Abstract
Background
F2‐isoprostanes, a biomarker of oxidant injury, increase with advancing chronic kidney disease (CKD) in humans. In cats, the relationship between CKD and oxidative stress is poorly understood.
Objectives
To determine whether cats with advancing CKD have increasing urinary F2‐isoprostanes.
Animals
Control cats without evidence of CKD (≥6 years old; n = 11), and cats with IRIS stage 1 (n = 8), 2 (n = 38), 3 (n = 21), and 4 (n = 10) CKD.
Methods
This was a prospective observational study. Urinary F2‐isoprostanes (specifically free 15‐F2t‐isoprostanes) normalized to urine creatinine (IsoPs) were compared among groups and tested for correlations with blood pressure, proteinuria, serum creatinine concentration, and urine specific gravity. The IsoPs also were compared between cats with and without hypertension or proteinuria, and in cats fed predominantly standard versus renal diets.
Results
Urinary IsoPs were increased, but not significantly, in cats with stage 1 CKD (median 263 pg/mg creatinine; range, 211–380) compared to controls (182 pg/mg; range, 80–348) and decreased significantly from stage 1 through advancing CKD (stage 2, 144 pg/mg; range, 49–608; stage 3, 102 pg/mg; range, 25–158; stage 4, 67 pg/mg; range, 26–117; P < .01). Urinary IsoPs were inversely correlated with serum creatinine (r = −0.66, P < .0001).
Conclusion and Clinical Importance
Urinary IsoPs are significantly higher in early CKD (stage 1) compared to cats with more advanced CKD. Additional studies are warranted to characterize oxidative stress in cats with stage 1 CKD and determine whether early antioxidant treatments have a protective effect on CKD progression.
Keywords: Biomarker, Feline, Oxidative stress, Prostanoids, Redox, Renal failure
Abbreviations
- ACE
angiotensin‐converting enzyme
- BCS
body condition score
- BHT
butylated hydroxytoluene
- BUN
blood (serum) urea nitrogen
- CBC
complete blood count
- CHEM
blood chemistry
- CKD
chronic kidney disease
- CV
coefficients of variation
- GFR
glomerular filtration rate
- GPx
glutathione peroxidase
- IRIS
International Renal Interest Society
- IsoP
15‐F2t‐isoprostane:creatinine ratio
- MS
mass spectrometry
- T4
total thyroxine
- UPC
urine protein:creatinine ratio
- USG
urine specific gravity
Oxidative stress is well documented in humans with chronic kidney disease (CKD).1, 2, 3, 4 Potential sources of renal oxidative stress include tubulointerstitial inflammation, systemic hypertension, and depletion or dysregulation of antioxidant pathways.5, 6, 7, 8 Various plasma biomarkers of oxidative stress, including F2‐isoprostanes, have been shown to correlate with the stages of progressive CKD and declining glomerular filtration rate (GFR) in humans.1, 3, 6, 9, 10, 11, 12 F2‐isoprostanes are formed in vivo by nonenzymatic, free radical‐catalyzed lipid peroxidation of arachidonic acid and are an established biomarker for oxidative injury in human patients across many disease states.13 F2‐isoprostanes also can be measured in the urine, where they are more stable and much less affected by ex vivo generation of prostanoids that can occur in plasma.14 Urinary F2‐isoprostanes have been considered equivalent to plasma measurements in most studies of patients with chronic disease states.15
Oxidative stress is not only a biomarker of CKD, but also has been shown to contribute to the pathogenesis and progression of CKD.16 Studies in rodents, nonhuman primates, and human patients with renal transplants have shown directly or indirectly that reactive oxygen species and oxidative stress likely contribute to the development and exacerbation of tubulointerstitial injury and interstitial fibrosis.17, 18, 19, 20, 21 F2‐isoprostanes in particular are produced locally in the kidneys and have potent bioactivity including renal arterial vasoconstriction.22 Furthermore, antioxidant supplementation has been shown in some models to decrease progression of renal tubulointerstitial injury, proteinuria, and hypertension.20, 23
Chronic kidney disease is the most common disease affecting geriatric cats, with prevalence estimates up to 80%.24 Despite this, relatively little is known about the factors that lead to the development and progression of CKD in cats. Based on evidence in human patients and in animal models, it seems likely that oxidative stress contributes to the progression of CKD in cats and could be a target for therapeutic intervention.16 In support of this hypothesis, serum malondialdehyde (MDA), a marker of lipid oxidation, and serum 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG), a marker of oxidative DNA damage, are present at higher concentrations in cats with CKD,25 and plasma antioxidant capacities were lower in 1 study.26 However, these studies did not consider stage of renal disease, and F2‐isoprostanes, which may have direct renal effects, were not evaluated. We previously found increased urinary F2‐isoprostanes (as measured by free 15‐F2t‐isoprostanes normalized to urine creatinine) in hyperthyroid cats with dilute urine (<1.035 USG, urine specific gravity),27 but these changes were reversible with treatment of hyperthyroidism, which implicated redox stress from the hyperthyroid state rather than underlying renal disease.
The primary aim of our study was to determine whether cats with CKD with advancing International Renal Interest Society (IRIS) stage have increased urinary F2‐isoprostanes, a measure of oxidative stress, compared to healthy older cats. A secondary aim was to determine whether urinary F2‐isoprostanes differ by criteria used for IRIS substaging.
