Abstract
Background
Spinal walking (SW) is described as the acquisition of an involuntary motor function in paraplegic dogs and cats without pain perception affected by a thoracolumbar lesion. Whereas spinal locomotion is well described in cats that underwent training trials after experimental spinal cord resection, less consistent information is available for dogs.
Hypothesis
Paraplegic dogs affected by a thoracolumbar complete spinal cord lesion undergoing intensive physical rehabilitation could acquire an autonomous SW gait under field conditions.
Animals
Eighty‐one acute paraplegic thoracolumbar dogs without pelvic limb pain perception.
Methods
Retrospective study of medical records of dogs selected for intensive rehabilitation treatment in paraplegic dogs with absence of pain perception on admission and during the whole treatment. Binary regression and multivariate logistic regression were used to analyze potential associations with the development of SW.
Results
Autonomous SW was achieved in 48 dogs (59%). Median time to achieve SW was of 75.5 days (range: 16–350 days). On univariate analysis, SW gait was associated with younger age (P = .002) and early start of physiotherapy (P = .024). Multivariate logistic regression showed that younger age (≤60 months) and lightweight (≤7.8 kg) were positively associated with development of SW (P = .012 and P < .001, respectively). BCS, full‐time hospitalization, and type and site of the lesion were not significantly associated with development of SW.
Conclusions
Dogs with irreversible thoracolumbar lesion undergoing intensive physiotherapic treatment can acquire SW. Younger age and lightweight are positively associated with the development of SW gait.
Keywords: dog, pain perception, paraplegia, physiotherapy
Abbreviations
- 95% CI
95% confidence interval
- ASCI
acute spinal cord injury
- BCS
body condition score
- CPG
central pattern generator
- CT
computed tomography
- IVDE
intervertebral disk extrusion
- MRI
magnetic resonance imaging
- No‐SW
no‐spinal walking
- OR
odds ratio
- ROC
receiver operating characteristic
- ROM
range of motion
- SD
standard deviation
- SW
spinal walking
- TL
thoracolumbar
- UWTM
under water treadmill
Acute spinal cord injuries (ASCI) in dogs are common and mainly result from intervertebral disk extrusion (IVDE) or traumatic injuries.1, 2 Recovery rate from ASCI is variable and depends on the severity of the spinal cord damage, rate of onset of clinical signs, and type of treatment.1, 3, 4 Considering the difficulty of objectively establishing the extent of the spinal cord damage, it is generally accepted to use the absence of pain perception as the most important indirect sign to asses complete functional spinal cord transection.1, 5 Absence of pain perception is classically associated with a severe to poor prognosis for functional recovery of voluntary locomotion.2, 6, 7, 8, 9 A recent study on paraplegic TL dogs with absent pain perception due to IVDE did not identify prognostic factors for any of the investigated variables, including age, corticosteroid administration, early surgical treatment, and severity of compression.10 Few studies described the treatment and the outcome of paraplegic dogs without pelvic limb pain perception,1, 4, 7, 11 and only one assessed the long‐term outcome (>6 months) describing a small percentage of dogs (11%) regaining the ability to walk without pain perception.1
Spinal walking (SW) is the term commonly used to define the acquisition of an involuntary motor function in paraplegic mammals affected by a complete thoracolumbar (TL) spinal cord lesion. SW is a reflex gait, resulting from complex dynamic interactions between the pelvic limb locomotor central pattern generator (CPG) and proprioceptive feedback from the body in the absence of superior control by the brain after complete spinal cord damage.12, 13 CPG is the network of interconnected interneurons in the spinal cord gray matter that modulates motor neuron activity for the generation of gait.5 Lumbar CPG, which displays a rostrocaudal excitability gradient for rhythmogenic capacity, influences the alpha‐motor neurons activity via short propriospinal pathways, located in the fasciculus proprius.5, 13
In experimental trials, spinal locomotion is well described in cats and showed that treadmill training exercise could improve the recovery of autonomous motor function after complete and incomplete spinal cord injury.14, 15, 16, 17, 18, 19, 20 Less consistent information is available for dogs.20, 21, 22, 23
To the authors' knowledge, there is no published information on the effect of physiotherapy in the development of successful spinal walking in dogs under field conditions.
