Abstract
Background
Diagnosis of protein loss into the gastrointestinal tract using noninvasive techniques is challenging. In people, scintigraphy not only is a sensitive tool to confirm protein‐losing enteropathy (PLE), but it also allows for localization of protein loss.
Hypothesis/Objectives
To investigate the feasibility of 99mTc‐labeled human serum albumin (HSA) scintigraphy in dogs with PLE in comparison with control dogs.
Animals
A total of 8 clinically healthy control research dogs and 7 client‐owned dogs with gastrointestinal clinical signs and hypoalbuminemia (serum albumin concentration <2.0 g/dL).
Methods
Prospective case–control study. After IV injection of 400 MBq freshly prepared 99mTc HSA (30 mg/dog), images of the abdomen were obtained 10, 60, 120, and 240 minutes postinjection. Additional images of the salivary and thyroid glands were obtained to rule out free 99mTc. A scan was considered positive for PLE when radiopharmaceutical exudation was detectable in the intestinal tract.
Results
Only 1 control dog showed exudation of the radiopharmaceutical into the intestinal tract. No free 99mTc was detected in any dog. In dogs with PLE, focal small intestinal and diffuse small intestinal radiopharmaceutical exudation into the bowel was detected in 2 and 3 dogs, respectively, whereas in 2 dogs, there was disagreement about whether radiopharmaceutical exudation was focal or diffuse.
Conclusion and Clinical Importance
99mTc‐labeled HSA scintigraphy was feasible to diagnose PLE in dogs.
Keywords: Enteral protein loss, Chronic enteric disease, Canine, Diagnostic imaging, Nuclear medicine
Abbreviations
- α‐1 PI
α1‐proteinase inhibitor
- CCECAI
canine chronic enteropathy clinical activity index
- HSA
human serum albumin
- MBq
megabecquerel
- PLE
protein‐losing enteropathy
- Tc
technetium
- WSAVA
World Small Animal Veterinary Association
Protein‐losing enteropathy (PLE) refers to a syndrome in which a gastrointestinal disease causes excessive nonselective protein loss into the intestinal tract, resulting in hypoproteinemia or hypoalbuminemia. In dogs, it is most commonly associated with chronic inflammatory enteropathy, lymphangiectasia, and intestinal lymphoma.1 Breed‐associated PLE has been reported for the Lundehund, Soft Coated Wheaten Terrier and Basenji.2, 3, 4 Vomiting, diarrhea, and weight loss are common clinical signs. Respiratory distress (due to pleural effusion), ascites and peripheral edema, seizures due to hypocalcemia, thromboembolic disease, as well as polyuria and polydipsia also may be present.1, 5, 6, 7, 8, 9, 10 Further evaluation is based on ultrasonographic examination of the abdomen and histopathologic analysis of intestinal biopsy samples collected during endoscopy or exploratory laparotomy to determine the underlying disease process.
Diagnosis of PLE is cumbersome because fecal samples must be shipped frozen to the laboratory for measurement of fecal α1‐proteinase inhibitor (α1‐PI) or technically demanding tests such as 51Cr‐albumin excretion must be conducted.11, 12 Fecal α1‐PI is synthesized in the liver and has a molecular mass similar to that of albumin (approximately 50,000 Daltons). Thus, when intestinal loss of albumin occurs, α1‐PI is lost as well, but not hydrolyzed by digestive or bacterial proteinases in the intestinal lumen. Therefore, fecal α1‐PI clearance or concentration can be used as an estimate of intestinal protein loss. There is no significant correlation between fecal α1‐PI and serum albumin concentration.13 Evaluation of fecal α1‐antitrypsin clearance and 99mTc‐HSA scintigraphy in PLE in people determined that scintigraphy had higher sensitivity (100 versus 46%) and negative predictive value (100 versus 63%) compared to fecal α1‐antitrypsin clearance.14 The latter test is time intensive due to the prolonged physical half‐life and therefore extended hospitalization. Finally, a positive laboratory test result cannot distinguish between focal and generalized protein loss.