Materials and Methods
Patients and Data Collection
Client‐owned cats presented to the University of Wisconsin Veterinary Medical Teaching Hospital (UW VMTH) or to Colorado State University Veterinary Teaching Hospital for diagnosed or suspected CKD were recruited for the study. Cats with adequate information for IRIS staging and substaging were eligible for enrollment. The IRIS staging was performed based on serum creatinine concentration at the time of enrollment. Cats placed in IRIS stage 1 were nonazotemic (serum creatinine concentration <1.6 mg/dL) with dilute urine (USG <1.035; iris‐kidney.com), and without evidence of other diseases affecting urine concentration. Enrolled cats were deemed to have stable CKD based on history, physical examination, and prior biochemical analyses if available. Cats with positive urine cultures or clinical evidence of acute or acute‐on‐chronic renal failure were not eligible. Furthermore, cats with concurrent hyperthyroidism, heart failure, liver disease, or systemic infectious, neoplastic, or immune‐mediated diseases were excluded, because these conditions could influence oxidative status independently of kidney disease.
For the control group, clinically healthy cats ≥6 years of age and on no medications except parasite prevention were recruited from the UW VMTH Primary Care service and from pets belonging to the UW VMTH staff. Age, breed, sex and neuter status, body weight, body condition score (BCS), current diet, and any drug therapies were recorded for all cats. Informed consent was obtained from all cat owners, and the study was approved by the Institutional Animal Care and Use Committee. Cats were recruited from August 2012 through April 2015.
Study Interventions
Both apparently healthy cats and cats with suspected CKD were screened by a physical examination, complete blood count (CBC), serum biochemical panel, and serum total thyroxine (T4) concentration. Serum biochemical panels were performed on a VITROS 5,1 FS system1 at UW‐Madison and on a Cobas C501 machine2 at Colorado State University; these systems have comparable reference ranges for serum creatinine in cats (0.9–2.3 mg/dL and 0.8–2.4 mg/dL, respectively). Urine was collected by cystocentesis for USG, urine protein:creatinine ratio (UPC), and urine culture. Blood pressure was measured by the Doppler method,3 with a forelimb with a #2 cuff while the cat was in sternal recumbency. Blood pressure was obtained before other interventions, and 5 systolic measurements were averaged for a final reading for each cat.
Additional urine (>2 mL) was collected and refrigerated immediately for determination of urinary F2‐isoprostane concentrations. Freshly prepared butylated hydroxytoluene (BHT) in 95% ethanol was added within an hour to each 2‐mL aliquot of urine to a final concentration of 0.005% BHT to prevent isoprostane oxidation. Urine was centrifuged to remove particulates, and the supernatants were frozen in aliquots at −80°C until shipping for analyses.
Urinary F2‐isoprostanes were quantified by a commercial laboratory4 by solid‐phase extraction on a C18 column followed by a competitive enzyme immunoassay5 that measures free 15‐F2t‐isoprostanes (ie, 8‐isoprostanes, 8‐epi‐PGF2α or 8‐iso‐PGF2α). Samples from CKD and control cats were run concurrently in the same assays along with within‐assay standard curves. Interassay coefficients of variation (CV) ranged from 6.3 to 10.2% for high and low standards over the duration of the study. All feline urine samples were screened in triplicate each at 1 : 5 and 1 : 10 dilutions in kit buffer (10× solution: 1 M phosphate solution containing 1% bovine serum albumin, 4 M sodium chloride, 10 mM EDTA, and 0.1% sodium azide), and %CV was calculated across all 6 values; individual samples with intra‐assay CV >20% were repeated. The linear range of quantification was 4–90 pg/mL; samples above this range were further diluted at 1 : 20 and 1 : 50. Dilutional parallelism was performed on 12 samples with concentrations >90 pg/mL at the 1 : 5 dilution. The CVs across individual results at dilutions of 1 : 10, 1 : 20, and 1 : 50 ranged from 4.5 to 18.0%, indicating good assay performance across these dilutions.28 Concentrations of 15‐F2t‐isoprostanes were normalized to urine creatinine (creat) to calculate free 15‐F2t‐isoprostane:creat ratios (IsoPs), as previously reported.29
Sample Size and Statistical Analyses
A sample size calculation predicted that 13 cats in each group would provide >80% power to detect, as significant, a 2‐fold change of urinary IsoPs between healthy cats and cats with CKD based on previous data in older healthy cats and cats with dilute urine after treatment with radioiodine.27 All variables were checked for normal distribution by a scatter plot, comparison of means and medians, and D'Agostino and Pearson omnibus normality test, before choosing statistical tests. Urinary free IsoPs were compared among all groups by a Kruskal–Wallis test followed by Dunn's multiple‐comparison tests, with P < .05 considered significant. The IsoPs were compared in cats with and without pathologic systemic hypertension (defined as systolic blood pressure ≥160 mmHg)30 or clinically relevant proteinuria (UPC >0.40)31 by a Mann–Whitney test and also were tested for correlations with serum creatinine, blood pressure, UPC, and USG across all cats with CKD by Spearman correlation tests. Finally, because renal diets supplemented with antioxidants such as omega‐3 fatty acids might affect measures of oxidative stress,20 urinary IsoPs were compared in cats fed predominantly standard diets versus renal diets by a Mann–Whitney test, and 15‐F2t‐isoprostane:creatinine ratio (IsoP) data among CKD stages were reanalyzed with censoring of cats fed renal diets.