The aim of this study was to evaluate the number of TL paraplegic dogs without pain perception that developed an autonomous SW gait after a period of intensive physical rehabilitation training. Attention was focused on the identification of potential parameters (age, weight, type and site of lesion, duration of clinical signs, hospitalization during physiotherapy, onset of physiotherapy) associated with involuntary pelvic limb motor function recovery.
Materials and Methods
Medical records of paraplegic dogs with TL spinal cord lesion due to acute IVDE or exogenous trauma, referred at the Physiotherapy and Rehabilitation Centre “Dog Fitness” (Reggio Emilia, Italy) between 2005 and 2014, were retrospectively reviewed.
Dogs were included in the study if they had a medical record documenting paraplegia and absent pelvic limb pain perception, after physical and neurological examinations by the referring neurologist and at the admission at the Physiotherapy and Rehabilitation Centre; a spinal cord lesion confirmed by magnetic resonance imaging (MRI) or computed tomography (CT); a complete protocol of physiotherapic rehabilitation treatment; and had absence of pelvic limb pain perception at the end of the physiotherapic rehabilitation treatment.
For the purpose of this study, absence of pain perception was defined as lack of a conscious response (e.g, crying, looking around, or similar reaction) to the application of heavy pressure to the pelvic limb digits with forceps.5 Dogs showing tail wagging in response to the owner's presence or voice were excluded by the study. SW was defined as the ability to develop an unassisted involuntary gait. Specifically, dogs were considered spinal walkers if they could walk for a potentially infinite period of time and, in case of falling, were able to regain unassisted the standing posture and continue to walk.
Clinical records of each dog included the following parameters: signalment, clinical presentation (by Olby scoring system24), type (acute IVDE or traumatic vertebral luxation/fracture) and site of the lesion, presence of surgery (hemilaminectomy in dogs with IVDE or stabilization in dogs with exogenous trauma), type of hospitalization regimen (full‐time or day‐hospital) during the physical rehabilitation and outcome.
A personalized physio therapy protocol was settled based on the specific needs of each dog, including the following five basic categories of exercises, differently arranged: passive range of motion (ROM) exercises, flexor reflex and crossed extensor reflex stimulation, active assisted exercises, electrostimulation (only in case of postspinal shock hypotonia) and hydrotherapy on underwater treadmill (UWTM).
Each physiotherapic treatment lasted approximately 60 minutes and was performed on all dogs every day, twice per day regardless of their status of hospitalization. UWTM was started on admittance or, in case of surgical management, at least 5 days after surgery at the speed of 1.8 km/h. UWTM bouts lasted 5 minutes at the beginning of the treatment and were progressively increased up to 15 minutes when dogs started to show pelvic limbs movements.
The duration of the cycle of physiotherapic treatment was tailored to each single dog depending on the physiotherapist's opinion in terms of deciding whether to continue or stop the treatment. Thus, the duration of the entire physiotherapic treatment was considered either the time elapsed between the start of the rehabilitation and the acquisition of independent spinal walking gait or the period between the start and the discontinuance of physio therapy due to the failure in achieving SW.
All statistical analyses were performed with a commercially available statistical data analysis program (MedCalc® version 12.2.1.0). Assessment of data for normality was calculated by applying the D'Agostino‐Pearson test. Data with normal distribution were expressed as mean and standard deviation (SD), while when normality is rejected, median with 95% confidence interval (95% CI) was used. Values of P < .05 were considered significant. A Wilcoxon signed‐rank test (P < .05) was used to consider the influence of the anatomic localization of the lesion on development of SW.
Dogs were divided into two groups: the spinal walking group (SW group), including dogs that achieved the ability to develop an autonomous unassisted involuntary locomotion, and the no‐spinal walking group (No‐SW group), including dogs that did not develop an autonomous unassisted involuntary locomotion.
In order to detect specific variables significantly associated with the development of SW gait, age, sex, weight, BCS, type of lesion (acute IVDE or trauma), hospitalization during the physical therapy treatment, and time between loss of pain perception and start of physiotherapic treatment were compared between the SW and No‐SW groups by a binary logistic regression. Variables that meet a cutoff of P < .15 at the univariate analysis were entered into a multivariate logistic regression. The odds ratio (OR) and 95% CI were calculated from the final model. A receiver operating characteristic (ROC) curve was used to select the optimum cutoff value of the variables to discriminate dogs with SW gait from dogs with No‐SW gait.
