Abstract
Background
Long‐term medical management of hypersomatotropism (HS) in cats has proved unrewarding. Pasireotide, a novel somatostatin analogue, decreases serum insulin‐like growth factor 1 (IGF‐1) and improves insulin sensitivity in cats with HS when administered as a short‐acting preparation.
Objectives
Assess once‐monthly administration of long‐acting pasireotide (pasireotide LAR) for treatment of cats with HS.
Animals
Fourteen cats with HS, diagnosed based on diabetes mellitus, pituitary enlargement, and serum IGF‐1 > 1000 ng/mL.
Methods
Uncontrolled, prospective cohort study. Cats received pasireotide LAR (6–8 mg/kg SC) once monthly for 6 months. Fructosamine and IGF‐1 concentrations, and 12‐hour blood glucose curves (BGCs) were assessed at baseline and then monthly. Product of fructosamine concentration and insulin dose was calculated as an indicator of insulin resistance (Insulin Resistance Index). Linear mixed‐effects modeling assessed for significant change in fructosamine, IGF‐1, mean blood glucose (MBG) of BGCs, insulin dose (U/kg) and Insulin Resistance Index.
Results
Eight cats completed the trial. Three cats entered diabetic remission. Median IGF‐1 (baseline: 1962 ng/mL [range 1051–2000 ng/mL]; month 6: 1253 ng/mL [524–1987 ng/mL]; P < .001) and median Insulin Resistance Index (baseline: 812 μmolU/L kg [173–3565 μmolU/L kg]; month 6: 135 μmolU/L kg [0–443 μmolU/L kg]; P = .001) decreased significantly. No significant change was found in mean fructosamine (baseline: 494 ± 127 μmol/L; month 6: 319 ± 113.3 μmol/L; P = .07) or MBG (baseline: 347.7 ± 111.0 mg/dL; month 6: 319.5 ± 113.3 mg/dL; P = .11), despite a significant decrease in median insulin dose (baseline: 1.5 [0.4–5.2] U/kg; 6 months: 0.3 [0.0–1.4] U/kg; P < .001). Adverse events included diarrhea (n = 11), hypoglycemia (n = 5), and worsening polyphagia (n = 2).
Conclusions and Clinical Importance
Pasireotide LAR is the first drug to show potential as a long‐term management option for cats with HS.
Keywords: Acromegaly, Cat, SOM230, Somatostatin
Abbreviations
- ALT
alanine aminotransferase
- BGC
blood glucose curve
- CT
computed tomography
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- GH
growth hormone
- HS
hypersomatotropism
- IGF‐1
insulin‐like growth factor 1
- LAR
long‐acting release
- MBG
mean blood glucose
- RI
reference interval
- RVC
Royal Veterinary College
- SD
standard deviation
- SSTR
somatostatin receptor
- SST
somatostatin
Hypersomatotropism (HS) is an increasingly recognized cause of diabetes mellitus (DM) in cats, with prevalence estimates among diabetic cats ranging from 17.8% in Switzerland and the Netherlands1 to 24.8% in the United Kingdom.2 Hypersomatotropism in cats usually results from excessive growth hormone (GH) secretion from a functional somatotroph adenoma in the pituitary gland.3 Growth hormone's insulin‐antagonistic effects cause most cats with HS to be diabetic, with many showing insulin resistance and persistent signs of poor glycemic control despite exogenous insulin administration.4 The mitogenic effects of GH excess, which are mediated by insulin‐like growth factor‐1 (IGF‐1),5 can ultimately lead to body tissue proliferation and the clinical syndrome of acromegaly. In cats, clinical acromegaly is associated with physical changes, which can include prognathism inferior, broadening of the face, cardiomyopathy, pancreatopathy, and degenerative arthropathy.6, 7
Treatment of HS in cats should aim to improve diabetic control and prevent progression of clinical acromegaly. Transsphenoidal hypophysectomy is the recommended first‐line treatment for acromegalic people.8 Hypophysectomy has successfully been used to treat HS in cats,9 , 1 but availability of surgery is limited. In people, medical treatment is used to control HS when surgery has failed, been declined, or is contraindicated, and commonly used drugs include long‐acting forms of the first‐generation somatostatin (SST) analogues, lanreotide2 and octreotide.3 , 10 First‐generation somatostatin analogue treatment leads to biochemical control of acromegaly in up to 54% of people, and pituitary adenoma shrinkage in 30–50% of people.10, 11 A minority of cats show a short‐term decrease in GH concentration when treated intravenously with octreotide,12 but long‐term medical management of HS in cats has otherwise proven unrewarding, and treatment with long‐acting octreotide, or l‐deprenyl, which is a monoamine oxidase‐B inhibitor, has failed to provide either clinical improvement and biochemical improvement.13 , 4
Somatostatin binds to 5 receptor subtypes, named somatostatin receptors (STTR) 1‐5, which are variably expressed in human pituitary tissue.14 Octreotide and lanreotide predominantly bind to STTR 215, 16, and low SSTR2 expression is a proposed reason for why some acromegalic people fail to respond to these treatments.14 Preliminary work on pituitary STTR expression in cats suggests that feline pituitary tissue expresses STTRs 1, 2, and 5 and that pituitaries of cats with HS show greater expression of STTRs 1 and 5 compared to STTR2.5 This finding could account for the poor response to octreotide treatment shown by cats with HS.4
Recently, a multireceptor‐binding STT analogue called pasireotide6 has been licensed as a long‐acting, once‐monthly treatment for acromegalic people. Pasireotide binds to a wider range of STTR subtypes than first‐generation STT analogues. Pasireotide can bind to STTRs 1, 2, 3, and 5, and shows a 30‐, 5‐ and 39‐times greater binding affinity for STTRs 1, 3, and 5, respectively, compared to octreotide, with a slightly lower affinity for STTR2.15 Pasireotide is effective in some acromegalic people who are resistant to first‐generation STT analogues,17 and short‐acting pasireotide has been shown to provide biochemical control, and improved insulin sensitivity, when used as a short‐term treatment in acromegalic cats.18 The current study was performed to assess whether long‐acting pasireotide (pasireotide LAR) could offer effective, long‐term treatment for diabetic cats with underlying HS.
Materials and Methods
This was an uncontrolled, 6‐month, prospective, longitudinal cohort study. The study was approved by the Ethics Committee of The Royal Veterinary College (Royal Veterinary College Ethical Approval Number URN2011 1120), and informed consent was obtained from all participating owners. The study population was formed from a convenience sample of cats diagnosed with HS at The Royal Veterinary College (RVC) Acromegalic Cat Clinic. Trial participation was offered to cats diagnosed with HS at this clinic between December 2011 and January 2014. Diagnosis of HS was based on the presence of DM, documented pituitary enlargement by computed tomography (CT), and a markedly increased serum IGF‐1 concentration of >1000 ng/mL. Serum IGF‐1 concentration was measured by a commercially available radioimmunoassay7 that had been previously validated in cats.19 This test has an upper limit of quantification of 2000 ng/mL, and values greater than this were assigned a value of 2000 ng/mL for the purpose of data analysis.