Results
One hundred and sixteen cats were screened, but 28 of these cats were not eligible for enrollment (9 had positive urine cultures, 8 had evidence of mild prerenal azotemia, 6 cats had disqualifying drug histories or laboratory abnormalities, 4 samples were thawed in transit and unsuitable for analysis, and 1 cat was too fractious to handle).
Therefore, 88 cats were evaluated, including 77 cats with CKD (8 cats in stage 1, 38 cats in stage 2, 21 cats in stage 3, and 10 cats in stage 4) and 11 control cats without evidence of CKD by IRIS staging. Domestic short‐, medium‐, and long‐hair breeds made up the majority of cats enrolled, and there were no significant differences in age among groups (Table 1). As expected, blood (serum) urea nitrogen (BUN) concentration, serum creatinine concentration, USG, and UPC were significantly different between control and CKD groups, and cats in stage 4 CKD had significantly lower BCS compared to healthy controls (P = .02; Table 1). There were no significant differences in systolic blood pressure detected among groups, but 9 cats with CKD were being treated for hypertension at the time of IsoP testing.
Table 1.
Clinical parameters for client‐owned cats with IRIS stage 1–4 chronic kidney disease (CKD), and for healthy older control cats, at the time of urinary isoprostane measurements
| Non‐CKD Controls (n = 11) | Cats with IRIS Stage 1 CKD (n = 8) | Cats with IRIS Stage 2 CKD (n = 38) | Cats with IRIS Stage 3 CKD (n = 21) | Cats with IRIS Stage 4 CKD (n = 10) | |
|---|---|---|---|---|---|
| Age (years) | 11.6a (6.3–20.8) | 13.9a (7.6–16.2) | 13.7a (3.5–19.4) | 14.7a (7.0–19.5) | 12.5a (4.7–16.4) |
| Sex | 5 FS | 5 FS | 17 FS | 10 FS | 3 FS |
| 6 MC | 3 MC | 21 MC | 11 MC | 7 MC | |
| Breed |
10 Domestic 1 Abyssinian |
6 Domestic 1 Burmese 1 Persian |
31 Domestic 3 Burmese 1 Tonkinese 1 Himalayan 1 Ragdoll 1 Orient SH |
18 Domestic 1 Tonkinese 1 Himalayan 1 Angora |
6 Domestic 1 Tonkinese 1 Balinese 1 Siamese 1 Orient SH |
| BW (kg) | 5.4a (4.4–8.1) | 4.5a (2.8–6.1) | 4.5a (2.8–7.9) | 4.2a (2.4–8.7) | 3.8a (3.2–6.2) |
| BCS (of 9) | 7 (4–8)a | 6 (5–7)a | 5 (3–9)a | 5 (2–8)a | 4 (3–6)b |
| BUN (mg/dL) | 23a (12–32) | 23a (16–34) | 33a (19–58) | 51b (38–107) | 97b (66–193) |
| Creatinine (mg/dL) | 1.1a (1.0–1.4) | 1.4a (0.8–1.5) | 2.3b (1.6–2.8) | 3.4c (2.9–4.8) | 6.7c (5.1–18.4) |
| USG | 1.044a (1.035–1.061) | 1.021a (1.008–1.029) | 1.015b (1.010–1.034) | 1.014b (1.008–1.026) | 1.009c (1.007–1.014) |
| UPC | 0.04a (0.02–0.14) | 0.06a (0.03–1.4) | 0.06a (0.01–0.59) | 0.16b (0.03–0.97) | 0.32b (0.09–1.76) |
| Systolic blood pressure (mmHg) | 150a (111–178) | 157a (130–190) | 153a (114–210) | 148a (105–220) | 138a (115–178) |
| Serum T4 concentrations (μg/dL) | 1.7 (1.4–2.6) | 1.8 (1.4–2.5) | 1.8 (0.9–3.5) | 1.8 (0.9–2.6) | 1.5 (0.9–2.0) |
FS, female spayed; MC, male castrated; BW, body weight; BCS, body condition score; BUN, blood (serum) urea nitrogen; USG, urine specific gravity; UPC, urine protein‐to‐creatinine ratio; IRIS, International Renal Interest Society; T4, total thyroxine.
All values are listed as medians with observed ranges.
Values with different superscripts letters vary significantly among groups (P < .05).
Urinary IsoPs were increased in cats with stage 1 CKD (median 263 pg/mg creat; range, 211–380) compared to mature control cats (median 182 pg/mg creat; range 80–348; Fig 1), but this difference did not reach significance with multiple testing. The 2 cats with the highest IsoP measurements in the control group (262 and 348 pg/mg creat) had USG values of 1.035. Among CKD groups, urinary IsoP values were significantly higher in stage 1 cats versus stage 2 (median 144 pg/mg creat; range, 49–608; P < .05), stage 3 (median 102 pg/mg/creat; range 25–158; P < .001), and stage 4 cats (median 67 pg/mg creat; range, 26–117, P < .001; Fig 1). In addition, IsoPs were significantly higher in stage 2 cats compared to both stages 3 and 4 (P < .01), and IsoPs in both stages 3 and 4 were significantly lower than in healthy controls (P < .01). Urinary IsoPs were inversely correlated with serum creatinine concentration (r = −0.66, P < .0001; Fig 2) and positively correlated with USG (r = 0.24, P = .038).
Figure 1.
