Abstract
Periodontal disease (PD) and atherosclerotic vascular disease (ASVD) are both chronic inflammatory diseases with a polymicrobial etiology and have been epidemiologically associated. The purpose is to examine whether periodontal bacteria that infect the periodontium can also infect vascular tissues and enhance pre-existing early aortic atherosclerotic lesions in LDLRnull mice. Mice were orally infected with intermediate bacterial colonizer Fusobacterium nucleatum for the first 12 weeks followed by late bacterial colonizers (Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia) for the remaining 12 weeks mimicking the human oral microbiota ecological colonization. Genomic DNA from all four bacterial was detected in gingival plaque by PCR, consistently demonstrating infection of mouse gingival surfaces. Infected mice had significant levels of IgG and IgM antibodies, alveolar bone resorption, and showed apical migration of junctional epithelium revealing the induction of PD. These results support the ability of oral bacteria to cause PD in mice. Detection of bacterial genomic DNA in systemic organs indicates hematogenous dissemination from the gingival pockets. Bacterial infection did not alter serum lipid fractions or serum amyloid A levels and did not induce aortic atherosclerotic plaque. This is the first study examining the causal role of periodontal bacteria in induction of ASVD in LDLRnull mice.
Keywords: Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Fusobacterium nucleatum, polymicrobial infection, LDLR−/− mice, periodontal disease, atherosclerosis
Major periodontal bacterial sequential infection in infected LDLR-deficient mice differs significantly from ApoE−/− mice where bacteria were closely associated with increased atherosclerosis.
INTRODUCTION
Cardiovascular disease (CVD) includes atherosclerosis in coronary, carotid and peripheral vessels, as well as associated high blood pressure. Plaque rupture and thrombosis in coronary atherosclerosis cause sudden arterial occlusion and myocardial infarction, carotid and cerebral arterial disease, as well as hemostasis in cardiac arrhythmias such as atrial fibrillation, cause thromboembolic strokes and peripheral arterial disease causes claudication and gangrene. Coronary atherosclerosis has significant associations with poor oral health and is a major global health problem and a leading cause of morbidity and mortality in North America. Epidemiological studies (Bahekar et al. 2007) have suggested periodontal disease (PD) as a risk factor for coronary heart diseases such as myocardial infarction and angina pectoris (Libby, Egan and Skarlatos 1997; Paquette, Brodala and Nichols 2007), but results have been inconsistent due to differences in study design, definition of PD, confounding factors and outcomes examined. PD and atherosclerosis share common risk factors such as old age, smoking, diabetes, hypertension, obesity, low socioeconomic status and genetic predisposition (Lockhart et al. 2012). Several clinical studies (Paquette, Brodala and Nichols 2007; Humphrey et al. 2008; Lockhart et al. 2012) also have reported associations between CVD and periodontal infections. For more than a century, a link between oral health and atherosclerosis has been proposed and recently confirmed by the American Heart Association, supporting published a statement supporting an association between PDs and atherosclerotic vascular disease (ASVD) but not a causal association (Lockhart et al. 2012).
The presence of genomic DNA from eight to nine oral bacteria including Porphyromonas gingivalis (Brodala et al. 2005), Treponema denticola (Cavrini et al. 2005), Tannerella forsythia (Lee, Jun and Choi 2014) and Fusobacterium nucleatum (Zaremba et al. 2007) in human aortic atherosclerotic lesions also revealed a definitive link between PD and ASVD. Among the eight to nine periodontal pathogens, P. gingivalis is most frequently detected followed by T. denticola, T. forsythia and F. nucleatum (Haraszthy et al. 2000; Fiehn et al. 2005). Recent studies investigated P. gingivalis, T. denticola, T. forsythia and F. nucleatum causative roles (invasion of gingiva, intravascular dissemination, invasion of aortic adventitia, alteration of lipid profile and atherosclerosis risk factors, inflammasome activation) in accelerating aortic atherosclerosis in vivo in ApoE−/− mice as a monoinfection in chronic infection model (Chukkapalli et al. 2014, 2015b; Velsko et al. 2014, 2015) or as synergistic multispecies polymicrobial infections in ApoE−/− mice (Rivera et al. 2013; Chukkapalli et al. 2015) and integrin β6−/− mice (Velsko et al. 2015).
Oral bacteria enter the bloodstream through severely inflamed gingival connective tissues and broken blood vessels (Hajishengallis 2015). Once in the blood stream, the bacteria can be carried throughout the body and into distant organs, and can attach and invade the endothelium of the vessel and initiate inflammation at sites distant from the oral cavity. Among the four well-characterized periodontal bacteria, only P. gingivalis and T. denticola demonstrated an active invasion of gingiva, aortic tissues and induction of aortic plaque, thus causally linking periodontitis and atherosclerosis (Chukkapalli et al. 2014; Velsko et al. 2014), whereas T. forsythia and F. nucleatum modulate systemic atherosclerosis risk and inflammatory factors without enhancing aortic plaque progression (Chukkapalli et al. 2015; Velsko et al. 2015). Periodontal pathogens such as P. gingivalis are believed to cause endothelial dysfunction leading to formation of plaques and potentially facilitating atherosclerotic plaque rupture to result in thrombosis (Brodala et al. 2005; Hajishengallis 2015). Among the most widely used mouse models of accelerated atherosclerosis are ApoEnull mice and LDLRnull mice. The low density lipoprotein receptor (LDLR) is a cell surface glycoprotein that plays a critical role in regulating the amount of cholesterol in the blood. These receptors are particularly abundant in the liver, which is the organ responsible for removing most excess cholesterol from the body (Li, Cam and Bu 2001). In terms of atherosclerotic plaque development, Apoe-deficient mice develop progressive lesions more characteristic of those observed in humans with and without high-fat diet supplementation. On the other hand, the lipid profile of Ldlr-deficient mice with a higher percentage of cholesterol carried in IDL/LDL particles more closely resembles the dyslipidemia in humans (Veniant, Withycombe and Young 2001). However, LDLR−/− mice have low serum cholesterol levels (200–300 mg dl−1) on regular chow diet (4% animal fat with <0.04% cholesterol) and very high levels (>2000 mg dl−1) when fed a high-fat diet (7.5% cocoa butter, 1.25% cholesterol, 7.5% casein and 0.5% sodium cholate). LDLR−/− mice develop small atherosclerotic lesions that develop slowly when fed normal chow (Getz and Reardon 2006). In humans, mutations in the genes coding for LDLR result in a disease called familial hypercholesterolemia, an autosomal dominant disorder (Soutar and Naoumova 2007).
