ABSTRACT
Vaccination with dendritic cells (DCs), the most potent professional antigen-presenting cells in the body, is a promising approach in cancer immunotherapy. However, tumors induce immunosuppression in their microenvironment that suppresses and impairs the function of DCs. Therefore, human clinical trials with DC therapy have often been disappointing. To improve the therapeutic efficacy and to overcome the major obstacles of DC therapy, we generated a novel adenovirus, Ad3-hTERT-CMV-hCD40L, which is fully serotype 3 and expresses hCD40L for induction of antitumor immune response. The specific aim is to enhance DCs function. Data from a human cancer patient indicated that this capsid allows effective transduction of distant tumors through the intravenous route. Moreover, patient data suggested that virally produced hCD40L can activate DCs in situ. The virus was efficient in vitro and had potent antitumor activity in vivo. In a syngeneic model, tumors treated with Ad5/3-CMV-mCD40L virus plus DCs elicited greater antitumor effect as compared with either treatment alone. Moreover, virally coded CD40L induced activation of DCs, which in turn, lead to the induction of a Th1 immune response and increased tumor-specific T cells. In conclusion, Ad3-hTERT-CMV-hCD40L is promising for translation into human trials. In particular, this virus could enable successful dendritic cell therapy in cancer patients.
KEYWORDS: CD40 ligand, dendritic cells, oncolytic adenovirus, T-cells, tumor microenvironment
Abbreviations
- ACK
ammonium-chloride-potassium lysis buffer
- Ad5
serotype 5 adenoviruses
- APCs
antigen-presenting cells
- ATAP
Advanced Therapy Access Program
- BD
Becton Dickinson
- BM
bone marrow
- CBA
cytometric bead array
- CTL
cytotoxic T lymphocyte
- DCs
dendritic cells
- DMEM
Dulbecco's modified Eagle's medium
- FCAP
flow cytometric analysis program
- GMCSF
granulocyte macrophage colony-stimulation factor
- hCD40L
human CD40 ligand
- hTERT
human telomerase reverse transcriptase
- IFN-gamma
interferon gamma
- IL-12
interleukin 12
- LPS
lipopolysaccharide
- mCD40L
murine CD40 ligand
- mGMCSF
murine granulocyte macrophage colony-stimulation factor
- NK
natural killer cells
- PAP
prostatic acid phosphatase
- RANTES
regulated on activation; normal T cell expressed and secreted
- SEAP
secreted embryonic alkaline phosphatase
- TAA
tumor-associated antigens
- Th1
T helper type 1 cells
- Th2
T helper type 2 cells
- TGF-β
transforming growth factor - β
- TME
tumor microenvironment
- TNF-α
tumor necrosis factor - α
- VEGF
vascular endothelial growth factor
Introduction
Oncolytic adenoviruses are modified so that they replicate in and lyse only cancer cells.1 This leads to the presentation of tumor-associated antigens (TAA) to immunological cells, which results in the activation of antitumor immune responses.2,3 Importantly, this occurs in the presence of danger signals, which is conducive to immunity versus tolerance.4 Thus, in essence oncolytic adenoviruses represent an individualized cancer vaccine for each patient. This occurs in situ, without the need to guess the relevant epitopes. If there is variation in epitopes in different tumor regions, oncolysis will still continue to present them. Unfortunately, adenovirus alone is not potent enough to initiate a powerful vaccine effect thus presenting the rationale for arming them with immunostimulatory molecules such as CD40L, a potent stimulator of dendritic cells (DCs).4-7
There are more than 50 identified serotypes of adenoviruses. Serotype 5 adenoviruses (Ad5) are commonly used in gene therapy applications.8 However, the Ad5 receptor is known to be downregulated in advanced tumors.9 To overcome this problem, Ad5/3 chimeric viruses featuring fiber knob from serotype 3 have been developed. This modification enhances transduction, but does not result in complete escape from immune responses against the Ad5 capsid as only the fiber is modified.10,11 Moreover, it is unknown if Ad5/3 is suitable for intravenous delivery which might have some advantages over the more typical intratumoral delivery.12 In this regard, the use of adenovirus based fully on serotype 3 is appealing,13 for example, because its primary receptor desmoglein 2 is highly expressed in advanced tumors.14 Oncolytic adenovirus Ad3-hTERT-E1A appears to be safe and effective in the laboratory as well as in cancer patients.15
CD40L is a transmembrane type II protein expressed on CD4+ T cells, whereas its receptor is predominantly expressed on antigen-presenting cells (APCs).16,17 DCs, which are professional APCs, express CD40 and get activated upon interaction with CD40L, which leads to priming of T cells followed by cytokine production and an immune response.18 Moreover, it initiates co-stimulatory signals that not only trigger T-lymphocyte expansion,16 but also enhances production of interleukin 12 (IL-12). IL-12 is important for cytotoxic T lymphocytes (CTL) engagement in antitumor immune response.19,20 Previously, both in vivo and in vitro studies have revealed that CD40L has also direct antitumor effects, such as induction of apoptosis and suppression of tumor cell proliferation.21-25
Ralph Steinman was the first who discovered DCs as one of the key cellular sensors. They are considered principle initiators of the adaptive immune response. DCs play an essential role in the regulation of several key immune events, with their unique ability to activate effector and generate memory T cells.26 Vaccination strategies involving DCs are emerging as promising approach.27 DC vaccinations aim to induce specific effector T cells against tumors and to create immunological memory to prevent tumor relapse.
In preclinical models, DCs pulsed with TAA have shown therapeutic TAA-specific immunity in different tumor types.27,28 In these studies, TAAs have been introduced in many forms including as protein, peptide, RNA, DNA or whole tumor lysate.29 Clinical trials of DCs-based vaccines have been applied for cancers such as renal, prostate cancer, lymphoma and melanoma.30-33 However, clinical outcomes have been variable. Less than impressive results have been attributed to the often significant dysregulation and impairment of the transferred DCs in the immunosuppressive tumor microenvironment (TME).34 To date, the only unequivocally successful implementation of this approach is sipuleucel-T, where GMCSF is utilized to stimulate APCs ex vivo against prostatic acid phosphatase (PAP). In this rather crude embodiment of a cancer vaccine, no tumor responses were seen, but survival of prostate cancer patients was nevertheless increased.35 However, long-term efficacy was not observed, and thus much work remains to fully capitalize clinically on the potential of the technology.
In the present study, we hypothesized that an armed oncolytic adenovirus could improve the efficacy of DC therapy. We constructed an oncolytic adenovirus based on serotype 3 and armed it with CD40L. Our novel virus Ad3-hTERT-CMV-hCD40L is based fully on Ad3 for enhanced tumor transduction through the intravenous route. It features the human telomerase reverse transcriptase (hTERT) promoter for tumor selective replication and CD40L for stimulation of immune cells and for direct killing of susceptible cells. Human data demonstrated successful delivery of Ad3 to distant tumor masses, and microarray analysis of human tumor biopsies indicated that CD40L can be used to stimulate DCs. Ad3-hTERT-CMV-hCD40L was tested in vitro and in vivo for specificity and efficacy. In syngeneic studies in immunocompetent model, Ad5/3-CMV-mCD40L showed potent antitumor activity and triggered significant antitumor immune response in combination with DC therapy.
