Abstract
Tyrosine kinase-activating mutations in Met have been observed in hereditary papillary renal carcinomas as well as in other cancers. These mutations have been examined in several in vitro systems, where they cause constitutive Met activation, focus formation, and cell motility, and are tumorigenic in xenografts. To study the influence of these mutations on tumorigenesis in vivo, we generated mice with targeted mutations in the murine met locus. The following five mouse lines with mutant Met were created: WT, D1226N, Y1228C, M1248T, and M1248T/L1193V. We observed that mice harboring D1226N, Y1228C, and M1248T/L1193V mutations developed a high frequency of sarcomas and some lymphomas, whereas the M1248T mice developed carcinomas and lymphomas. Of considerable interest, we observed trisomy of chromosome 6 and duplication of the mutant met allele in a majority of the tumors, similar to what has been reported in patients with hereditary renal papillary carcinomas. These results demonstrate that activating Met mutations and met amplification play key roles in promoting tumorigenesis in vivo. Moreover, our findings show that different mutations in the Met kinase domain can influence the types of cancers that develop.
Met is the tyrosine kinase receptor for hepatocyte growth factor/scatter factor (HGF/SF). Met is expressed predominantly in epithelial cells, whereas HGF/SF is expressed in a paracrine manner by mesenchymal cells (1, 2). Signaling through the Met receptor is crucial for embryonic development as well as for mediating cell growth, motility, invasion, and angiogenesis (3–6). Met and HGF/SF are well known oncogenes, and inappropriate Met and/or HGF/SF expression is associated with multiple human solid tumors and poor clinical prognosis (7) (www.vai.org/vari/metandcancer). Several distinct germ-line and somatic activating mutations in Met have been observed in hereditary papillary renal carcinoma (HPRC) (8, 9) as well as in other carcinomas (10–12). HPRC accounts for ≈10% of renal cancers and is characterized by multifocal, bilateral papillary tumors (13). Although Met is a dominant oncogene, patients with germ-line missense mutations in the kinase domain have incomplete penetrance and develop HPRC late in life (8). The long tumor latency may be due to the requirement of duplication of the mutant allele, which is frequently observed in patients with HPRC (14, 15). These events suggest that Met mutations require at least a “second hit” for tumors to progress.
Although the oncogenic potential of these Met mutations has been demonstrated through in vitro studies (16, 17), their causal role for in vivo tumorigenesis in mouse models has not been demonstrated. To directly address the physiological role of activating Met mutations, we generated mice with targeted mutations in the murine met locus, representative of both somatic and germ-line activating mutations present in HPRC. We found that the different activating Met mutations predispose mice to diverse tumor types, including sarcomas, lymphomas, and carcinomas, some of which are metastatic. Moreover, similar to HPRC, we also discovered trisomy of chromosome 6 and nonrandom duplication of the mutant met allele in the majority of tumors. Our results lead us to conclude that met amplification and genetic instability are necessary for tumor progression within the presence of activating Met mutations.
Materials and Methods
Generation of met Mutant Mice. A cDNA clone containing the coding region from the SphI site in exons 14 (3,051 bp) through 20 was inserted into the pSXLDUE vector containing a poly(A) sequence. The bacterial neomycin (neo) gene was inserted 3′ of the poly(A) tail, and the herpes simplex virus thymidine kinase (tk) gene was ligated downstream of the 3′ recombination region to allow for counterselection against random integration of the construct. Mutations were introduced into individual constructs by PCR [M1248T (ATG → ACG), Y1228C (TAC → TGC), D1226N (GAC → AAC), and M1248T/L1193V (ATG → ACG/CTT → GTT)] and verified by sequencing. The SpeI linearized construct was electroporated into embryonic stem cells and injected into blastocysts by using methods described in refs. 18 and 19. Germ-line transmission was confirmed by Southern hybridization on tail DNA and PCR genotyping (for details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site). Experiments using mice were approved by the Van Andel Research Institute Institutional Animal Care and Use Committee.
Immunoprecipitation and Western Blot Analyses. Approximately 100 mg of liver tissue from adult male mice was homogenized in RIPA buffer [20 mM Tris (pH 7.5)/150 mM NaCl/0.1% SDS/1% Non-idet P-40/50 mM sodium fluoride/1 mM sodium orthovanadate/1 mM EDTA/0.5% deoxycholate/Complete Proteinase Inhibitor Mixture Tablets (Roche Diagnostics)]. Protein concentrations were quantified by using the DC Protein Assay (Bio-Rad). For immunoprecipitation, 1 mg of protein was incubated overnight with anti-Met antibody (B-2, Santa Cruz Biotechnology) in 1 ml of RIPA buffer at 4°C. Immune complexes were collected with protein G-Sepharose beads (Amersham Pharmacia) and washed three times in RIPA buffer. Immune complexes were eluted in 30 μl of Laemmli sample buffer (pH 6.8) (Sigma) and boiled for 10 min before being loaded on to a gel. Immunoprecipitated lysates were separated on a 0.8% Tris-glycine gel (Invitrogen), transferred onto a poly(vinylidene difluoride) membrane (Invitrogen), and examined by Western analysis using an anti-Met antibody (B-2, Santa Cruz Biotechnology). Western blot analyses were performed as described in ref. 20.