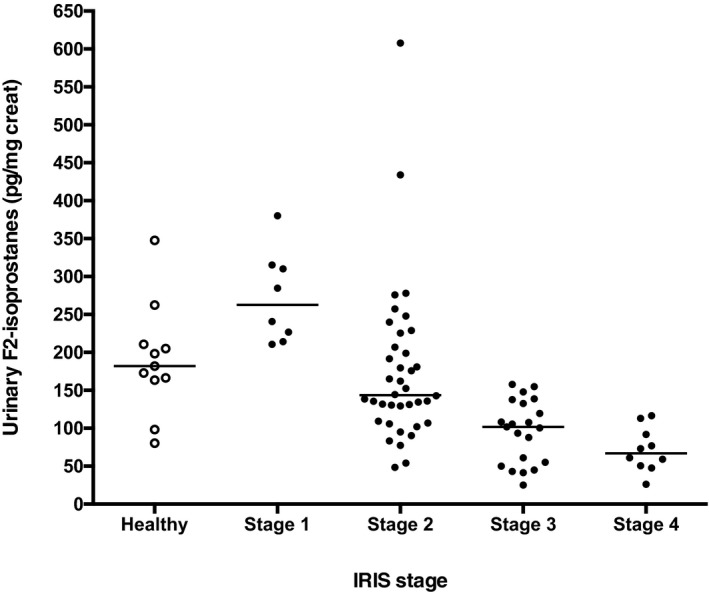
Urinary 15‐F2t‐isoprostane:creatinine ratios (IsoPs) in healthy mature cats and cats with chronic kidney disease (CKD) International Renal Interest Society (IRIS) stages 1–4. IsoPs were significantly higher in IRIS stage 1 cats compared to stages 2, 3, and 4 (P < .05 to P < .001), and higher in stage 2 cats compared to stages 3 and 4 (P < .01). In addition, IsoPs in cats with stage 3 and 4 CKD were significantly lower than in healthy controls (P < .01).
Figure 2.
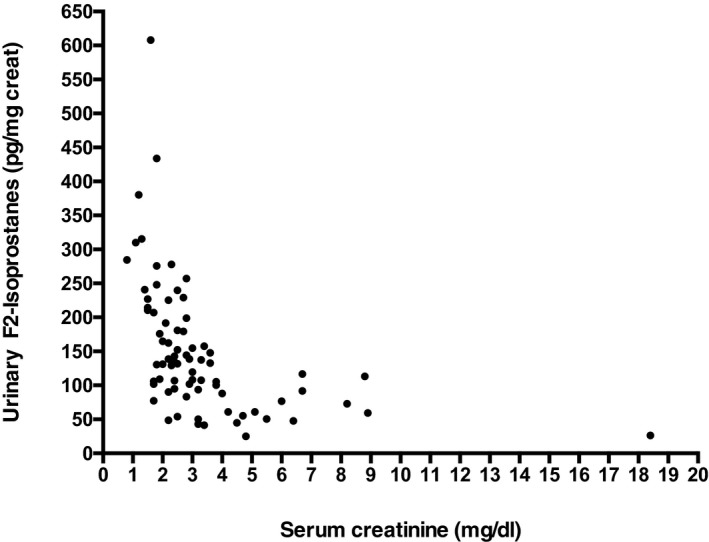
Inverse correlation between serum creatinine and urinary IsoPs in cats with International Renal Interest Society stage 1–4 chronic kidney disease. r = −0.66, P < .0001.
When evaluated by criteria used for IRIS substaging, there were no differences in urinary IsoPs between CKD cats with hypertension (median, 133 pg/mg creat; range, 25–608; n = 24) and without hypertension (median, 132 pg/mg creat; range, 26–434; n = 51; P = .67), based on blood pressure at the time of urine sampling. Two of the 77 CKD cats did not have blood pressure measured. Furthermore, there was no significant correlation between IsoPs and systolic blood pressure (r = 0.07, P = .52). The data were reanalyzed after censoring those cats that were on angiotensin‐converting enzyme (ACE) inhibitors or amlodipine at the time of sampling (2 cats in the hypertensive group and 6 cats in the nonhypertensive group). The IsoP values between these untreated CKD cats still were not different between those with hypertension (median, 133 pg/mg creat; range, 25–608; n = 22) and without hypertension (median, 134 pg/mg creat; range, 26–434; n = 45; P = .82).
Urine protein:creatinine ratios were available for 76 of the 77 cats with CKD. No significant differences were detected in IsoPs between cats with CKD and proteinuria (median, 100 pg/mg creat; range, 26–310; n = 10) and CKD without proteinuria (median, 136 pg/mg creat; range, 25–608; n = 66; P = .09), but the number of proteinuric cats was small. Further, there was no correlation between IsoP and UPC (r = −0.16, P = .17) in this population.
Because renal diets may be supplemented with antioxidants that could affect redox measurements, we performed an analysis to compare urine IsoPs in CKD cats being fed primarily standard commercial diets (>75% of intake; n = 41) to those fed primarily diets formulated for renal disease (>75% of intake; n = 30). Six cats were censored because of insufficient dietary history or ingestion of a varied combination of renal and standard diets. This censoring included the 2 cats with the highest IsoP concentrations in the population, each with stage 2 CKD (Fig 1). In this subpopulation, there was no significant difference detected in urinary IsoPs in the renal diet group (median, 115 pg/mg creat; range, 26–278) compared to the standard diet group (median, 134 pg/mg creat; range, 41–380; P = .22; Fig 3). We also reanalyzed urinary IsoPs by IRIS stage only in the 41 CKD cats fed standard commercial diets. These findings were similar to those of the larger population, with urinary IsoPs being significantly higher in IRIS stage 1 cats compared to CKD cats in stages 3 and 4 (these groups were combined to maintain an adequate sample size; P < .001; Fig 4). Furthermore, IsoPs still were significantly lower in stages 3 and 4 compared to healthy controls (P < .01). Therefore, it is unlikely that antioxidant‐supplemented renal diets were the reason for lower IsoPs in higher stages of CKD.
