Abstract
Background
Development of hypocalcemia after treatment of hyperparathyroidism results in increased costs and risk of poorer outcomes. Previous studies have shown conflicting data about predictors of hypocalcemia after these procedures.
Hypothesis/Objectives
The objective of this study was to investigate whether ionized calcium (iCa) concentrations before treatment are predictive of hypocalcemia or its clinical signs after surgical removal or heat ablation in dogs with primary hyperparathyroidism.
Animals
Fifty‐four dogs with primary hyperparathyroidism (29 female, 25 male; 49 retrospective, 5 prospective).
Methods
Dogs were enrolled if they met the inclusion criteria: persistent hypercalcemia (iCa >1.41 mmol/L) due to primary hyperparathyroidism and absence of preemptive calcitriol treatment. All dogs were treated with parathyroidectomy (n = 37) or percutaneous ultrasound‐guided heat ablation (n = 17). After treatment, iCa was monitored twice daily until plateau or intervention.
Results
There was a moderate correlation between before‐treatment hypercalcemia and after‐treatment hypocalcemia. The prospective study was terminated due to ethical concerns given findings in the retrospective section. All dogs were placed into groups according to their pretreatment iCa: 1.46–1.61 mmol/L, 1.62–1.71 mmol/L, iCa 1.72–1.81 mmol/L, or >1.81 mmol/L. After treatment, the mean lowest iCa for each group, respectively, was 1.19, 1.18, 1.13, and 1.01 mmol/L. There was a significant association between higher group and proportion of dogs with iCa <1.00 mmol/L (P = .014).
Conclusions and Clinical Importance
This study demonstrates a moderate correlation between iCa concentration before treatment and hypocalcemia after treatment. Dogs with higher initial iCa concentrations should be treated to prevent rapid decline and development of clinical hypocalcemia.
Keywords: Ablation, Calcitriol, Endocrinology, Parathyroid glands, Parathyroidectomy
Abbreviations
- CBC
Complete blood count
- iCa
ionized calcium
- PTH
parathyroid hormone
- PTHrp
parathyroid hormone‐related protein
- SC
Subcutaneously
- tCa
total calcium
- VMTH
Veterinary Medical Teaching Hospital
Primary hyperparathyroidism is a condition in which hypercalcemia develops secondary to autonomous and excessive production of parathyroid hormone (PTH).1, 2 In dogs, this disorder is most often caused by a functioning solitary parathyroid chief cell adenoma and less commonly by the autonomous synthesis and secretion of PTH from either parathyroid adenomatous hyperplasia (usually isolated to 1 gland) or a solitary parathyroid carcinoma.3, 4, 5 A small percentage of affected dogs have been described as having 2 abnormal parathyroid glands concurrently or sequentially.5, 6, 7 The most common clinical signs associated with the disease include polyuria, polydipsia, muscle weakness, poor appetite, weight loss, and lower urinary tract signs due to cystolithiasis, infection, or both. Clinical signs in this disease can be subtle, as suggested by more than one third of primary hyperparathyroid dog owners who do not observe any clinical signs.8 Lack of clinical signs due to primary hyperparathyroidism is also recognized in a large percentage of affected people.9 The authors’ clinical impressions are that resolution of hypercalcemia due to primary hyperparathyroidism in dogs likely improves their quality of life, even when signs are not obvious, as has been suggested in asymptomatic people.10
The goals in treating primary hyperparathyroidism are to resolve the clinical signs, restore a healthy calcium and phosphorous balance, and eliminate risk of adverse events stemming from continued hypercalcemia. Two methods have been described for treating primary hyperparathyroidism: surgical extirpation of autonomously functioning parathyroid tissue and percutaneous ultrasound‐guided ablation (with either heat or ethanol).3, 11, 12, 13 Both surgery and percutaneous ablation have been associated with resolution of hyperparathyroidism and hypercalcemia.13
However, studies in both humans and dogs have shown that hypocalcemia or iatrogenic hypervitaminosis D after treatment are risk factors for morbidity and death when managing dogs after treatment for primary hyperparathyroidism.9, 11, 12, 14, 15, 16, 17, 18 Therefore, the ability to accurately predict hypocalcemia after treatment would be valuable and would allow prophylactic administration of calcitriol (vitamin D) only to predisposed dogs. Vitamin D in appropriate doses can prevent or minimize the severity of hypocalcemia without having an effect on the initial resolution of hypercalcemia.19 Dogs with total serum calcium concentrations (tCa) above 15 mg/dL might be predisposed and those below 14 mg/dL are unlikely to develop clinically consequential hypocalcemia after treatment.20 Studies investigating prevalence of hypocalcemia after treatment have had mixed results. One study suggested association between severity of hypercalcemia before treatment and hypocalcemia after treatment,19 but in other studies hypocalcemia after treatment was difficult to predict.21, 22