Survival Curves. Survival was determined by following the respective cohorts of animals daily to determine mortality. Cohort sizes were as follows: WT, n = 36; WTmu/+, n = 13; M1248T/L1193V, n = 71; Y1228C, n = 59; D1226N-1, n = 119; D1226N-2, n = 72; and M1248T, n = 49. We analyzed survival by using the Kaplan–Meier survival function. Pairwise comparisons of survival curves were performed with a log-rank test.
Tumor Analysis. Mice that died or were moribund were subjected to necropsy. Tumor samples were surgically isolated and directly fixed in 4% paraformaldehyde/PBS for at least 48 h. Fixed tissues were dehydrated, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin/eosin.
FISH. Tumor touch preps were prepared by imprinting slightly thawed tissues onto glass slides and air-drying them. The slides were fixed in methanol/acetic acid (3:1) for 20 min, equilibrated in 2× saline/sodium citrate at 60°C for 45 min, digested with 0.005% pepsin at 37°C for 10 min, and washed with 1× PBS for 5 min. Slides were placed in 1% formaldehyde for 10 min at room temperature, washed with 1× PBS for 5 min, and dehydrated in an ethanol series (70%, 85%, 95%) for 2 min each. Slides were denatured in 70% formamide/4× saline/sodium citrate at 73°C for 5 min, washed in a cold ethanol series (70%, 85%, 95%) for 2 min each, and air dried. Probes were denatured at 73°C for 5 min, added to the slide, and hybridized overnight at 37°C. Posthybridization washes were with 2× saline/sodium citrate at 73°C for 2.5 min, cooled in 4× saline/sodium citrate/0.1% Tween 20, and rinsed in H2O. Slides were air-dried and then counterstained with anti-fade DAPI.
Bacterial artificial chromosome clone RP23–416H6 (located within A2 of chromosome 6) was labeled with Spectrum Green (Vysis, Downers Grove, IL), and clone RP24–462C10 (located within D3 of chromosome 6) was labeled with Spectrum Orange (Vysis) by using nick translation. Clone RP23–151I21 (located within A1 of chromosome 8) and clone RP23–23D5 (located within B3 of chromosome 16) were labeled with either Spectrum Green or Orange depending on the comparison. At least three spleens were analyzed as controls from each line.
Image acquisition was performed with a charge-coupled device camera (VDS vossKühler Gmbh, Osnabrück, Germany) mounted on an Olympus BX51 epifluorescence microscope by using fish-view software (Version 2.1, Applied Spectral Imaging, Vista, CA). Hybridization signals were scored for at least 200 nuclei per slide.
Real-Time PCR Analysis. Genomic DNA from tumor and control samples was extracted and RNase-treated with the Wizard Genomic DNA Purification Kit (Promega). RT-PCR was performed with the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) according to the manufacturer's instructions. A 6-carboxyfluorescein (FAM)-labeled TaqMan MGB probe (5′-CACCAAAGGACCACAC-3′) and primers (forward, 5′-CGCAGAAGTTCACCACCAAGTC-3′, and reverse, 5′-CGTCATGAGCTCCCAGAGGAG-3′) were specifically designed by using primer express v1.5a (Applied Biosystems). Standards were prepared with 2, 20, 100, and 200 ng of +/+, mu/+, and mu/mu genomic DNA and amplified by using TaqMan Universal PCR Master Mix (PE Biosystems, Foster City, CA) according to the following PCR conditions: 50°C for 2 min and 95°C for 10 min, followed by 35 cycles of 92°C for 15 s, and 58°C for 1 min. The threshold cycle (CT) for each sample was determined and used to generate individual standard curves for +/+, mu/+, and mu/mu samples. For tumor tissues and controls (mu/+ and mu/mu), 100 ng of genomic DNA was amplified, and the copy number of the mutant met allele was determined by using the linear regression equations from the standard curves. The copy number of met was determined by using the manufacturer's calculation where the mass of the mouse haploid genome is 3.3 pg (Applied Biosystems).
Results
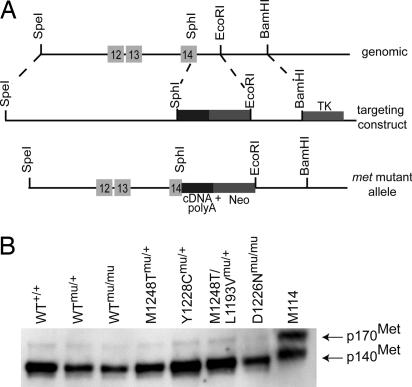
Generation of Mice with Targeted met Mutations. To study the tumorigenic potential in vivo of Met mutations when expressed from the endogenous met locus, we generated mice with activating missense met mutations that correspond to mutations detected in HPRC (8, 9). Mice were generated with a murine met construct (mu) containing the 3′ portion of exons 14 (bases 31–225) through 20 and a simian virus 40 poly(A) tail (Fig. 1A). Five mouse lines were generated on a C57BL/6J;129/SV background and are referred to according to their mutation as follows: D1226N, Y1228C, M1248T, and a met locus containing a highly active in vitro double mutation M1248T/L1193V. A WT met line was generated to control for locus modifications. Germ-line transmission of the mutant alleles was confirmed by Southern blot hybridization and PCR genotyping (see Fig. 6A, which is published as supporting information on the PNAS web site), and the mutations were verified by sequencing (data not shown). Based on RT-PCR and immunoblotting, the levels of met RNA and protein expression are equivalent in the WT and mutant mice (Figs. 1B and 6B). Phosphorylated Met was observed at equal, but extremely low, levels in the liver extracts of each of the lines (data not shown).