On the day of admission, all cats received a physical examination, echocardiography, abdominal ultrasonography, urinalysis, and measurement of CBC, serum biochemistry, fructosamine concentration, and IGF‐1 concentration. Cats were excluded if these tests revealed relevant underlying disease that might affect successful trial completion. This included diseases with poor prognosis and diseases for which treatment was likely to interfere with trial commitments. Cats then received pituitary imaging under sedation by contrast‐enhanced CT, as previously described,20 and a dorsoventral pituitary height of >4 mm was considered to be consistent with enlargement.21 Maximal pituitary height was recorded for each cat. Other recorded baseline data included signalment, bodyweight, and whether phenotypic signs of acromegaly were present. Cats then underwent a 12‐hour blood glucose curve (BGC) while hospitalized using their usual insulin type and dose. Blood glucose curves were carried out a minimum of 12 hours after the above investigations and were performed by a handheld glucometer previously validated for cats.8 Curves consisted of a reading taken every 2 hours over a 12‐hour period, starting at the time of morning food and insulin administration.
Cats received 8 mg/kg of pasireotide LAR by subcutaneous injection before discharge and once monthly thereafter for a planned 6‐month follow‐up period. Pasireotide LAR dose was based on manufacturer's recommendations. The majority of included cats received 3 days of short‐acting pasireotide treatment9 between collection of their baseline data and their first pasireotide LAR dose. This short‐acting pasireotide was given as part of a separate, previously reported trial.18 Baseline data in these cats, including their baseline BGC, were recorded before any pasireotide, either short‐ or long‐acting, was administered.
Monthly re‐examination appointments and pasireotide LAR administration were carried out at the RVC Acromegalic Cat Clinic. Owners were asked to perform a 12‐hour BGC on their cat at home, or at their primary veterinary practice, in the 5 days before each re‐examination. At each re‐examination, cats received a physical examination, and fructosamine and IGF‐1 measurement in addition to pasireotide LAR administration. Adjustments in insulin dose were made based on the clinical opinion of the clinical trial team. Cats who remained normoglycemic without insulin or oral antihyperglycemic medications (with the exception of low‐carbohydrate diet) for a minimum of 4 weeks were classified as being in diabetic remission. Cats received a repeated CBC, serum biochemistry measurement, and abdominal ultrasound examination at the month 6 time point, or sooner if clinically indicated, to monitor for abnormalities that might be related to pasireotide treatment. Suspected adverse events, the reason for any trial withdrawals, and any alterations made in pasireotide LAR dosage were recorded. Cats remaining in the study at the end of the 6‐month period received repeated pituitary imaging by contrast‐enhanced CT at the month 6 time point to assess for change in pituitary size. Change in phenotypic signs of acromegaly was not evaluated as part of this study. It was thought this would be challenging to assess because phenotypic signs of feline acromegaly are often only subtle when judged by CT.20
Statistical Analysis
Average values for variables were reported as mean ± standard deviation (SD) for normally distributed data and as median with range for non‐normal data. Normality was judged by histograms, normal plots, and Shapiro‐Wilk tests.
A linear mixed‐effects model with first‐order autoregressive covariance structure was used to assess for significant change in fructosamine concentration, IGF‐1 concentration, insulin dose given at each twice‐daily insulin administration (U/kg), and mean blood glucose (MBG) concentration during BGCs over the 6‐month trial. Similar to the insulin sensitivity product assessed in the previous short‐acting pasireotide trial,18 for the purpose of this study, the product of fructosamine and twice‐daily insulin dose (U/kg) was calculated as a surrogate indicator of insulin resistance, referred to as the Insulin Resistance Index. This enabled documentation of improvements in insulin sensitivity by integrating glycemic changes with exogenous insulin dose changes. Change in this variable over the duration of the trial was also assessed by a linear mixed‐effects model with first‐order autoregressive covariance structure, and values were logarithmically transformed before analysis to meet the model's assumptions. For variables that showed a significant change, Fisher's least significant difference post hoc comparison was used to assess pairwise differences between each time point and month 0.
The maximal pituitary height at month 0 and month 6 time points in cats completing the study was compared by a Wilcoxon signed‐rank test. Significance for all tests was set at P < .05, and all analyses were performed by commercial statistical software packages.10 , 11
Results
Thirty‐three cats examined during the study period were eligible for inclusion. Of these, 8 owners declined trial participation and 11 owners decided to manage their cat's HS by hypophysectomy. Fourteen cats were therefore enrolled onto the trial. Twelve of these cats received 3 days of short‐acting pasireotide before receiving their first dose of pasireotide LAR.18 The remaining 2 cats were enrolled after the short‐acting pasireotide trial had ceased recruiting participants and so received only pasireotide LAR.
Cat Characteristics at Enrollment
The study population consisted of 11 domestic shorthairs, 1 domestic longhair, 1 Persian, and 1 Burmese. Ten cats were neutered males and 4 were neutered females. All cats had clinical signs of poor diabetic control, and 12 of the 14 cats had phenotypic changes consistent with acromegaly on physical examination. Mean age at enrollment was 9.9 ± 2.2 years, and mean bodyweight was 5.8 ± 1.1 kg. Complete blood cell counts revealed 1 cat to have a stress leukogram, 1 cat to have mild, non‐regenerative anemia (PCV 21%; reference interval (RI) 24–45%), and 1 cat to have a moderate, mature neutrophilia, but revealed no other abnormalities. Serum biochemistry analyses at trial entry revealed no abnormalities, apart from hyperglycemia in all cats. Abdominal ultrasound findings noted at trial entry included bilateral adrenomegaly (n = 9), bilateral renomegaly (n = 8), hyperechoic hepatic parenchyma (n = 6), hepatomegaly (n = 5), and suspected pancreatopathy (n = 4), with most cats having more than 1 abnormality. For the purpose of this trial, adrenomegaly was defined as an adrenal width of >4.8 mm, as proposed by a previous study.22
Cats had been receiving insulin treatment for a median of 231 (63–122) days before trial enrollment. All cats were receiving twice‐daily (q12h) insulin with 10 cats receiving porcine lente insulin,12 2 cats receiving protamine zinc insulin13 , and 2 cats receiving insulin glargine.14 No cats changed insulin type at the time of starting pasireotide LAR, but 6 cats changed insulin type later in the study period. Five cats were changed from porcine lente to glargine treatment, and 1 cat was changed from protamine zinc insulin to porcine lente insulin. These changes in insulin treatment occurred a median of 3 months (2–4 months) into the trial period.