To avoid symptomatic hypocalcemia after treatment evidenced by muscle cramping, pain, tetany, convulsions, or death, primary hyperparathyroid dogs are often treated preemptively with vitamin D. Such treatment can be helpful as described, but overdose of vitamin D can cause both hypercalcemia and hyperphosphatemia, mineralization of soft tissues, and acute kidney injury. Once initiated, treatment with calcitriol is typically continued for 2–6 months until normal parathyroid gland function returns and requires relatively expensive and frequent monitoring to determine and maintain the appropriate dose. The ability to accurately predict which dogs should receive preemptive calcitriol should help avoid the need for emergency calcium infusions, which carry a risk of cardiac arrhythmias or tissue sloughing and necrosis when extravasated or administered SC.
The objectives of this planned retrospective and prospective study were to investigate correlations between plasma ionized calcium concentrations (iCa) before and after treatment and to evaluate the relationship between plasma iCa concentrations and incidence of adverse events. We hypothesized that the greater the hypercalcemia before treatment, the greater the likelihood of consequential hypocalcemia after treatment. Other possible factors before treatment associated with clinically consequential hypocalcemia after treatment were studied. We hypothesized that there would be no association between serum PTH concentrations before treatment and hypocalcemia after treatment.
Materials and Methods
The computerized medical record system at the University of California, Davis, William R Pritchard Veterinary Medical Teaching Hospital (VMTH), was used for the retrospective analysis. The search was directed at identifying dogs diagnosed as having primary hyperparathyroidism treated with parathyroidectomy or reported as having parathyroid heat ablation between January 1, 2004, and July 31, 2014. Concurrently, dogs were prospectively enrolled between August 1, 2014, and February 28, 2015. The prospective study was terminated for ethical reasons due to compelling data from the retrospective group that supported our hypothesis.
Eligibility requirements were identical for both retrospective and prospective cohorts. Dogs were included only if a diagnosis of primary hyperparathyroidism had been suspected on the basis of compatible history, clinical signs, and results of a physical examination, CBC, urinalysis, and serum biochemical analyses. Dogs in which primary hyperparathyroidism had been diagnosed were included only if iCa had been >1.41 mmol/L (reference range, 1.12–1.41 mmol/L) at least twice during a period of >14 days before treatment. Each dog must have been successfully treated at the VMTH with either surgery or percutaneous heat ablation. Twice daily iCa measurements must have been obtained until (1) an after‐treatment nadir was identified or (2) calcitriol treatment was initiated due to observation of signs compatible with hypocalcemia or the iCa concentration was arbitrarily considered dangerously low. All iCa values (both serum and plasma) were recorded.
Nonparathyroid disorders that could result in hypercalcemia must have been excluded before treatment. In addition, dogs were included only if abdominal radiography or ultrasonography and thoracic radiography had been performed within 30 days before treatment and no evidence of nonparathyroid neoplasia had been seen. Dogs were included only if 1 or more cervical masses had been observed at surgery or by means of ultrasonography. Diagnosis must have been confirmed on the basis of resolution of hypercalcemia within 3 days of either treatment. For dogs that underwent surgery, histological changes in the tissue removed must have been consistent with parathyroid adenoma, parathyroid carcinoma, or parathyroid hyperplasia. Dogs were excluded if they received glucocorticoids in the 30 days before or after diagnosis of primary hyperparathyroidism and its treatment. No dog could have received preemptive treatment with calcium or vitamin D, could have incomplete medical records, or have evidence of nonparathyroid gland neoplastic disease or bowel disease that could disrupt calcium and phosphorous regulation.