Results
Transduction of human tumor metastases with intravenously administered adenovirus serotype 3
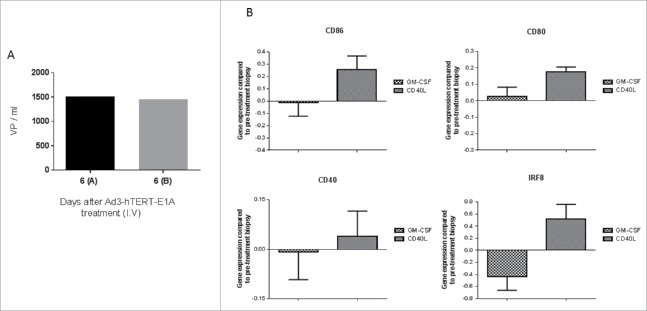
Breast cancer patient R367 was treated intravenously with Ad3-hTERT-E1A (CGTG-201).15 Tumor biopsies on day 6 after treatment showed the presence of virus genomes (Fig. 1A), indicating that intravenous administration of Ad3 leads to transduction of distant tumors. A Day 0 biopsy was not available but is has been shown that ATAP patients are negative for this virus at baseline.15
Figure 1.
Human data provides the rationale for construction of Ad3-hTERT-CMV-hCD40L. (A) A breast cancer patient was treated with Ad3-hTERT-E1A (CGTG-201) virus intravenously (I.V). Presence of virus in tumor biopsies was measured with qPCR 6 d later. (B) Immunomodulatory effects of hCD40L coding adenovirus on intratumoral DCs in cancer patients. Gene expression of DC maturation and differentiation markers were analyzed from tumor biopsies of patients treated with hCD40L and GMCSF coding adenoviruses.
DC activation in patients following treatment with CD40L encoding oncolytic adenovirus
Cancer patients were treated with oncolytic adenoviruses either coding for CD40L or GMCSF in ATAP. Post-treatment tumor biopsies from these patients showed that hCD40L coding adenoviruses induced upregulation of genes associated with DC maturation and differentiation (Fig. 1B).
Ad3-hTERT-CMV-hCD40L virus expresses functional CD40L and efficiently kills tumor cells in vitro
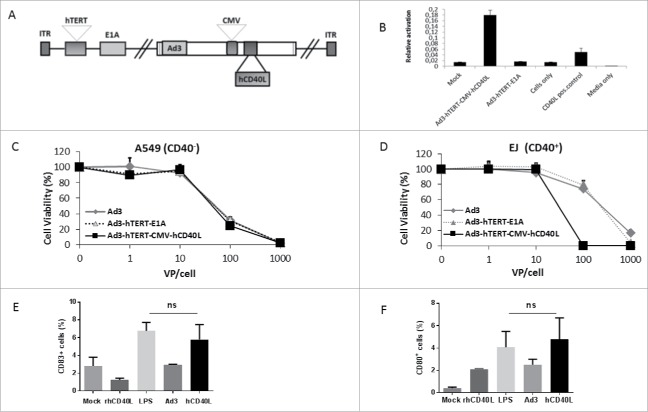
As Ad3 serotype seemed amenable to systemic delivery and virally coded CD40L appeared to induce beneficial gene expression profiles in human tumors, it was attractive to construct an oncolytic adenovirus 3 expressing human CD40L. hCD40L was inserted in the E3 region under a CMV promoter. Moreover, the TATA box (the endogenous promoter) in front of the E1A region was replaced with an hTERT promoter for tumor selectivity. Structure was confirmed by sequencing (Fig. 2A).
Figure 2.
Functionality of Ad3-hTERT-CMV-hCD40L in vitro. (A) Schematic presentation of Ad3-hTERT-CMV-hCD40L. (B) Functionality of virally produced hCD40L was confirmed by studying NF-κB/AP-1 activation in Ramos-Blue cells. The assay was repeated three times (in triplicates each time). Oncolytic potency of Ad3-hTERT-CMV-hCD40L in (C) A549 (CD40−) and (D) EJ (CD40+) cells. MTS assay was used to analyze infected cell lines. Cell viability was assessed relative to uninfected mock cells. (E, F) Capability of virally produced hCD40L to induce dendritic cell maturation. The assay was done in triplicate. Lipopolysaccharide; LPS, recombinant hCD40L protein; rhCD40L, supernatant collected from A549 cells infected with Ad3-hTERT-E1A and with Ad3-hTERT-CMV-hCD40L; Ad3, hCD40L, respectively.
To assess the functionality of Ad3-encoded hCD40L in vitro, we used human Ramos-Blue cells stably expressing an NF-kB/AP-1-inducible SEAP construct. This cell line is responsive to human CD40L and expresses alkaline phosphatase when activated. A549 cells were infected with virus expressing hCD40L. Supernatant was collected and filtered through 0.02 µm filter. The concentration of hCD40L in the filtered supernatant was confirmed (Fig. S3). The assay was run in triplicate and we observed several fold increase in NF-kB/AP-1 in Ramos-Blue cells only with Ad3-hTERT-CMV-hCD40L virus compared with other groups (Fig. 2B). This demonstrates that Ad3-hTERT-CMV-hCD40L expresses functional CD40L.
In order to study the oncolytic potency of the constructed viruses in A549 cells (Fig. 2C) and EJ cells (Fig. 2D), cytotoxicity assays were performed. Ad3-hTERT-CMV-hCD40L was shown to be more potent in killing EJ (CD40+) cells compared with control viruses. In these cells, the mechanism of cell killing could be due to both hCD40L-induced apoptosis and Ad-induced oncolysis.6 As expected, there was no difference between the hCD40L-armed Ad3 and the backbone Ad3 virus in A549 (CD40−) cells, indicating that the presence of transgene does not affect the oncolytic potency of virus.
Maturation of human DCs ex vivo
In order to assess the functionality of Ad3-encoded hCD40L ex vivo, we infected A549 cells with virus expressing hCD40L and unarmed virus. Supernatant was collected and filtered twice, and then added on immature human DCs which were obtained after culturing CD14+ monocyte-enriched peripheral blood mononuclear cells (PBMCs) in 10% RPMI in the presence of GMCSF and IL-4 for 5 d. The assay was done in triplicate and we observed increase in maturation makers in a group treated with virally produced hCD40L present in supernatant. Moreover, we did not find any significant difference between LPS and virally produce hCD40L-treated groups, suggesting the strong ability of hCD40L expressed by Ad3-hTERT-CMV-hCD40L to induce DC maturation (Fig 2E and 2F).
Oncolytic efficacy of Ad3 viruses in immunodeficient mice
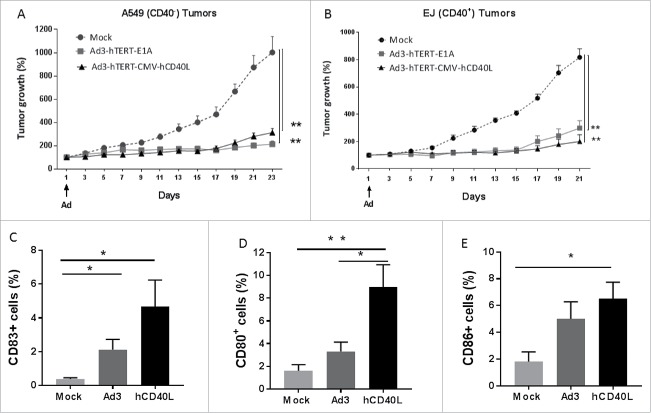
Human adenovirus does not replicate in murine tissue and human CD40L is not active in mice.6 Therefore, we studied antitumor mechanisms in immunodeficient mice-bearing human xenografts, while immunocompetent mouse models, featuring murine CD40L, were used for immunological studies. Oncolytic potency of the constructed adenoviruses was tested in immunodeficient nude mice-bearing human bladder cancer (EJ) and lung cancer (A549) xenografts. Following intravenous injection, both Ad3-hTERT-E1A and Ad3-hTERT-CMV-hCD40L were able to significantly reduce tumor growth when compared with controls receiving PBS (p < 0.01, Fig. 3). At the end of the experiment tumors were collected and in vivo expression of hCD40L was confirmed (Fig. S1). The low concentration of hCD40L might be due to the fact that tumors were already quite small at the time of collection. Moreover, the virus was injected only once in the start of the experiment. There were no sign of any apparent toxicity during the experiment.