Fig. 1.
Generation of met mutant mice. (A) Targeting strategy used to insert met cDNA into the met locus. The met expression cassette contains exons 14–20 and poly(A) sequences. Homologous recombination (dashed lines) resulted in the targeted allele. The following mutations were inserted during recombination: M1248T (ATG → ACG), Y1228C (TAC → TGC), D1226N (GAC → AAC), and a double mutation, M1248T/L1193V (ATG → ACG/CTT → GTT). WT met mutant mice also were created. (B) Western blot analysis of Met expression in livers from adult mice. M114 cells (NIH 3T3 cells stably transfected with murine met) were used as a positive control.
Male and female heterozygous mice were intercrossed to determine the viability of homozygous mutant animals. Both homozygous WTmu/mu and D1226Nmu/mu mice were obtained, but only heterozygous offspring were produced from the other met mutant lines. This result was not unexpected given that the D1226N mutation had weak activity in in vitro studies (16, 17). Approximately 130 embryos at various developmental stages were examined from each line. For reasons not yet apparent, viable homozygous embryos were not observed in the Y1228C and M1248T lines or the M1248T/L1193V line after embryonic day 6.5 or 13.5, respectively.
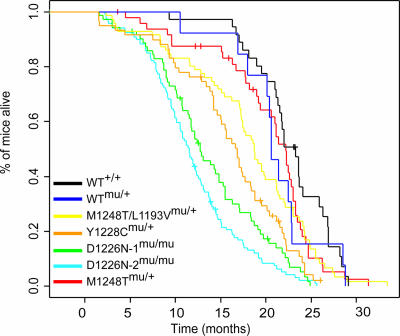
Survival Analysis of met Mutant Mice. To determine the effect of activating Met mutations on the lifespan of the met mutant mice, pairwise Kaplan–Meier analysis was performed on each line (Fig. 2). Median survival times (months) for each line were as follows: WT+/+, 22.1 ± 4.3; WTmu/+, 20.5 ± 4.7; M1248T/L1193Vmu/+, 19.0 ± 5.3; Y1228Cmu/+, 15.9 ± 6.4; D1226N-1mu/mu, 12.5 ± 6.0; D1226N-2mu/mu, 10.6 ± 6.7; and M1248Tmu/+, 18.3 ± 5.8. There was no significant difference in survival between WT+/+ and WTmu/+ mice (P = 0.3). The M1248Tmu/+ and M1248T/L1193Vmu/+ mice also had no significant difference in survival compared with WTmu/+ mice (P = 0.9 and P = 0.4, respectively). This result was unexpected, given that M1248T and M1248T/L1193V are the most active mutations in focus formation and tumorigenic assays in nude mice (16, 17). Although the M1248Tmu/+ and M1248T/L1193Vmu/+ mice live a normal lifespan, neoplasias generally were discovered in the older mice upon necropsy. By contrast, the Y1228Cmu/+ mice had a significantly shorter lifespan than WTmu/+ mice (P = 0.03). The D1226Nmu/mu mice had the shortest lifespan of all of the mutated lines. Only eight D1226Nmu/+ mice were aged and had a lifespan of 20 ± 6.6 months. Because of the long lifespan of these mice, D1226Nmu/mu mice were used in the following studies. The met mutant lines are not congenic, and, therefore, the influence of genetic background on the tumor profiles cannot be excluded. However, it is important to note that two D1226Nmu/mu lines originating from separate founders had no statistical difference in their lifespan (P = 0.07) or tumor profile, suggesting that our results are not due to background effects.
Fig. 2.
Survival analysis of met mutant mice showing Kaplan–Meier survival curves of met mutant and WT mice.
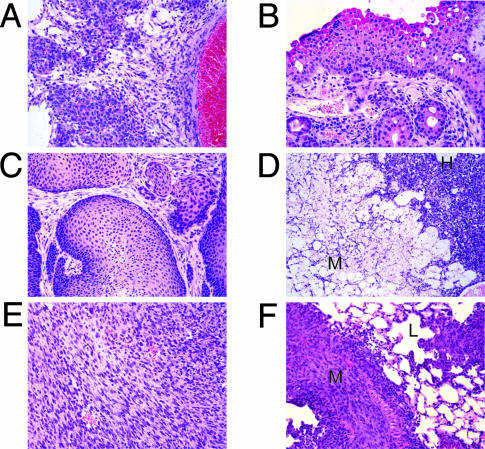
met Mutant Mice Develop Unique Tumor Phenotypes. We performed histological analysis on ≈18 mice from each line (Tables 1 and 2). Surprisingly, different lines of met mutant mice presented different types of tumors. The most striking difference noted was between the D1266Nmu/mu, Y1228Cmu/+, and M1248T/L1193Vmu/+ lines, which showed a high frequency of sarcomas and rare carcinomas (observed in FISH studies), and the M1248Tmu/+ mice, which developed carcinomas but not sarcomas. All met mutant lines except D1226Nmu/mu developed lymphomas. D1226Nmu/mu mice developed only sarcomas, primarily hemangiosarcomas. The hemangiosarcomas detected in our mice were histologically unique compared with hemangiosarcomas observed in WT mice. The hemangiosarcomas in met mutant mice contained small-caliber blood vessels with moderately pleiomorphic endothelial cells, cavernous blood vessels, and palisading epithelioid-like cells (Fig. 3A). The reason for this unique histological characteristic is not known. The two D1226Nmu/mu lines were combined in the histological studies because similar tumor profiles were observed in each line. In the Y1228Cmu/+ mice, 63% developed sarcomas, whereas 32% developed lymphomas. Although murine carcinomas are rare, several, such as a transitional cell and a squamous cell carcinoma, were observed in the M1248Tmu/+ mice (Fig. 3 B and C). M1248T/L1193Vmu/+ mice presented with various sarcomas, including hemangiosarcomas, fibrosarcomas, a leiomyosarcoma, and a spindle-like sarcoma, but only two lymphomas. In addition, some of the mutant met mice presented with multiple cancers. For example, one M1248Tmu/+ mouse had a transitional cell carcinoma and histiocytic sarcoma, and a Y1228Cmu/+ mouse had a contiguous hemangiosarcoma and myxoma (Fig. 3D). Several other mice with contiguous tumors were identified (Table 3). In addition, we observed a Y1228Cmu/+ mouse with a metastatic hemangiosarcoma within the lung and liver, and a M1248T/L1193Vmu/+ mouse presented with metastatic fibrosarcoma in the lung and liver (Fig. 3 E and F). Finally, all mutant lines with the exception of the D1226Nmu/mu line showed infrequent to moderate incidence of kidney defects including glomerulonephritis and hydronephrosis (data not shown).