Data gathered from the medical records included signalment, history of urolithiasis, date of diagnosis, date of treatment, surgery versus ablation treatment, laboratory and imaging results. Diagnostics recorded before treatment included urine specific gravity, serum, or plasma concentrations of iCa, tCa, phosphorus, urea nitrogen, creatinine, PTH, and parathyroid hormone‐related protein (PTHrP), when available. Measurements of cervical masses identified on ultrasound examinations performed at the VMTH were recorded. Results recorded after treatment included lowest iCa and lowest tCa (if assessed). All adverse events related to the surgery or hypocalcemia were included for analysis.
The date of initial diagnosis with hypercalcemia or urolithiasis was designated the date of disease onset, and the date of treatment was designated as the date of resolution. The duration of disease was estimated by determining the interval between these 2 dates.
Procedures
Routine methods were used to perform serum biochemical analyses, CBCs, and urinalyses. Serum tCa concentrations were determined by means of colorimetric evaluation.1 Serum2 and plasma3 , 4 iCa concentrations were determined by means of ion‐selective electrode analysis.8 Serum PTH concentrations were determined by use of a previously validated whole PTH assay system.5 , 8 All serum chemistry assays were performed in the clinical chemistry laboratory of the VMTH. Ultrasonography of the neck (ie, cervical ultrasonography) was performed with a 10‐MHz, linear, phased‐array transducer and a standard ultrasonography machine.6
Statistical Analysis
To evaluate the relationship between iCa before and after treatment, polynomial regression and Pearson's correlation coefficient were performed. The Cochran‐Armitage test was used to examine trends in proportions of treated dogs developing hypocalcemia across ordered groups of dogs based on iCa concentration before treatment. The Mann–Whitney U‐test was used to evaluate the relationship between duration of known hyperparathyroidism and incidence of after‐treatment clinical signs associated with hypocalcemia. Values of P < .05 were considered significant. All calculations were performed by StatXact 107 and Stata IC/13.18 . Means (±SD) were estimated for data compatible with a normal distribution in individual groups, and medians (ranges) were estimated for non‐normally distributed data in individual groups.
Results
Fifty‐four dogs seen between January 1, 2004, and February 28, 2015, met the inclusion criteria (29 female [28 spayed] and 25 male dogs [24 neutered]). Forty‐nine dogs were retrospectively evaluated and 5 prospectively (Table 1). Their ages ranged from 3 to 15 years old (mean age 10.4 years). There were 38 purebred dogs and 16 mixed breed dogs. Pure bred dogs included 6 Golden Retrievers, 3 Huskies, 2 each of German shepherd dog, Jack Russell terrier, keeshond, Labrador retriever, malamute, Pit bull terrier, and shih tzu, and 1 each of boxer, Brittany spaniel, bull terrier, cocker spaniel, Dalmatian, English springer spaniel, fox terrier, German short haired pointer, miniature dachshund, old English sheepdog, toy poodle, pug, Staffordshire terrier, Weimaraner, and West Highland white terrier.
Table 1.
Five dogs were prospectively enrolled between December 1, 2014, and February 28, 2015
| Signalment | Highest iCa (mmol/L) | Highest tCa (mg/dL) | PTH (pmol/L) | Procedure | Lowest iCa (mmol/L) | Clinical Signs |
|---|---|---|---|---|---|---|
| 9 year MC Husky | 1.62 | 13.6 | 3.70 | Surgery | 1.14 | None |
| 3 year MC Newfoundland/X | 1.66 | 13.5 | 3.30 | Surgery | 0.74 | None |
| 12 year FS Golden Retriever | 1.87 | 12.6 | 2.90 | Surgery | 0.82 | None |
| 9 year FS Jack Russell Terrier | 1.91 | 15.8 | 7.30 | Surgery | 0.75 | Facial pruritus |
| 12 year MC Cocker Spaniel | 1.91 | 14.5 | 3.50 | Ablation then surgery | 1.11 | None |
iCa, ionized calcium; tCa, total calcium; PTH, parathyroid hormone.