Figure 3.
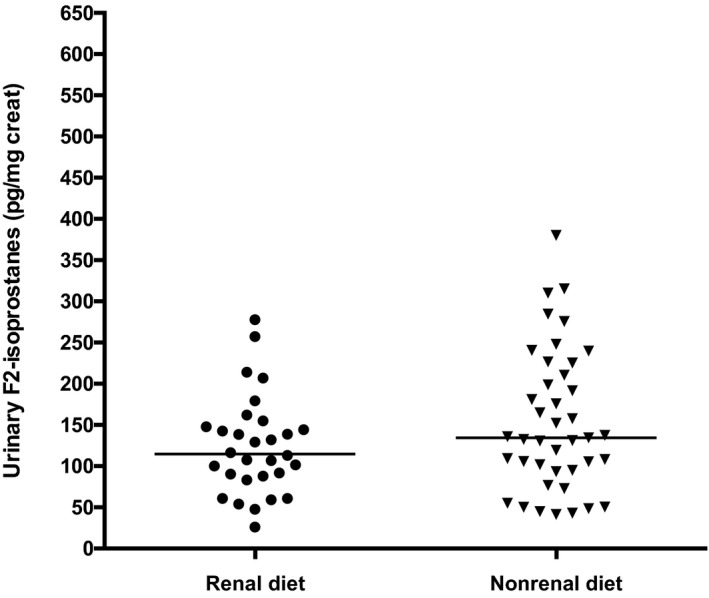
Urinary IsoPs in cats with chronic kidney disease reportedly fed primarily standard commercial diets compared to those fed primarily renal‐formulated diets. P = .22 between groups.
Figure 4.
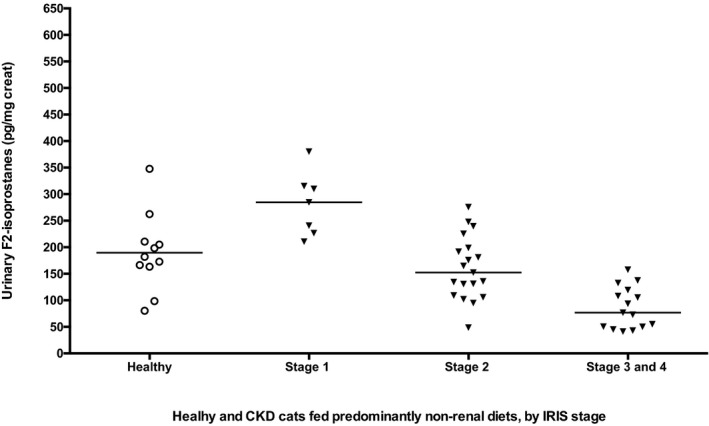
Urinary IsoPs in healthy mature cats and cats with chronic kidney disease (CKD) International Renal Interest Society (IRIS) stages 1–4, including only those cats fed predominantly (>75%) standard commercial nonrenal diets. As found in the larger population, IsoPs were significantly higher in IRIS stage 1 cats compared to stages 3 and 4 (P < .001), and IsoPs in cats with stage 3 and 4 CKD were significantly lower than in healthy controls (P < .01).
Discussion
F2‐isoprostanes are a well‐studied indicator of lipid peroxidation and redox stress and have been shown in many studies to increase with advancing CKD in humans.1, 3, 6, 10, 11, 12 In addition, redox stress appears to contribute to disease progression in animal models of CKD,16, 17, 18, 19, 20 making it a possible therapeutic target. We therefore measured urinary F2‐isoprostanes in cats with CKD, with the hypothesis that this marker of oxidative stress would increase by IRIS stage in this species as in humans.
In contrast to our hypothesis, however, we found that urinary IsoPs were highest in early (stage 1) CKD in cats compared to stage 2 and significantly decreased with progressive IRIS stage. In addition, urinary IsoPs concentrations were strongly and inversely correlated with serum creatinine concentration. These findings together suggest that renal oxidative stress, if accurately reflected by urinary IsoPs, may actually be highest in the early stages of CKD in cats.
These findings appear to be in contrast to those obtained in human patients with moderate‐to‐severe CKD, in whom plasma F2‐isoprostanes were significantly increased in many studies.1, 7, 10, 11, 12 Urinary and plasma F2‐isoprostanes have been considered to be equivalent markers of chronic (but not acute) oxidative stress,15 but our different findings in cats with CKD could be because of sampling urine rather than plasma. In support of this, a large cohort study (Framingham Offspring) showed significantly lower urinary IsoPs in 291 human patients with CKD when compared to more than 3,000 non‐CKD subjects.32 In addition, a cross‐sectional study in more than 600 elderly men found a positive relationship between GFR and urinary IsoPs,33 similar to our inverse correlation between serum creatinine concentrations and IsoPs in cats. However, neither of these longitudinal studies in humans investigated early CKD as a separate category.
