubMed] [Google Scholar]
- 31.Taramelli D, Fossati G, Mazzocchi A, Delia D, Ferrone S, Parmiani G. Classes I and II HLA and melanoma-associated antigen expression and modulation on melanoma cells isolated from primary and metastatic lesions. Cancer Res 1986; 46:433-9; PMID:3079589 [PubMed] [Google Scholar]
- 32.Jo YS, Lee JC, Li S, Choi YS, Bai YS, Kim YJ, Lee IS, Rha SY, Ro HK, Kim JM et al.. Significance of the expression of major histocompatibility complex class II antigen, HLA-DR and -DQ, with recurrence of papillary thyroid cancer. Int J Cancer Journal international du cancer 2008; 122:785-90; PMID:17957790; http://dx.doi.org/ 10.1002/ijc.23167 [DOI] [PubMed] [Google Scholar]
- 33.Michel S, Linnebacher M, Alcaniz J, Voss M, Wagner R, Dippold W, Becker C, von Knebel Doeberitz M, Ferrone S, Kloor M. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer Journal international du cancer 2010; 127:889-98; PMID:20013806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez J, Lopez-Nevot MA, Cabrera T, Oliva R, Esquivias J, Ruiz-Cabello F, Garrido F. Class I and II HLA antigen distribution in normal mucosa, adenoma and colon carcinoma: relation with malignancy and invasiveness. Exp Clin Immunogenet 1987; 4:144-52; PMID:2856203 [PubMed] [Google Scholar]
- 35.McDougall CJ, Ngoi SS, Goldman IS, Godwin T, Felix J, DeCosse JJ, Rigas B. Reduced expression of HLA class I and II antigens in colon cancer. Cancer Res 1990; 50:8023-7; PMID:2123744 [PubMed] [Google Scholar]
- 36.Sconocchia G, Eppenberger-Castori S, Zlobec I, Karamitopoulou E, Arriga R, Coppola A, Caratelli S, Spagnoli GC, Lauro D, Lugli A et al.. HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia 2014; 16:31-42; PMID:24563618; http://dx.doi.org/ 10.1593/neo.131568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh MD, Dent OF, Young JP, Wright CM, Barker MA, Leggett BA, Bokey L, Chapuis PH, Jass JR, Macdonald GA. HLA-DR expression is associated with better prognosis in sporadic Australian clinicopathological Stage C colorectal cancers. Int J Cancer Journal international du cancer 2009; 125:1231-7; PMID:19462453; http://dx.doi.org/ 10.1002/ijc.24484 [DOI] [PubMed] [Google Scholar]
- 38.Wolkersdorfer T, Fussel M, Kiesslich T, Neureiter D, Berr F, Aust D, Wolkersdörfer GW. MHC class II genotype- and MHC class I and II phenotype-related parameters in sporadic colorectal cancer. Oncol Rep 2011; 26:1165-71; PMID:21874254 [DOI] [PubMed] [Google Scholar]
- 39.Lovig T, Andersen SN, Thorstensen L, Diep CB, Meling GI, Lothe RA, Rognum TO. Strong HLA-DR expression in microsatellite stable carcinomas of the large bowel is associated with good prognosis. Br J Cancer 2002; 87:756-62; PMID:12232760; http://dx.doi.org/ 10.1038/sj.bjc.6600507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasman A, Andersson E, Marklund L, Tertipis N, Hammarstedt-Nordenvall L, Attner P, Nyberg T, Masucci GV, Munck-Wikland E, Ramqvist T et al.. HLA class I and II expression in oropharyngeal squamous cell carcinoma in relation to tumor HPV status and clinical outcome. PloS One 2013; 8:e77025; PMID:24130830; http://dx.doi.org/ 10.1371/journal.pone.0077025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuss DE, Mucha J, Holtkamp N, Muller U, Berlien HP, Mautner VF, Ehemann V, Platten M, Scheffzek K, von Deimling A. Functional MHC class II is upregulated in neurofibromin-deficient Schwann cells. J Invest Dermatol 2013; 133:1372-5; PMID:23303456; http://dx.doi.org/ 10.1038/jid.2012.488 [DOI] [PubMed] [Google Scholar]