Results
Eighty‐one dogs met the inclusion criteria and were included in the study. Figure 1 describes the process of selection of the cases. Descriptive data of the general population are detailed in Table 1. Table 2 reports the descriptive data of the SW and No‐SW groups.
Figure 1.
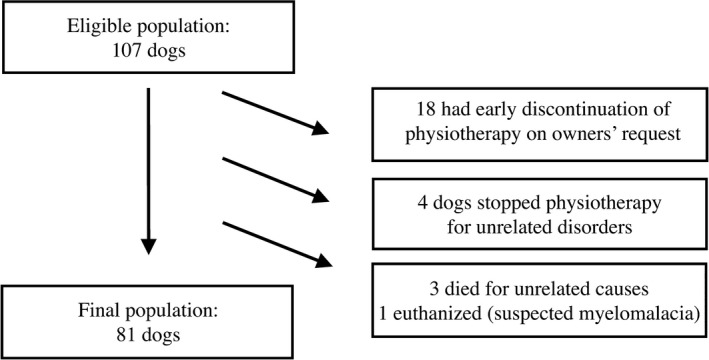
Selection of the dogs' population.
Table 1.
Data of the general population
| Dogs | 81 |
| Breeds (most represented) | Mixed breed (n = 29; 36%), Dachshund (n = 16; 25%), Miniature poodle (n = 6; 7%), Yorkshire Terrier (n = 5; 6%), Cocker Spaniel (n = 5; 6%) |
| Age | m: 60 months (range: 8–144; 95% CI: 48–66) |
| Sex | Male: 41 (50.6%) (C: 4; 3.7%) |
| Female: 30 (37%) (N: 7; 8.6%) | |
| Weight | m 8 kg (range: 2.3–29.6; 95% CI: 8–9.79) |
| ≤10 kg: 49; >10 kg: 32 | |
| BCS | m: 5 (range: 3–8; 95% CI: 5–5) |
| Dogs with IVDE | 54 (66%) |
| Dogs with traumatic injuries | 27 (34%) |
| Dogs with full time hospitalized | 65 (80%) |
| Delay in onset of physiotherapya | m: 12 days (range: 2–1260; 95% CI: 8.21–19.38) |
| Duration of physiotherapy | m: 74 days (range 10–370; 95% CI: 60.41–81.79) |
Days between the onset of paraplegia and the beginning of physical therapy.
m, median; BCS, body condition score; C, castrated, N, neutered.
Table 2.
Spinal Walking (SW) and No‐Spinal Walking (No‐SW) groups
| Spinal Walking (SW) Group | No‐spinal Walking (No‐SW) Group | |
|---|---|---|
| Dogs | 48 (59%) | 33 (41%) |
| Breed (most represented) | Mixed breed (n = 17; 35%), Dachshund (n = 8; 17%), Miniature poodle (n = 5; 10%) | Mixed breed (n = 13; 39%), Dachshund (n = 8; 24%), Beagle (n = 2; 6%) |
| Age | m: 54 months (95% CI: 48–60) | m: 72 months (95% CI: 52.93–84) |
| Sex | Males: 23 (C: 2) | Males: 21 (C: 1) |
| Females: 25 (N: 5) | Females: 12 (N: 2) | |
| Weight | Range: 7–29.6 kg | |
| m 7.3 kg (95% CI: 7–8.26) | m 10 kg (95% CI: 8.24–12.59) | |
| ≤10 kg: 33; >10 kg: 15 | ≤10 kg: 16; >10 kg: 17 | |
| BCS | m: 5 (95% CI: 5–5) | m: 5 (95% CI: 5–5) |
| Dogs affected by IVDE | 34 (71%) | 20 (61%) |
| Surgical management | 27 (56%) | 20 (61%) |
| Medical management | 7 (15%) | 0 |
| Traumatic injuries | 14 (29%) | 13 (39%) |
| Surgical management | 4 (8%) | 10 (30%) |
| Medical management | 10 (21%) | 3 (9%) |
| Hospitalization | 39 (81%) | 26 (79%) |
| Delay in onset of physiotherapya | m: 9.5 days (95% CI: 6–12.77) | m: 23 days (95% CI: 11.41–60) |
| Duration of physiotherapy | m: 75.5 days (95% CI: 58–94.84) | m: 69 (95% CI: 46.64–92.95) |
Days between neurological deficit onset and the beginning of physical therapy.
m, median; BCS, body condition score; C, castrated, N, neutered.