Table 1 shows the number of cats remaining in the trial at each time point and average values for variables during the trial. Before starting pasireotide treatment, median q12h insulin dose was 1.5 U/kg (0.4–5.2 U/kg), median IGF‐1 concentration was 1962 ng/mL (1051–2000 ng/mL), and mean fructosamine concentration was 494 ± 127 μmol/L. Mean blood glucose (MBG) concentration during 12‐hour BGCs before pasireotide treatment was 347.7 ± 111.0 mg/dL (19.3 ± 6.2 mmol/L). Median maximal pituitary height on CT at enrollment was 7.2 mm (5.5–12.0 mm).
Table 1.
Number of cats remaining in the study at each time point and average values for variables monitored during the trial
| Time Point (month) | Number of Cats Remaining in Study | IGF‐1 Concentration (ng/mL)a | Twice‐Daily Insulin Dose (U/kg)a | Fructosamine Concentration (μmol/L)b | MBG During BGCs (mg/dL)b | Insulin Resistance Index (μmolU/L kg)a |
|---|---|---|---|---|---|---|
| 0 | 14 |
1962 (1051–2000) n = 14 |
1.5 (0.4–5.2) n = 14 |
494 ± 127 n = 14 |
347.7 ± 111.0 n = 13 |
812 (173–3565) n = 14 |
| 1 | 12 |
909 (105–2000)*** n = 12 |
1.3 (0.1–1.3)*** n = 12 |
437 ± 126 n = 12 |
302.2 ± 159.8 n = 12 |
155 (43‐774)*** n = 12 |
| 2 | 10 |
836 (415–1937)*** n = 10 |
0.4 (0.0–1.5)*** n = 10 |
406 ± 116 n = 9 |
260.6 ± 128.8 n = 9 |
99 (0–601)** n = 9 |
| 3 | 9 |
1076 (386–2000)** n = 9 |
0.2 (0.0–1.7)*** n = 9 |
483 ± 152 n = 8 |
283.7 ± 154.4 n = 9 |
146 (0–850)** n = 9 |
| 4 | 9 |
1437 (841–1613)* n = 9 |
0.1 (0.0–1.2)*** n = 9 |
370 ± 143 n = 9 | 191.9 ± 115.1 n = 6 | 40 (0–830) *** n = 9 |
| 5 | 8 |
971 (520–1665)*** n = 8 |
0.3 (0.0–1.0)*** n = 8 |
399 ± 132 n = 8 |
213.2 ± 96.3 n = 6 |
29 (0–522)** n = 8 |
| 6 | 8 |
1253 (524–1987)** n = 8 |
0.3 (0.0–1.4)*** n = 8 |
416 ± 125 n = 8 |
319.5 ± 113.3 n = 6 |
135 (0–443)** n = 8 |
Indicates variable described using median (range).
Indicates variable described using mean ± standard deviation. n = number of patients with available measurement at that time point. IGF‐1, insulin‐like growth factor‐1; MBG, mean blood glucose; BGC, blood glucose curve. *, ** and *** indicate a statistically significant difference compared to Month 0 value (* indicates P < .05, ** indicates P < .01, *** indicates P < .001).
Response to Treatment
Three cats (21%) entered diabetic remission. A fourth cat stopped insulin treatment for 15 days but then became hyperglycemic again so did not meet criteria for remission. In the 3 cats that achieved remission, insulin was discontinued 89 days (n = 2) and 91 days (n = 1) after their first pasireotide LAR dose (Fig 1). All cats that achieved remission continued to receive once‐monthly pasireotide LAR, and all completed the 6‐month trial without a recurrence of DM. After trial completion, 2 cats remained in remission until death 479 and 580 days after starting pasireotide LAR, and the third cat is alive and in diabetic remission at the time of writing, 1420 days after starting pasireotide LAR treatment.
Figure 1.
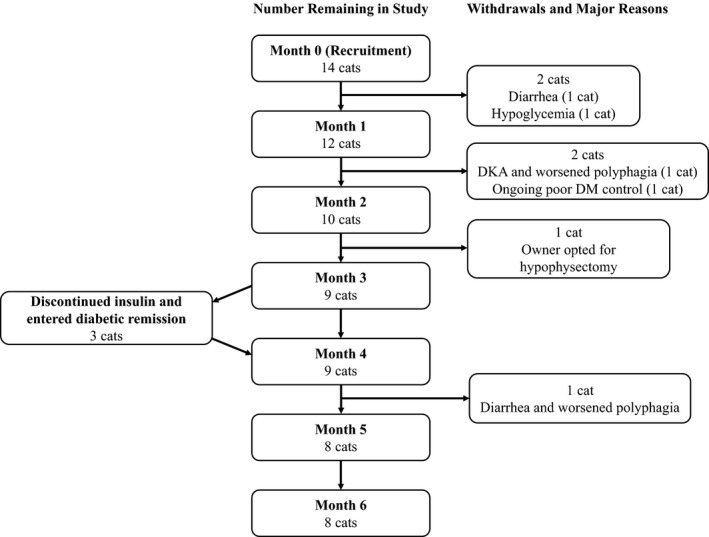
Flow diagram showing the number of cats remaining in the study at each time point, and the timing of study withdrawals and diabetic remission. Major reasons for study withdrawals are also shown. DKA, diabetic ketoacidosis; DM, diabetes mellitus.
A significant decrease was detected in IGF‐1 concentration (P=<.001), q12h insulin dose (P=<.001), and Insulin Resistance Index (P = .001) over the trial period. Post hoc analysis revealed a statistically significant difference between month 0 and all subsequent time points for all 3 variables (P‐values shown in Table 1 and Figures 2, 3, and 4).
Figure 2.
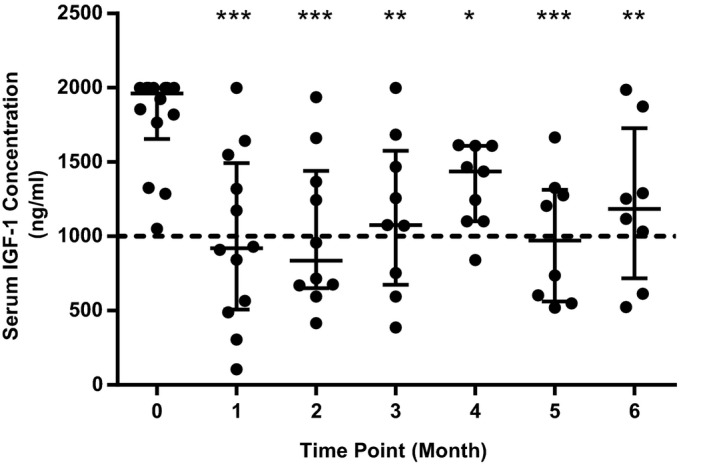
Scatterplot showing individual serum IGF‐1 concentrations at each study time point. Central horizontal bar indicates median value with error bars indicating interquartile range. Dashed line shows recommended cutoff for screening for HS in cats (1000 ng/mL). *, ** and *** indicate a statistically significant difference compared to Month 0 value (* indicates P < .05, ** indicates P < .01, *** indicates P < .001).