Higher urinary IsoPs in cats with stage 1 as compared to stage 2 CKD may reflect early renal inflammation and local redox stress, followed by progression in later stages to fibrosis, loss of nephrons, and loss of cells that generate these prostanoids. Although urinary IsoPs have been used in many studies as a biomarker of systemic oxidative stress, it is unclear what proportion of these prostanoids reflects local (renal) versus systemic oxidative stress.22, 34 Our findings also could reflect differences in response to oxidative stress in cats as a species compared to humans. For example, humans with advanced CKD show lower plasma activities of glutathione peroxidase (GPx), lower concentrations of its cofactor selenium, and lower reduced‐to‐oxidized glutathione (GSH:GSSG) ratios.6, 8, 35, 36 In contrast, cats with stage 4 CKD show a modest but significant increase in plasma GPx activities, with no decreases in serum selenium concentrations, and higher GSH:GSSG ratios.26, 37 In addition, humans with CKD often die of cardiovascular complications (related to systemic inflammation and oxidative stress), rather than of direct metabolic complications of uremia,38, 39 but this has not been observed in cats. Together these data suggest that, compared to humans, cats with CKD may show a different adaptive response to oxidative stress.
When we examined our study population by criteria used for IRIS substaging, there were no significant differences in IsoPs in cats with and without hypertension (133 and 132 pg/mg creat, respectively). However, blood pressures were measured in the hospital, and stress could have led to a false diagnosis of hypertension in some cats. In addition, cats were not necessarily tested at the time of first documentation of hypertension, and some of the cats were being treated with amlodipine or ACE inhibitors at the time of sampling. Hypertension and renal oxidative stress are related, with angiotensin II leading to generation of superoxide radicals and F2‐isoprostanes,40 and it is unclear how ACE inhibitors or amlodipine might affect renal IsoP generation and in what time frame. When we reanalyzed the data after censoring cats that were on antihypertensive drugs at the time of sampling, median IsoP values were essentially unchanged and were still not different between cats with and without hypertension. A future study should examine the effect of drugs such as ACE inhibitors on urinary IsoPs in cats with CKD by a controlled prospective design.
With regard to proteinuria, urinary IsoP values in cats with UPCs >0.40 were not significantly different compared to those with lower UPCs. In the previously cited cross‐sectional study of 600 elderly men, urinary IsoPs were negatively correlated with albumin:creatinine ratios.33 In our study, a posthoc sample size calculation indicated that 83 proteinuric and 83 nonproteinuric CKD cats would be needed to determine whether urinary IsoPs might differ significantly by UPC. Follow‐up studies utilizing additional markers of oxidative stress in a larger population of cats with CKD, before treatment for hypertension or proteinuria, are needed to further investigate the relationships among proteinuria, hypertension, and oxidative stress in cats.
Our study had several limitations. We used an immunoassay for IsoP measurements, rather than 1 of several mass spectrometry (MS) methods that are considered gold standards,41 but MS methods are more expensive and labor‐intensive,41 and suboptimal for veterinary use. In a previous study in 20 cats, the same Cayman ELISA (with slightly different preparative steps) correlated significantly with gas chromatography/negative ion chemical ionization‐MS, but the correlation was somewhat weak (r = 0.678), with the ELISA measuring lower concentrations than MS (fixed bias), and sometimes missing low concentrations that were detectable by MS (proportional bias).42 In our study, we did not have the same sensitivity problem and were able to detect urinary IsoPs in all cats. However, we acknowledge that our ELISA results cannot be directly compared to results using MS methods, because MS may focus on a single analyte (with different MS‐based assays measuring different F2‐isomers),41 whereas immunoassays may detect >1 structurally related F2‐isomer derived from arachidonic acid (which may or may not have biologic activities).
We had relatively small groups of cats with IRIS stage 1 and 4 kidney disease, because cats in these 2 stages are more difficult to identify and recruit. Although we had adequate power to detect differences in IsoPs among CKD stages, our study was underpowered to detect a significant difference between stage 1 CKD and healthy cats after correcting for multiple comparisons. Even with aggressive screening of apparently healthy older cats for stage 1 CKD over a period of 32 months, many cats ultimately were categorized as stage 2. Our screening experience suggests that stage 1 may be a relatively transient phase of CKD progression in cats, at least as measured by serum creatinine concentration. A posthoc sample size calculation for ANOVA targeted 17 cats in each group to detect the difference observed between healthy and stage 1 CKD cats as significant, with 80% power. Subsequent studies also should incorporate plasma symmetric dimethylarginine (SDMA) measurements, which may represent a more sensitive biomarker for early loss of GFR. Doing so may allow identification of more cats with stage 1 CKD, because SDMA concentration can become abnormal before the serum creatinine concentration threshold of 1.6 mg/dL.43
Body condition score also was a potential source of bias in our study, because overweight cats (mean BCS of 8/9) have significantly higher urinary IsoP concentrations than cats with normal BCS (mean 5/9).29 Only these stage 4 CKD cats had significantly lower BCS than healthy controls, but there was wide variability within groups, and it is possible that loss of fat may have contributed to lower IsoP values in cats with advancing CKD. In addition, cats with later stage CKD can lose substantial muscle mass, and this could have underestimated the degree of nephron loss and led to IRIS misclassification in some cats.44 This limitation could be addressed by the inclusion of SDMA measurements in subsequent studies. In addition, we focused on only a single measure of oxidative stress, and the inclusion of plasma IsoPs or other markers of systemic oxidative stress would strengthen follow‐up studies.