SW Group
At the end of the cycle of physiotherapic treatment, 48 dogs (59%) developed an autonomous spinal locomotion. Of the 48 SW dogs, 31 (64%) underwent surgery. The spinal cord lesion was situated between Th4‐Th5 and L2‐L3. The most frequently affected site (20 dogs) was at the level of Th12‐Th13 (Fig 2).
Figure 2.
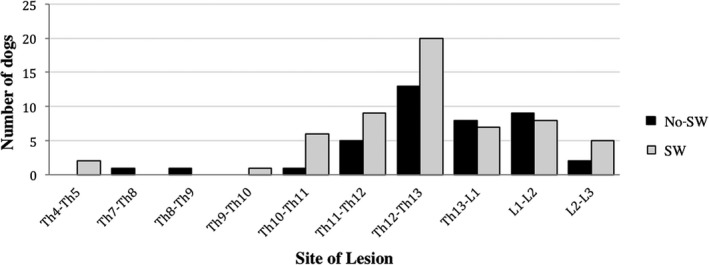
Distribution of different site of lesion in SW and No‐SW dogs. SW: Spinal walking group; No‐SW: group of dogs that did not achieve spinal walking. Figure shows that the most frequently site of lesion was at the level of Th12‐Th13 in both groups (20 and 13 dogs in SW and No‐SW dogs, respectively). No significant association was found between the site of lesion and the development of SW (P = .93). Nine dogs of “SW group” had multiple sites of lesions; 6 dogs of “No‐SW group” had multiple sites of lesion.
Median time between loss of pain perception and the beginning of physical therapy was 9.5 days (range: 2–210), and the median duration of physiotherapic treatment was 75.5 days (range: 16–350). Thirty‐nine dogs (81%) had full‐time hospitalization.
The median duration between the start of physiotherapic treatment and the ability to stand up unassisted, recorded in 29 dogs, was 20 days (range 2–150).
No‐SW Group
At the end of the cycle of physiotherapic treatment, 33 dogs (41%) did not develop an autonomous spinal locomotion. Of the 33 No‐SW dogs, 30 (91%) underwent surgery. The spinal cord lesion was situated between Th7‐Th8 and L2‐L3. The most frequently affected site (13 dogs) was at the level of Th12‐Th13 (Fig 2).
Median time between loss of pain perception and the beginning of physio therapy was 23 days (range: 2–1260), and the median duration of physiotherapic treatment was 69 days (range: 10–370). 26 No‐SW dogs (79%) had full‐time hospitalization.
On univariate analysis, comparison between the SW and the No‐SW groups showed that age was significantly lower in SW group (P = .002; OR = 4.14; 95% CI: 1.61–10.66). Early start of physiotherapy was positively associated with achievement of SW gait (P = .024; OR = 2.81; 95% CI: 1.12–7.01).
Weight (P = .089), BCS (P = .051), full‐time hospitalization (P = .78), type (P = .33), and site of the lesion (P = .93) were not significantly associated with development of SW.
By a multivariate logistic regression, younger age and lower weight were significantly associated with development of SW (P = .012 OR = 5.66; 95% CI: 1.69–18.94 and P < .001, OR = 5.09; 95% CI: 1.54–16.78, respectively). ROC curve showed that dogs aging ≤60 months and dogs weighting ≤7.8 kg had higher possibilities to achieve SW (95% CI: 0.53–0.75; sensitivity: 72.9%; specificity: 60.6% and 95% CI: 0.63–0.83; sensitivity: 54.2%; specificity: 93.9%, respectively) (Figs 3 and 4).
Figure 3.