Figure 3.
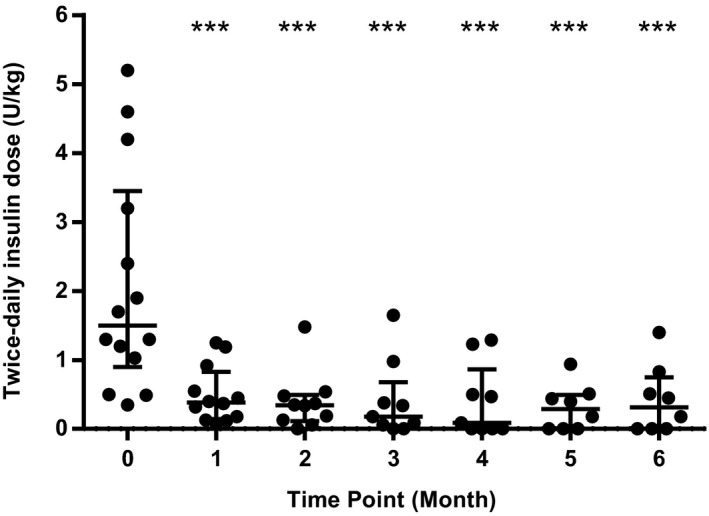
Scatterplot showing individual twice‐daily insulin doses at each study time point. Central horizontal bar indicates median value with error bars indicating interquartile range. *** indicate a statistically significant difference compared to Month 0 value with P < .001.
Figure 4.
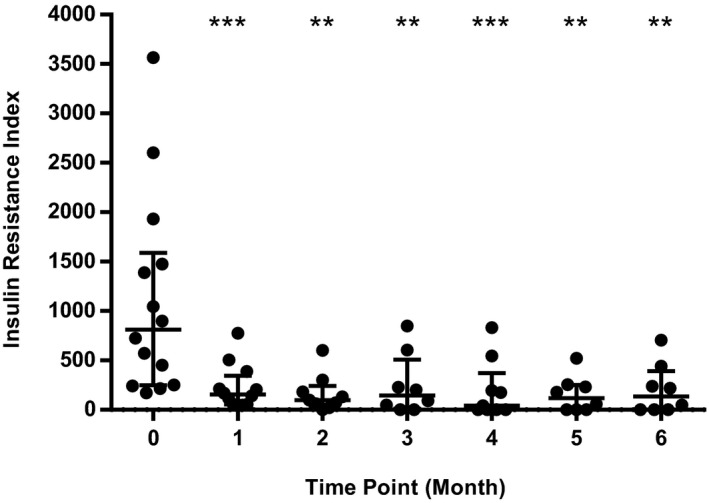
Scatterplot showing individual Insulin Resistance Index values (fructosamine × q12h insulin dose in U/kg) at each study time point. Central horizontal bar indicates median value with error bars indicating interquartile range. ** and *** indicate a statistically significant difference compared to Month 0 value (** indicates P < .01, *** indicates P < .001).
There was no significant change in serum fructosamine (P = .07) or MBG during BGCs (P = .11) over the study period (Table 1 and Figs 5 and 6). In the 8 cats that completed the 6‐month trial, maximal pituitary height on CT examination changed by a median of −0.5 mm (−1.8 to +1.3 mm) (P = .16).
Figure 5.
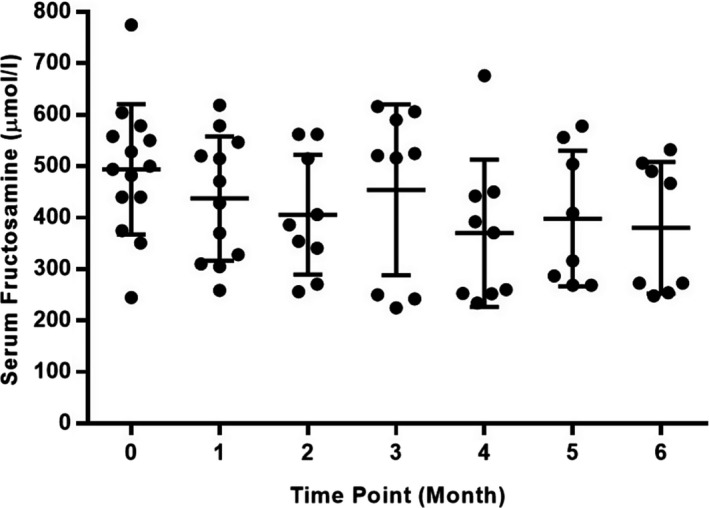
Scatterplot showing individual serum fructosamine concentrations at each study time point. Central horizontal bar indicates mean value with error bars indicating 1 standard deviation.
Figure 6.
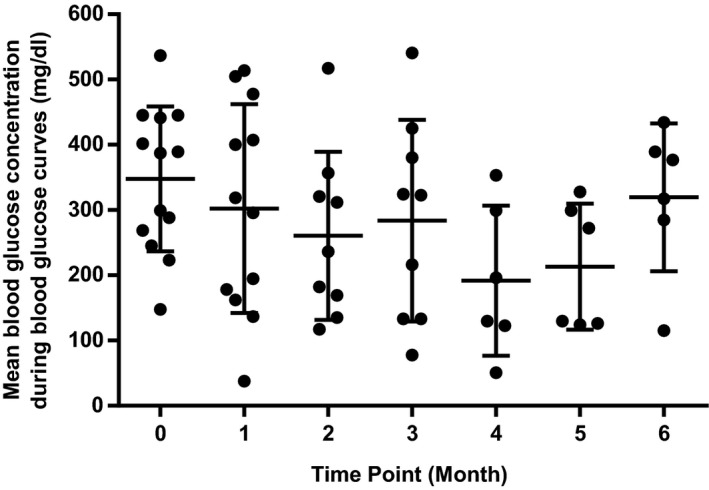
Scatterplot showing individual mean blood glucose values during blood glucose curves at each study time point. Central horizontal bar indicates mean value with error bars indicating 1 standard deviation.
Adverse Events
Various adverse effects were recorded. Diarrhea, of varying severity, was the most common adverse effect and was experienced by 11 cats (76%). Ten of these cats developed diarrhea within 1 month of their first pasireotide LAR dose, and 1 cat developed diarrhea within 2 months of starting pasireotide LAR treatment. In 2 cats, diarrhea was transient and resolved within 2 months of starting treatment. Four cats had their diarrhea treated with one, or a combination, of exclusion diet treatment (n = 4), pancreatic enzyme supplementation (n = 3), parenteral cobalamin injections (n = 1), and soluble fiber supplementation (n = 1). Two cats had their monthly pasireotide LAR dose decreased to 6 mg/kg in the hope of improving their diarrhea. These management changes caused a mild‐to‐negligible improvement. One cat experienced 2 hypovolemic episodes that were suspected to be secondary to bouts of diarrhea. Severe diarrhea was a reason for trial withdrawal in 2 cats (Fig 1). Six hypoglycemic events were documented in the study population and occurred in 5 cats with 1 cat experiencing 2 episodes. Five of the 6 episodes were associated with clinical signs. Other adverse effects were worsening polyphagia in 2 cats, a transient injection site reaction in 1 cat, and delayed hair regrowth in 1 cat.