Table 1. Histopathological analysis of met mutant mice.
| Tumor/lesion | M1248Tmu/+ | Y1228Cmu/+ | M1248T/L1193Vmu/+ | D1226Nmu/+ | WTmu/+ |
|---|---|---|---|---|---|
| Sarcomas | |||||
| Hemangiosarcoma | — | 10 | 4* | 13 | — |
| Fibrosarcoma | — | 1 | 1* | 2* | — |
| Myxoma | — | 1† | — | — | — |
| Leiomyosarcoma | — | — | 1 | — | — |
| Spindle-like | — | — | 1 | — | — |
| Lymphomas | |||||
| Histiocytic sarcoma | 5 | 5 | 1 | — | 1 |
| Lymphoblastic | — | — | 1 | — | 2 |
| Lymphocytic | 1 | 1 | — | — | — |
| Follicular center | 1 | — | — | — | — |
| Carcinomas | |||||
| Transitional cell | 1 | — | — | — | — |
| Squamous cell | 1 | — | — | — | — |
| Lung adenocarcinoma | 1 | — | — | — | 1 |
| Lung adenoma | 1 | — | — | — | 1 |
| Basosquamous adenocarcinoma | 1 | — | — | — | — |
| Hepatoma | 1 | — | 1 | — | — |
| Nonremarkable | 1 | 0 | 3 | 3 | 4 |
Some mice contained more than one tumor. Some mice also had pathological phenotypes that were not reported in this study; therefore, these mice were not included in the “nonremarkable pathology” group. Lung adenomas and hepatomas may have been observed due to the old age of the mice.
Tumors in which metastatic lesions were observed.
The myxoma was contiguous with a hemangiosarcoma (shown in Fig. 3D).
Table 2. Tumor incidence in met mutant mice.
| Mutant | Total mice | Survival range, months | Tumor incidence, % |
|---|---|---|---|
| M1248Tmu/+ | 17 | 4-29 | 58.8 |
| Y1228Cmu/+ | 19 | 1-23 | 89.5 |
| M1248T/L1193Vmu/+ | 18 | 3-27 | 50.0 |
| D1226Nmu/mu | 18 | 2-19 | 83.8 |
| WTmu/+ | 9 | 10-28 | 44.4 |
Some mice contained more than one tumor. Only malignant tumors were included in the tumor incidence calculation (i.e., lung adenomas were not included).
Fig. 3.
met mutant mice develop unique tumor profiles. (A) Hemangiosarcoma observed s.c. in a D1226Nmu/mu mouse. (B) Transitional cell carcinoma of the ureter observed in a M1248Tmu/+ mouse. (C) Squamous cell carcinoma of the anus observed in a M1248Tmu/+ mouse. (C) Adjacent myxoma (M) and hemangiosarcoma (H) observed in a Y1228Cmu/+ mouse. (E and F) Primary fibrosarcoma from a M1248T/L1193Vmu/+ mouse (E) and the metastatic lesion (M) in the lung (L) (F). (Hematoxylin/eosin images, ×200 magnification.)
Table 3. Relative copy number of met using dual-color FISH in met mutant tumors, showing percentage of nuclei with amplification or loss.