- 42.Tomasi TB, Magner WJ, Wiesen JL, Oshlag JZ, Cao F, Pontikos AN, Gregorie CJ. MHC class II regulation by epigenetic agents and microRNAs. Immunol Res 2010; 46:45-58; PMID:19771399; http://dx.doi.org/ 10.1007/s12026-009-8128-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Respa A, Bukur J, Ferrone S, Pawelec G, Zhao Y, Wang E, Marincola FM, Seliger B. Association of IFN-gamma signal transduction defects with impaired HLA class I antigen processing in melanoma cell lines. Clin Cancer Res 2011; 17:2668-78; PMID:21248298; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenzweig SD, Holland SM. Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev 2005; 203:38-47; PMID:15661020; http://dx.doi.org/ 10.1111/j.0105-2896.2005.00227.x [DOI] [PubMed] [Google Scholar]
- 45.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell 2002; 109:S21-33; PMID:11983150; http://dx.doi.org/ 10.1016/S0092-8674(02)00696-7 [DOI] [PubMed] [Google Scholar]
- 46.Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, Johnson NA, Zhao Y, Telenius A, Neriah SB et al.. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 2011; 471:377-81; PMID:21368758; http://dx.doi.org/ 10.1038/nature09754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surmann EM, Voigt AY, Michel S, Bauer K, Reuschenbach M, Ferrone S, von Knebel Doeberitz M, Kloor M. Association of high CD4-positive T cell infiltration with mutations in HLA class II-regulatory genes in microsatellite-unstable colorectal cancer. Cancer Immunol Immunother 2014; 64:357-66; PMID:25445815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez T, Mendez R, Del Campo A, Aptsiauri N, Martin J, Orozco G, Pawelec G, Schadendorf D, Ruiz-Cabello F, Garrido F. Patterns of constitutive and IFN-gamma inducible expression of HLA class II molecules in human melanoma cell lines. Immunogenetics 2007; 59:123-33; PMID:17180681; http://dx.doi.org/ 10.1007/s00251-006-0171-9 [DOI] [PubMed] [Google Scholar]
- 49.God JM, Cameron C, Figueroa J, Amria S, Hossain A, Kempkes B, Bornkamm GW, Stuart RK, Blum JS, Haque A. Elevation of c-MYC disrupts HLA class II-mediated immune recognition of human B cell tumors. J Immunol 2015; 194:1434-45; http://dx.doi.org/ 10.4049/jimmunol.1402382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yazawa T, Ito T, Kamma H, Suzuki T, Okudela K, Hayashi H, Horiguchi H, Ogata T, Mitsui H, Ikeda M et al.. Complicated mechanisms of class II transactivator transcription deficiency in small cell lung cancer and neuroblastoma. Am J Pathol 2002; 161:291-300; PMID:12107114; http://dx.doi.org/ 10.1016/S0002-9440(10)64181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang ES, Kim DW, Hwang JH, Jung HS, Suh JM, Park YJ, Chung HK, Song JH, Park KC, Park SH et al.. Regulation of signal transducer and activator of transcription 1 (STAT1) and STAT1-dependent genes by RET/PTC (rearranged in transformation/papillary thyroid carcinoma) oncogenic tyrosine kinases. Mol Endocrinol 2004; 18:2672-84; PMID:15297606; http://dx.doi.org/ 10.1210/me.2004-0168 [DOI] [PubMed] [Google Scholar]
- 52.Lu Y, Tschickardt ME, Schmidt BJ, Blanck G. IFN-gamma inducibility of class II transactivator is specifically lacking in human tumour lines: relevance to retinoblastoma protein rescue of IFN-gamma inducibility of the HLA class II genes. Immunol Cell Biol 1997; 75:325-32; PMID:9315472; http://dx.doi.org/ 10.1038/icb.1997.50 [DOI] [PubMed] [Google Scholar]
- 53.Xi H, Blanck G. The IRF-2 DNA binding domain facilitates the activation of the class II transactivator (CIITA) type IV promoter by IRF-1. Mol Immunol 2003; 39:677-84; PMID:12493643; http://dx.doi.org/ 10.1016/S0161-5890(02)00214-6 [DOI] [PubMed] [Google Scholar]