Repeated CBC results at month 6 revealed no abnormalities, apart from mild eosinophilia in 1 cat. Repeated serum biochemical analyses at month 6 also revealed no abnormalities, apart from moderate hypercholesterolemia in 1 cat, and a mild‐to‐moderate increase in serum alanine aminotransferase (ALT) activity in 2 cats (126 U/L and 274 U/L; RI 20–100 U/L). Both cats with ALT elevation remained clinically well, apart from diarrhea in 1 cat, and neither showed other biochemical indicators of hepatic dysfunction. One cat with severe diarrhea underwent repeated serum biochemistry measurement at the month 2 time point, but this revealed no abnormalities. The only notable changes on repeated abdominal ultrasonography at month 6, compared to trial entry, were intestinal muscularis layer thickening in 2 cats, and gall bladder sediment in 1 cat. Both cats with muscularis layer thickening experienced diarrhea as an adverse effect of pasireotide LAR treatment. The cat with gall bladder sediment was 1 of 2 cats that developed an increased serum ALT activity.
Trial Completion and Withdrawals
Eight cats (57%) completed the trial. Trial withdrawals occurred after a median of 2 months (1–5 months). Figure 1 shows the timing of each withdrawal and the major reasons for which cats stopped trial treatment. Withdrawals occurred for a variety of reasons with some having more than 1 contributing factor. Reasons included severe diarrhea (n = 2), worsened polyphagia (n = 2), failure of diabetic control to improve (n = 1), and euthanasia after a hypoglycemic event (n = 1) or episode of diabetic ketoacidosis (n = 1). One cat was withdrawn from the study after the owner decided to manage its HS by hypophysectomy rather than pasireotide LAR.
Discussion
This study found that once‐monthly pasireotide LAR treatment was associated with a significant reduction in IGF‐1 concentration in diabetic cats with HS, suggesting that pasireotide LAR significantly improves excessive GH production from feline pituitary somatotrophinomas. Several pasireotide‐treated cats achieved long‐standing diabetic remission, which rarely occurs when diabetic cats with HS are treated with routine diabetic management alone.23 Cats receiving pasireotide LAR experienced a significant decrease in insulin requirement and estimated insulin resistance, although serum fructosamine concentration and MBG did not significantly change over the study period.
Previous attempts at long‐term medical management of HS in cats have proven unsuccessful,13 , 4 making pasireotide LAR the first drug to show potential as a long‐term management option for feline HS. Octreotide is the only other long‐acting STT analogue to have been assessed as a treatment for feline HS, but was found to be ineffective.4 Together, these findings are similar to those of recent human trials, which have shown pasireotide LAR to be superior to long‐acting octreotide treatment.17, 24 This superiority has been attributed to pasireotide's wide receptor range and binding affinity, which allow it to accommodate variations in STTR expression in patients’ somatotrophinomas. Octreotide predominantly binds to STTR2, with lesser affinity for STTR3 and 5, whereas pasireotide binds to STTRs 1, 2, 3, and 5 and also shows a substantially greater binding affinity for STTRs 1, 3, and 5 compared to octreotide.15 The biochemical improvement shown by cats in this study, and the poor previous experience with octreotide treatment, supports that the pituitaries of cats with HS have greater expression of STTRs 1 and 5 compared to STTR2.5
That several cats in the current study achieved diabetic remission supports that pasireotide LAR offers a viable medical treatment option for HS in cats. Diabetic cats with HS are most likely to achieve remission when treated with a therapy that successfully addresses their excess GH production.3 Diabetic remission can occur in cats whose HS is treated by radiotherapy25 or hypophysectomy,9 with a recent study reporting a 78% remission rate among cats who survived more than 17 days after hypophysectomy surgery.1 In contrast, treatment that addresses a cat's DM alone, such as insulin and low‐carbohydrate diet, typically provides inadequate glycemic control and little chance of remission.23 The presence of remission among cats in the current trial therefore supports that pasireotide LAR effectively reduces GH secretion from somatotrophinomas in cats and improves insulin sensitivity in treated cats. Pituitary radiotherapy for HS in cats requires frequent anesthesia and treated cats can have an unpredictable response to treatment.25 Hypophysectomy seems more predictably successful and requires only a single anesthetic, but is offered at reasonably few veterinary centers, is associated with a postoperative fatality rate of approximately 14% and requires lifelong, post‐operative hormone supplementation.1 Pasireotide LAR could therefore provide a viable alternative option for control of HS in cats that are poor surgical or anesthetic candidates, or have no access to hypophysectomy. However, the common occurrence of diarrhea, need for regular injections, and lack of information regarding ideal dosing frequency still pose challenges to its use.
This study did not identify a significant difference in MBG, or fructosamine concentration, over the duration of the trial. Lack of change in MBG could be secondary to how BGCs, from which MBG was calculated, were performed just before each cat's next monthly pasireotide LAR injection. As the LAR is designed to last 1 month, it is likely that each cat's previous pasireotide LAR treatment was waning by this time leading to worsening diabetic control at the end of each monthly treatment period and no apparent improvement in MBG. The once‐monthly dosing frequency used in this trial was based on recommendations from pasireotide LAR treatment in people, and further work would be necessary to establish the optimum dosing frequency in cats. Mean blood glucose results were also likely to be artificially increased toward the end of the study because 2 owners whose cats went into diabetic remission stopped performing monthly blood glucose curves, opting instead to monitor their cat with intermittent blood glucose measurements. Serum fructosamine concentration is generally accepted to reflect cats’ glycemic control over the preceding 1–2 weeks26 and so sampling at the end of each monthly treatment period is less likely to account for the lack of change in serum fructosamine among recruited cats. This may instead have been caused by many cats already having a fructosamine concentration consistent with good‐to‐moderate glycemic control at study entry, though only when receiving relatively high dosages of exogenous insulin.
The fact that fructosamine and MBG remained unchanged, despite a significant decrease in insulin dose, suggests that pasireotide LAR is associated with improved insulin sensitivity in treated cats. A simple Insulin Resistance Index was used to illustrate this in the current trial and showed a significant decrease at all time points compared to time of enrollment. This index has not been previously evaluated in cats, but the use of reference methods for estimating insulin resistance, such as the hyperinsulinemic euglycemic clamp or frequently sampled intravenous glucose tolerance test, was thought to be too invasive to justify their use on client‐owned cats. Simpler methods for evaluating insulin sensitivity, such as the Homeostasis Model Assessment (HOMA) and Quantitative Insulin Sensitivity Check Index (QUICKI), have been used in diabetic cats.27 However, the fasting insulin and glucose values required for these calculations were not available for cats in this study.