Few reports have described the imaging findings in dogs with PLE. The intestinal hyperechoic mucosal striations seen ultrasonographically in a series of 23 dogs were associated histologically with lacteal dilatation in 96% and clinically with PLE in 78% of the dogs.15 A pilot study using Doppler examinations for pattern recognition and feature extraction of celiac and cranial mesenteric arterial waveforms was performed in 11 dogs with chronic enteropathy. It detected a lack of increase in mean diastolic flow, abnormal arterial waveform shapes, and suboptimal increases in diastolic blood flow during digestion.16
The first successful scintigraphic examination of human patients with PLE was performed in 1986.17 Radiopharmaceuticals bound to a protein accumulate in the gastrointestinal tract because protein loss occurs into the digestive tract resulting in increased radioactivity in the intestinal lumen on scintigraphic imaging. In 1997, a scintigraphic evaluation of 4 dogs with PLE was performed using 111indium‐labeled transferrin.18 Although it appeared to be a feasible method for evaluating dogs with suspected PLE, costs and prolonged isolation of the dogs due to the long physical half‐life of 111In were considerable limitations. 99mTc‐labeled human serum albumin (HSA) scans are recommended as screening test for PLE in human patients and are considered safe in children.19, 20 Furthermore, differentiation between focal and diffuse intestinal protein loss is possible.21 99mTc‐HSA or 111In‐labeled protein scans are highly sensitive in diagnosing and localizing enteral protein loss (90.1 versus 81.1%) in people22 with radiopharmaceutical exudation visible in the gastrointestinal tract in human patients with PLE.
The aim of our study was to assess the feasibility of 99mTc‐labeled HSA scintigraphy to detect protein loss into the gastrointestinal tract of clinically healthy dogs in comparison with dogs with PLE.
Materials and Methods
The study was approved by the state ethics and welfare committee of Hessia (number 63/2011). Owner consent allowing for sedation and anesthesia during scintigraphy, followed by endoscopy or surgery, was obtained before inclusion into the study.
Clinically healthy, clinic‐owned beagle dogs were used as controls, based on normal physical examination and CBC, serum biochemistry profile, urinalysis and urine protein/creatinine ratio, pre‐ and postprandial bile acid concentrations, and ACTH stimulation test as well as unremarkable abdominal ultrasonographic examination and deworming treatment 1 week before scintigraphy.
From October 2011 to December 2013, client‐owned dogs with PLE were prospectively enrolled. To meet the inclusion criteria, PLE had to be diagnosed based on the presence of clinical signs (eg, weight loss, chronic diarrhea >3 weeks, edema, ascites) and hypoalbuminemia (serum albumin concentration <2.0 g/dL). All dogs had the following tests performed to assess the cause of the hypoalbuminemia: canine chronic enteropathy clinical activity index (CCECAI) scores,23 CBC, serum biochemistry profile, urinalysis and urine protein/creatinine ratio, pre‐ and postprandial bile acids, ACTH stimulation test, thromboelastography, serum cobalamin concentration, canine‐specific pancreatic lipase concentration, fecal examination (flotation, sedimentation), and abdominal ultrasonography. Anti‐inflammatory and immunosuppressive medication had to be discontinued for at least 1 month before inclusion.