Our previous experimentation in ApoEnull mice (Chukkapalli et al. 2014, 2015a,b; Velsko et al. 2014, 2015) designed to examine the causal associations between PD and atherosclerosis has given us clues about the role of polymicrobial synergy in atherosclerotic plaque formation which led us to explore this causal association in an alternative mouse model for atherosclerosis to assess any similarities for differences between ApoEnull and LDLRnull mouse atheroma models. This study was initiated specifically to investigate the role of four well-characterized oral bacteria in the progression of atherosclerosis using LDLRnull mice. The focus of this study was to examine whether synergistic polybacterial infection can enhance pre-existing but minimal aortic atherosclerotic lesions in LDLRnull mice on normal chow diets.
MATERIALS AND METHODS
Periodontal bacterial strains and growth conditions
Fusobacterium nucleatum ATCC 49256, Porphyromonas gingivalis ATCC 53977, Treponema denticola ATCC 35404 and Tannerella forsythia ATCC 43037 were used for gingival infection in this study. These strains were routinely cultured in an anaerobic environment at 37°C in a coy anaerobic chamber (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015a,b; Velsko et al. 2014, 2015). Bacterial concentrations were determined using the Petroff-Hausser bacterial counting chamber, and cells were resuspended in RTF (reduced transport fluid) at 1010 cells per mL for mouse gingival infection. For topical gingival polymicrobial infection, P. gingivalis was mixed with an equal quantity of T. denticola for 5 min; subsequently, T. forsythia was added to the culture tubes and cells were mixed thoroughly and allowed to interact for an additional 5 min. Porphyromonas gingivalis (Pg), T. denticola (Td) and T. forsythia (Tf) were then mixed with an equal volume of 8% CMC in phosphate buffered saline (PBS), and this mixture was used for gingival infection (5 × 109 bacteria per mL) in LDLRnull mice (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
Sequential infection of LDLRnull mice
Male LDLRnull B6.129S7-Ldlr tm1Her/J mice (8 weeks old) were purchased from the Jackson Laboratories (Bar Harbor, ME. USA) and were housed in microisolator plastic cages, fed standard chow and water ad libitum. These mice were randomly distributed into two groups. Group 1 mice were infected with F. nucleatum (Fn) followed by Pg/Td/Tf as polymicrobial infection (n = 12) and group II mice were sham infected (n = 12). All mouse procedures were performed in accordance with the approved protocol guidelines by the IACUC of the University of Florida (IACUC Protocol # 201004539). Kanamycin (500 μg ml−1) was administered in drinking water for consecutive 3 days to the mice, prior to the gingival infection. The mouse oral cavity was also swabbed with 0.12% chlorhexidine gluconate (Peridex: 3M ESPE Dental Products, St. Paul, MN, USA) to inhibit endogenous microorganisms and to enhance subsequent colonization of human periodontal bacteria (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
In order to mimic the sequential colonization of human periodontal bacteria in the mice oral cavity, a sequential infection model was adopted comprising infection with F. nucleatum (intermediate colonizer) first, followed by infection with P. gingivalis, T. denticola and T. forsythia (late colonizers). The experimental infection scheme (Fig. 1) consisted of total eight infection cycles (4 days a week every third week for 24 weeks). For the first 12 weeks of infection (Fig. 1), group I mice were periodontally infected with 109F. nucleatum cells in RTF-8% CMC. The multispecies polymicrobial inoculum (5 × 109 combined bacteria per mL; 1 × 109 cells in 0.2 mL per mouse; 3.3 × 108P. gingivalis; 3.3 × 108T. denticola and 3.3 × 108T. forsythia) was administered topically to gingival surface to infection group for the remaining 12 weeks mimicking the human ecological microbial colonization. Sham-infected mice (n = 12) were inoculated with sterile 8% CMC suspended in RTF (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
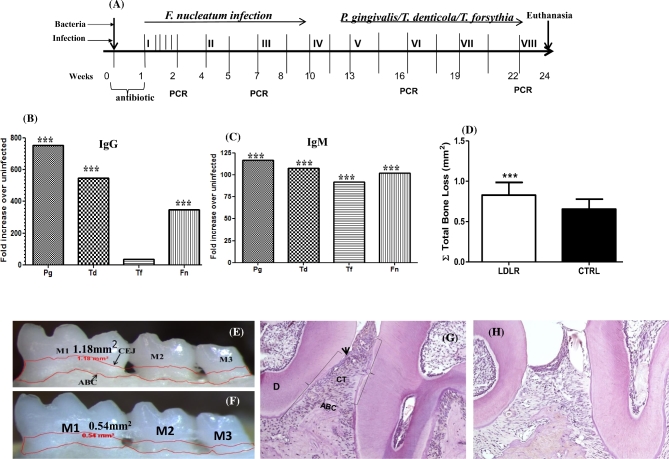
Figure 1.