This study revealed pasireotide LAR treatment to be associated with various adverse effects, the most common being diarrhea. Diarrhea is a common adverse effect of all long‐acting STT analogue therapies in people17, 24 and is caused by STT's widespread actions in the gastrointestinal tract, which include suppression of gastrointestinal exocrine secretions, inhibition of gastric and gallbladder emptying, and alterations in intestinal motility.16 Alopecia is a recognized adverse effect of pasireotide LAR treatment in people24, and 1 cat in the current study showed delayed fur regrowth. This might have been secondary to pasireotide treatment. Alternatively, this cat could have been affected by concurrent hyperadrenocorticism, which is associated with DM, insulin resistance and the presence of a pituitary adenoma, and has been reported to occur alongside hypersomatotropism in cats.28 This cat did not receive pituitary–adrenal axis testing and did not have adrenomegaly detected on abdominal ultrasound examination. Hypoglycemia was the second most common adverse event in the present study, whereas hyperglycemia and development of DM are frequent adverse effects in people receiving pasireotide LAR.29 Pasireotide‐associated hyperglycemia is thought to result from decreases in insulin and incretin secretion.30 In people, STT decreases insulin secretion by binding to SSTR5 on pancreatic β‐cells,31 and pasireotide's high affinity for STTR5 therefore reduces insulin secretion in treated patients. The mechanism by which pasireotide decreases incretin secretion is currently unknown. The frequency of hypoglycemia in the current study and the fact that 3 cats entered diabetic remission suggest that STT's effect on insulin and incretin secretion are mediated differently in cats compared to people, or that improved insulin sensitivity likely outweighs any decrease in insulin and incretin secretion in treated cats.
Two cats in the current study developed a mild‐to‐moderate increase in ALT activity while receiving pasireotide LAR, and a third cat developed gall bladder sediment. Increased serum ALT activity occurs in up to 5% of acromegalic people treated with pasireotide LAR,32 whereas cholelithiasis and gall bladder abnormalities are more common adverse effects of long‐acting STT analogue treatment in people. Gall bladder stones or sediment develop in up to 45% of acromegalic people treated with long‐acting STT analogues, and often occur in the absence of liver enzyme elevations.24, 33 Several factors contribute to the predisposition for cholelithiasis among acromegalic people receiving long‐acting STT analogue treatment. Somatostatin and its analogues reduce meal‐stimulated cholecystokinin secretion from the small intestine, which decreases gall bladder emptying.34 Also, acromegalic people receiving octreotide are shown to have changes in bile composition, which predispose to cholelithiasis, including increased biliary cholesterol content and alterations in bile acid concentrations.35 All cats who developed increased serum ALT activity, or ultrasonographic biliary tract abnormalities, in this study remained clinically well. However, it would nevertheless be advisable to monitor cats receiving pasireotide LAR by intermittent bloodwork and abdominal ultrasonography until the long‐term tolerability of pasireotide LAR in cats has been better established.
Pasireotide LAR causes a significant reduction in tumor volume in up to 80% of treated people.24 This is thought to be caused by STT, and STT analogues, having both direct and indirect effects on tumor growth. Somatostatin analogues induce cell cycle arrest and apoptosis through direct interaction with STTRs on tumor cells.36, 37 Indirect effects include decreasing secretion of growth‐promoting hormones and factors, such as GH and IGF‐1, and by decreasing angiogenesis in the local tumor environment.38 Octreotide decreased tumor vascularity in a small series of people with acromegaly,39 which might be caused by its ability to decrease vascular endothelial growth factor expression.40 The failure to detect a significant change in pituitary height in the current study might be due to only a small number of cats completing the 6‐month treatment period. Furthermore, pituitary tumor shrinkage is shown to increase with time in people who receive STT treatment over several years41 and so the relatively short duration of this trial may have been insufficient to observe a difference. A larger sample size and longer cat follow‐up could therefore be required to demonstrate whether pasireotide LAR treatment is associated with tumor shrinkage in acromegalic cats.
This study has a number of limitations. People with HS are often monitored using both GH and serum IGF‐1 concentration, whereas only serum IGF‐1 concentration was measured in this study. This was firstly due to lack of a commercially available assay for feline GH. Secondly, GH release is pulsatile with a serum half‐life of less than 20 minutes in humans,42 whereas IGF‐1 is more constantly produced and has a half‐life of approximately 15 hours.8 IGF‐1 concentration is therefore the recommended screening test for HS in people, rather than single GH measurement.43 IGF‐1 is the main mediator for GH's mitogenic effects,5 so an IGF‐1 concentration within normal limits is a treatment goal in people with HS because this represents clinical control of acromegaly.8 Interestingly, many of the cats in the current study failed to achieve an IGF‐1 concentration below the recommended screening value for HS (<1000 ng/mL)4 during the 6‐month trial (Fig 2), so might still have subclinical HS, despite pasireotide LAR treatment. Increasing pasireotide LAR dose, or dosing frequency, might result in more cats achieving a normalized IGF‐1 value, but it is possible that adverse effects might become dose‐limiting in many cats. Another limitation is that most included cats took part in a 3‐day trial examining the effect of short‐acting pasireotide treatment before their first injection of pasireotide LAR.18 Short‐acting pasireotide's pharmacodynamics have not been studied in cats, but it has a half‐life of approximately 12 hours in healthy people.44 If the rate of elimination is similar in cats, previous short‐acting pasireotide treatment is unlikely to have affected any patient values during this study. A proportion of cats also changed insulin type during the study, and it is possible this may have contributed to minor changes in these cats’ diabetic control over the 6‐month period. Nevertheless, insulin treatment alone is rarely successful in providing adequate glycemic control in diabetic cats with HS, regardless of the insulin type,23 and it is therefore unlikely that any change in insulin type was responsible for the changes in diabetic control observed in the study population.
This study used a convenience sample formed from diabetic cats diagnosed with HS at the RVC Acromegalic Cat Clinic. All owners were offered hypophysectomy for management of their cat's HS, as an alternative to trial enrollment, at the initial examination. It is possible that cats whose owners opted for trial inclusion systematically differed from those who underwent hypophysectomy and that this resulted in selection bias when forming the study population. Treatment with pasireotide LAR was provided at no cost to participating cats, and this financial incentive is likely to have had a major influence on many owners’ decision to partake in the trial. However, it is possible that other factors, such as large pituitary tumor size, might have caused clinicians to less strongly recommend hypophysectomy in certain cats. In support of this theory, a study of cats that underwent hypophysectomy at the same hospital over the same time period revealed them to have a smaller median pituitary height (6.0 mm; range 4.0–10.6 mm) than cats in the current study.1 Pituitary mass volume is unrelated to IGF‐1 concentration in cats with HS.45 However, cats with larger tumors could be predisposed to deterioration and early trial withdrawal because of neurological compromise from their mass.4 Although this potentially could have negatively biased this study's observed response to pasireotide, no cats in the current trial were excluded due to neurological deterioration.