- 54.Chou SD, Khan AN, Magner WJ, Tomasi TB. Histone acetylation regulates the cell type specific CIITA promoters, MHC class II expression and antigen presentation in tumor cells. Int Immunol 2005; 17:1483-94; PMID:16210330; http://dx.doi.org/ 10.1093/intimm/dxh326 [DOI] [PubMed] [Google Scholar]
- 55.Satoh A, Toyota M, Ikeda H, Morimoto Y, Akino K, Mita H, Suzuki H, Sasaki Y, Kanaseki T, Takamura Y et al.. Epigenetic inactivation of class II transactivator (CIITA) is associated with the absence of interferon-gamma-induced HLA-DR expression in colorectal and gastric cancer cells. Oncogene 2004; 23:8876-86; PMID:15467734; http://dx.doi.org/ 10.1038/sj.onc.1208144 [DOI] [PubMed] [Google Scholar]
- 56.Radosevich M, Song Z, Gorga JC, Ksander B, Ono SJ. Epigenetic silencing of the CIITA gene and posttranscriptional regulation of class II MHC genes in ocular melanoma cells. Invest Ophthalmol Vis Sci 2004; 45:3185-95; PMID:15326139; http://dx.doi.org/ 10.1167/iovs.04-0111 [DOI] [PubMed] [Google Scholar]
- 57.Seguin-Estevez Q, De Palma R, Krawczyk M, Leimgruber E, Villard J, Picard C, Tagliamacco A, Abbate G, Gorski J, Nocera A et al.. The transcription factor RFX protects MHC class II genes against epigenetic silencing by DNA methylation. J Immunol 2009; 183:2545-53; http://dx.doi.org/ 10.4049/jimmunol.0900376 [DOI] [PubMed] [Google Scholar]
- 58.Kanaseki T, Ikeda H, Takamura Y, Toyota M, Hirohashi Y, Tokino T, Himi T, Sato N. Histone deacetylation, but not hypermethylation, modifies class II transactivator and MHC class II gene expression in squamous cell carcinomas. J Immunol 2003; 170:4980-5; http://dx.doi.org/ 10.4049/jimmunol.170.10.4980 [DOI] [PubMed] [Google Scholar]
- 59.LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol 2004; 34:1513-25; PMID:15162420; http://dx.doi.org/ 10.1002/eji.200424964 [DOI] [PubMed] [Google Scholar]
- 60.Morimoto Y, Toyota M, Satoh A, Murai M, Mita H, Suzuki H, Takamura Y, Ikeda H, Ishida T, Sato N, Inactivation of class II transactivator by DNA methylation and histone deacetylation associated with absence of HLA-DR induction by interferon-gamma in haematopoietic tumour cells. Br J Cancer 2004; 90:844-52; PMID:14970863; http://dx.doi.org/ 10.1038/sj.bjc.6601602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zika E, Ting JP. Epigenetic control of MHC-II: interplay between CIITA and histone-modifying enzymes. Curr Opin Immunol 2005; 17:58-64; PMID:15653312; http://dx.doi.org/ 10.1016/j.coi.2004.11.008 [DOI] [PubMed] [Google Scholar]
- 62.Hu JM, Li L, Chen YZ, Liu C, Cui X, Yin L, Yang L, Zou H, Pang L, Zhao J et al.. HLA-DRB1 and HLA-DQB1 methylation changes promote the occurrence and progression of Kazakh ESCC. Epigenetics 2014; 9:1366-73; PMID:25437052; http://dx.doi.org/ 10.4161/15592294.2014.969625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leite FA, Lira RC, Fedatto PF, Antonini SR, Martinelli CE Jr., de Castro M, Neder L, Ramalho LN, Tucci S Jr, Mastelaro MJ et al.. Low expression of HLA-DRA, HLA-DPA1, and HLA-DPB1 is associated with poor prognosis in pediatric adrenocortical tumors (ACT). Pediatr Blood Cancer 2014; 61:1940-8; PMID:25156210; http://dx.doi.org/ 10.1002/pbc.25118 [DOI] [PubMed] [Google Scholar]