| Mutant line | Tumor no. | Pathology | Trisomy chr. 6, % | Tetrasomy chr. 6, % | Amplification of met locus, % | Loss of chr. 6, % | Loss of met locus, % |
|---|---|---|---|---|---|---|---|
| Y1228C | |||||||
| 1 | Undifferentiated sarcoma | 45.2 | 0 | 0 | 0 | 0.5 | |
| 2 | Hemangiosarcoma | 14.9 | 12.9 | 0 | 2 | 1 | |
| 3 | Hemangiosarcoma | 6.9 | 7.8 | 0 | 3.9 | 2 | |
| 4 | Hemangiosarcoma | 17.5 | 0 | 1.7 | 1.1 | 0 | |
| 5 | Hemangiosarcoma | 10.2 | 0 | 2.7 | 5.8 | 8.8 | |
| 6 | Lymphoblastic lymphoma | 6 | 0 | 1.5 | 0 | 2 | |
| 7 | Hemangiosarcoma | 2.3 | 0 | 0 | 2.3 | 7.9 | |
| 8 | Hemangiosarcoma/myxoma | 12.2 | 1.5 | 0 | 2 | 3 | |
| M1248T/L1193V | |||||||
| 1 | Hemangiosarcoma | 4.4 | 0 | 5.2 | 1.5 | 3.7 | |
| 2 | Pancreatic ductal carcinoma | 21 | 0.5 | 2 | 0.5 | 0 | |
| 3 | Histiocytic sarcoma/hemangiosarcoma | 3.4 | 0 | 1.1 | 3.4 | 5.2 | |
| 4 | Lymphoblastic lymphoma | 19.7 | 0 | 3 | 0.4 | 0 | |
| 5 | Hemangiosarcoma | 19.9 | 5 | 1.5 | 2 | 0 | |
| 6 | Lymphoblastic lymphoma | 15.3 | 5.1 | 1.4 | 0.9 | 0 | |
| 7 | Leiomyosarcoma/histiocytic sarcoma | 11.2 | 1.5 | 0 | 7.8 | 5.3 | |
| M1248T | |||||||
| 1 | Histiocytic sarcoma | 6.5 | 17.9 | 2 | 0 | 1.5 | |
| 2 | Lymphoblastic lymphoma | 3.5 | 0 | 1 | 1 | 3.5 | |
| 3 | Lymphoma | 8 | 0 | 1.5 | 1 | 1 | |
| 4 | Lymphoblastic lymphoma | 0 | 0 | 0 | 0 | 1 | |
| 5 | Lymphocytic lymphoma | 11.7 | 0 | 1 | 1 | 1 | |
| 6 | Histiocytic sarcoma | 45.5 | 0.5 | 2 | 0 | 0.5 | |
| 7 | Adenosquamous carcinoma | 57 | 0 | 0.5 | 1 | 0 | |
| D1226N | |||||||
| 1 | Hemangiosarcoma | 14.5 | 0 | 0 | 3 | 2.5 | |
| 2 | Hemangiosarcoma | 5.1 | 0 | 0 | 3.9 | 3.5 | |
| 3 | Hemangiosarcoma | 10.4 | 5.9 | 0 | 1 | 2.5 | |
| 4 | Fibrosarcoma | 5.7 | 1.7 | 4.5 | 0 | 1.7 | |
| 5 | Hemangiosarcoma | 2 | 0 | 4 | 1.5 | 1 | |
| 6 | Undifferentiated sarcoma | 8.4 | 0 | 11.8 | 3.9 | 3.4 | |
| 7 | Hemangiosarcoma | 7.5 | 0 | 0 | 4.5 | 2 | |
| 8 | Undifferentiated sarcoma | 9.1 | 0 | 9.6 | 4.8 | 2.4 |
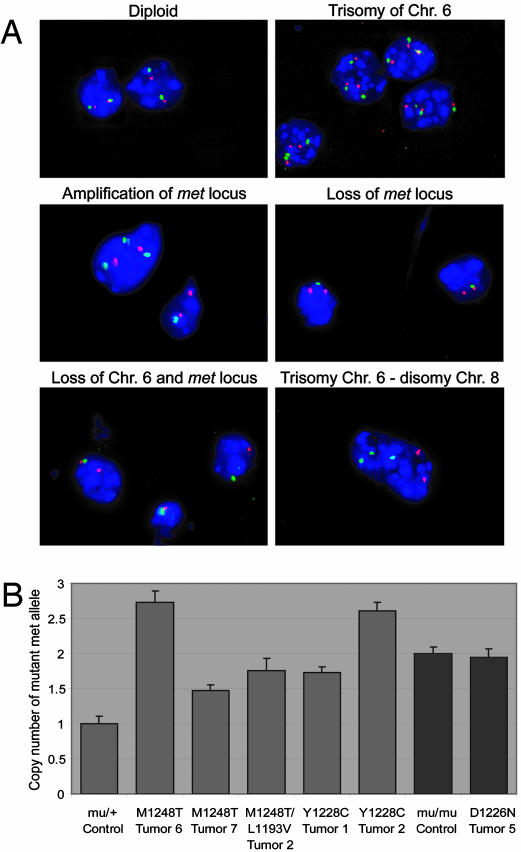
Trisomy of Chromosome 6 is Observed in met Mutant Tumors. In HPRC, nonrandom duplication of the mutant met allele is shown to be a factor for tumor development (14, 15). To determine whether the met locus was duplicated in tumor samples from met mutant mice, we performed dual-color interphase FISH on touch preparations of tumors and normal tissues from each of the met lines by using a bacterial artificial chromosome clone specific for the met locus in band A2 of chromosome 6 (RP23-416H6) as well as a control probe for band D3 in chromosome 6 (RP24-462C10). The control probe allowed us to distinguish between duplication of chromosome 6 and localized amplification of the met locus. Strikingly, cells with trisomy and tetrasomy of chromosome 6 were observed in 97% and 30% of the met mutant tumors examined (n = 30), respectively (Fig. 4A and Table 3). By contrast, normal tissues examined from each met mutant line had polyploidy in <2.1% of their nuclei (Table 4). Moreover, when the same tumors were examined with markers for chromosomes 8 and 16, no changes in the copy number of chromosome 16 were observed, and only two tumors exhibited more than two copies of chromosome 8 (Fig. 4A). Cells with amplification of only the met locus, but not the chromosome 6D3 region, were noted in half of the tumors examined, albeit at a lower frequency than the trisomic and tetrasomic cells, and were most common in D1226Nmu/mu tumors. Interestingly, the loss of one copy of chromosome 6 and/or loss of only the met locus also was observed in several tumors that also contained chromosome 6 polyploidy. We suspect that the WT met allele is lost in the nuclei where one met locus is observed. Collectively, these results demonstrate that tumor formation in met mutant mice is associated with nonrandom polyploidization of chromosome 6 and the met locus.