Scintigraphy
To perform abdominal scintigraphy, 400 megabecquerel (MBq) 99mTc‐HSA1 was prepared according to the manufacturer's instructions and incubated for 30 minutes at room temperature and 1 vial (30 mg HSA) was injected IV via the cephalic vein to avoid interference with the abdominal scan. Ventral and right lateral images were obtained 10, 60, 120, and 240 minutes after injection of the radiopharmaceutical. The dogs were either lightly sedated using diazepam or heavily sedated using diazepam and propofol to effect. Static images were acquired for a total of 5 minutes at each time point. Images were obtained with a large‐field‐of‐view gamma camera2 fitted with a low‐energy all‐purpose collimator, integrating a work station equipped with dedicated software3. A 256 × 256 × 16 matrix was used. The possibility of in vivo breakdown of the radiopharmaceutical and false‐positive localization of free pertechnetate was monitored by visualizing the stomach during all studies and by visualization of salivary and thyroid glands 240 minutes after injection. All scans were evaluated independently by 2 diplomates in veterinary diagnostic imaging (NO, KvP) experienced with reading scintigraphic images with regard to the presence of intestinal radiopharmaceutical exudation, 1 of them blinded regarding the status of the dogs. Location of exudation was defined as small intestinal if seen ventrally and right cranially or large intestinal if seen longitudinal dorsally and along the left lateral abdominal wall. The intensity of the radiopharmaceutical exudation was graded visually and compared to liver activity, as follows: 1 = mild exudation (less than liver); 2 = moderate exudation (equal to liver); 3 = marked exudation (more than liver). After scintigraphy, intestinal tissue samples were obtained either via gastroduodenoscopy and ileocolonoscopy (all control dogs and 4 dogs with PLE) or surgically (3 dogs with PLE) by full thickness biopsies. All histopathologic examinations were performed by 1 board‐certified veterinary pathologist according to the World Small Animal Veterinary Association (WSAVA) recommendations.24
Results
Eight healthy beagle dogs were included; they ranged in age from 1.8 to 3.5 years (median, 2.4 years), 6 were female and 2 were male, and all were castrated or spayed.
Seven dogs with PLE were included. Mild‐to‐moderate ascites was present in 4 dogs with PLE. Urine protein/creatinine ratio and pre‐ and postprandial serum bile acid results were within the reference range in all dogs. The ACTH stimulation test result was diagnostic for atypical hypoadrenocorticism in 1 dog. Canine‐specific pancreatic lipase concentration was high in 3 dogs (range, 382–434 μg/L; reference range, <200 μg/L). Fecal examination was positive for Giardia sp. (coproantigen ELISA) in 2 dogs and for Trichuris vulpis (fecal flotation) in 1 dog. All dogs with PLE were in a hypercoagulable state as detected by thromboelastography. Hypocobalaminemia (range, 96–281 pg/mL; reference range, 300–800 pg/mL) was found in 4 dogs. Abdominal ultrasonographic examination detected ascites in 4 dogs and hyperechoic mucosal striations in 3 dogs. In 1 dog, focal loss of wall layering was detected in the jejunum.
In the healthy control dogs, all scans detected normal radiopharmaceutical accumulation in the liver, spleen, kidneys, urinary bladder, and large vessels. No radiopharmaceutical accumulation was observed in the stomach, salivary glands, thyroid glands, and the intestinal tract in all but 1 control dog (Fig 1). Moderate radiopharmaceutical exudation in the small intestine was recognized by the diplomate blinded to the status of the dogs, but not by the other diplomate. Moderate lymphoplasmacytic mucosal infiltration of the duodenum and ileum was present in 2 control dogs.
Figure 1.