- 64.Gerner MY, Casey KA, Mescher MF. Defective MHC class II presentation by dendritic cells limits CD4 T cell help for antitumor CD8 T cell responses. J Immunol 2008; 181:155-64; PMID:18566380; http://dx.doi.org/ 10.4049/jimmunol.181.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chamuleau ME, Souwer Y, Van Ham SM, Zevenbergen A, Westers TM, Berkhof J, Meijer CJ, van de Loosdrecht AA, Ossenkoppele GJ. Class II-associated invariant chain peptide expression on myeloid leukemic blasts predicts poor clinical outcome. Cancer Res 2004; 64:5546-50; PMID:15313888; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1350 [DOI] [PubMed] [Google Scholar]
- 66.Phipps-Yonas H, Cui H, Sebastiao N, Brunhoeber PS, Haddock E, Deymier MJ, Klapper W, Lybarger L, Roe DJ, Hastings KT. Low GILT expression is associated with poor patient survival in diffuse large B-cell lymphoma. Front Immunol 2013; 4:425; PMID:24409177; http://dx.doi.org/ 10.3389/fimmu.2013.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Luijn MM, van den Ancker W, Chamuleau ME, Zevenbergen A, Westers TM, Ossenkoppele GJ, van Ham SM, van de Loosdrecht AA. Absence of class II-associated invariant chain peptide on leukemic blasts of patients promotes activation of autologous leukemia-reactive CD4+ T cells. Cancer Res 2011; 71:2507-17; PMID:21310823; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-3689 [DOI] [PubMed] [Google Scholar]
- 68.Ghorpade DS, Holla S, Sinha AY, Alagesan SK, Balaji KN. Nitric oxide and KLF4 protein epigenetically modify class II transactivator to repress major histocompatibility complex II expression during Mycobacterium bovis bacillus Calmette-Guerin infection. J Biol Chem 2013; 288:20592-606; PMID:23733190; http://dx.doi.org/ 10.1074/jbc.M113.472183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohmura-Hoshino M, Matsuki Y, Aoki M, Goto E, Mito M, Uematsu M, Kakiuchi T, Hotta H, Ishido S. Inhibition of MHC class II expression and immune responses by c-MIR. J Immunol 2006; 177:341-54; PMID:16785530; http://dx.doi.org/ 10.4049/jimmunol.177.1.341 [DOI] [PubMed] [Google Scholar]
- 70.Thibodeau J, Bourgeois-Daigneault MC, Huppe G, Tremblay J, Aumont A, Houde M, Bartee E, Brunet A, Gauvreau ME, de Gassart A et al.. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol 2008; 38:1225-30; PMID:18389477; http://dx.doi.org/ 10.1002/eji.200737902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esteban F, Ruiz-Cabello F, Concha A, Perez-Ayala M, Sanchez-Rozas JA, Garrido F. HLA-DR expression is associated with excellent prognosis in squamous cell carcinoma of the larynx. Clin Exp Metastasis 1990; 8:319-28; PMID:2350918; http://dx.doi.org/ 10.1007/BF01810678 [DOI] [PubMed] [Google Scholar]
- 72.Kunihiro M, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Combined expression of HLA-DR antigen and proliferating cell nuclear antigen correlate with colorectal cancer prognosis. Oncology 1998; 55:326-33; PMID:9663422; http://dx.doi.org/ 10.1159/000011870 [DOI] [PubMed] [Google Scholar]
- 73.Lazaris AC, Theodoropoulos GE, Davaris PS, Panoussopoulos D, Nakopoulou L, Kittas C, Golematis BC. Heat shock protein 70 and HLA-DR molecules tissue expression. Prognostic implications in colorectal cancer. Dis Colon Rectum 1995; 38:739-45; PMID:7607036; http://dx.doi.org/ 10.1007/BF02048033 [DOI] [PubMed] [Google Scholar]
- 74.Morita M, Tanaka K, Kawanishi H, Tsuji M, Ookusa T, Takada H, Okamura A, Hioki K. Immunohistochemically demonstrated expression of HLA-DR antigen in colorectal adenocarcinomas and its relation to clinicopathological features. J Surg Oncol 1995; 59:233-8; PMID:7630170 [DOI] [PubMed] [Google Scholar]
- 75.Hilders CG, Houbiers JG, van Ravenswaay Claasen HH, Veldhuizen RW, Fleuren GJ. Association between HLA-expression and infiltration of immune cells in cervical carcinoma. Lab Invest 1993; 69:651-9; PMID:8264228 [PubMed] [Google Scholar]