Another source of bias was the fact that these client‐owned cats were fed a variety of diets. Renal diets for cats are often supplemented with omega‐3 fatty acids, and omega‐3 fatty acids have been shown to decrease urinary IsoP concentrations in human patients45 as well as to decrease systemic inflammation and renal injury in rodent nephrectomy models.20 We attempted to control for diet effects by comparing IsoPs in cats that were fed predominantly renal‐formulated versus standard commercial diets and by reanalyzing the data only in cats on standard diets, and our findings were unchanged. However, individual renal and standard diets can vary in omega‐3 fatty acid content, and clinically quantifying dietary intake can be subjective, so we may have missed unrecognized diet effects. In addition, we did not collect information on how long each cat had been fed the current diet. The ideal way to study this question would be with a longitudinal or cross‐sectional study of markers of oxidative stress in cats acclimated to a standardized diet, which might be difficult in client‐owned cats.
In conclusion, our results show a significant increase in urinary F2‐isoprostane concentrations in IRIS stage 1 CKD cats compared to stage 2 CKD, followed by declining IsoPs to below control values with advancing IRIS stages. These findings raise the question of whether renal oxidative stress is contributing to early progression of CKD in cats, or whether it is merely an epiphenomenon of early renal damage. Follow‐up studies are needed to evaluate the extent of oxidative stress during stage 1 CKD in cats, and to determine the therapeutic value of antioxidant supplementation early in the course of CKD in cats.
Acknowledgments
The authors thank Helen Schultz, Terri Gregson, Nicole Hoffman, Angela Daugherty, and Leigh Davis for assistance with sample collection.
Conflict of Interest Declaration: The research was supported by the Waltham Foundation and by the Companion Animal Fund at the University of Wisconsin‐Madison.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
This work was performed at the University of Wisconsin‐Madison, School of Veterinary Medicine and Colorado State University, College of Veterinary Medicine & Biomedical Sciences.
Preliminary data were presented in abstract form at the Annual Meeting of the American College of Veterinary Internal Medicine, 2014 Nashville, TN.
Footnotes
Ortho Clinical Diagnostics, Raritan, NJ
Roche Diagnostics, Indianapolis, IN
Park Medical Electronics, Las Vegas, NV
Cayman Chemical Company, Ann Arbor, MI
8‐Isoprostane EIA kit, Cayman Chemical Company
References
- 1. Handelman GJ, Walter MF, Adhikarla R, et al. Elevated plasma F2‐isoprostanes in patients on long‐term hemodialysis. Kidney Int 2001;59:1960–1966. [DOI] [PubMed] [Google Scholar]
- 2. Locatelli F, Canaud B, Eckardt KU, et al. Oxidative stress in end‐stage renal disease: An emerging threat to patient outcome. Nephrol Dial Transplant 2003;18:1272–1280. [DOI] [PubMed] [Google Scholar]
- 3. Terawaki H, Yoshimura K, Hasegawa T, et al. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int 2004;66:1988–1993. [DOI] [PubMed] [Google Scholar]
- 4. Karamouzis I, Sarafidis PA, Karamouzis M, et al. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol 2008;28:397–404. [DOI] [PubMed] [Google Scholar]
- 5. Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens 2004;13:93–99. [DOI] [PubMed] [Google Scholar]
- 6. Yilmaz MI, Saglam M, Caglar K, et al. The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 2006;47:42–50. [DOI] [PubMed] [Google Scholar]
- 7. Cottone S, Mule G, Guarneri M, et al. Endothelin‐1 and F2‐isoprostane relate to and predict renal dysfunction in hypertensive patients. Nephrol Dial Transplant 2009;24:497–503. [DOI] [PubMed] [Google Scholar]
- 8. Kuchta A, Pacanis A, Kortas‐Stempak B, et al. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press Res 2011;34:12–19. [DOI] [PubMed] [Google Scholar]
- 9. Witko‐Sarsat V, Friedlander M, Nguyen Khoa T, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol 1998;161:2524–2532. [PubMed] [Google Scholar]
- 10. Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 2004;65:1009–1016. [DOI] [PubMed] [Google Scholar]
- 11. Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis 2006;48:752–760. [DOI] [PubMed] [Google Scholar]
- 12. Ramos LF, Shintani A, Ikizler TA, et al. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol 2008;19:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Czerska M, Zielinski M, Gromadzinska J. Isoprostanes—a novel major group of oxidative stress markers. Int J Occup Med Environ Health 2016;29:179–190. [DOI] [PubMed] [Google Scholar]
- 14. Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: Physiology, pharmacology and clinical implications. Trends Pharmacol Sci 2002;23:360–366. [DOI] [PubMed] [Google Scholar]
- 15. Halliwell B, Lee CY. Using isoprostanes as biomarkers of oxidative stress: Some rarely considered issues. Antioxid Redox Signal 2010;13:145–156. [DOI] [PubMed] [Google Scholar]
- 16. Brown SA. Oxidative stress and chronic kidney disease. Vet Clin North Am Small Anim Pract 2008;38:157–166, vi. [DOI] [PubMed] [Google Scholar]