(A) Schematic diagram of the experimental design illustrating infections, gingival plaque sampling, euthanasia and tissue collection. Mice were sequentially administered an oral infection comprising first four cycles of infection with F. nucleatum (infection I through infection IV) followed by a polybacterial infection with P. gingivalis, T. denticola and T. forsythia (infection V through infection VIII). Gingival plaque samplings are indicated by PCR at week 1, 7, 16 and 19 following initial infection. Blood and tissue specimens were collected after euthanasia following 24 weeks of infection. (B) Serum IgG response in LDLRnull mice after 24 weeks of sequential polybacterial infection with F. nucleatum, P. gingivalis, T. denticola and T. forsythia (n = 12). The graph shows the response to gingival infection with periodontal bacteria expressed as fold increase over sham-infected mice (n = 12). (C) Serum IgM levels specific to F. nucleatum, P. gingivalis, T. denticola and T. forsythia after 24 weeks of bacterial infection. The graph shows the response to gingival infection expressed as fold increase over sham-infected mice (n = 12). (D) Total horizontal ABR measurements in infected mice, sham-infected LDLRnull mice (n = 12) (***P < 0.001). Each bar indicates the mean horizontal ABR. Measurements were made between the CEJ and ABC of three molar teeth by two independent individuals blinded to the treatment group. Error bars indicate standard deviations. (E) Representative image of the mandible lingual surface depicting the horizontal ABR following polybacterial infection in LDLRnull mice. (F) Representative image of mandible lingual surface following sham infection in LDLRnull mice. Measureable bone resorption denoted by the area inside the red line. M1, first molar; M2, second molar; M3, third molar; Black arrows represent CEJ and ABC, respectively. (G) Representative image of the infected LDLRnull mice gingival tissue demonstrating enhanced apical migration of JE, epithelial hyperplasia and infiltration of inflammatory cells in connective tissue, and bone resorption lacuna in ABC. D—dentin, arrow head indicates CEJ and ABC, brackets indicate migration of JE and CT—connective tissue. (H) Representative image of sham-infected LDLRnull mice gingival tissue showing no signs of epithelial migration, infiltration of inflammatory cells and visible inflammation in CT.
Gingival plaque sampling
To determine gingival colonization of bacteria in infected LDLRnull mice, a total of four post-infection gingival plaque samples (two samples from intermediate colonizer and two samples from late colonizers) were collected 3 days after each infection 1, 3, 6 and 7. The gingival plaque samples were collected using a sterile veterinary cotton swab. The molar teeth and surrounding gingival tissue were swabbed and immersed in TE buffer. The presence of F. nucleatum genomic DNA was determined after infections 1 and 3, during the first 12 weeks of infection. For the remaining 12 weeks of infection with late colonizers, the presence of P. gingivalis, T. denticola and T. forsythia genomic DNA was determined after 6 and 7 infection cycles (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
Detection of bacterial genomic DNA in gingival plaque samples
Polymerase chain reaction (PCR) was performed to detect the presence of the bacterial species in the gingival plaque samples. Colony PCR was carried out with a Bio-Rad thermal cycler and by using 16S rRNA species-specific oligonucleotide forward and reverse primers: P. gingivalis forward 5΄- GGT AAG TCA GCG GTG AAA CC-3΄, reverse 5΄- ACG TCA TCC ACA CCT TCC TC-3΄; T. denticola forward 5΄-TAATACCGAATGTGCTCATTTACAT-3΄, reverse 5΄-CTGCCATATCTCTATGTCATTGCTCTT-3΄; T. forsythia forward 5΄-AAAACAGGGGTTCCGCATGG-3΄, reverse 5΄-TTCACCGCGGACTTAACAGC-3΄; F. nucleatum forward 5΄-TAAAGCGCGTCTAGGTGGTT-3΄, reverse 5΄-ACAGCTTTGCGAC-TCTCTGT-3΄. PCR was performed in a 50 μl reaction mixture containing Phusion® High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA), template DNA and 0.2 μM of oligonucleotide primers. Genomic DNA extracted from these four strains served as positive controls and PCR performed with no template DNA served as negative control. PCR products were separated by 1.5% agarose gel electrophoresis and the bands were visualized using a BioRad Gel Doc XR/Chemidoc Gel Documentation System (BioRad, CA, USA) (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
Blood and tissue specimen collection from LDLRnull mice
Following eight oral bacterial infection cycles, mice were euthanized and blood was collected by cardiac puncture. Serum was separated, stored at −20°C for bacterial specific immunoglobulin G (IgG) and immunoglobulin M (IgM) antibody, serum lipid profile and serum amyloid A (SAA, acute-phase inflammatory marker) analyses. The aorta, heart, lungs, spleen, liver and kidneys were also collected to determine the levels of hematogenous dissemination of oral bacteria from gingival tissue. In addition, the mouse maxillae and mandibles were also collected for evaluation of alveolar bone resorption (ABR) by morphometric analysis (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
Serum antibody analysis
Serum collected from the mice was used to determine IgG and IgM antibody levels against each of the four bacteria using a standard enzyme-linked immunosorbent assay (ELISA) protocol. Mice serum antibody concentrations were determined using a gravimetric standard curve that consisted of six mouse IgG and IgM concentrations (Sigma-Aldrich, St. Louis, MO, USA) (Rivera et al. 2013). Mean antibody titer values of infected mice were divided by mean antibody titer values of sham-infected mice, and the quotient represents the fold change in mean specific antibody titer produced by polybacterial infection. Graphs demonstrate mean fold-change in specific antibody titer of infected mice. Statistically significant differences between mean specific antibody titers of infected and sham-infected groups were determined by unpaired two-tailed Student's t test, and are indicated above fold-change values on the graph. (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