The median iCa and serum tCa concentrations before treatment from all 54 dogs was 1.74 mmol/L (interval: 1.46–2.82 mmol/L, reference interval 1.12–1.41 mmol/L) and 13.1 mg/dL (interval: 12.1–15.8 mg/dL, reference interval: 9.7–11.5 mg/dL before 2011 and 9.6–11.2 from January 1, 2011 on), respectively. The median serum PTH concentration was 5.70 pmol/L (interval: 1.30–39.40 pmol/L, reference interval: 0.5–5.8 pmol/L, n = 50). Parathyroid hormone‐related protein results were available for 26 dogs, 21 of which were undetectable. Five dogs had values that were detectable but within the reference range (0.0–1.0 pmol/L) and none had evidence of nonparathyroid neoplasia. There was no significant correlation between before‐treatment PTH concentrations and lowest after‐treatment iCa (r = 0.092, P = .51).
The median serum creatinine, blood urea nitrogen, and phosphate concentrations before treatment were within normal limits at 1.0 mg/dL (interval: 0.3–4.6 mg/dL, reference interval: 0.8–1.5 mg/dL), 17 mg/dL (interval 6–63 mg/dL, reference interval: 11–33 mg/dL), and 3.0 mg/dL (interval: 0.8–5.1 mg/dL, reference interval: 2.6–5.2 mg/dL), respectively. The median urine specific gravity was 1.016 (interval: 1.004–1.050, n = 44). Eight dogs had cystic calculi, all were removed via cystotomy or voiding urohydropropulsion, and stone analysis was available in 6, revealing calcium containing stones (calcium oxalate or apatite) in each. Two dogs had evidence of chronic kidney disease and were azotemic. Each had compelling evidence for concurrent primary hyperparathyroidism: presence of hypercalcemia (iCa of 1.46 and 2.08 mmol/L, respectively), low‐end reference range serum phosphate concentration, a solitary parathyroid nodule identified in each dog via ultrasound and hypercalcemia resolving within 48 hours of treatment (dog 1: creatinine 3.2 mg/dL, iCa 1.78 mmol/L and phosphorus 4.6 mg/dL, dog 2: creatinine 4.6 mg/dL, iCa 1.46 mmol/L and phosphorus 4.0 mg/dL). These dogs’ azotemia remained unchanged after treatment.
Parathyroid nodules were defined as either an internal, external, or ectopic structure found to be compatible with sonographic appearance of the parathyroid >3 mm in diameter. A solitary nodule in the area of a parathyroid gland was identified with cervical ultrasound in 45 dogs, 2 nodules were observed in 8 dogs (6 on contralateral sides), and 4 nodules were identified in 1 dog.
All 54 dogs had resolution of their hypercalcemia within 48 hours of treatment. Eighteen dogs were treated with ultrasound‐guided heat ablation, 17 successfully. One dog that did not respond to percutaneous heat ablation was then successfully treated surgically. All 37 dogs undergoing surgery had resolution of their hypercalcemia. Thirty‐two of 37 dogs had a solitary parathyroid mass removed and 5 dogs each had 2 masses removed. One dog with a solitary parathyroid mass identified via ultrasound had bilateral adenomas identified at surgery. Of the 8 dogs with 2 nodules seen via ultrasound, 2 dogs had a single adenoma and 1 dog had a single carcinoma identified surgically, 3 dogs had both a solitary adenoma and a solitary parathyroid gland described as adenomatous hyperplasia identified, and 2 had multiple areas of adenomatous hyperplasia identified. Two masses, a single parathyroid adenoma and contralateral thyroid cyst, were found in the dog with 4 masses seen via ultrasound.
Of the 54 dogs in this study, 10 were given either calcitriol, calcium, or both. Five hypocalcemic dogs developed clinical signs consistent with tetany and each was started on treatment to prevent progression. Five hypocalcemic dogs were given calcitriol, calcium, or both due to worrisome hypocalcemia, but none of these had clinical signs at the time that treatment was begun. Data from these dogs were excluded after these interventions.