Figure 3.
Antitumor efficacy in vivo. (A) A549 (CD40−) and (B) EJ (CD40+) tumors were inoculated subcutaneously in the right flank of immunodeficient nude mice. Virus was injected intravenously once (as indicated with an arrow) at 1×1010 VP/mouse (n = 7 mice per group). Tumor growth was monitored after every other day. Tumor growth is expressed as percentage increase from first day of virus injection. **p < 0.01. Ad3-hTERT-CMV-hCD40L induces DC maturation: A549 tumors were injected subcutaneously in immunodeficient SCID mice (n = 5 mice per group). Virus at 1×108 VP and 1×106 DCs were injected intratumorally three times, alternatively. Proportion of dendritic cells expressing (C) CD83 (D) CD80 and (E) CD86 markers on the surface were analyzed from tumors samples by FACS. Data presented as mean ± SEM.*p ≤ 0.5, **p ≤ 0.01.
hCD40L armed Ad induces maturation of human DCs in immunodeficient mice
In order to study the ability of our clinically relevant adenovirus 3 coding hCD40L (Ad3-hTERT-CMV-hCD40L) to induce maturation of immature DCs in vivo, we used immunodeficient SCID mice-bearing lung cancer (A549) xenograft. In addition to Ad3-hTERT-E1A, Ad3-hTERT-CMV-hCD40L and PBS, immature human DCs were injected intratumorally. Phenotypic markers of matured DCs were analyzed with FACS and analyses of tumor samples showed that the Ad3-hTERT-CMV-hCD40L significantly enhances DC maturation (Figs. 3C, D and F).
Combining CD40L coding Ad with adoptive T-cell therapy in immunocompetent mice does not seem to enhance antitumor efficacy
In order to assess the impact of adenovirus expressing CD40L on adoptive T-cell therapy, a promising emerging form of immunotherapy, we used replication deficient Ad5/3-CMV-mCD40L in immunocompetent mice. This virus codes for mouse CD40L as human CD40L is not active in mice.6 Moreover, mice are non-permissive for human adenovirus replication, which restricts the utility of experiments with oncolytic adenoviruses in syngeneic murine tumor models. To mimic virus replication-induced inflammation, we gave multiple intratumoral viral injections. The B16.OVA model was used to assess the impact of adenovirus treatment in combination with adoptive T-cell therapy. Therefore, B16.OVA melanoma tumor-bearing mice were injected intratumorally with PBS, Ad5/3-luc or Ad5/3-CMV-mCD40L with or without OT-1 adoptive cell therapy given I.P. However, we did not find any statistically significant differences between the groups (Fig. 4A).
Figure 4.
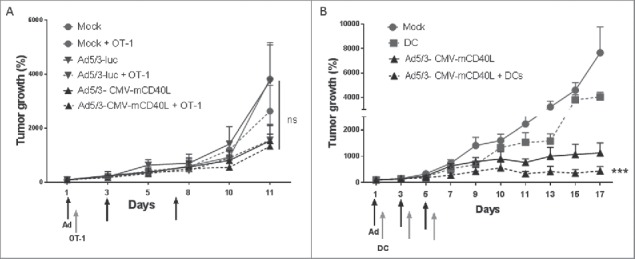
Ad5/3-CMV-mCD40L enhances DC therapy but not adoptive T-cell therapy. (A) B16.OVA and (B) B16.F10 tumors were injected subcutaneously in immunocompetent C57BL/6 mice. Viruses was injected intratumorally three times at 2×108 VP. (A) CD8-enriched OT-1 T cells (1.5 × 106 cells) were injected intraperitoneally once and (B) dendritic cells (DCs, 1×106 cells) were injected intratumorally three times between virus injections (arrows). Tumor growth was monitored every other day. Virus and DCs in combination significantly reduced tumor growth as compared with other groups. Data presented as mean ± SEM. Tumor growth is expressed as percentage increase from first day of virus injection. ***p < 0.001.
CD40L armed Ad enhances the antitumor efficacy of adoptively transferred DCs in immunocompetent mice
As CD40L seemed to be able to activate DCs in human tumors, we hypothesized that CD40L armed Ad could be used to enhance the efficacy of adoptive DC therapy, a promising cancer vaccination approach which has struggled due to low clinical efficacy. As a tumor model for DC therapy, we chose to use the parental model lacking OVA to avoid immune response related to artificial nature of the OVA antigen. Previously, we have not detected significant differences in CD8+ T cells specific for the xenoantigen chicken ovalbumin expressed by the B16.OVA cells.6,36 Instead, we have seen responses in T-cell clones against “natural epitopes” such as gp100 and TRP-2 36 suggesting that the “more natural” B16.F10 model without OVA may be more appropriate in many cases. Therefore, immunocompetent mice-bearing B16.F10 melanoma tumors were injected intratumorally with PBS, Ad5/3-CMV-mCD40L, antigen-pulsed DCs, or with both Ad5/3-CMV-mCD40L and antigen-pulsed DCs. Prior to adoptive transfer, the maturation status of cultured and in vitro activated DCs was analyzed with flow cytometry. The transferred DC population expressed high levels of maturation markers CD86 and CD80 (data not shown). When these DCs were injected into recipient mice, the cells alone had only a minimal inhibitory effect on tumor growth in vivo, whereas the combination of Ad coding CD40L and DCs showed significantly increased antitumor efficacy as compared with other groups (p < 0.001, Fig. 4B).
CD40L armed Ad combined with DC therapy induces antitumor immune responses
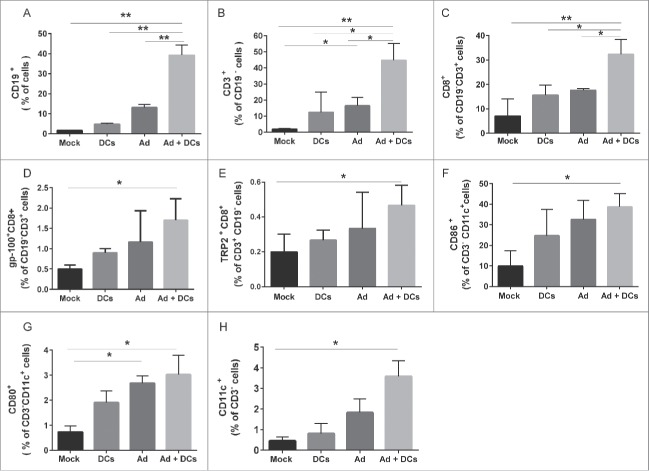
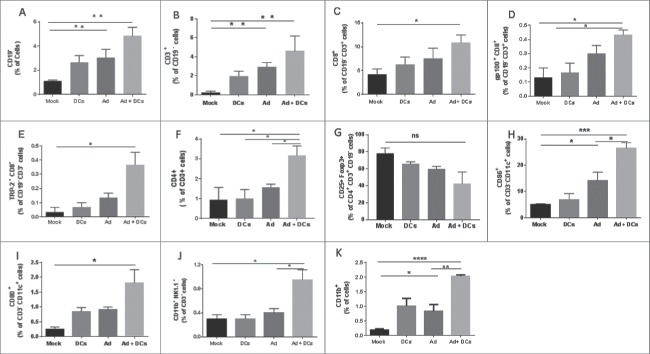
CD40L has an effect on APCs and especially DCs which in turn are required for initiation of a Th1 type immune response. To gain a more in depth understanding of the underlying mechanism, we performed flow cytometric analysis of tumor-draining and non-draining lymph nodes (Fig. 5) and tumors (Fig. 6) to assess differences in immune cell populations possibly contributing to the observed antitumor efficacy. Analysis of tumor draining and non-draining lymph nodes revealed enhanced immune responses following combination therapy, as levels of B and T lymphocytes were significantly increased in the adenovirus plus DC group (Fig. 5). Pentamer analysis showed a statistically significant increase in CD8+ T cells specific for endogenous tumor antigens TRP-2 and gp100 in combination group. In addition, the presence of high levels of IFN-gamma, TNF-α and RANTES in combination treated tumors suggested that a Th1 type immune response was induced (Fig. S2). Moreover, analyses of tumor samples revealed that the combination augmented accumulation of tumor-specific CD8+ T cells, CD4+ T cells, NK cells and mature DCs at the tumor site (Fig. 6). The data indicates that expression of CD40L in the TME can enhance DC maturation, which is a crucial parameter of DC function, thus leading to the activation of other immune cells. This creates an ideal opportunity to overcome immunosuppressive TMEs.