Morphometric analysis of ABR
The horizontal area ABR was measured by histomorphometry. The maxilla and mandibles collected from mice post euthanasia were immersed in 3% (vol/vol) hydrogen peroxide for 2–3 h after autoclaving and defleshing. Digital images of both buccal and lingual root surfaces of all molar teeth were captured under a 10× stereo dissecting microscope (SteReo Discovery V8; Carl Zeiss Microimaging, Inc, Thornwood, NY, USA), after superimposition of buccal and lingual cusps to ensure reproducibility and consistency. The line tool was used to measure the horizontal ABR from the cemento-enamel junction (CEJ) to the alveolar bone crest (ABC). The surface perimeters of CEJ and ABC were traced using the calibrated line tool (AxioVision LE 29A software version 4.6.3.). Two examiners who were blind to the study performed all measurements twice at separate times (Kesavalu et al. 2007; Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
Histology of gingival tissue
The maxillas were collected from five mice on sacrifice and the tissues were fixed in 10% neutral buffered formalin followed by decalcification in PBS containing 0.4 M EDTA and 2% formaldehyde, embedded in paraffin, and sectioned. Sections were later stained for histological analysis using hematoxylin and eosin (H&E) and were scanned with a ScanScope CS system (Aperio, Vista, CA). The scanned slides were later viewed at a 200× magnification with ImageScope viewing software (Aperio). Evidence of inflammation was determined as previously described (Bainbridge et al. 2010; Velsko et al. 2014, 2015)
Detection of bacterial genomic DNA in distal organs
Bacterial genomic DNA was isolated from heart, liver, kidney, lungs, spleen and aorta tissue samples by following a standardized protocol using the Qiagen DNeasy Blood and Tissue kit. The genomic DNA isolated was used for bacterial species-specific PCR as previously described (Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
Serum lipid profile and SAA measurements
Sera (30 μl each) from infected (n = 6) and sham-infected (n = 6) mice were analyzed for serum lipoprotein levels using the method of Liposearch analysis which is based on gel permeation principle of high pressure liquid chromatography at Skylight Biotech Inc. (Akita, Japan) (Chukkapalli et al. 2014, 2015). SAA levels in the infected and sham-infected mice were measured using a standardized ELISA protocol using a kit designed by Kamiya Biomedical, Seattle, WA (Chukkapalli et al. 2014, 2015).
Morphometric analysis of aortic atherosclerosis
The heart, aortic arch and thoracic aorta were harvested from the mice after euthanasia. Each sample was then fixed in 10% neutral buffered formalin, processed and paraffin embedded. Paraffin embedded samples were cut transversely into cross sections of 5 μm thick cryosections on the Leica EG 1160 Cryostat (Leica Microsystems Inc., Bannockburn, IL, USA), 8–12 cross sections taken along the length of each segment. Sections were stained with H&E. Morphometric analysis was performed using an Olympus DP7 color video camera attached to an Olympus BX51 microscope (Olympus America, Center Valley, PA, USA). The images were captured and analyzed with the Image Pro system MC 6.0 software program (Olympus America), with measurements adjusted to the microscopic objective (Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
Statistical analysis
Statistical analyses of bacterial-specific serum antibody levels, ABR, serum lipid profiles and SAA were performed by using Graph Pad Prism Software for Windows, version 5. A value of P < 0.05 was considered significant. Antibody analysis and ABR data are presented in figures as means ± standard deviations (SD). Aortic plaque histology measurements were analyzed by analysis of variance with the Statview program and post hoc Fisher's PLSD analysis with bar graphs represented as mean ± standard error. An unpaired two-tailed Student's t-test was used to compare two independent groups (Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
RESULTS
Periodontal bacterial infection of the LDLRnull mice
During 24 weeks of bacterial infection, LDLRnull mice were monitored for the presence of human periodontal bacteria (Fusobacterium nucleatum, Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia) by PCR using 16S rRNA bacterial-specific primers. Gingival plaque samples were obtained from both infected and sham-infected mice. This was done to confirm colonization/infection of the bacterial inocula. In the first 12 weeks of monoinfection, F. nucleatum was detected in the gingival surface 9 out of 12 mice by the seventh week of infection (Table 1). In the remaining 12 weeks of polybacterial infection (19th week of infection), 7 out of 12 infected mice and 5 out of 12 mice were also positive for P. gingivalis and T. denticola, respectively (Table 1). In contrast, none of the 12 mice were positive for T. forsythia (Table 1). No sham-infected mice were positive for F. nucleatum, P. gingivalis, T. denticola or T. forsythia, at any of the time points examined (Table 1).
Table 1.
Distribution of LDLRnull mice gingival plaque samples positive for bacterial genomic DNA by PCR.
| Bacterial infection | Positive gingival plaque samples | |||
|---|---|---|---|---|
| Number of weeks of infections | 1 weeka | 7 weeksa | 16 weeksb | 19 weeksb |
| Infected micec (n = 12) | ||||
| Fusobacterium nucleatum | 1 | 9 | NI | NI |
| Porphyromonas gingivalis | NI | NI | 5 | 7 |
| Treponema denticola | NI | NI | 3 | 5 |
| Tannerella forsythia | NI | NI | 0 | 0 |
| Sham-infected miced (n = 12) | 0 | 0 | 0 | 0 |
Indicate time points at which the LDLRnull mice were infected only with F. nucleatum (intermediate colonizer).
Indicate time points at which the LDLRnull mice were infected only with a polybacterial inoculum of P. gingivalis, T. denticola and T. forsythia (late colonizers).