Twenty of the 54 dogs had at least 1 iCa <1.12 mmol/L and 14 of those 20 had a least 1 plasma iCa concentration <1.05 mmol/L after treatment. Ten of those 14 dogs had at least 1 iCa concentration <1.00 mmol/L after treatment (Table 2). Overall, there was a moderate correlation between higher iCa before treatment and lower iCa after treatment (r = −0.416, P = .008—Fig 1).
Table 2.
Highest ionized calcium (iCa) before treatment versus lowest iCa after treatment (all represent group means and are measured in mmol/L)
| Group | iCa (mmol/L) | |||
|---|---|---|---|---|
| ≤1.61 | 1.62–1.71 | 1.72–1.81 | ≥1.82 | |
| No. of dogs | 12 | 15 | 14 | 13 |
| iCa before treatment | 1.56 | 1.66 | 1.77 | 1.97 |
| iCa after treatment | 1.19 | 1.18 | 1.13 | 1.01 |
| No. <1.05 | 1 | 1 | 4 | 8 |
| No. <1.0 | 1a | 1a | 2a | 6a |
| No. <0.9 | 1 | 1 | 1 | 4 |
Indicates significance (P = .0144).
Figure 1.
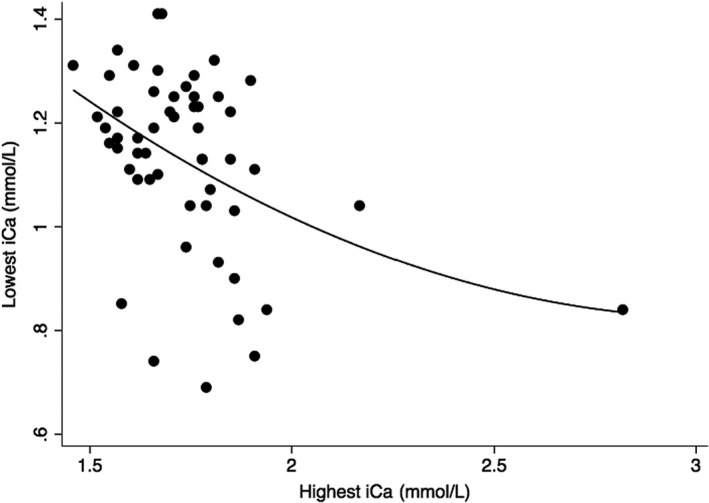
Scatterplot graphing the highest iCa before treatment against the lowest iCa after treatment. The smoothing line is from polynomial regression with a correlation coefficient of −0.416 and P‐value of .008.
Each of the 54 dogs was arbitrarily placed into 1 of 4 groups based on before‐treatment iCa concentration: 1.46–1.61 mmol/L (Group 1, 12 dogs); 1.62–1.71 mmol/L (Group 2, 15 dogs); 1.72–1.81 mmol/L (Group 3, 14 dogs); and ≥1.82 mmol/L (Group 4, 13 dogs). After treatment, the mean (±SD) lowest iCa concentrations for each of the 4 groups were the following: 1.19 mmol/L (±0.13—Group 1), 1.18 mmol/L (±0.16—Group 2), 1.13 mmol/L (±0.17—Group 3), and 1.01 mmol/L (±0.18—Group 4). Of the 10 dogs with at least 1 iCa <1.00 mmol/L, 1 was from Group 1, 1 was from the Group 2, 2 were from Group 3, and 6 were from Group 4. Combining groups 1 and 2, 2 of 27 dogs (7%) had at least 1 documented iCa concentration <1.00 mmol/L. Combining groups 3 and 4, 8 of 27 dogs (30%) had at least 1 documented iCa concentration <1.00 mmol/L. There was a significant association between higher group number and proportion of dogs with hypocalcemia <1.00 mmol/L (P = .014). Six dogs were noted to have adverse events after treatment including weakness, collapse, or tremors (1 dog each in groups 1, 2, and 3), facial pruritus (1 dog in Group 4), seizures (1 dog in Group 3) and sudden death (1 dog in Group 4). This dog's iCa was 1.76 mmol/L before treatment, last measured at 1.22 mmol/L 6 hours before death, 2 days after treatment. Necropsy was performed, but cause of death was not determined; therefore, hypocalcemia remained a differential.