- 76.Moretti S, Pinzi C, Berti E, Spallanzani A, Chiarugi A, Boddi V, Reali UM, Giannotti B. In situ expression of transforming growth factor beta is associated with melanoma progression and correlates with Ki67, HLA-DR and beta 3 integrin expression. Melanoma Res 1997; 7:313-21; PMID:9293481; http://dx.doi.org/ 10.1097/00008390-199708000-00006 [DOI] [PubMed] [Google Scholar]
- 77.Pollack MS, Livingston PO. HLA and DR antigen frequencies in melanoma patients: possible relation to disease prognosis. Tissue antigens 1985; 26:262-5; PMID:3878014; http://dx.doi.org/ 10.1111/j.1399-0039.1985.tb00970.x [DOI] [PubMed] [Google Scholar]
- 78.Swanson PE, Wick MR. HLA-DR (Ia-like) reactivity in tumors of bone and soft tissue: an immunohistochemical comparison of monoclonal antibodies LN3 and LK8D3 in routinely processed specimens. Mod Pathol 1990; 3:113-9; PMID:1691491 [PubMed] [Google Scholar]
- 79.Zhou Q, Xu J, Zhao J, Zhang S, Pan W. Downregulation of CD99 and upregulation of human leukocyte antigen class II promote tumor aggravation and poor survival in patients with osteosarcomas. Onco Targets Ther 2014; 7:477-84; PMID:24711704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Degener T, Momburg F, Moller P. Differential expression of HLA-DR, HLA-DP, HLA-DQ and associated invariant chain (Ii) in normal colorectal mucosa, adenoma and carcinoma. Virchows Arch A Pathol Anat Histopathol 1988; 412:315-22; PMID:3125670; http://dx.doi.org/ 10.1007/BF00750257 [DOI] [PubMed] [Google Scholar]
- 81.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 82.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:21436444; http://dx.doi.org/ 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 83.von Knebel Doeberitz M, Kloor M. Towards a vaccine to prevent cancer in Lynch syndrome patients. Familial Cancer 2013; 12:307-12; PMID:23760517; http://dx.doi.org/ 10.1007/s10689-013-9662-7 [DOI] [PubMed] [Google Scholar]
- 84.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007; 109:5346-54; PMID:17327412; http://dx.doi.org/ 10.1182/blood-2006-10-051318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207:651-67; PMID:20156973; http://dx.doi.org/ 10.1084/jem.20091921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zanetti M. Tapping CD4 T cells for cancer immunotherapy: the choice of personalized genomics. J Immunol 2015; 194:2049-56; http://dx.doi.org/ 10.4049/jimmunol.1402669 [DOI] [PubMed] [Google Scholar]
- 87.Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H, Triebel F, Charron D, Aoudjit F, Al-Daccak R et al.. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol 2011; 186:5173-83; http://dx.doi.org/ 10.4049/jimmunol.1002050 [DOI] [PubMed] [Google Scholar]
- 88.Win SJ, Ward VK, Dunbar PR, Young SL, Baird MA. Cross-presentation of epitopes on virus-like particles via the MHC I receptor recycling pathway. Immunol Cell Biol 2011; 89:681-8; PMID:21221122; http://dx.doi.org/ 10.1038/icb.2010.161 [DOI] [PubMed] [Google Scholar]