Figure 5.
Host immune responses in C57Bl/6 syngeneic murine models. Percentage of (A) CD19+ B cells, (B) CD3+ T cells (C) CD8+ CD3+ T cells (D) gp100+ CD8+ T cells (E) TRP-2+ CD8+ T cells (F) CD86+ CD11c+ cells (G) CD80+ CD11c+ cells (H) CD11c+ CD3− cells in lymph nodes (tumor-draining and non-draining) which were processed into single-cell suspensions, stained with antibodies and analyzed by flow cytometry. Error bars, SE **,*p < 0.05. Mock (PBS), dendritic cells (DCs) alone, Ad5/3-CMV-mCD40L (Ad) alone and Ad5/3-CMV-mCD40L plus DCs (Ad+ DCs).
Figure 6.
Immune responses in tumor microenvironment. Percentage of (A) CD19+ B cells, (B) CD3+ (CD19−) T cells (C) CD8+ (CD19−) CD3+ T cells (D) gp100+ CD8+ (CD19−) CD3+ T cells (E) TRP−2+ CD8+ (CD19−) CD3+ T cells (F) CD4+ CD3+ T cells (G) CD25+ Foxp3+ (CD4+ CD3+) regulatory T cells (H) CD86+ CD11c+ CD3− cells (I) CD80+ CD11c+ CD3− cells (J) CD11b+ NK1.1+ CD3− (K) CD11b+ cells in tumors samples which were processed into single-cell suspensions, stained with antibodies and analyzed by flow cytometry. Error bars, SE ***p < 0.001. **,*p < 0.05. Mock (PBS), dendritic cells (DCs) alone, Ad5/3-CMV-mCD40L (Ad) alone and Ad5/3-CMV-mCD40L plus DCs (Ad+ DCs).
Discussion
A major obstacle limiting the efficacy of cancer immunotherapies in general and DC therapy in particular is the immunosuppressive microenvironment typically found in advanced solid tumors.37-39 Immunosuppression is mediated by a number of molecules such as VEGF and TGF-β, which impair many types of antitumor immune response, including the maturation of DCs.38,39 Consequently, the microenvironment is skewed in a Th2 type direction resulting in impaired DC function.
CD40–CD40L interaction is required for successful activation of APCs.40 This stimulates APCs and especially DCs to produce IL-12, which is a potent immune-stimulatory cytokine required for initiation of Th1 responses.16,41 CD40L mediated activation of DCs has been reported to result in antitumor immunity in mice.16,42 Upon interaction with mCD40L, DCs upregulate the expression of co-stimulatory receptors and increase the production of cytokines. This is important because the lack of co-stimulatory signals from APCs causes a defect in T cell priming and expansion and may even induce T cell apoptosis.43,44 Therefore, to enhance therapeutic efficacy of DC vaccination, CD40L is an attractive candidate. The present study demonstrates that DC vaccination in combination with adenovirus expressing mCD40L can significantly improve the antitumor immune response over single-agent treatments.
Oncolytic adenoviruses have a good safety profile in humans, their stability in vivo and ability to infect wide range of tumors make them appealing for use in cancer therapy.4,45 In this study, we report the construction of a novel Ad3 virus that expresses human CD40L. Serotype 3 was selected over the more common serotype 5 because human data from ATAP (Fig 1A) and based on a previous publication indicating that the Ad3 capsid allows effective intravenous delivery.15
To make virus replication tumor selective, the endogenous promoter of E1A was replaced with the promoter of the catalytic subunit of human telomerase (hTERT) which is active in most human tumors.46-48 To allow high level expression of CD40L in both tumor cells and the TME where DCs are located, a CMV promoter was placed in the E3 region to drive the CD40L transgene (2A). Ad3-hTERT-CMV-hCD40L retains E3-gp19K, an adenoviral protein whose function is reduction of adenovirus recognition by cytotoxic T-cells. This molecule inhibits the formation of TAP-MHCI complex by interfering with the transport of MHCI to the plasma membrane.49,50 Consequently, adenovirus is able to replicate in a prolific manner in tumor cells since infected cells are not killed by anti-viral T-cells.
In our approach, expression of virally produced CD40L boosts the antitumor immune response in a paracrine manner as CD40L is released from dying tumor cells. The monomeric configuration of hCD40L used here was selected because it is not secreted as much as trimeric forms or constructs with specific secretory domains. Our approach results in additional tumor selectivity as infected normal cells (which are not lysed when a tumor selective oncolytic adenovirus is used) do not burst and spill out transgene products, resulting in less CD40L in normal tissues. While recombinant CD40L has been rather safe when used systemically in human trials, liver toxicity has limited its use to hematological malignancies.51
CD40L is known to stimulate and recruit APCs, especially DCs, which in turn activate cytotoxic T-cells and modulate the TME in a Th1 direction.6 In theory, this might have resulted in indirect enhancement of adoptive T-cell therapy (through DCs), but this was not seen, suggesting that DCs may have limited relevance in this approach, at least in the B16.OVA model. Further studies are needed to elaborate this in more detail and in other models of adoptive T-cell transfer (Fig. 4).
The data reported here with Ad3-hTERT-CMV-hCD40L is compatible with previous reports of the ability of CD40L to induce apoptosis of CD40+ tumors.6 However, previous work has also revealed the ability of CD40L to induce APC activation not only CD40+ but also in CD40− tumors, suggesting the potential use of virus for tumor immunotherapy regardless of CD40 status.6,7,52 This is in accordance with our study in which Ad3-hTERT-CMV-hCD40L (Figs. 3C–E) as well as virally produced hCD40L (Figs. 2E and F) has shown to induce DC maturation.
In the correlative studies done on tissue specimens collected from mice treated with DCs and adenovirus, CD40L increased the expression of T-helper type 1 (Th1) cytokines, TNF-α, IFN gamma and RANTES in tumors (Fig. S2). Moreover, significantly enhanced cytotoxic T cell responses were observed in the Ad + DC group (Figs. 5 and 6). Also, the intratumoral level of NK cells was increased in mice treated with the Ad + DC combination. The underlying mechanism is likely related to the interaction of virally produced CD40L with DCs leading to production of IL-12, which in turn activates NK cells. Conversely, activated NK cells may in turn promote maturation of DCs.53
Upon maturation, DCs migrate to regional lymph nodes, which is a key step in induction of antitumor immune responses.38 In the current study, there was significant increase in the number of DCs migrated to lymph nodes in mice treated with the combination.54 TNF-α is a proinflammatory cytokine known to enhance the migration of DCs to lymph nodes.55 Our data are in accordance with this notion, as we saw enhanced production of TNF-α in virus injected tumors in conjunction with increased migration of DCs to lymph nodes. Taken together, our data suggests that CD40L virus injection prior to DC therapy increases the efficacy of DC vaccination through T cell mediated antitumor immune mechanisms.