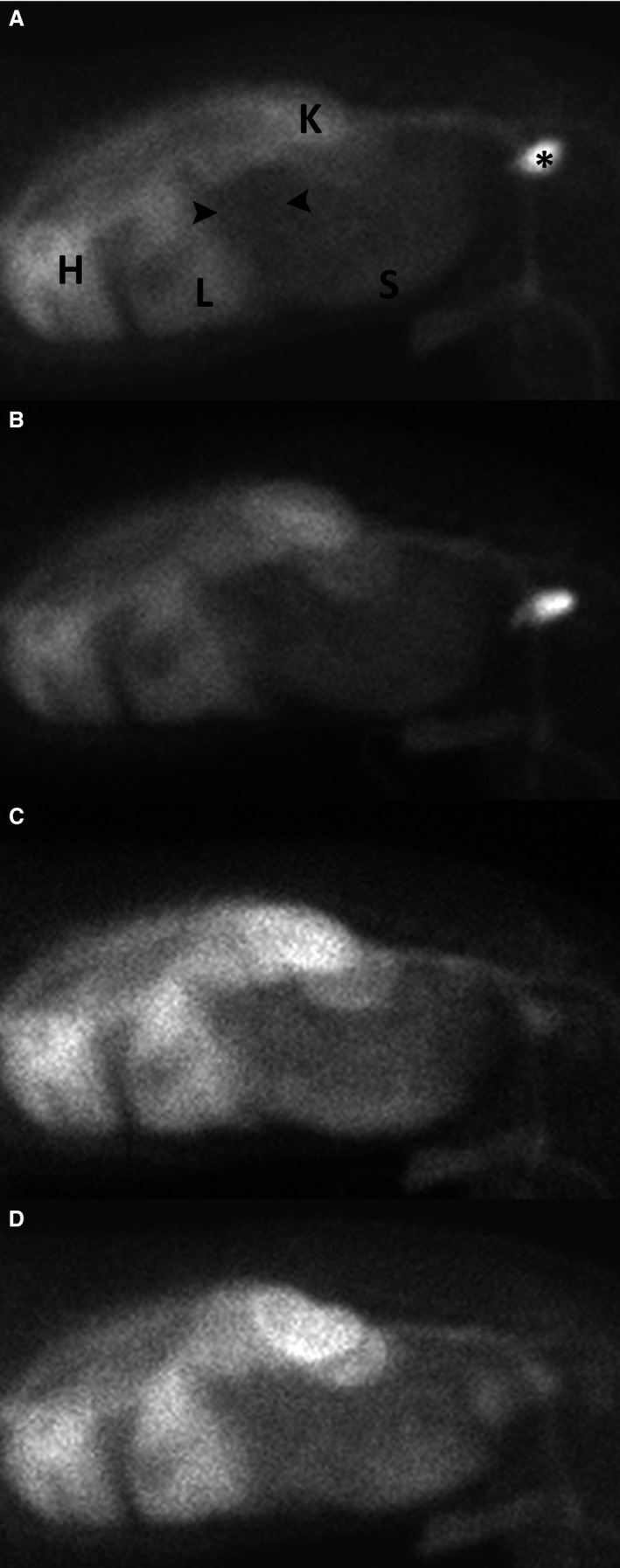
Right lateral static scintigrams of a healthy control dog 10 minutes (A), 1 hour (B), 2 hours (C), and 4 hours (D) after intravenous injection of 99mTc‐HSA. Radiopharmaceutical activity is noted within the heart (H) and major blood vessels, kidneys (K), liver (L), and spleen. Note the void of activity within the liver compatible with the gallbladder and caudal to the liver (black arrowheads) compatible with the stomach. HSA, human serum albumin.
Both diplomates detected radiopharmaceutical exudation in the intestinal tract in all dogs with PLE in addition to normal radiopharmaceutical accumulation in the liver, spleen, kidneys, urinary bladder, and large vessels. No free pertechnetate accumulation was present in stomach, salivary glands, and thyroid gland in dogs with PLE. Radiopharmaceutical exudation was diffuse small intestinal (Fig 2), focal small intestinal or large intestinal (Fig 3) and could be seen as soon as 10 minutes postinjection. The best time points to visualize radiopharmaceutical exudation varied among individual dogs and were at 10 minute (n = 1), 60 minute (n = 1), 120 minute (n = 1), 240 minute (n = 3), and at all time points equally (n = 1). There was agreement regarding diffuse versus localized and small versus large intestinal radiopharmaceutical exudation between both diplomates in all but 2 dogs with PLE. Mild, moderate, and marked exudation was seen in the intestinal tract in 2, 2, and 3 dogs, respectively. Full thickness biopsy specimens with subsequent histologic examination were surgically obtained in 3 dogs with PLE, including 2 dogs with focal tracer exudation in which a partial enterectomy also was performed. Partial enterectomy was performed in 1 dog 24 hours after scintigraphy with intraoperative tracer measurement at the site of highest accumulation and in the other dog based on palpation of the intestinal tract.
Figure 2.