Indicate LDLRnull mice were sequentially infected for 24 weeks with F. nucleatum for the first 12 weeks, followed by a polybacterial infection of P. gingivalis, T. denticola and T. forsythia. Samples were analyzed using appropriate bacterial species-specific PCR primers with positive and negative controls.
Oral plaque samples were collected from sham-infected mice periodically and were examined for the presence of bacterial genomic DNA of F. nucleatum, P. gingivalis, T. denticola and T. forsythia. NI, no infection with bacteria.
Serum antibody response specific to the individual bacterial species
Bacteria-specific serum IgG and IgM levels were evaluated in mice sera from both infected and sham-infected mice by ELISA. All of 24 weeks infected mice had statistically significant higher levels of IgG compared to sham-infected mice (P < 0.001) with respect to F. nucleatum, P. gingivalis and T. denticola (Fig. 1B). In contrast, antibodies to T. forsythia did not reach significance (Fig. 1B) compared to the sham-infected mice. Similarly, mice infected with all four pathogens induced significant IgM antibodies than sham-infected mice (Fig. 1C). These data strongly suggest that all four bacteria were adhered and colonized in the gingival surface, invaded gingival epithelium and induced bacterial specific IgG and IgM antibodies. In addition, T. forsythia was colonized and induced significant IgM antibodies even though the gingival plaque samples were not positive (below threshold levels or due to difficulty in plaque sampling) for genomic DNA.
Alveolar bone resorption
The progression of PD resulting from polymicrobial infection was examined by measuring the area between CEJ and ABC on buccal and palatal surfaces of the molars to analyze the effects of polymicrobial infection. The findings demonstrated significantly higher (P < 0.001) palatal horizontal ABR in both the mandible and maxilla of polymicrobial infected mice, when compared to sham-infected mice (Fig. 1D). The significant ABR in the infected mice (Fig. 1E) than sham-infected mice (Fig. 1F) indicates that the chronic gingival infection effectively induced significant PD in LDLR−/− mice.
Gingival inflammation
On histological examination of gingival tissue sequential infected LDLR−/− mice showed enhanced apical migration of junctional epithelium (JE), epithelial hyperplasia, inflammatory cellular infiltration in connective tissue and bone resorption lacuna in ABC (Fig. 1G) compared to sham-infected LDLR−/− mice (Fig. 1H) strongly indicating induction of PD to bacterial infection.
Systemic invasion of the periodontal pathogens in the distal organs
Oral bacteria have the ability to enter the blood stream from inflamed gingival connective tissues during brushing, flossing and dental procedure resulting in low-level, transient bacteremia. Thus, to evaluate whether local gingival topical application of these bacteria can result in gingival adherence, invasion and multiplication in gingival epithelium and in intravascular dissemination, the presence of bacterial genomic DNA was evaluated in multiple distant organs. As shown in Table 2, PCR analysis of heart, aorta, liver, kidney and lung samples revealed the presence of all four bacterial genomic DNA (P. gingivalis > T. denticola > F. nucleatum > T. forsythia) in the infected mice. These results clearly indicate that oral bacteria were able to be transported from the gingival tissue and invade or infect distant organs via systemic circulation.
Table 2.
Distribution of systemic infection in internal organs of LDLRnull mice.
| Bacterial infection | Positive systemic tissue samples | |||||
|---|---|---|---|---|---|---|
| Number of organs sampled by PCR | ||||||
| Heart | Aorta | Liver | Spleen | Kidney | Lung | |
| (n = 11) | (n = 8) | (n = 11) | (n = 5) | (n = 11) | (n = 11) | |
| Fusobacterium nucleatum | 3 | 1 | 1 | 0 | 3 | 2 |
| Porphyromonas gingivalis | 6 | 4 | 3 | 0 | 1 | 4 |
| Treponema denticola | 5 | 3 | 1 | 0 | 0 | 1 |
| Tannerella forsythia | 3 | 1 | 1 | 0 | 0 | 0 |
| Sham infection | 0 | 0 | 0 | 0 | 0 | 0 |
To analyze intravascular infection of systemic organs (heart, aorta, liver, spleen, kidney, lungs), total DNA from each perspective organ was isolated and examined for four bacterial genomic DNA by PCR using appropriate species-specific primers.
Serum lipid fractions and SAA levels
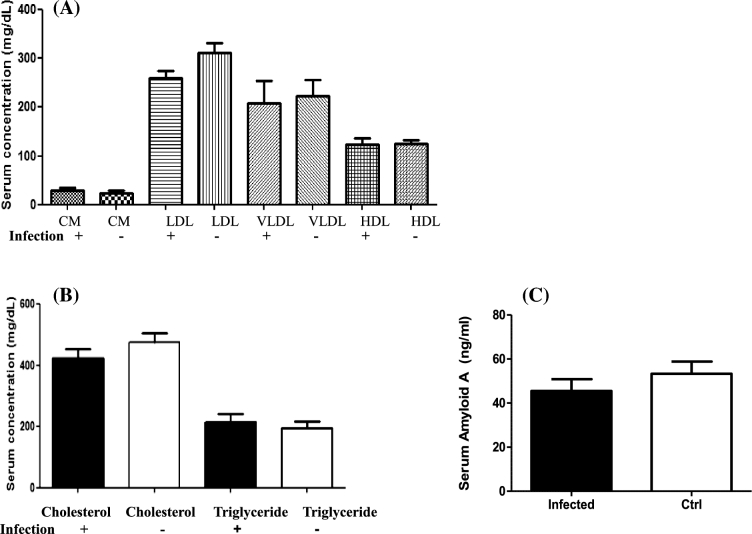
Evaluation of serum lipoprotein fractions and atherosclerotic risk factors was done following oral infection in LDLRnull mice for 24 weeks. We observed no significant increase in the LDL or VLDL levels as seen in Fig. 2A. The LDL levels in the infected and sham-infected mice were only within 200–300 mg dl−1. The total cholesterol level (Fig. 2B) was lower in the infected mice than the sham-infected mice, but this did not reach significance. Furthermore, when the SAA levels were evaluated, it was observed that the bacterial infection lowered the SAA levels in the LDLRnull mice compared to sham-infected mice (Fig. 2C).