In summary, we report significant antitumor immune responses when adenovirus coding for CD40L was used to facilitate adoptively transfer of DCs. Key mechanisms include the maturation and migration of DCs to lymph nodes and induction of cytotoxic T cell responses. Our findings provide rationale for clinical investigation of Ad3-hTERT-CMV-hCD40L as an enabling technology for DC vaccination in patients with currently incurable solid tumors.
Materials and methods
Cells lines
Human bladder cancer EJ cell line was kindly provided by A.G. Eliopoulos (University of Crete Medical School and Laboratory of Cancer Biology, Heraklion, Crete, Greece). Human embryonic kidney 293 cells, Human lung adenocarcinoma cancer A549 cells and Mouse melanoma B16.F10 cells were obtained from American Type Culture Collection (ATCC; LGS standards and B16.F10 cells from Manassas, VA, USA). Murine melanoma B16 cells expressing ovalbumin (OVA) was kindly provided and authenticated by Richard Vile (Rochester). A549 and EJ cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM). B16.OVA and B16.F10 cells were cultured in RPMI at 37°C and 5% CO2. Culturing conditions for all the cell lines were supplemented with 10% FBS, 1% L-Glutamine, 1% Pen/Strep solution. Growth medium for B16.OVA was also supplemented with 5 mg/mL G418 (Roche, Basel, Switzerland).
Virus construction
Viruses Ad3-hTERT-E1A, Ad3-hTERT-CMV-hCD40L, Ad5/3-Luc1 and Ad5/3-CMV-mCD40L were constructed according to the adenovirus preparation techniques.56-58 Construction of Ad5/3-Luc1,59 Ad5/3-CMV-mCD40L6 and Ad3-hTERT-E1A have been previously reported.13
Ad3-hTERT-CMV-hCD40L construction
The Ad3-hTERT-CMV-hCD40L vector was generated based on pWEA-Ad3-GFP vector.16 The 13.2 kb FseI/NotI fragment of pWEA-Ad3-GFP containing the 5′end of Ad3 genome was cloned into a modified pBluescript KS(−) vector (the multiple cloning site between SacI and XbaI were modified as SacI-PmeI-MluI-FseI-SalI-NoT-XbaI) (pBS-Ad3–5′end). The 6.5 kb EcoRI/FseI fragment containing CMV-GFP-PA transgene cassette and 3′end of Ad3 genome was cloned out (pWEA-Ad3–3′end-CMV-GFP).
The 1.1 kb fragment containing Ad3 5′end, hTERT promoter and part of Ad3 E1 region was amplified from pKBS2-hTERT,17 the following primers were used: forward, 5′- GTCAGTTTAAACTTAGGCCGGCCCTATCTATATAATATACCTTATAGATGGAATGG-3′; reverse, 5′- CTTCATCAGCAGCTAGCAGCATAGAATCAG-3′. The FseI/NheI fragment of pBS-Ad3–5′end containing the first ∼800 bp of Ad3 genome were replaced by the PmeI/NheI digested PCR fragment (pBS-Ad3–5′end-hTERT). The hCD40L fragment was amplified by PCR, with the following primer, forward, 5′-TAGCTGCTAGCATGATCGAAACATACAAC-3′; reverse, 5′-GTCAATTTGGGCCCTCAGAGTTTGAGTAAGCCAA-3′. The GFP gene in the pWEA-Ad3–3′end-CMV-GFP was replaced by the hCD40L gene (pWEA-Ad3–3′end-CMV-hCD40L).
The final Ad3-hTERT-CMV-hCD40L genome were cloned together by joining of ∼13.4 kb fragment containing 5′end of Ad3 genome and hTERT, ∼16.7 kb NotI/EcoRI fragment of Ad3 genome and ∼6.5 kb fragment containing Ad3 3′end genome and CMV-hCD40L (pWEA-Ad3-hTERT-CMV-hCD40L), the ligation was packaged into phages using Gigapack III Plus Packaging Extract (Stratagene, La Jolla, CA) and propagated. The recombinant Ad3-hTERT-CMV-hCD40L viral genome was released by FseI digestion and transfected into 293 cells using a standard calcium phosphate method. After a first round of rescuing on 293 cells, HeLa cells were infected with the cell lysate containing Ad3-hTERT-CMV-hCD40L virus for further virus propagation.
Ad3-hTERT-E1A virus in human tumor metastases
A breast cancer patient participating in the Advanced Therapy Access Program (ATAP)15 was safely treated with Ad3-hTERT-E1A (CGTG-201) at 4 × 1012 VP intravenously. Tumor biopsies were collected 6 d later and presence of virus was detected with qPCR. All patients reported here were treated with oncolytic adenovirus in the ATAP, which was a personalized therapy program ongoing 2007–2012.60 Before treatment in ATAP, patients had solid tumors that were refractory to standard treatments and no major organ dysfunctions were present. A written informed consent was received from all of the patients participating in the program. Analysis of the data reported here was approved by the Helsinki University Central Hospital Operative Ethics Committee (HUS 62/13/03/02/2013).
CD40L functionality in vitro
A549 cells were infected with Ad3-hTERT-E1A or Ad3-hTERT-CMV-hCD40L and supernatant was collected and filtered (0.02 µm, Whatman 6809–1002) after 48 h. Filtered supernatants were used to stimulate Ramos-Blue cells. Ramos-Blue is a B-lymphocyte cell line stably expressing NFkB/AP-1-inducible SEAP (secreted embryonic alkaline phosphatase) reporter gene. When stimulated by CD40L, they produce SEAP in the supernatant which can be detected using QUANTI-Blue (InvivoGen), a reagent that turns purple/blue in the presence of SEAP. Levels of activation were read with a microplate reader at the wavelength of 450 nm. Concentration of hCD40L in the filtered supernatant was analyzed with CBA Flex set. Recombinant hCD40L protein at a concentration of 2 ng (Abcam, ab51956) was used as positive control.
Microarray from human tumor biopsies treated with CD40L and GMCSF viruses
Patients participating in ATAP were treated with oncolytic adenovirus encoding either human CD40L (n = 3) or another immunostimulatory molecule, granulocyte macrophage colony-stimulation factor (GMCSF) (n = 11). mRNA expression levels were analyzed from tumor biopsies as reported previously.61
MTS assay
10,000 A549 and EJ cells/well were plated in 100 uL of 2% growth media. After 24 h, both cell lines were infected in triplicates with Ad3, Ad3-hTERT-E1A or Ad3-hTERT-CMV-hCD40L in different concentrations (1–1,000 VP/cell). Viability of cells was determined after 6 d with MTS cytotoxicity assay according to manufacturer´s instructions (Cell Titer 96 AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI).
Generation of PBMCs-derived human DCs
Human PBMCs were isolated from buffy coat of healthy donor by lymphoprep (StemCell technologies) gradient density separation under sterile conditions. Cells were washed with PBS twice, erythrocytes were lysed with ACK red blood cell lysis buffer (Sigma, St Louis, MO. A10492–01). CD14+ magnetic beads (Miltenyi Biotec, 130–050–201) were used to isolate human CD14+ cells (according to the manufacturer instructions) from fresh PBMCs. 4.5 × 106 Magnetic microbeads separated CD14+ monocyte-enriched PBMCs were cultured in 10 mL of 10% RPMI in the presence of 1,000 U GMCSF and 20 ng IL-4 in T25 flask for 5 d at 37°C in order to get immature DCs.