- 17. Hahn S, Kuemmerle NB, Chan W, et al. Glomerulosclerosis in the remnant kidney rat is modulated by dietary alpha‐tocopherol. J Am Soc Nephrol 1998;9:2089–2095. [DOI] [PubMed] [Google Scholar]
- 18. Djamali A. Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. Am J Physiol Renal Physiol 2007;293:F445–F455. [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Seok YM, Jung KJ, et al. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol 2009;297:F461–F470. [DOI] [PubMed] [Google Scholar]
- 20. An WS, Kim HJ, Cho KH, et al. Omega‐3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol 2009;297:F895–F903. [DOI] [PubMed] [Google Scholar]
- 21. Okamura DM, Pennathur S. The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biol 2015;6:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morrow JD. The isoprostanes—unique products of arachidonate peroxidation: Their role as mediators of oxidant stress. Curr Pharm Des 2006;12:895–902. [DOI] [PubMed] [Google Scholar]
- 23. Arellano‐Mendoza MG, Vargas‐Robles H, Del Valle‐Mondragon L, et al. Prevention of renal injury and endothelial dysfunction by chronic l‐arginine and antioxidant treatment. Ren Fail 2011;33:47–53. [DOI] [PubMed] [Google Scholar]
- 24. Brown CA, Elliott J, Schmiedt CW, et al. Chronic kidney disease in aged cats: Clinical features, morphology, and proposed pathogeneses. Vet Pathol 2016;53:309–326. [DOI] [PubMed] [Google Scholar]
- 25. Yu S, Paetau‐Robinson I. Dietary supplements of vitamins E and C and beta‐carotene reduce oxidative stress in cats with renal insufficiency. Vet Res Commun 2006;30:403–413. [DOI] [PubMed] [Google Scholar]
- 26. Keegan RF, Webb CB. Oxidative stress and neutrophil function in cats with chronic renal failure. J Vet Intern Med 2010;24:514–519. [DOI] [PubMed] [Google Scholar]
- 27. Branter E, Drescher N, Padilla M, et al. Antioxidant status in hyperthyroid cats before and after radioiodine treatment. J Vet Intern Med 2012;26:582–588. [DOI] [PubMed] [Google Scholar]
- 28. Andreasson U, Perret‐Liaudet A, van Waalwijk van Doorn LJ, et al. A practical guide to immunoassay method validation. Front Neurol 2015;6:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeusette I, Salas A, Iraculis N, et al. Increased urinary F2‐isoprostane concentrations as an indicator of oxidative stress in overweight cats. Intern J Appl Res Vet Med 2009;7:36–42. [Google Scholar]
- 30. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 31. Syme HM, Markwell PJ, Pfeiffer D, et al. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006;20:528–535. [DOI] [PubMed] [Google Scholar]
- 32. Upadhyay A, Larson MG, Guo CY, et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant 2011;26:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nerpin E, Helmersson‐Karlqvist J, Riserus U, et al. Inflammation, oxidative stress, glomerular filtration rate, and albuminuria in elderly men: A cross‐sectional study. BMC Res Notes 2012;5:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu JH, Ward NC, Indrawan AP, et al. Effects of alpha‐tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes. Clin Chem 2007;53:511–519. [DOI] [PubMed] [Google Scholar]
- 35. Zachara BA, Trafikowska U, Adamowicz A, et al. Selenium, glutathione peroxidases, and some other antioxidant parameters in blood of patients with chronic renal failure. J Trace Elem Med Biol 2001;15:161–166. [DOI] [PubMed] [Google Scholar]
- 36. Puchades MJ, Saez G, Munoz MC, et al. Study of oxidative stress in patients with advanced renal disease and undergoing either hemodialysis or peritoneal dialysis. Clin Nephrol 2013;80:177–186. [DOI] [PubMed] [Google Scholar]
- 37. Krofic Zel M, Tozon N, Nemec Svete A. Plasma and erythrocyte glutathione peroxidase activity, serum selenium concentration, and plasma total antioxidant capacity in cats with IRIS stages I‐IV chronic kidney disease. J Vet Intern Med 2014;28:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cottone S, Lorito MC, Riccobene R, et al. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol 2008;21:175–179. [PubMed] [Google Scholar]
- 39. Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr Opin Nephrol Hypertens 2003;12:593–598. [DOI] [PubMed] [Google Scholar]
- 40. Araujo M, Wilcox CS. Oxidative stress in hypertension: Role of the kidney. Antioxid Redox Signal 2014;20:74–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Milne GL, Sanchez SC, Musiek ES, et al. Quantification of F2‐isoprostanes as a biomarker of oxidative stress. Nat Protoc 2007;2:221–226. [DOI] [PubMed] [Google Scholar]
- 42. Soffler C, Campbell VL, Hassel DM. Measurement of urinary F2‐isoprostanes as markers of in vivo lipid peroxidation: A comparison of enzyme immunoassays with gas chromatography‐mass spectrometry in domestic animal species. J Vet Diagn Invest 2010;22:200–209. [DOI] [PubMed] [Google Scholar]
- 43. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014;28:1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, l‐carnitine, and medium‐chain triglycerides. Vet J 2014;202:588–596. [DOI] [PubMed] [Google Scholar]
- 45. Mori TA, Puddey IB, Burke V, et al. Effect of omega 3 fatty acids on oxidative stress in humans: GC‐MS measurement of urinary F2‐isoprostane excretion. Redox Rep 2000;5:45–46. [DOI] [PubMed] [Google Scholar]