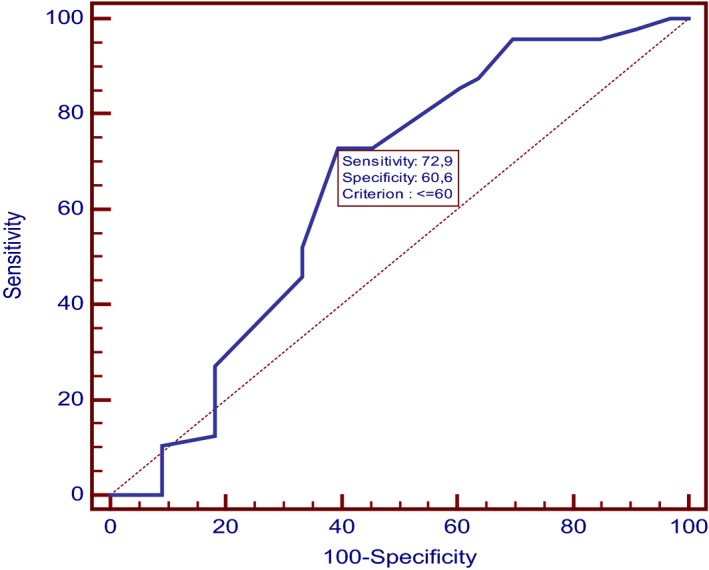
ROC curve of age. Figure shows the receiver operating characteristic (ROC) curve used to select the optimum cutoff value of age to discriminate dogs with SW gait from dogs with No‐SW gait. It shows that dogs aging equal or less than 60 months had higher possibilities to achieve SW, with a sensitivity of 72.9% and a specificity of 60.6%. (95% CI: 0.53–0.75).
Figure 4.
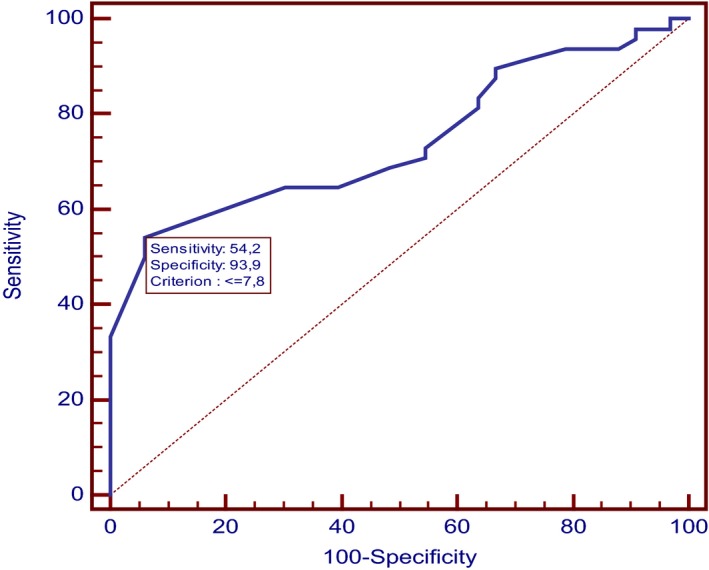
ROC curve of weight. Figure shows the receiver operating characteristic (ROC) curve used to select the optimum cutoff value of weight to discriminate dogs with SW gait from dogs with No‐SW gait. It shows that dogs weighting equal or less than 7.8 kg had higher possibilities to achieve SW, with a sensitivity of 54.2% and a specificity of 93.9%. (95% CI: 0.63–0.83).
Discussion
In veterinary literature, SW is rarely described in dogs4, 20, 21, 22, 23 and most of studies report SW gait development in training cats experimentally spinalized.14, 15, 16, 19, 20
This study shows that paraplegic dogs with absent pain perception undergoing intensive physiotherapic treatment have good chance (59% in our population) to develop an unassisted involuntary gait. Our study found that younger age (≤60 months on ROC curve analysis) was positively associated with development of SW. Literature does not provide comparable data, except for experimental studies. In the eighties, it was demonstrated that adult dogs could develop SW gait after experimental mid‐thoracic spinal cord transection, disputing the earlier statements that functional recovery of locomotion in TL spinalized animals depended upon their very young age at the time of transection.22 A recent study on the outcome of paraplegic dogs without pain perception did not find any significant association with age.10 In another study, younger age was associated with faster rather than better recovery of the motor function.1
Our analysis showed that lightweight dogs (≤7.8 kg on ROC curve analysis) have a significantly better possibility of developing SW. Despite the empiric belief that lightweight dogs with acute spinal cord lesion have a better outcome, literature does not provide specific information concerning the association between weight and outcome, including development of SW. It is worth mentioning that one 26 kg dog with IVDE developed SW after appropriate physio therapy, indicating that even larger dogs, occasionally, can achieve SW gait.