Inclusion of a control group would have helped to support that the observed changes in recruited cats were directly due to pasireotide treatment, rather than another explanatory factor. Inclusion of a control group was considered ethically controversial because cats with HS typically experience persistent signs of poor diabetic control when treated with routine DM management alone.23 The fact that diabetic cats with HS predictably show a poor response to routine diabetic treatment supports that the changes observed in the current trial are most likely to be related to pasireotide treatment. It is also possible that some suspected adverse events recorded in this study were actually unrelated to pasireotide treatment. However, diarrhea was the most common adverse event in this study and is also a common adverse effect of long‐acting STT analogue treatment in people.17, 24 This similarity and the fact that several other adverse effects observed in this trial are also reported in people receiving pasireotide LAR suggest that most adverse effects in this study were caused by pasireotide treatment. Finally, the pharmacokinetics of pasireotide LAR in cats are currently unknown and require future investigation in order to establish the optimum dosing protocol for pasireotide LAR as a treatment for HS in cats.
In conclusion, this study shows pasireotide LAR to be the first drug to show potential as a long‐term management option for diabetic cats with HS, with treated cats showing a significantly decreased IGF‐1, improved insulin sensitivity, and some cats achieving diabetic remission. Diarrhea is a significant adverse effect and further work is necessary to establish the optimum dosing strategy to provide effective diabetic control while limiting adverse effects.
Acknowledgments
We thank Dr. Yu‐Mei Chang for statistical assistance, cats and owners, and staff of the RVC Clinical Investigation Centre. RVC manuscript number CSS_01339.
Conflict of Interest Declaration: H.A. Schmid is an employee of Novartis International AG, which manufactures long‐acting pasireotide (Signifor® LAR) for treatment of hypersomatotropism in people.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This research was performed in The Department of Clinical Science and Services at the Royal Veterinary College.
This study received no grants, but pasireotide LAR was provided free of charge by Novartis International AG.
This research was presented as a poster research abstract at the 2014 ACVIM Forum, Nashville, Tennessee.
Footnotes
Kenny P, Scudder C, Keyte S, et al. Treatment of Feline Hypersomatotropism – efficacy, morbidity and mortality of hypophysectomy [abstract]. J Vet Int Med. 2015; 29(4):1271
Somatuline® Depot, Ipsen Biopharmaceuticals Inc., Basking Ridge, NJ
Sandostatin® LAR®, Novartis International AG, Basel, Switzerland
Timian J and Lunn KF. Evaluation of a long‐acting somatostatin receptor ligand for the treatment of feline acromegaly. 2012 ACVIM Forum Research Abstracts Program. J Vet Int Med 2012; 26(3): 757
Scudder C, Mirczuk SM, Richardson K, et al. Feline hypersomatotropism as a spontaneous animal model of acromegaly: molecular analyses of somatostatin receptor expression in GH‐secreting adenomas in the cat (Felis catus) [abstract]. ENDO2016, Boston, MA
Signifor® LAR, Novartis International AG, Basel, Switzerland
IGF‐1 RIA‐CT, Mediagnost®, Reutlingen, Germany
Alphatrak2®, Abbott Animal Health, Chicago, IL
Signifor®, Novartis International AG, Basel, Switzerland
SPSS™, IBM Corporation, Armonk, New York
GraphPad Prism®, GraphPad Software Inc, California
Caninsulin®, Intervet UK Ltd, Milton Keynes, UK
Hypurin® Bovine PZI, Wockhardt UK Ltd, Wrexham, UK
Lantus®, Sanofi, Paris, France
References
- 1. Schaefer S, Kooistra HS, Riond B, et al. Evaluation of insulin‐like growth factor‐1, total thyroxine, feline pancreas‐specific lipase and urinary corticoid‐to‐creatinine ratio in cats with diabetes mellitus in Switzerland and the Netherlands. J Feline Med Surg 2016. Aug 30. pii: 1098612X16664390. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niessen SJ, Forcada Y, Mantis P, et al. Studying cat (Felis catus) diabetes: Beware of the acromegalic imposter. PLoS ONE 2015;10:e0127794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reusch C. Disorders of Growth Hormone In: Feldman E, Nelson R, Reusch C, Scott‐Moncrieff C, eds. Canine and Feline Endocrinology, 4th ed St. Louis, MO: Elsevier; 2015:37–76. [Google Scholar]
- 4. Niessen SJ, Petrie G, Gaudiano F, et al. Feline acromegaly: An underdiagnosed endocrinopathy? J Vet Intern Med 2007;21:899–905. [DOI] [PubMed] [Google Scholar]
- 5. Laron Z. Insulin‐like growth factor 1 (IGF‐1): A growth hormone. Mol Pathol 2001;54:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Myers JA, Lunn KF, Bright JM. Echocardiographic findings in 11 cats with acromegaly. J Vet Intern Med 2014;28:1235–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peterson ME, Taylor RS, Greco DS, et al. Acromegaly in 14 cats. J Vet Intern Med 1990;4:192–201. [DOI] [PubMed] [Google Scholar]
- 8. Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:3933–3951. jc20142700. [DOI] [PubMed] [Google Scholar]
- 9. Meij BP, Auriemma E, Grinwis G, et al. Successful treatment of acromegaly in a diabetic cat with transsphenoidal hypophysectomy. J Feline Med Surg 2010;12:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shanik MH, Cao PD, Ludlam WH. Historical response rates of somatostatin analogs in the treatment of acromegaly: A systematic review. Endocr Pract 2016;22:350–356. [DOI] [PubMed] [Google Scholar]
- 11. Feelders RA, Hofland LJ, van Aken MO, et al. Medical therapy of acromegaly: Efficacy and safety of somatostatin analogues. Drugs 2009;69:2207–2226. [DOI] [PubMed] [Google Scholar]
- 12. Slingerland LI, Voorhout G, Rijnberk A, et al. Growth hormone excess and the effect of octreotide in cats with diabetes mellitus. Domest Anim Endocrinol 2008;35:352–361. [DOI] [PubMed] [Google Scholar]
- 13. Abraham LA, Helmond SE, Mitten RW, et al. Treatment of an acromegalic cat with the dopamine agonist L‐deprenyl. Aust Vet J 2002;80:479–483. [DOI] [PubMed] [Google Scholar]
- 14. Hofland LJ, Lamberts SW. Somatostatin receptors in pituitary function, diagnosis and therapy. Front Horm Res 2004;32:235–252. [DOI] [PubMed] [Google Scholar]