Fig. 4.
FISH of met mutant tumors. (A) Relative copy number of met was measured by using dual-color interphase FISH on tumor touch preps. Photomicrographs with RP23–416H6 met probe (green), RP24–462C10 probe (red), and DAPI counterstaining are shown as follows: diploid nuclei containing two RP23–416H6 and two RP24–462C10 signals (Top Left); trisomy of chromosome (Chr.) 6 (Top Right); duplication of only the met locus (Middle Left); loss of the met locus (Middle Right); and loss of one copy of Chr. 6 (Bottom Left). (Bottom Right) A photomicrograph of interphase FISH with RP23–416H6 probe (Chr. 6, green), RP23–151I21 probe (Chr. 8, red), and DAPI counterstaining; trisomy of Chr. 6 and disomy of Chr. 8 are present. (B) The number of mutant met alleles was determined by using quantitative PCR. M1248T tumors 6 and 7, M1248T/L1193V tumor 2, and Y1228C tumors 1 and 2 all had significant levels of Chr. 6 trisomy based on FISH analysis (Table 3). The mutant met allele was significantly increased in comparison with the mu/+ control. Based on FISH, D1226N tumor 5 had no Chr. 6 trisomy, and no increase in mutant met was observed by quantitative PCR when compared with the mu/mu control. P values for each comparison are as follows: M1248T tumor 6, P = 2.4 × 10–5; M1248T tumor 7, P = 0.001; M1248T/L1193V tumor 2, P = 0.002; Y1228C tumor 1, P = 0.0001; Y1228C tumor 2, P = 4.0 × 10–6; and D1226N tumor 5, P = 0.7.
Table 4. Relative copy number of met using dual-color FISH in normal tissues, showing percentage of nuclei with amplification or loss.
| Mutant line | Trisomy chr. 6, % | Tetrasomy chr. 6, % | Amplification of met locus, % | Loss of chr. 6, % | Loss of met locus, % |
|---|---|---|---|---|---|
| Y1228C | 2.1 | 0.6 | 1.0 | 0.0 | 1.9 |
| M1248T/L1193V | 0.9 | 0.3 | 1.3 | 0.6 | 1.5 |
| M1248T | 1.8 | 0.3 | 1.4 | 0.3 | 1.9 |
| D1226N | 1.0 | 0.0 | 1.2 | 0.7 | 2.8 |
At least three spleens were used as controls for each met mutant line. chr., chromosome.
The Mutant met Allele Is Duplicated in Mutant met Tumors. To determine whether the mutant met allele was being amplified in the murine tumors, we performed quantitative PCR with a probe specific to the mutant met allele. Five tumors with significant chromosome 6 trisomy and one tumor without trisomy were examined (Fig. 4B). Each of the tumors with trisomy had significant increases in the copy number of the mutant met allele when compared with the nontumor heterozygous control. Because the tumor extracts examined contained a heterogeneous mixture of normal and tumor cells, the copy numbers for the mutant allele did not equal absolute values. Note that a control tumor lacking trisomy 6 had no increase in the mutant allele when compared to the nontumor mu/mu control. These results demonstrate that duplication of the mutant met allele is required for tumorigenesis.
Discussion
Activating mutations in the tyrosine kinase domain of Met are associated with HPRC. An essential role for these mutations in the etiology of HPRC is supported by the observation that ectopic expression of the same Met mutations in cultured cell lines causes constitutive Met activation, focus formation, cell motility, and tumorigenesis (16, 17). Here, we show that these mutations, under the control of the endogenous met promoter, are responsible for spontaneous tumorigenesis in vivo. These results provide convincing evidence that activating Met mutations play a key role in promoting in vivo tumorigenesis.
Interestingly, the met mutant mice we generated in this study showed remarkable differences in tumor profiles. Why the D1226Nmu/mu, Y1228Cmu/+, and M1248T/L1193Vmu/+ lines develop a high frequency of sarcomas and rarely carcinomas, whereas the M1248Tmu/+ mice develop carcinomas but not sarcomas, is not apparent. We hypothesize that these differences arise because of structural changes that distinctly alter the ability of Met to recruit accessory proteins into an active signaling complex. Indeed, the D1226N and Y1228C mutations lie directly adjacent within the activation loop (Fig. 5) and have similar tumor profiles, with both lines developing hemangiosarcomas and fibrosarcomas (Table 1). By contrast, M1248T lies within the P+1 loop of the activation domain (furthest from D1226N and Y1228C) and promotes a distinct tumor profile compared with the other mutations. It is particularly interesting that mice carrying both the M1248T and L1193V mutations develop a combination of lymphomas and sarcomas similar to those in the D1226N mu/+ and Y1228Cmu/+ mice. Given that L1193 lies near the activation loop, these results suggest that structural changes at or about the activation loop are “dominant” to activating mutations within the P+1 loop. It is also noteworthy that mice with activating Met mutations, particularly M1248T, developed a high incidence of carcinomas; whereas most age-related human cancers have epithelial origins and develop into carcinomas, mice normally develop mesenchymal tumors such as sarcomas and lymphomas (21). Remarkably, although these mice showed a significant incidence of glomerulonephritis and severe hydronephrosis, they did not develop HPRC. Similar disparities between mice and humans have been reported for other cancers; e.g., mice deficient in the von Hippel–Lindau tumor suppressor, which is linked to clear cell renal carcinomas in humans, also fail to develop renal cancers (22, 23). However, we cannot rule out that the C57BL/6J;129/SV background suppresses the development of renal carcinomas.