The median duration of disease was 101 days (interval: 14–714 days). Although there was no significant correlation between duration of disease and lower after‐treatment iCa (r = 0.107, P = .44), dogs who developed adverse events related to hypocalcemia had significantly longer known duration of disease (median 312 days) than dogs who did not display clinical signs of hypocalcemia (median 92 days, P = .048). No adverse events were documented in any dog whose duration of disease was <100 days. Conversely, the 2 dogs with most profound adverse events (seizure and sudden death) had a known duration disease of 452 and 393 days, respectively.
Discussion
Management of most dogs with hyperparathyroidism after treatment is often uneventful and rewarding. However, managing some dogs can be challenging if life‐threatening hypocalcemia develops or if vitamin D overdose occurs while attempting to avoid hypocalcemia.18, 23 The decision to supplement with vitamin D is often based on clinician preference or anecdotal evidence because standard recommendations do not exist and there have been conflicting results in previous studies investigating the relationship between findings before treatment and the development of hypocalcemia after treatment.19, 21, 22
In this study, we aimed to evaluate dogs both retrospectively and prospectively to gather a large cohort for examining the relationship between findings before treatment and hypocalcemia after treatment, as well as adverse events. Because dogs treated at the VMTH were typically pretreated with calcitriol if their tCa exceeded 15 mg/dL or if their known duration of disease was >6 months, only 1 dog enrolled retrospectively in this study had a tCa ≥15 mg/dL and none were >16 mg/dL. In 1 previous study, dogs with higher before‐treatment total calcium (median 16.8 mg/dL) had a higher risk of developing hypocalcemia than those with lower before‐treatment total calcium (13.6 mg/dL).19 The lower before‐treatment calcium concentrations for dogs in this study would be expected to be associated with a decreased likelihood of after‐treatment hypocalcemia. In spite of this limitation, our results demonstrate that there is an association between higher before‐treatment iCa and incidence of after‐treatment hypocalcemia. Given this clear association, the prospective arm of our study was discontinued out of concern for animal welfare due to the higher risk of developing hypocalcemia in dogs with higher before‐treatment iCa.
If the study population are arbitrarily separated by pretreatment iCa into mild (iCa <1.66 mmol/L), moderate (iCa 1.66–1.75 mmol/L), and severe (>1.75 mmol/L), these data suggest that dogs with mild pretreatment hypercalcemia are at relatively low risk of developing after‐treatment hypocalcemia and do not warrant prophylactic administration of calcitriol. Dogs with severe hypercalcemia before treatment are at risk of developing clinically consequential hypocalcemia and many, if not all, would likely benefit from preemptive administration of calcitriol. Dogs with moderate hypercalcemia might or might not benefit from its administration and the decision regarding the initiation of vitamin D treatment should be left to the clinician. The clinician, in turn, should make this decision based in part on owner acceptance of frequent laboratory assessments and the ability to intensively and aggressively monitor the dog and serum calcium concentrations. These guidelines must be interpreted for each hospital, clinician and laboratory, as measurement protocols and references ranges can vary.
The association between iCa before and after treatment reported in this study challenges the findings of 2 other studies.21, 22 In 1 large study, the authors did not find a relationship between higher iCa before treatment and lower iCa after treatment, but the monitoring of their dogs was sporadic and could have underestimated subclinical hypocalcemia.22 In our study, 20 dogs became hypocalcemic, 14 below the established cutoff of 1.05 mg/dL, although only 6 developed clinical signs. The study was designed to retrospectively enroll dogs that had iCa monitoring at least every 12 hours until the values plateaued or until calcitriol treatment was initiated in order to maximize our ability to identify dogs who developed hypocalcemia. Additionally, by only including dogs seen by the VMTH, we minimized variability in sample handling on the measurement of iCa.