Figure 2.
Changes in systemic risk factors of atherosclerosis after sequential bacterial infection in LDLRnull mice. (A) Polybacterial infection-induced alterations in serum lipoprotein faction levels in LDLRnull mice (n = 6). CM indicates chylomicron, VLDL indicates very low-density lipoprotein, LDL indicates low-density lipoprotein and HDL indicates high-density lipoprotein. Data points and error bars represent means and standard deviations for infected compared to control mice. (B) Polybacterial infection-induced alteration in serum total cholesterol and triglyceride levels in the serum of LDLRnull mice (n = 6). Data points and error bars represent means and standard deviations for infected compared to sham-infected mice. (C) Polybacterial infection-induced alteration in serum levels of SAA in LDLRnull mice (n = 6). Data points and error bars represent means and standard deviations for infected compared to sham-infected mice.
Histomorphometric analysis of atherosclerotic plaque
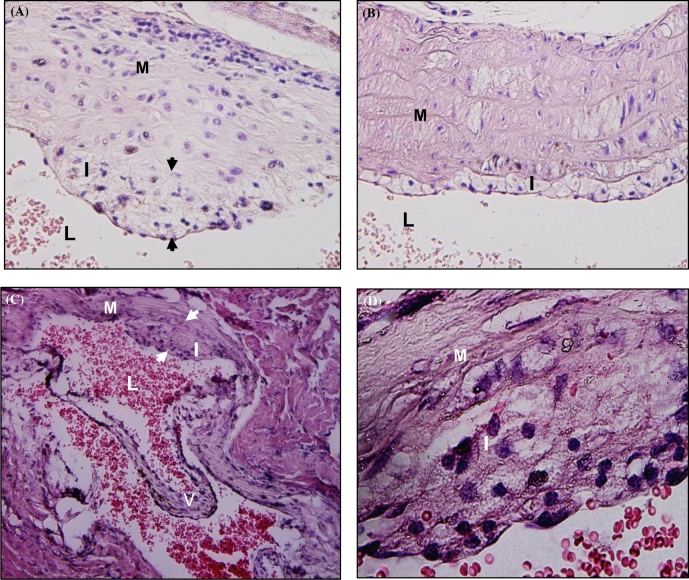
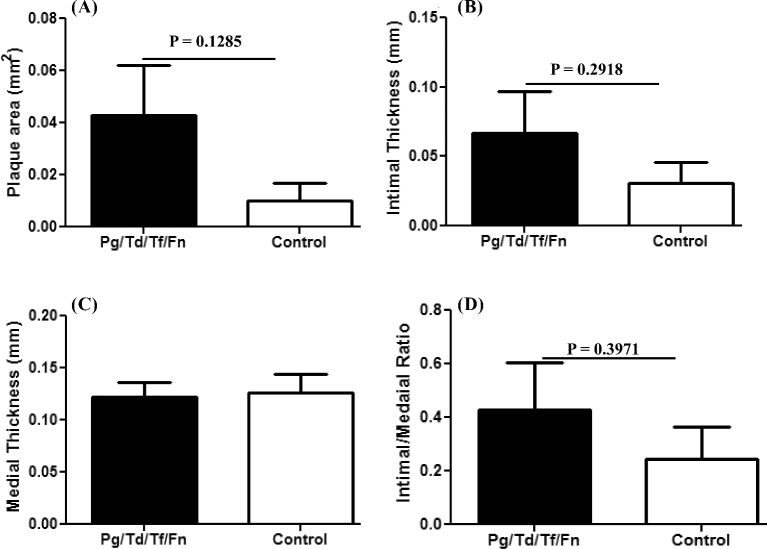
Sequential colonization of four oral bacteria over 24 weeks of chronic infection did not induce increased atherosclerotic plaque in the aortic arch and thoracic aorta of LDLRnull mice (Figs 3A–B and 4A–D). One out of 10 sham-infected mice had small plaque in the aortic root valve (Fig. 3C and 3D).
Figure 3.
Atherosclerotic plaque in the H&E stained aortic leaf of sham-infected LDLRnull mice. Panel A is a representative image of plaque in infected LDLRnull mice at 24 weeks of infection at a lower magnification -20× and B is a representative image of plaque in infected LDLRnull mice at a higher magnification 100×. Panel C is a representative image of plaque in sham-infected LDLRnull mice at 24 weeks of infection at a lower magnification 20× and panel D is a representative image of plaque in sham-infected LDLRnull mice at a higher magnification 100×. White arrowheads indicate plaque margin; I indicates intimal layer; M indicates medial layer; V indicates valve; and L indicates lumen.
Figure 4.
Chronic sequential infection induced alteration in the aorta of LDLRnull mice. (A) Bar graphs of morphometric analysis of mean aortic plaque area in the sequential-infected and sham-infected LDLRnull mice, (B) intimal layer thickness, (C) medial layer thickness and (D) intimal/medial layer thickness ratios. (n = 10 for all graphs).