Fig. 5.
Model of activating mutations within the Met kinase domain. The M1248T mutation is located within the P+1 loop (teal), whereas the D1226N and Y1228C mutations lie within the activation loop (purple). The weaker L1193V mutation (light blue) lies near catalytic residues (green). The model is based on the crystal structure of the insulin receptor (29) as derived from Birchmeier et al. (7). [Reproduced with permission from Nature Reviews (www.nature.com/reviews) Molecular Cell Biology (7) (Copyright 2004, Macmillan Magazines Ltd.).]
Nonrandom duplication of the mutant met allele has been reported in HPRC as a requirement for progression (14, 15). We found that a majority of tumors from the mutant mouse lines show similar nonrandom duplication of the mutant met allele. This finding suggests that amplification of the mutant allele is the second hit necessary for tumor development. We observed trisomy of chromosome 6 and duplication of the mutant met allele in each of the met mutant lines and in a majority of tumors of epithelial or mesenchymal origin. The presence of this amplification event in the met mutant mice is noteworthy considering that mouse cancer models often lack the chromosomal alterations that are highly prevalent in human cancers (21). The requirement of this duplication event may explain the long tumor latency that is observed in the heterozygous met mutant mice. This tumor latency mimics the late age of onset observed in patients with HPRC (24). Interestingly, similar results have been observed in patients harboring germ-line mutations in the kinase domain of Ret, a protooncogene structurally similar to Met. Patients with ret mutations develop multiple endocrine neoplasia type 2, and genetic analysis of pheo-chromocytomas from patients with multiple endocrine neoplasia type 2 has shown either duplication of the mutant ret allele or loss of the WT allele in the tumors (25). Because of the low percentage of nuclei with loss of the met locus in our tumors, we were unable to determine which allele was lost by quantitative PCR. Based on the results from patients with Ret mutations, we suspect that the WT allele is lost in the few cases in which the mutant allele is not amplified. The recapitulation of this cytogenetic event confirms the value of this mouse model for understanding the role of mutationally activated Met in tumor development and metastasis in diverse cellular environments.
The met oncogene is involved in the progression and metastasis of numerous human cancers (7). Therefore, understanding the distinct signaling activities of mutationally activated receptor tyrosine kinases is crucial for the development of successful cancer therapeutics. This observation was made abundantly evident in a recent study of the efficacy of the Met inhibitor SU11274 on various mutant forms of Met (26). SU11274 was found to potently inhibit human equivalents of the murine Met M1248T and H1092Y mutants but was ineffective against the L1193V and Y1228H mutants (26). Similarly, Gleevec has been shown to be ineffective in patients with point mutations in the kinase domains of Bcr-Abl or Kit (27, 28). Our met mutant mice will provide a valuable model to test the effectiveness of Met inhibitors on tumors containing activating mutations present in human cancers.
Supplementary Material
Acknowledgments
We thank R. Sigler for pathology expertise; the Bioinformatics Special Program and the Cytogenetics Program for technical assistance; B. Eagleson and the Lab Animal Resources staff; N. Duesbery for critical reading of the manuscript; Y. Zhang for sequencing analysis; D. Diegel for construction of the necropsy database; R. Hay, M. Hassen, Y. Jimenez, and K. Obeng for assistance with tissue processing; and D. Petillo, M. Gustafson, S. Moshkovitz, M. Reed, J. DeGroot, and J. Schoumans for technical assistance. We gratefully acknowledge the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor for support (Michigan Animal Models Consortium Grant 085P1000815 and Michigan Center for Biological Information Grant 085P1000819).
Author contributions: C.G. and G.V.W. designed research; C.G., Y.S., J.K., L.-M.W., L.T., and M.F. performed research; C.B. and P.S. contributed new reagents/analytic tools; R.B. analyzed data; and C.G. wrote the paper.
Abbreviations: HGF/SF, hepatocyte growth factor/scatter factor; HPRC, hereditary papillary renal carcinoma; mu, murine met construct.