Experimental studies showed that the functional experience obtained in cats by step training (on a treadmill at the greatest speeds possible) facilitated or reinforced the capacity of the adult lumbar spinal cord to generate full weight‐bearing stepping after the elimination of supraspinal connectivity.15 The experimental results of different authors suggest that the recovery of motor function in cats seems to be improved by the level and types of exposure of assisted training after the injury.14, 15, 18, 19 Consequently, exercise is considered to play an important beneficial role in promoting motor recovery after ASCI.19 While confirmed on univariate analysis, our multivariate analysis failed to confirm the association between early start of physiotherapy and development of SW. Nevertheless, the high percentage of dogs developing SW suggests that, also under field conditions, specific motor training could play an important role in facilitating the CPG in the development an involuntary motor function. Ethical reasons and the retrospective nature of our study did not allow us having a control group of dogs, thus preventing the possibility to demonstrate the real influence of physiotherapy. In a study that assessed the long‐term outcome of paraplegic dogs without pelvic limb pain perception,1 the small number of dogs (n = 7) which developed a SW gait took longer time when compared to our SW group. Possible explanations include the lack of clearly mentioned physiotherapic treatment. Further studies should be encouraged to demonstrate our belief that physiotherapy could play a crucial role in the development of SW.
In our study, the site of the lesion was not significantly associated with the outcome. In the seventies, the results of a study influenced the general opinion for many years, stating that SW could develop only if the lesion was not cranial to the 13th thoracic vertebra, to avoid the atrophy of paravertebral muscles.21 Further experimental study contradicted these results, showing that 7 of 9 dogs with a complete transection of the T9‐T10th spinal cord segment developed SW gait.22 Our results support these latter experimental findings also under field conditions. Unexpectedly, five dogs with spinal cord lesions at the presumed level of the CPG (L2‐L3) developed SW gait. Unfortunately, their number is too small to draw suitable conclusions.
Future studies are needed to appropriately investigate some specific physiotherapic parameters, which may be relevant in facilitating the achievement of SW gait. Authors believe that UWTM plays an important role in facilitating paraplegic dogs to start reflex pelvic limbs movements and, consequently, develop SW. In our clinical experience, almost all dogs undergoing UWTM physiotherapy develop pelvic limbs movements several days before than in the normal treadmill. Nevertheless, specific studies are necessary to compare physiotherapic treatment protocols with or without UWTM, to confirm the anectodal statement that rehabilitation centers using UWTM have increased development of SW. In addition, cage restriction may play an important role in order to avoid dogs learning wrong behavior, such as freely dragging on their pelvic limbs, which could affect the development of correct involuntary limbs movements.
Besides the lack of a control group, our study has several limitations. Authors are aware that multiple analyses of small datasets are liable to false discovery and the conclusions require suitable cautions because they could not fit to other datasets. The retrospective nature of the study did not prevent the possibility to have bias in the case selection, including the relative paucity of large breed dogs and the lower number of cases with lumbar injuries. Furthermore, inclusion criteria excluded dogs that developed myelomalacia, possibly affecting the percentage of dogs which gained SW. Finally, physiotherapic treatment of the No‐SW group was stopped according to the subjective physiotherapist's judgment on the lack of response.
In conclusion, our study demonstrates that a percentage of paraplegic dogs without pain perception undergoing a cycle of intensive physio therapy treatment can acquire SW gait.
Veterinarians and owners, when facing a catastrophic event such as an ASCI leading to paraplegia without pain perception, should be aware of the possibility that their pets can develop SW gait and encouraged to act consequently. Further studies are necessary to objectively assess the role of intensive physiotherapy in the acquisition of SW gait in paraplegic dogs without pain perception.
Acknowledgments
The practical work (neurological assessment and physiotherapy) was made at the “Dog Fitness” Veterinary Physiotherapy and Rehabilitation Centre, Reggio Emilia, Italy; processing and analysis of the data were made at the Department of Veterinary Medical Sciences, University of Bologna, Italy. Authors thank all clinicians and physiotherapist who contributed to clinical management of dogs enrolled in the study.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Fundings: No grant support.
The results of this study were presented at the 28th ECVN‐ESVN Symposium, Amsterdam, the Netherlands, September 18–19, 2015.