- 89.Li Y, Wang LX, Pang P, Twitty C, Fox BA, Aung S, Urba WJ, Hu HM. Cross-presentation of tumor associated antigens through tumor-derived autophagosomes. Autophagy 2009; 5:576-7; PMID:19333005; http://dx.doi.org/ 10.4161/auto.5.4.8366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lopez-Albaitero A, Mailliard R, Hackman T, Andrade Filho PA, Wang X, Gooding W, Ferrone S, Kalinski P, Ferris RL. Maturation pathways of dendritic cells determine TAP1 and TAP2 levels and cross-presenting function. J Immunother 2009; 32:465-73; PMID:19609238; http://dx.doi.org/ 10.1097/CJI.0b013e3181a1c24e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alatrash G, Ono Y, Sergeeva A, Sukhumalchandra P, Zhang M, St John LS, Yang TH, Ruisaard K, Armistead PM, Mittendorf EA et al.. The role of antigen cross-presentation from leukemia blasts on immunity to the leukemia-associated antigen PR1. J Immunother 2012; 35:309-20; PMID:22495388; http://dx.doi.org/ 10.1097/CJI.0b013e31824b3b14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lorenzi S, Mattei F, Sistigu A, Bracci L, Spadaro F, Sanchez M, Spada M, Belardelli F, Gabriele L, Schiavoni G. Type I IFNs control antigen retention and survival of CD8alpha(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J Immunol 2011; 186:5142-50; PMID:21441457; http://dx.doi.org/ 10.4049/jimmunol.1004163 [DOI] [PubMed] [Google Scholar]
- 93.Altomonte M, Fonsatti E, Visintin A, Maio M. Targeted therapy of solid malignancies via HLA class II antigens: a new biotherapeutic approach?. Oncogene 2003; 22:6564-9; PMID:14528281; http://dx.doi.org/ 10.1038/sj.onc.1206960 [DOI] [PubMed] [Google Scholar]
- 94.Bhatia A, Kumar Y. Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Expert Rev Clin Immunol 2014; 10:41-62; PMID:24325346; http://dx.doi.org/ 10.1586/1744666X.2014.865519 [DOI] [PubMed] [Google Scholar]
- 95.Booman M, Douwes J, Glas AM, Riemersma SA, Jordanova ES, Kok K, Rosenwald A, de Jong D, Schuuring E, Kluin PM. Mechanisms and effects of loss of human leukocyte antigen class II expression in immune-privileged site-associated B-cell lymphoma. Clin Cancer Res 2006; 12:2698-705; PMID:16675561; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-2617 [DOI] [PubMed] [Google Scholar]
- 96.Cycon KA, Mulvaney K, Rimsza LM, Persky D, Murphy SP. Histone deacetylase inhibitors activate CIITA and MHC class II antigen expression in diffuse large B-cell lymphoma. Immunology 2013; 140:259-72; PMID:23789844; http://dx.doi.org/ 10.1111/imm.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.del Campo AB, Carretero J, Aptsiauri N, Garrido F. Targeting HLA class I expression to increase tumor immunogenicity. Tissue antigens 2012; 79:147-54; PMID:22309256; http://dx.doi.org/ 10.1111/j.1399-0039.2011.01831.x [DOI] [PubMed] [Google Scholar]
- 98.Holling TM, Schooten E, Langerak AW, van den Elsen PJ. Regulation of MHC class II expression in human T-cell malignancies. Blood 2004; 103:1438-44; PMID:14563641; http://dx.doi.org/ 10.1182/blood-2003-05-1491 [DOI] [PubMed] [Google Scholar]
- 99.Janitz M, Reiners-Schramm L, Muhlethaler-Mottet A, Rosowski M, Lauster R. Analysis of the sequence polymorphism within class II transactivator gene promoters. Exp Clin Immunogenet 2001; 18:199-205; PMID:11872950; http://dx.doi.org/ 10.1159/000049198 [DOI] [PubMed] [Google Scholar]
- 100.Liu S, Ren S, Howell P, Fodstad O, Riker AI. Identification of novel epigenetically modified genes in human melanoma via promoter methylation gene profiling. Pigment Cell Melanoma Res 2008; 21:545-58; PMID:18627528; http://dx.doi.org/ 10.1111/j.1755-148X.2008.00484.x [DOI] [PubMed] [Google Scholar]
- 101.Meissner M, Whiteside TL, Kaufmann R, Seliger B. CIITA versus IFN-gamma induced MHC class II expression in head and neck cancer cells. Arch Dermatol Res 2009; 301:189-93; PMID:19104823; http://dx.doi.org/ 10.1007/s00403-008-0922-6 [DOI] [PubMed] [Google Scholar]