Virally produced hCD40L induces DCs maturation
To study the effect of virally produced hCD40L induces DCs maturation. A549 cells were infected with Ad3-hTERT-CMV-hCD40L and with Ad3-hTERT-E1A, supernatants were collected and filtered (0.02 µm filter) to avoid the presence of virus and then re-filtered. 1×105 immature DCs were cultured with re-filtered supernatants for 48 h. Lipopolysaccharide LPS (100 ng) (Sigma, L4391–1MG) and recombinant hCD40L protein (2 ng) (Abcam, ab51956) were used as positive control. Cells were stained with antibodies and analyzed by flow cytometry. The assay was done in triplicates.
Generation of bone marrow-derived DCs
Bone marrow (BM) was collected from mouse femur and tibia under sterile conditions. Suspension of BM was filtered and erythrocytes were lysed with ACK red blood cell lysis buffer (Sigma, St Louis, MO. A10492–01). Cells were centrifuged and re-suspended in 10% RPMI. Cells were plated at 2.5 × 105 cells per mL in 24-well plate in the presence of 40 ng/mL mGMCSF (Peprotech, 315–03). On day 3, 75% of media was replaced with fresh complete media containing mGMCSF. On day 6, cells were incubated with 50 μg/mL B16.F10 cell lysate for 24 h, followed by maturation with 50 ng/mL LPS (Sigma, L4391–1MG) in 10% RPMI for 17–24 h. Phenotypic makers of matured DCs were analyzed with FACS.
Animal experiments
The experimental animal committee of the University of Helsinki (Helsinki, Finland) and the Provincial Government of Southern Finland had approved the protocols for all the animal experiments. Mice were 4- to 5-weeks old (Taconic) and quarantined for one week before beginning the experiments.
Immunodeficient nude (NMRI) mice received subcutaneous injections of 106 A549 or EJ cells in the flank. When the size of tumors reached approximately 5 × 5 mm, mice were divided into groups (n = 7 mice/group). Mice were treated with one intravenous injection of 1 × 1010 VP/mouse of Ad3-hTERT-E1A, Ad3-hTERT-CMV-hCD40L or PBS through tail vein. Tumor growth was measured every other day. Presence of hCD40L in the tumor samples and blood was analyzed with Flex set (BD Cytometric Bead Array human Flex Sets; BD Biosciences).
To study the maturation of DC in vivo, 5 × 106 A549 cells (n = 5 mice/group) were injected subcutaneously (one tumor per mouse) in SCID mice. When tumors become injectable, PBS (50 µL) or 108 VP/tumor Ad3-hTERT-E1A, Ad3-hTERT-CMV-hCD40L were injected on days 0, 2, 4 and 1×106 immature DCs (prepared as described previously) were injected on days 1, 3, 5. Viruses and DCs were injected intratumorally. Two days after the last treatment, mice were euthanized and tumors were collected.
Immunocompetent female C57BL/6 mice were implanted with 2.5 × 105 B16.OVA cells subcutaneously. When tumors become injectable, mice (n = 8 mice/group) were treated intratumorally with either PBS (50 µL) or 109 VP/tumor Ad5/3-luc and Ad5/3-CMV-mCD40L on days 1, 3 and 7 and intraperitoneally with 1.5 × 106 CD8-enriched OT-1 T-cells on day 1. Enrichment of CD8a splenocytes was performed as previously reported.36 Briefly, spleen from C57BL/6-Tg (TcraTcrb) 1100Mjb/J (OT-1) mice were mashed and treated with lysis buffer (ACK). Mouse CD8+ (Ly-2) microbeads (Miltenyi Biotech) were used to enrich CD8a+ T cells, which were then expanded in supplemented growth media for 1 week.
Immunocompetent female C57BL/6 mice were implanted with 2.5 × 105 B16.F10 cells subcutaneously. Mice were treated intratumorally three times either with 50 µL PBS, Ad5/3-CMV-mCD40L virus alone (at 2 × 108 VPs/tumor), DCs (106 cells) alone or as a combination of both virus and DCs. Virus was injected on days 1, 3 and 5, whereas DCs were injected on days 2, 4 and 6. Tumor growth was measured with every other day using electronic caliper. In the end of experiment, tumors and lymph nodes from B16.F10 tumor-bearing mice were mashed, filtered and cultured for overnight. Immune Cells and pentamers detecting T cell receptors specific for residues TRP-2 180–188, gp100 25–33 (Proimmune, Oxford, UK) (Table 1) were analyzed with fluorescence-activated cell-sorting (FACS) and results were analyzed with BD software (BD Biosciences). Cytokines analysis was done with CBA Flex set cytokine beads (BD) on BD Accuri C6 flow cytometer with FCAP array software according to manufacturer's instructions.
Table 1.
Antibodies used in the experiments.
| Anitbody | Catalog number | Company |
|---|---|---|
| CD19-PE | 12–0193–82 | e-bioscience |
| CD3 APC | 553066 | BD |
| CD3 PE-Cy7 | 560591 | BD Pharmigen |
| Anti-mouse CD8b FITC | 11–0083–85 | e-bioscience |
| Anti-mouse CD4 PerCP-Cy5.5 | 550954 | BD |
| Anti-Mouse CD25 PE | 12–0251–82 | e-bioscience |
| Anti-Mouse/Rat Foxp3 APC | 17–5773–82 | eBiosciences |
| 1333-H-2Db-KVPRNQDWL-gp100-Pentamer-APC | F1333–4A | Proimmune |
| 185-H-2Kb-SVYDFFVWL-TRP-2 Pentamer-APC | F185–4A-E | Proimmune |
| Anti-Mouse NK1.1 FITC | 11–5941–82 | eBiosciences |
| Anti-mouse CD11b PerCP-Cy5.5 | 550993 | BD Pharmigen |
| CD11c FITC 0.5 mg | 553801 | BD |
| CD11b PerCP-Cy™ 5.5 0.1 mg | 550993 | BD |
| CD80 PerCP-Cy™ 5.5 0.05 mg | 560526 | BD Biosciences |
| CD86 PE 0.2 mg | 553692 | BD |
| Anti-human CD86 PE Antibody | 305405 | Biolegend |
| Anti-human CD80 FITC Antibody | 305205 | Biolegend |
| Anti-Human CD83 APC (HB15) | 17–0839–41 | e-bioscience |
Statistics
For statistical analysis of data, two tailed Student's t-test using Graph pad Prism 6 (graph Pad Prism Software Inc., San Diego, CA) and for tumor growth analysis SPSS version 21 were used. When the p values were <0.05, difference was considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Minna Oksanen, Susanna Gronberg-Vaha-Koskela and Iulia Diaconu from University of Helsinki, Finland and Lameris Roeland and Marta Lopez Gonzalez from VU University Amsterdam, The Netherland for excellent expert assistance. We thank Majlen Fazer and Paul Ehrnrooth for their donation. This study was supported by Jane and Aatos Erkko Foundation, HUCH Research Funds (EVO), Sigrid Juselius Foundation, Biocentrum Helsinki, Biocenter Finland, Finnish Cancer Organizations, University of Helsinki and TILT Biotherapeutics Ltd. A.H. is Jane and Aatos Erkko Professor of Oncology at the University of Helsinki. A.H. is shareholder in Targovax ASA. A.H. is employee and shareholder in TILT Biotherapeutics Ltd. A.H. is consultant for Amgen Inc.