References
- 1.Stoker, M., Gherardi, E., Perryman, M. & Gray, J. (1987) Nature 327, 239–242. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenberg, E., Meyer, D., Weidner, K. M. & Birchmeier, C. (1993) J. Cell Biol. 123, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bladt, F., Riethmacher, D., Isenmann, S., Aguzzi, A. & Birchmeier, C. (1995) Nature 376, 768–771. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt, C., Bladt, F., Goedecke, S., Brinkmann, V., Zschiesche, W., Sharpe, M., Gherardi, E. & Birchmeier, C. (1995) Nature 373, 699–702. [DOI] [PubMed] [Google Scholar]
- 5.Uehara, Y., Minowa, O., Mori, C., Shiota, K., Kuno, J., Noda, T. & Kitamura, N. (1995) Nature 373, 702–705. [DOI] [PubMed] [Google Scholar]
- 6.Rubin, J. S., Bottaro, D. P. & Aaronson, S. A. (1993) Biochim. Biophys. Acta 1155, 357–371. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G. F. (2003) Nat. Rev. Mol. Cell Biol. 4, 915–925. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt, L., Duh, F.-M., Chen, F., Kishida, T., Glenn, G., Choyke, P., Scherer, S. W., Zhuang, Z., Lubensky, I., Dean, M., et al. (1997) Nat. Genet. 16, 68–73. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt, L., Junker, K., Nakaigawa, N., Kinjerski, T., Weirich, G., Miller, M., Lubensky, I., Neumann, H. P., Brauch, H., Decker, J., et al. (1999) Oncogene 18, 2343–2350. [DOI] [PubMed] [Google Scholar]
- 10.Lee, J. H., Han, S. U., Cho, H., Jennings, B., Gerrard, B., Dean, M., Schmidt, L., Zbar, B. & Vande Woude, G. F. (2000) Oncogene 19, 4947–4953. [DOI] [PubMed] [Google Scholar]
- 11.Park, W. S., Dong, S. M., Kim, S. Y., Na, E. Y., Shin, M. S., Pi, J. H., Kim, B. J., Bae, J. H., Hong, Y. K., Lee, K. S., et al. (1999) Cancer Res. 59, 307–310. [PubMed] [Google Scholar]
- 12.Ma, P. C., Kijima, T., Maulik, G., Fox, E. A., Sattler, M., Griffin, J. D., Johnson, B. E. & Salgia, R. (2003) Cancer Res. 63, 6272–6281. [PubMed] [Google Scholar]
- 13.Kovacs, G., Akhtar, M., Beckwith, B. J., Bugert, P., Cooper, C. S., Delahunt, B., Eble, J. N., Fleming, S., Ljungberg, B., Medeiros, L. J., et al. (1997) J. Pathol. 183, 131–133. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, J., Palmedo, G., von Knobloch, R., Bugert, P., Prayer-Galetti, T., Pagano, F. & Kovacs, G. (1998) Oncogene 17, 733–739. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang, Z., Park, W. S., Pack, S., Schmidt, L., Vortmeyer, A. O., Pak, E., Pham, T., Weil, R. J., Candidus, S., Lubensky, I. A., et al. (1998) Nat. Genet. 20, 66–69. [DOI] [PubMed] [Google Scholar]
- 16.Jeffers, M., Schmidt, L., Nakaigawa, N., Webb, C. P., Weirich, G., Kishida, T., Zbar, B. & Vande Woude, G. F. (1997) Proc. Natl. Acad. Sci. USA 94, 11445–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffers, M., Fiscella, M., Webb, C. P., Anver, M., Koochekpour, S. & Vande Woude, G. F. (1998) Proc. Natl. Acad. Sci. USA 95, 14417–14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessarollo, L. (2001) Methods Mol. Biol. 158, 47–63. [DOI] [PubMed] [Google Scholar]
- 19.Bonin, A., Reid, S. W. & Tessarollo, L. (2001) Methods Mol. Biol. 158, 121–134. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, Y.-W., Wang, L.-M., Jove, R. & Vande Woude, G. F. (2002) Oncogene 21, 217–226. [DOI] [PubMed] [Google Scholar]
- 21.Rangarajan, A. & Weinberg, R. A. (2003) Nat. Rev. Cancer 3, 952–959. [DOI] [PubMed] [Google Scholar]
- 22.Gnarra, J. R., Ward, J. M., Porter, F. D., Wagner, J. R., Devor, D. E., Grinberg, A., Emmert-Buck, M. R., Westphal, H., Klausner, R. D. & Linehan, W. M. (1997) Proc. Natl. Acad. Sci. USA 94, 9102–9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, W., Tessarollo, L., Hong, S. B., Baba, M., Southon, E., Back, T. C., Spence, S., Lobe, C. G., Sharma, N., Maher, G. W., et al. (2003) Cancer Res. 63, 5320–5328. [PubMed] [Google Scholar]
- 24.Zbar, B., Glenn, G., Lubensky, I., Choyke, P., Walther, M. M., Magnusson, G., Bergerheim, U. S., Pettersson, S., Amin, M. & Hurley, K. (1995) J. Urol. 153, 907–912. [PubMed] [Google Scholar]
- 25.Huang, S. C., Koch, C. A., Vortmeyer, A. O., Pack, S. D., Lichtenauer, U. D., Mannan, P., Lubensky, I. A., Chrousos, G. P., Gagel, R. F., Pacak, K. & Zhuang, Z. (2000) Cancer Res. 60, 6223–6226. [PubMed] [Google Scholar]
- 26.Berthou, S., Aebersold, D. M., Schmidt, L. S., Stroka, D., Heigl, C., Streit, B., Stalder, D., Gruber, G., Liang, C., Howlett, A. R., et al. (2004) Oncogene 23, 5387–5393. [DOI] [PubMed] [Google Scholar]
- 27.Roumiantsev, S., Shah, N. P., Gorre, M. E., Nicoll, J., Brasher, B. B., Sawyers, C. L. & Van Etten, R. A. (2002) Proc. Natl. Acad. Sci. USA 99, 10700–10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Silva, C. M. & Reid, R. (2003) Pathol. Oncol. Res. 9, 13–19. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard, S. R., Wei, L., Ellis, L. & Hendrickson, W. A. (1994) Nature 372, 746–754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.