Another goal of this study was to evaluate the relationship between other factors before treatment and hypocalcemia after treatment. To estimate the duration of disease, we assigned the date of first diagnosis as the start date and the date of treatment as the date of resolution of primary hyperparathyroidism. This method undoubtedly underestimated the duration of disease. Many dogs in this study had no clinical signs before diagnosis, so there is a strong possibility that they could have been affected by the disease for much longer than estimated. Determining the true duration of disease would be difficult. We attempted to investigate the association between duration of primary hyperparathyroidism and incidence of hypocalcemia or adverse events after treatment but were unable to find a significant correlation between these 2 factors. Similarly, serum PTH concentrations before treatment did not correlate with iCa concentrations after treatment.
A limitation of this study was the use of both serum and plasma iCa values due to the nature of the retrospective arm of the study. Factors known to affect iCa measurement include sample pH and heparin concentration in whole blood samples. Serum samples sent to an external laboratory were adjusted to correct to a sample pH of 7.4.2 Heparinized whole blood was collected in lithium heparin Microtainers9 and run as soon as possible (within 5–10 minutes) to minimize effects of aerobic conditions. Nevertheless, the ex vivo handling of the whole blood might decrease measured iCa concentrations. The mixed sample population likely represents what would be expected in many veterinary clinics.
The data from this study suggest that there is a moderate correlation between higher hypercalcemia before treatment and lower iCa concentrations after treatment. There was no association between PTH concentration or duration of disease before treatment and development of hypocalcemia after the operation.
Acknowledgments
The study was supported by a gift from the late Ruth Johnston, San Mateo, CA, a former client of the UC Davis VMTH.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This project was presented as an abstract at the 2015 ECVIM‐Companion Animal Congress in Lisbon, Portugal.
Footnotes
Hitachi 717, Roche Laboratories, Indianapolis, IN
634 Ca/pH Analyzer, Bayer/Ciba‐Corning Diagnostics Corp, East Walpole, MA
ABL 705, Radiometer Medical A/S, Copenhagen, Denmark
ABL 815, Radiometer Medical A/S
Parathyroid hormone assays from the Michigan State University's Diagnostic Center for Population and Animal Health
HDI 5000, ATL Ultrasound, Philips Medical Systems Co Inc, Bothell, WA
Cytel Software Corporation, Cambridge, MA
StataCorp LP, College Station, TX
Becton, Dickinson and Company, Franklin Lakes, NJ
References
- 1. Krook L. Spontaneous hyperparathyroidism in the dog. Acta Pathol Microbiol Scand 1957;41(Suppl 122):27–37. [PubMed] [Google Scholar]
- 2. Bilezikian JP. Hypercalcemic states In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York, NY: Raven Press; 1992:493–522. [Google Scholar]
- 3. Berger B, Feldman EC. Primary hyperparathyroidism in dogs: 21 cases (1976–1986). J Am Vet Med Assoc 1987;191:350–356. [PubMed] [Google Scholar]
- 4. Weir EC, Norrdin RW, Barthold SW, et al. Primary hyperparathyroidism in a dog: Biochemical, bone histomorphometric, and pathologic findings. J Am Vet Med Assoc 1986;189:1471–1474. [PubMed] [Google Scholar]
- 5. DeVries SE, Feldman EC, Nelson RW, et al. Primary parathyroid gland hyperplasia in dogs: Six cases (1982–1991). J Am Vet Med Assoc 1993;202:1132–1136. [PubMed] [Google Scholar]