- 15. Bruns C, Lewis I, Briner U, et al. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 2002;146:707–716. [DOI] [PubMed] [Google Scholar]
- 16. Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999;20:157–198. [DOI] [PubMed] [Google Scholar]
- 17. Gadelha MR, Bronstein MD, Brue T, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): A randomised, phase 3 trial. Lancet Diabetes Endocrinol 2014;2:875–884. [DOI] [PubMed] [Google Scholar]
- 18. Scudder CJ, Gostelow R, Forcada Y, et al. Pasireotide for the medical management of feline hypersomatotropism. J Vet Intern Med 2015;29:1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Church DB, Watson AD, Emslie DR, et al. Effects of proligestone and megestrol on plasma adrenocorticotrophic hormone, insulin and insulin‐like growth factor‐1 concentrations in cats. Res Vet Sci 1994;56:175–178. [DOI] [PubMed] [Google Scholar]
- 20. Lamb CR, Ciasca TC, Mantis P, et al. Computed tomographic signs of acromegaly in 68 diabetic cats with hypersomatotropism. J Feline Med Surg 2014;16:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tyson R, Graham JP, Bermingham E, et al. Dynamic computed tomography of the normal feline hypophysis cerebri (Glandula pituitaria). Vet Radiol Ultrasound 2005;46:33–38. [DOI] [PubMed] [Google Scholar]
- 22. Combes A, Pey P, Paepe D, et al. Ultrasonographic appearance of adrenal glands in healthy and sick cats. J Feline Med Surg 2013;15:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niessen SJ, Church DB, Forcada Y. Hypersomatotropism, acromegaly, and hyperadrenocorticism and feline diabetes mellitus. Vet Clin North Am Small Anim Pract 2013;43:319–350. [DOI] [PubMed] [Google Scholar]
- 24. Colao A, Bronstein MD, Freda P, et al. Pasireotide versus octreotide in acromegaly: A head‐to‐head superiority study. J Clin Endocrinol Metabol 2014;99:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunning MD, Lowrie CS, Bexfield NH, et al. Exogenous insulin treatment after hypofractionated radiotherapy in cats with diabetes mellitus and acromegaly. J Vet Intern Med 2009;23:243–249. [DOI] [PubMed] [Google Scholar]
- 26. Reusch C. Feline Diabetes Mellitus In: Feldman EC, Nelson RW, Reusch C, Scott‐Moncrieff C, eds. Canine and Feline Endocrinology, 4th ed St. Louis, MI: Elsevier Saunders; 2015:258–314. [Google Scholar]
- 27. Slingerland LI, Robben JH, van Haeften TW, et al. Insulin sensitivity and beta‐cell function in healthy cats: Assessment with the use of the hyperglycemic glucose clamp. Horm Metab Res 2007;39:341–346. [DOI] [PubMed] [Google Scholar]
- 28. Meij BP, van der Vlugt‐Meijer RH, van den Ingh TS, et al. Somatotroph and corticotroph pituitary adenoma (double adenoma) in a cat with diabetes mellitus and hyperadrenocorticism. J Comp Pathol 2004;130:209–215. [DOI] [PubMed] [Google Scholar]
- 29. Colao A, Auriemma RS, Pivonello R. The effects of somatostatin analogue therapy on pituitary tumor volume in patients with acromegaly. Pituitary 2016;19:210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Henry RR, Ciaraldi TP, Armstrong D, et al. Hyperglycemia associated with pasireotide: Results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metabol 2013;98:3446–3453. [DOI] [PubMed] [Google Scholar]
- 31. Zambre Y, Ling Z, Chen MC, et al. Inhibition of human pancreatic islet insulin release by receptor‐selective somatostatin analogs directed to somatostatin receptor subtype 5. Biochem Pharmacol 1999;57:1159–1164. [DOI] [PubMed] [Google Scholar]
- 32. Novartis [internet]. Signifor LAR Safety Profile. http://hcp.novartis.com/products/signifor-lar/acromegaly/safety-profile/#adverse-reaction-profile. Accessed: 25th July 2016.
- 33. Petersenn S, Bollerslev J, Arafat AM, et al. Pharmacokinetics, pharmacodynamics, and safety of pasireotide LAR in patients with acromegaly: A randomized, multicenter, open‐label, phase I study. J Clin Pharmacol 2014;54:1308–1317. [DOI] [PubMed] [Google Scholar]
- 34. Dowling RH. Review: Pathogenesis of gallstones. Aliment Pharmacol Ther 2000;14(Suppl 2):39–47. [DOI] [PubMed] [Google Scholar]
- 35. Hussaini SH, Murphy GM, Kennedy C, et al. The role of bile composition and physical chemistry in the pathogenesis of octreotide‐associated gallbladder stones. Gastroenterology 1994;107:1503–1513. [DOI] [PubMed] [Google Scholar]
- 36. Hubina E, Nanzer AM, Hanson MR, et al. Somatostatin analogues stimulate p27 expression and inhibit the MAP kinase pathway in pituitary tumours. Eur J Endocrinol 2006;155:371–379. [DOI] [PubMed] [Google Scholar]
- 37. Theodoropoulou M, Zhang J, Laupheimer S, et al. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3‐kinase signaling and inducing Zac1 expression. Cancer Res 2006;66:1576–1582. [DOI] [PubMed] [Google Scholar]
- 38. Reubi JC, Laissue JA. Multiple actions of somatostatin in neoplastic disease. Trends Pharmacol Sci 1995;16:110–115. [DOI] [PubMed] [Google Scholar]
- 39. Sathyapalan T, Lowry M, Turnbull LW, et al. Mechanism of action of octreotide in acromegalic tumours in vivo using dynamic contrast‐enhanced magnetic resonance imaging. Pituitary 2007;10:233–236. [DOI] [PubMed] [Google Scholar]
- 40. Kurosaki M, Saegert W, Abe T, et al. Expression of vascular endothelial growth factor in growth hormone‐secreting pituitary adenomas: Special reference to the octreotide treatment. Neurol Res 2008;30:518–522. [DOI] [PubMed] [Google Scholar]
- 41. Cozzi R, Montini M, Attanasio R, et al. Primary treatment of acromegaly with octreotide LAR: A long‐term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkage. J Clin Endocrinol Metabol 2006;91:1397–1403. [DOI] [PubMed] [Google Scholar]
- 42. Jaffe CA, Ocampo‐Lim B, Guo W, et al. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest 1998;102:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Freda PU, Katznelson L, van der Lely AJ, et al. Long‐acting somatostatin analog therapy of acromegaly: A meta‐analysis. J Clin Endocrinol Metabol 2005;90:4465–4473. [DOI] [PubMed] [Google Scholar]
- 44. Ma P, Wang Y, van der Hoek J, et al. Pharmacokinetic‐pharmacodynamic comparison of a novel multiligand somatostatin analog, SOM230, with octreotide in patients with acromegaly. Clin Pharmacol Ther 2005;78:69–80. [DOI] [PubMed] [Google Scholar]
- 45. Berg RI, Nelson RW, Feldman EC, et al. Serum insulin‐like growth factor‐I concentration in cats with diabetes mellitus and acromegaly. J Vet Intern Med 2007;21:892–898. [DOI] [PubMed] [Google Scholar]