DISCUSSION
Numerous observational studies have supported the association between PD and ASVD but not a causal association (Paquette, Brodala and Nichols 2007; Humphrey et al. 2008; Lockhart et al. 2012). Detection of genomic DNA from nine periodontal bacteria in inflammatory atherosclerotic lesions further suggested the association between periodontal bacteria and atherosclerosis (Lockhart et al. 2012). Our laboratory has focused on better elucidation of a causal association of periodontal bacteria and ASVD and our previous studies examined the causal role of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia and Fusobacterium nucleatum in induction of atherosclerosis using a hyperlipidemic ApoEnull mouse model and integrin β6−/− mouse model (Velsko et al. 2014, 2015; Chukkapalli et al. 2014, 2015). In view of LDLR's critical role in regulation of the amount of cholesterol in the circulating blood, we explored whether the periodontal bacteria can induce atherosclerosis in an LDLR−/− mouse model. Furthermore, we tried to understand the role of sequential polymicrobial infection in promoting oral bacterial colonization/infection in gingival tissue, vascular inflammation and atherosclerosis. We chose to examine a chronic infection model of mice rather than a short-term infection, as this provides the best index for observing the natural means by which periodontal infection can affect the distal organs involved in atherogenesis.
A previous study from our laboratory (Rivera et al. 2013) involved a polymicrobial infection of only the late colonizers P. gingivalis, T. denticola and T. forsythia of the subgingiva in ApoEnull mice. In our current study, four well-characterized periodontal bacteria F. nucleatum (intermediate colonizer), P. gingivalis, T. denticola and T. forsythia (late colonizers) were sequentially infected in LDLRnull mice, mimicking the human oral bacterial colonization. The findings indicate that 67% of LDLRnull mice had gingival surface colonized with F. nucleatum, 58% with P. gingivalis and 42% with T. denticola, and additional infections were performed in order to simulate conditions equivalent to chronic infection (Chukkapalli et al. 2014, 2016). ABR is a major characteristic of periodontitis and these LDLRnull mice exhibited significant ABR and gingival inflammation. These results were also similar to the ABR observed in the ApoEnull mice (Rivera et al. 2013; Chukkapalli et al. 2014, 2015; Velsko et al. 2014).
The polymicrobial infection significantly elicited bacterial species-specific serum IgG (P. gingivalis > T. denticola > F. nucleatum) and IgM antibodies to each of the four bacteria in LDLRnull mice (P. gingivalis > T. denticola > F. nucleatum > T. forsythia). These observations in infected LDLRnull mice indicate that there is a clear indication of a robust colonization of these pathogens and an induction of pathogen-specific humoral immune response. Our detection of bacterial genomic DNA in distant organs clearly indicates that these bacteria have the ability to invade gingival epithelium and disseminate hematogenously similar to that observed in ApoEnull mice (Rivera et al. 2013; Chukkapalli et al. 2015). Furthermore, the presence of bacterial genomic DNA especially in the aorta and heart samples indicates that these bacteria invaded aortic vessel and maybe involved in causing vascular wall lesions and promoting the initiation of atheromatous plaque.
A high serum level of LDL is a risk factor for atherosclerotic plaque development. According to Getz and Reardon (2006), when LDLRnull mice are fed normal chow diet, these mice exhibit modest levels of atherosclerotic plaque and have total cholesterol levels within the range 200–300 mg dl−1. Our study findings were similar to these prior observations as the cholesterol levels observed in the infected mice lie in aforementioned range, indicating that infection does not alter the serum lipid profile levels in LDLRnull mice.
Our previous experiments with P. gingivalis as a monobacterial infection in ApoEnull mice demonstrated that although the serum levels of lipid molecules are not altered significantly by chronic infection, P. gingivalis gingival infection can trigger atheroma plaque formation (Velsko et al. 2014). This observation indicates that the oral infection may be involved in altering expression of certain genes in the aortic vessel involved in promoting atherosclerosis. Moreover, in this study with the bacterial infection, we did not also observe any alteration in the acute phase inflammatory marker SAA levels in LDLRnull mice. In contrast, our experimentation in ApoEnull mice (Rivera et al. 2013; Chukkapalli et al. 2015; Velsko et al. 2015) with the polymicrobial infection altered both the serum lipid and SAA levels together with increasing atheroma formation.
In LDLRnull mice, atherosclerotic plaque is most often seen in the aortic root and throughout the aorta (Getz and Reardon 2006). In this study, we did not observe significantly increased atherosclerotic plaque and in fact minimal plaque growth in the thoracic aorta or in the aortic arch. These observations are parallel previous studies (Ishibashi et al. 1994; Sehayek et al. 2001; Teupser, Persky and Breslow 2003) which have shown that LDLRnull mice maintained on a chow diet exhibit minimal if any atherosclerosis. Furthermore, Sanan et al. (1998) have reported the atherosclerotic lesion development in LDLR−/− mice independent of consumption of a high-fat, high cholesterol diet when there is high-level expression of human apoB-100 in LDLR−/− mice. These observations signify the need to look for molecules which tandemly are associated with LDLR.
In conclusion, the results clearly demonstrate that (i) periodontal bacteria colonized the gingival pockets; (ii) periodontal infection-elicited significant IgG and IgM immune response; (iii) polymicrobial infection-induced significant ABR; (iv) presence of bacterial genomic DNA in heart, aorta, liver and lungs indicating hematogenous dissemination: (v) bacterial infection did not alter the serum lipid profiles and acute-phase protein SAA; (vi) bacterial infection did not enhance aortic plaque formation in infected LDLR deficient mice; and (vii) bacterial infection in infected LDLR-deficient mice differs significantly from ApoE−/− mice where PD bacteria were closely associated with increased atherosclerosis. Overall, this is the first study that has examined the role of sequential polybacterial infection in atherosclerosis progression in LDLRnull mice. Furthermore, studies are needed to understand the influence of alternate molecular pathways, genetic susceptibility and high-fat diet in bacterial PD induction of atherosclerosis on the chronic inflammation-induced bacterial infection.