- 6. Wisner ER, Nyland TG. Ultrasonography of the thyroid and parathyroid glands. Vet Clin North Am Small Anim Pract 1998;28:973–991. [DOI] [PubMed] [Google Scholar]
- 7. Wisner ER, Penninck D, Biller DS, et al. High resolution parathyroid sonography. Vet Radiol Ultrasound 1997;38:462–466. [DOI] [PubMed] [Google Scholar]
- 8. Feldman EC, Hoar B, Pollard R, et al. Pretreatment clinical and laboratory findings in dogs with primary hyperparathyroidism: 210 cases (1987–2004). J Am Vet Med Assoc 2005;227:756–761. [DOI] [PubMed] [Google Scholar]
- 9. Silverberg SJ, Shane E, Jacobs TP, et al. A 10‐year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 1999;341:1249–1255. [DOI] [PubMed] [Google Scholar]
- 10. Utiger RD. Treatment of primary hyperparathyroidism. N Engl J Med 1999;341:1301–1302. [DOI] [PubMed] [Google Scholar]
- 11. Long CD, Goldstein RE, Hornof WJ, et al. Percutaneous ultrasound‐guided chemical parathyroid ablation for treatment of primary hyperparathyroidism in dogs. J Am Vet Med Assoc 1999;215:217–221. [PubMed] [Google Scholar]
- 12. Pollard RE, Long CD, Nelson RW, et al. Percutaneous ultrasonographically guided radiofrequency heat ablation for treatment of primary hyperparathyroidism in dogs. J Am Vet Med Assoc 2001;218:1106–1110. [DOI] [PubMed] [Google Scholar]
- 13. Rasor L, Pollard R, Feldman EC. Retrospective evaluation of three treatment methods for primary hyperparathyroidism in dogs. J Am Anim Hosp Assoc 2007;43:70–77. [DOI] [PubMed] [Google Scholar]
- 14. Wisner ER, Nyland TG, Feldman EC, et al. Ultrasonographic evaluation of the parathyroid glands in hypercalcemic dogs. Vet Radiol Ultrasound 1993;34:108–111. [Google Scholar]
- 15. Matwichuk CL, Taylor SM, Wilkinson AA, et al. Use of technetium Tc99 m sestamibi for detection of a parathyroid adenoma in a dog with primary hyperparathyroidism. J Am Vet Med Assoc 1996;209:1733–1736. [PubMed] [Google Scholar]
- 16. Matwichuk CL, Taylor SM, Daniel GB, et al. Double‐phase parathyroid scintigraphy in dogs using technetium‐99 m‐sestamibi. Vet Radiol Ultrasound 2000;41:461–469. [DOI] [PubMed] [Google Scholar]
- 17. Feldman EC, Wisner ER, Nelson RW, et al. Comparison of results of hormonal analysis of samples obtained from selected venous sites versus cervical ultrasonography for localizing parathyroid masses in dogs. J Am Vet Med Assoc 1997;211:54–57. [PubMed] [Google Scholar]
- 18. Schenck PA, Chew DJ. Investigation of hypercalcaemia and hypocalcaemia In: Mooney CT, Peterson ME, eds. BSAVA Manual of Canine and Feline Endocrinology, 4th ed Quedgeley, Gloucester, UK: British Small Animal Veterinary Association; 2012:221–233. [Google Scholar]
- 19. Gear RN, Neiger R, Skelly BJ, et al. Primary hyperparathyroidism in 29 dogs: Diagnosis, treatment, outcome and associated renal failure. J Small Anim Pract 2005;46:10–16. [DOI] [PubMed] [Google Scholar]
- 20. Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction, 3rd ed St. Louis, MO: WB Saunders Co; 2003. [Google Scholar]
- 21. Arbaugh M, Smeak D, Monnet E. Evaluation of preoperative serum concentrations of ionized calcium and parathyroid hormone as predictors of hypocalcemia following parathyroidectomy in dogs with primary hyperparathyroidism: 17 cases (2001–2009). J Am Vet Med Assoc 2012;241:233–236. [DOI] [PubMed] [Google Scholar]
- 22. Milovancev M, Schmiedt CW. Preoperative factors associated with postoperative hypocalcemia in dogs with primary hyperparathyroidism that underwent parathyroidectomy: 62 cases (2004–2009). J Am Vet Med Assoc 2013;242:507–515. [DOI] [PubMed] [Google Scholar]
- 23. Chew DJ, Nagode LA. Treatment of Hypoparathyroidism In: Bonagura JD, ed. Kirk's Current Veterinary Therapy, XIII ed Philadelphia, PA: W. B. Saunders Company; 2000:340–345. [Google Scholar]