- 102.Meissner M, Whiteside TL, van Kuik-Romein P, Valesky EM, van den Elsen PJ, Kaufmann R, Seliger B. Loss of interferon-gamma inducibility of the MHC class II antigen processing pathway in head and neck cancer: evidence for post-transcriptional as well as epigenetic regulation. Br J Dermatol 2008; 158:930-40; PMID:18284388; http://dx.doi.org/ 10.1111/j.1365-2133.2008.08465.x [DOI] [PubMed] [Google Scholar]
- 103.Radwan FF, Zhang L, Hossain A, Doonan BP, God JM, Haque A. Mechanisms regulating enhanced human leukocyte antigen class II-mediated CD4 + T cell recognition of human B-cell lymphoma by resveratrol. Leukemia & lymphoma 2012; 53:305-14; PMID:21854084; http://dx.doi.org/ 10.3109/10428194.2011.615423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Safaeian M, Johnson LG, Yu K, Wang SS, Gravitt PE, Hansen JA, Carrington M, Schwartz SM, Gao X, Hildesheim A et al.. Human leukocyte antigen Class I and II alleles and cervical adenocarcinoma. Front Oncol 2014; 4:119; PMID:24995157; http://dx.doi.org/ 10.3389/fonc.2014.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skibola CF, Akers NK, Conde L, Ladner M, Hawbecker SK, Cohen F, Ribas F, Erlich HA, Goodridge D, Trachtenberg EA et al.. Multi-locus HLA class I and II allele and haplotype associations with follicular lymphoma. Tissue Antigens 2012; 79:279-86; PMID:22296171; http://dx.doi.org/ 10.1111/j.1399-0039.2012.01845.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strobel P, Chuang WY, Chuvpilo S, Zettl A, Katzenberger T, Kalbacher H, Rieckmann P, Nix W, Schalke B, Gold R et al.. Common cellular and diverse genetic basis of thymoma-associated myasthenia gravis: role of MHC class II and AIRE genes and genetic polymorphisms. Ann N Y Acad Sci 2008; 1132:143-56; PMID:18567864; http://dx.doi.org/ 10.1196/annals.1405.018 [DOI] [PubMed] [Google Scholar]
- 107.van den Ancker W, van Luijn MM, Chamuleau ME, Kelder A, Feller N, Terwijn M, Zevenbergen A, Schuurhuis GJ, van Ham SM, Westers TM et al.. High class II-associated invariant chain peptide expression on residual leukemic cells is associated with increased relapse risk in acute myeloid leukemia. Leuk Res 2014; 38:691-3; PMID:24731748; http://dx.doi.org/ 10.1016/j.leukres.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 108.van den Elsen PJ, van der Stoep N, Vietor HE, Wilson L, van Zutphen M, Gobin SJ. Lack of CIITA expression is central to the absence of antigen presentation functions of trophoblast cells and is caused by methylation of the IFN-gamma inducible promoter (PIV) of CIITA. Hum Immunol 2000; 61:850-62; PMID:11053628; http://dx.doi.org/ 10.1016/S0198-8859(00)00159-2 [DOI] [PubMed] [Google Scholar]
- 109.van Eggermond MC, Boom DR, Klous P, Schooten E, Marquez VE, Wierda RJ, Holling TM, van den Elsen PJ. Epigenetic regulation of CIITA expression in human T-cells. Biochem Pharmacol 2011; 82:1430-7; PMID:21664896; http://dx.doi.org/ 10.1016/j.bcp.2011.05.026 [DOI] [PubMed] [Google Scholar]
- 110.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HM et al.. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015; 35:S185-98; PMID:25818339 [DOI] [PubMed] [Google Scholar]
- 111.Xiao X, Liu L, Li WJ, Liu J, Chen DJ. HLA-A, HLA-B, HLA-DRB1 polymorphisms and risk of cervical squamous epithelial cell carcinoma: a population study in China. Asian Pac J Cancer Prev 2013; 14:4427-33; PMID:23992015; http://dx.doi.org/ 10.7314/APJCP.2013.14.7.4427 [DOI] [PubMed] [Google Scholar]
- 112.Zhukov VF, Vasil'chenko MI, Proletarskii AV. Surveillance of prostate adenoma patients administering selective alpha-1a-adrenoblocker. Voen Med Zh 2004; 325:44-7; PMID:15188540 [PubMed] [Google Scholar]