Acknowledgments
We thank Dr Donghang Zheng for assisting the technical aspect of specimen processing.
FUNDING
This study was supported by NIH National Institute for Dental and Craniofacial Research NIDCR R01DE020820. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest. None declared.
REFERENCES
- Bahekar AA, Singh S, Saha S et al. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J 2007;154:830–7. [DOI] [PubMed] [Google Scholar]
- Bainbridge B, Verma RK, Eastman C et al. Role of Porphyromonas gingivalis phosphoserine phosphatase enzyme SerB in inflammation, immune response, and induction of alveolar bone resorption in rats. Infect Immun 2010;78:4560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodala N, Merricks EP, Bellinger DA et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscl Thromb Vas 2005;25:1446–51. [DOI] [PubMed] [Google Scholar]
- Cavrini F, Sambri V, Moter A et al. Molecular detection of Treponema denticola and Porphyromonas gingivalis in carotid and aortic atheromatous plaques by FISH: report of two cases. J Med Microbiol 2005;54:93–6. [DOI] [PubMed] [Google Scholar]
- Chukkapalli SS, Rivera MF, Velsko IM et al. Invasion of oral and aortic tissues by oral spirochete Treponema denticola in ApoE(−/−) mice causally links periodontal disease and atherosclerosis. Infect Immun 2014;82:1959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli SS, Rivera MF, Velsko IM et al. Chronic oral infection with major periodontal bacteria Tannerella forsythia modulates systemic atherosclerosis risk factors and inflammatory markers. Pathog Dis 2015a;73, DOI: 10.1093/femspd/ftv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli SS, Velsko IM, Rivera-Kweh MF et al. Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in ApoEnull mice. PLoS One 2015b;10:e0143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli SS, Velsko IM, Rivera-Kweh MF et al. Global TLR2 and 4 deficiency in mice impacts bone resorption, inflammatory markers and atherosclerosis to polymicrobial infection. Mol Oral Microbiol 2016, DOI: 10.1111/omi.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn NE, Larsen T, Christiansen N et al. Identification of periodontal pathogens in atherosclerotic vessels. J Periodontol 2005;76:731–6. [DOI] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscl Thromb Vas 2006;26:242–9. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 2015;15:30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszthy VI, Zambon JJ, Trevisan M et al. Identification of periodontal pathogens in atheromatous plaques. J Periodontol 2000;71:1554–60. [DOI] [PubMed] [Google Scholar]
- Humphrey LL, Fu R, Buckley DI et al. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med 2008;23:2079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Goldstein JL, Brown MS et al. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest 1994;93:1885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavalu L, Sathishkumar S, Bakthavatchalu V et al. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun 2007;75:1704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HR, Jun HK, Choi BK. Tannerella forsythia BspA increases the risk factors for atherosclerosis in ApoE(−/−) mice. Oral Dis 2014;20:803–8. [DOI] [PubMed] [Google Scholar]
- Li Y, Cam J, Bu G. Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol Neurobiol 2001;23:53–67. [DOI] [PubMed] [Google Scholar]
- Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation 1997;96:4095–103. [DOI] [PubMed] [Google Scholar]
- Lockhart PB, Bolger AF, Papapanou PN et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation 2012;125:2520–44. [DOI] [PubMed] [Google Scholar]
- Paquette DW, Brodala N, Nichols TC. Cardiovascular disease, inflammation, and periodontal infection. Periodontol 2000 2007;44:113–26. [DOI] [PubMed] [Google Scholar]
- Rivera MF, Lee JY, Aneja M et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS One 2013;8:e57178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanan DA, Newland DL, Tao R et al. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a). P Natl Acad Sci USA 1998;95:4544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehayek E, Ono JG, Duncan EM et al. Hyodeoxycholic acid efficiently suppresses atherosclerosis formation and plasma cholesterol levels in mice. J Lipid Res 2001;42:1250–6. [PubMed] [Google Scholar]
- Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Card 2007;4:214–25. [DOI] [PubMed] [Google Scholar]
- Teupser D, Persky AD, Breslow JL. Induction of atherosclerosis by low-fat, semisynthetic diets in LDL receptor-deficient C57BL/6J and FVB/NJ mice: comparison of lesions of the aortic root, brachiocephalic artery, and whole aorta (en face measurement). Arterioscl Thromb Vasc 2003;23:1907–13. [DOI] [PubMed] [Google Scholar]
- Velsko IM, Chukkapalli SS, Rivera MF et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One 2014;9:e97811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velsko IM, Chukkapalli SS, Rivera-Kweh MF et al. Fusobacterium nucleatum alters atherosclerosis risk factors and enhances inflammatory markers with an atheroprotective immune response in ApoEnull mice. PLoS One 2015a;10:e0129795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velsko IM, Chukkapalli SS, Rivera-Kweh MF et al. Periodontal pathogens invade gingiva and aortic adventitia and elicit inflammasome activation in alphavbeta6 integrin-deficient mice. Infect Immun 2015b;83:4582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniant MM, Withycombe S, Young SG. Lipoprotein size and atherosclerosis susceptibility in Apoe(−/−) and Ldlr(−/−) mice. Arterioscl Thromb Vasc 2001;21:1567–70. [DOI] [PubMed] [Google Scholar]
- Zaremba M, Gorska R, Suwalski P et al. Evaluation of the incidence of periodontitis-associated bacteria in the atherosclerotic plaque of coronary blood vessels. J Periodontol 2007;78:322–7. [DOI] [PubMed] [Google Scholar]