References
- 1.Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett 2007; 254:178-216; PMID:17383089; http://dx.doi.org/ 10.1016/j.canlet.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nettelbeck DM. Cellular genetic tools to control oncolytic adenoviruses for virotherapy of cancer. J Mol Med (Berlin, Germany) 2008; 86:363-77; PMID:18214411; http://dx.doi.org/ 10.1007/s00109-007-0291-1 [DOI] [PubMed] [Google Scholar]
- 3.Liu TC, Thorne SH, Kirn DH. Oncolytic adenoviruses for cancer gene therapy. Method Mol Biol (Clifton, NJ) 2008; 433:243-58; PMID:18679628; http://dx.doi.org/23162778 10.1007/978-1-59745-237-3_15 [DOI] [PubMed] [Google Scholar]
- 4.Cerullo V, Vaha-Koskela M, Hemminki A. Oncolytic adenoviruses: A potent form of tumor immunovirotherapy. Oncoimmunology 2012; 1:979-81; PMID:23162778; http://dx.doi.org/ 10.4161/onci.20172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol 2009; 21:265-72; PMID:19524453; http://dx.doi.org/ 10.1016/j.smim.2009.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK, Bramante S, Parviainen S, Kanerva A, Loskog AS et al.. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res 2012; 72:2327-38; PMID:22396493; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2975 [DOI] [PubMed] [Google Scholar]
- 7.Pesonen S, Diaconu I, Kangasniemi L, Ranki T, Kanerva A, Pesonen SK, Gerdemann U, Leen AM, Kairemo K, Oksanen M et al.. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res 2012; 72:1621-31; PMID:22323527; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3001 [DOI] [PubMed] [Google Scholar]
- 8.Kanerva A, Hemminki A. Adenoviruses for treatment of cancer. Annal Med 2005; 37:33-43; PMID:15902845; http://dx.doi.org/ 10.1080/07853890410018934 [DOI] [PubMed] [Google Scholar]
- 9.Kanerva A, Hemminki A. Modified adenoviruses for cancer gene therapy. Int J Cancer 2004; 4:475-80; PMID:15122578; http://dx.doi.org/18401431 10.1002/ijc.20129 [DOI] [PubMed] [Google Scholar]
- 10.Sarkioja M, Pesonen S, Raki M, Hakkarainen T, Salo J, Ahonen MT, Kanerva A, Hemminki A. Changing the adenovirus fiber for retaining gene delivery efficacy in the presence of neutralizing antibodies. Gene Ther 2008; 15:921-9; PMID:18401431; http://dx.doi.org/ 10.1038/gt.2008.56 [DOI] [PubMed] [Google Scholar]
- 11.Mastrangeli A, Harvey BG, Yao J, Wolff G, Kovesdi I, Crystal RG, Falck-Pedersen E. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther 1996; 7:79-87; PMID:8825871; http://dx.doi.org/ 10.1089/hum.1996.7.1-79 [DOI] [PubMed] [Google Scholar]
- 12.Hemminki O, Parviainen S, Juhila J, Turkki R, Linder N, Lundin J, Kankainen M, Ristimaki A, Koski A, Liikanen I et al.. Immunological data from cancer patients treated with Ad5/3-E2F-Delta24-GMCSF suggests utility for tumor immunotherapy. Oncotarget 2015; 6:4467-81; PMID:25714011; http://dx.doi.org/ 10.18632/oncotarget.2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemminki O, Bauerschmitz G, Hemmi S, Lavilla-Alonso S, Diaconu I, Guse K, Koski A, Desmond RA, Lappalainen M, Kanerva A et al.. Oncolytic adenovirus based on serotype 3. Cancer Gene Ther 2011; 18:288-96; PMID:21183947; http://dx.doi.org/ 10.1038/cgt.2010.79 [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB et al.. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med 2011; 17:96-104; PMID:21151137; http://dx.doi.org/ 10.1038/nm.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemminki O, Diaconu I, Cerullo V, Pesonen SK, Kanerva A, Joensuu T, Kairemo K, Laasonen L, Partanen K, Kangasniemi L et al.. Ad3-hTERT-E1A, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer. Mol Ther 2012; 20:1821-30; PMID:22871667; http://dx.doi.org/ 10.1038/mt.2012.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annual Rev Immunol 1998; 16:111-35; PMID:9597126; http://dx.doi.org/ 10.1146/annurev.immunol.16.1.111 [DOI] [PubMed] [Google Scholar]
- 17.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol (Baltimore, Md : 1950) 1993; 151:2497-510; PMID:8103067 [PubMed] [Google Scholar]
- 18.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukocyte Biol 2000; 67:2-17; PMID:10647992 [DOI] [PubMed] [Google Scholar]
- 19.Loskog AS, Fransson ME, Totterman TT. AdCD40L gene therapy counteracts T regulatory cells and cures aggressive tumors in an orthotopic bladder cancer model. Clin Cancer Res 2005; 11:8816-21; PMID:16361570; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1817 [DOI] [PubMed] [Google Scholar]
- 20.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ Jr.. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol (Baltimore, Md : 1950) 1998; 161:2094-8; PMID:9725199 [PubMed] [Google Scholar]
- 21.Eliopoulos AG, Dawson CW, Mosialos G, Floettmann JE, Rowe M, Armitage RJ, Dawson J, Zapata JM, Kerr DJ, Wakelam MJ et al.. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr Virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene 1996; 13:2243-54; PMID:8950992 [PubMed] [Google Scholar]
- 22.Tong AW, Papayoti MH, Netto G, Armstrong DT, Ordonez G, Lawson JM, Stone MJ. Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clin Cancer Res 2001; 7:691-703; PMID:11297266 [PubMed] [Google Scholar]
- 23.Eliopoulos AG, Davies C, Knox PG, Gallagher NJ, Afford SC, Adams DH, Young LS. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol 2000; 20:5503-15; PMID:10891490; http://dx.doi.org/ 10.1128/MCB.20.15.5503-5515.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano A, Longo DL, Taub DD, Ferris DK, Young LS, Eliopoulos AG, Agathanggelou A, Cullen N, Macartney J, Fanslow WC et al.. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood 1999; 93:2999-3007; PMID:10216096 [PubMed] [Google Scholar]
- 25.Davies CC, Mason J, Wakelam MJ, Young LS, Eliopoulos AG. Inhibition of phosphatidylinositol 3-kinase- and ERK MAPK-regulated protein synthesis reveals the pro-apoptotic properties of CD40 ligation in carcinoma cells. J Biol Chem 2004; 279:1010-9; PMID:14581487; http://dx.doi.org/ 10.1074/jbc.M303820200 [DOI] [PubMed] [Google Scholar]
- 26.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/ 10.1038/nrc3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annual Rev Immunol 2000; 18:245-73; PMID:10837059; http://dx.doi.org/ 10.1146/annurev.immunol.18.1.245 [DOI] [PubMed] [Google Scholar]
- 28.Proudfoot O, Pouniotis D, Sheng KC, Loveland BE, Pietersz GA. Dendritic cell vaccination. Expert Rev Vaccines 2007; 6:617-33; PMID:17669014; http://dx.doi.org/ 10.1586/14760584.6.4.617 [DOI] [PubMed] [Google Scholar]
- 29.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annual Rev Med 1999; 50:507-29; PMID:10073291; http://dx.doi.org/ 10.1146/annurev.med.50.1.507 [DOI] [PubMed] [Google Scholar]
- 30.Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A 2002; 99:931-6; PMID:11792864; http://dx.doi.org/9500607 10.1073/pnas.022634999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4:328-32; PMID:9500607; http://dx.doi.org/ 10.1038/nm0398-328 [DOI] [PubMed] [Google Scholar]
- 32.Tjoa BA, Erickson SJ, Bowes VA, Ragde H, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, Boynton AL, Murphy GP. Follow-up evaluation of prostate cancer patients infused with autologous dendritic cells pulsed with PSMA peptides. Prostate 1997; 32:272-8; PMID:9288186; http://dx.doi.org/ 10.1002/(SICI)1097-0045(19970901)32:4%3c272::AID-PROS7%3e3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 33.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996; 2:52-8; PMID:8564842; http://dx.doi.org/ 10.1038/nm0196-52 [DOI] [PubMed] [Google Scholar]