References
- 1. Olby NJ, Levine J, Harris T, et al. Long‐term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001). J Am Vet Med Assoc 2003;222:762–769. [DOI] [PubMed] [Google Scholar]
- 2. Brisson BA. Intervertebral disc disease in dogs. Vet Clin Small Anim 2010;40:829–858. [DOI] [PubMed] [Google Scholar]
- 3. Ferreira AJA, Correia JHD, Jaggy A. Thoracolumbar disc disease in 71 paraplegic dogs: Influence of rate of onset and duration of clinical signs on treatment results. J Small Anim Pract 2002;43:158–163. [DOI] [PubMed] [Google Scholar]
- 4. Butterworth SJ, Denny HR. Follow‐up study of 100 cases with thoracolumbar disc protrusions treated by lateral fenestration. J Small Anim Pract 1991;32:443–447. [Google Scholar]
- 5. De Lahunta A. General sensory system: General proprioception and general somatic afferent In: De Lahunta A., Glass E., Kent M., ed. Veterinary Neuroanatomy and Clinical Neurology, 4th ed. Philadelphia: Saunders Elsevier; 2015;237–256 [Google Scholar]
- 6. Duval J, Dewey C, Roberts R, Aron D. Spinal cord swelling as a myelographic indicator of prognosis: A retrospective study in dogs with intervertebral disc disease and loss of deep pain perception. Vet Surg 1996;25:6–12. [DOI] [PubMed] [Google Scholar]
- 7. Scott HW. Hemilaminectomy for the treatment of thoracolumbar disc disease in the dog: A follow‐up study of 40 cases. J Small Anim Pract 1997;38:488–494. [DOI] [PubMed] [Google Scholar]
- 8. Cudia SP, Duval JM. Thoracolumbar intervertebral disk disease in large, nonchondrodystrophic dogs: A retrospective study. J Am Anim Hosp Assoc 1997;33:456–460. [DOI] [PubMed] [Google Scholar]
- 9. Ruddle TL, Allen DA, Schertel ER, et al. Outcome and prognostic factors in non‐ambulatory Hansen type I intervertebral disc extrusions: 308 cases. Vet Comp Orthop Traumatol 2006;19:29–34. [PubMed] [Google Scholar]
- 10. Jeffery ND, Barker AK, Hu HZ, et al. Factors associated with recovery from paraplegia in dogs with loss of pain perception in the pelvic limbs following intervertebral disk herniation. J Am Vet Med Assoc 2016;248:386–394. [DOI] [PubMed] [Google Scholar]
- 11. Scott HW, McKee WM. Laminectomy for 34 dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception. J Small Anim Pract 1999;40:417–422. [DOI] [PubMed] [Google Scholar]
- 12. Edgerton VR, Tillakaratne NJK, Bigbee AJ, et al. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 2004;27:145–167. [DOI] [PubMed] [Google Scholar]
- 13. Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 2006;29:279–306. [DOI] [PubMed] [Google Scholar]
- 14. Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full‐weight‐ bearing stepping in the adult spinal cat. Exp Neurol 1986;92:421–435. [DOI] [PubMed] [Google Scholar]
- 15. De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight‐bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol 1998;80:83–91. [DOI] [PubMed] [Google Scholar]
- 16. De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J Neurophysiol 1999;81:85–94. [DOI] [PubMed] [Google Scholar]
- 17. Edgerton VR, de Leon RD, Harkema SJ, et al. Retraining the injured spinal cord. J Physiol 2001;533:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyce VS, Tumolo M, Fischer I, et al. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J Neurophysiol 2007;98:1988–1996. [DOI] [PubMed] [Google Scholar]
- 19. Battistuzzo CR, Callister RJ, Callister R, Galea MP. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma 2012;29:1600–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sherrington CS. Flexion‐reflex of the limb, crossed extension‐reflex, and reflex stepping and standing. J Physiol 1910;40:28–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blauch B. Spinal reflex walking in the dog. Vet Med Small Anim Clin 1977;72:169–173. [PubMed] [Google Scholar]
- 22. Handa Y, Naito A, Watanabe S, et al. Functional recovery of locomotive behavior in the adult spinal dog. Tohoku J Exp Med 1986;148:373–384. [DOI] [PubMed] [Google Scholar]
- 23. Naito A, Shimizu Y, Handa Y. Analyses of airstepping movement in adult spinal dogs. Tohoku J Exp Med 1990;162:41–48. [DOI] [PubMed] [Google Scholar]
- 24. Olby NJ, De Risio L, Muñana KR, et al. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res 2001;62:1624–1628. [DOI] [PubMed] [Google Scholar]