- 34.Gervais A, Leveque J, Bouet-Toussaint F, Burtin F, Lesimple T, Sulpice L, Patard JJ, Genetet N, Catros-Quemener V. Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency. Breast Cancer Res BCR 2005; 7:R326-35; PMID:15987427; http://dx.doi.org/ 10.1186/bcr1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB et al.. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Eng J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 36.Tahtinen S, Gronberg-Vaha-Koskela S, Lumen D, Merisalo-Soikkeli M, Siurala M, Airaksinen AJ, Vaha-Koskela M, Hemminki A. Adenovirus improves the efficacy of adoptive T-cell therapy by recruiting immune cells to and promoting their activity at the tumor. Cancer Immunol Res 2015; 3:915-25; PMID:25977260; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0220-T [DOI] [PubMed] [Google Scholar]
- 37.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5:263-74; PMID:15776005; http://dx.doi.org/ 10.1038/nrc1586 [DOI] [PubMed] [Google Scholar]
- 38.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 2005; 5:296-306; PMID:15803149; http://dx.doi.org/ 10.1038/nri1592 [DOI] [PubMed] [Google Scholar]
- 39.Lim DS, Kim JH, Lee DS, Yoon CH, Bae YS. DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors. Cancer Immunol Immunother CII 2007; 56:1817-29; PMID:17443323; http://dx.doi.org/ 10.1007/s00262-007-0325-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev 1996; 153:85-106; PMID:9010720; http://dx.doi.org/ 10.1111/j.1600-065X.1996.tb00921.x [DOI] [PubMed] [Google Scholar]
- 41.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 1996; 184:747-52; PMID:8760829; http://dx.doi.org/ 10.1084/jem.184.2.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature 1995; 378:617-20; PMID:8524395; http://dx.doi.org/ 10.1038/378617a0 [DOI] [PubMed] [Google Scholar]
- 43.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med 1994; 180:1263-72; PMID:7523569; http://dx.doi.org/ 10.1084/jem.180.4.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peguet-Navarro J, Dalbiez-Gauthier C, Rattis FM, Van Kooten C, Banchereau J, Schmitt D. Functional expression of CD40 antigen on human epidermal Langerhans cells. J Immunol (Baltimore, Md: 1950) 1995; 155:4241-7; PMID:7594581 [PubMed] [Google Scholar]
- 45.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M, Guse K et al.. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther 2010; 18:1874-84; PMID:20664527; http://dx.doi.org/ 10.1038/mt.2010.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujiwara T, Urata Y, Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Curr Cancer Drug Targets 2007; 7:191-201; PMID:17346111; http://dx.doi.org/ 10.2174/156800907780058835 [DOI] [PubMed] [Google Scholar]
- 47.Huang P, Watanabe M, Kaku H, Kashiwakura Y, Chen J, Saika T, Nasu Y, Fujiwara T, Urata Y, Kumon H. Direct and distant antitumor effects of a telomerase-selective oncolytic adenoviral agent, OBP-301, in a mouse prostate cancer model. Cancer Gene Ther 2008; 15:315-22; PMID:18274558; http://dx.doi.org/ 10.1038/cgt.2008.3 [DOI] [PubMed] [Google Scholar]
- 48.Davis JJ, Wang L, Dong F, Zhang L, Guo W, Teraishi F, Xu K, Ji L, Fang B. Oncolysis and suppression of tumor growth by a GFP-expressing oncolytic adenovirus controlled by an hTERT and CMV hybrid promoter. Cancer Gene Ther 2006; 13:720-3; PMID:16485010; http://dx.doi.org/ 10.1038/sj.cgt.7700944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgert HG, Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell 1985; 41:987-97; PMID:3924414; http://dx.doi.org/ 10.1016/S0092-8674(85)80079-9 [DOI] [PubMed] [Google Scholar]
- 50.Andersson M, Paabo S, Nilsson T, Peterson PA. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell 1985; 43:215-22; PMID:2934137; http://dx.doi.org/ 10.1016/0092-8674(85)90026-1 [DOI] [PubMed] [Google Scholar]
- 51.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ et al.. Phase I study of recombinant human CD40 ligand in cancer patients. J Clinical Oncol 2001; 19:3280-7; PMID:11432896; http://dx.doi.org/ 10.1200/JCO.2001.19.13.3280 [DOI] [PubMed] [Google Scholar]
- 52.Sun Y, Peng D, Lecanda J, Schmitz V, Barajas M, Qian C, Prieto J. In vivo gene transfer of CD40 ligand into colon cancer cells induces local production of cytokines and chemokines, tumor eradication and protective antitumor immunity. Gene Ther 2000; 7:1467-76; PMID:11001366; http://dx.doi.org/ 10.1038/sj.gt.3301264 [DOI] [PubMed] [Google Scholar]
- 53.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 2002; 195:327-33; PMID:11828007; http://dx.doi.org/ 10.1084/jem.20010938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin-Fontecha A, Baumjohann D, Guarda G, Reboldi A, Hons M, Lanzavecchia A, Sallusto F. CD40L+ CD4+ memory T cells migrate in a CD62P-dependent fashion into reactive lymph nodes and license dendritic cells for T cell priming. J Exp Med 2008; 205:2561-74; PMID:18838544; http://dx.doi.org/ 10.1084/jem.20081212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med 2003; 198:615-21; PMID:12925677; http://dx.doi.org/ 10.1084/jem.20030448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vardouli L, Lindqvist C, Vlahou K, Loskog AS, Eliopoulos AG. Adenovirus delivery of human CD40 ligand gene confers direct therapeutic effects on carcinomas. Cancer Gene Ther 2009; 16:848-60; PMID:19461675; http://dx.doi.org/ 10.1038/cgt.2009.31 [DOI] [PubMed] [Google Scholar]
- 57.Bauerschmitz GJ, Ranki T, Kangasniemi L, Ribacka C, Eriksson M, Porten M, Herrmann I, Ristimaki A, Virkkunen P, Tarkkanen M et al.. Tissue-specific promoters active in CD44+CD24-/low breast cancer cells. Cancer Res 2008; 68:5533-9; PMID:18632604; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5288 [DOI] [PubMed] [Google Scholar]
- 58.Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM, Barnes MN, Alvarez RD, Siegal GP, Curiel DT et al.. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther 2002; 5:695-704; PMID:12027553; http://dx.doi.org/ 10.1006/mthe.2002.0599 [DOI] [PubMed] [Google Scholar]
- 59.Kanerva A, Zinn KR, Peng KW, Ranki T, Kangasniemi L, Chaudhuri TR, Desmond RA, Wang M, Takayama K, Hakkarainen T et al.. Noninvasive dual modality in vivo monitoring of the persistence and potency of a tumor targeted conditionally replicating adenovirus. Gene therapy 2005; 12:87-94; PMID:15385953; http://dx.doi.org/ 10.1038/sj.gt.3302387 [DOI] [PubMed] [Google Scholar]
- 60.Hemminki A. Portrait of a leader in immunotherapeutics: oncolytic viruses for treatment of cancer. Hum Vaccin Immunotherap 2012; 8:1018-21; PMID:22914447; http://dx.doi.org/ 10.4161/hv.21699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taipale K, Liikanen I, Juhila J, Turkki R, Tahtinen S, Kankainen M, Vassilev L, Ristimaki A, Koski A, Kanerva A et al.. Chronic activation of innate immunity correlates with poor prognosis in cancer patients treated with oncolytic adenovirus. Mol Ther 2016; 24:175-83; PMID:26310629; http://dx.doi.org/ 10.1038/mt.2015.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.