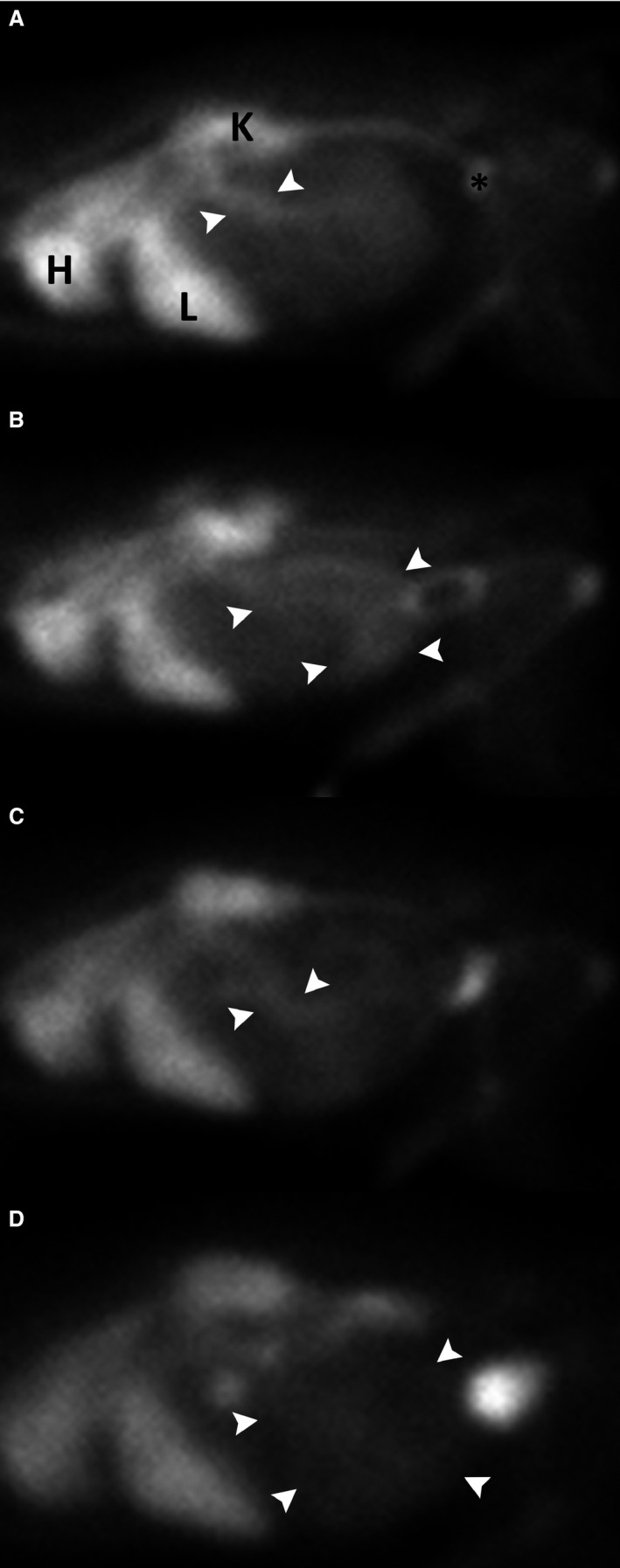
Right lateral static scintigrams of a dog (Papillon, male, 11 years old) with diffuse small intestinal radiopharmaceutical exudation 10 minutes (A), 1 hour (B), 2 hours (C), and 4 hours (D) after intravenous injection of 99mTc‐HSA. Radiopharmaceutical activity is noted within the heart (H) and major blood vessels, kidneys (K), liver (L), and spleen. The level of intestinal activity (white arrowheads) is increased relative to the heart, liver, and kidneys. Views a and c suggest focally increased radiopharmaceutical exudation, whereas on views b and d radiopharmaceutical loss is evident throughout the intestinal tract which underlines the importance of subsequent monitoring of radiopharmaceutical activity over time in order to differentiate between focal and diffuse losses, equivalent to surgical versus nonsurgical candidates. Note the variable size and position of the activity void caudal to the liver compatible with the stomach. HSA, human serum albumin.
Figure 3.
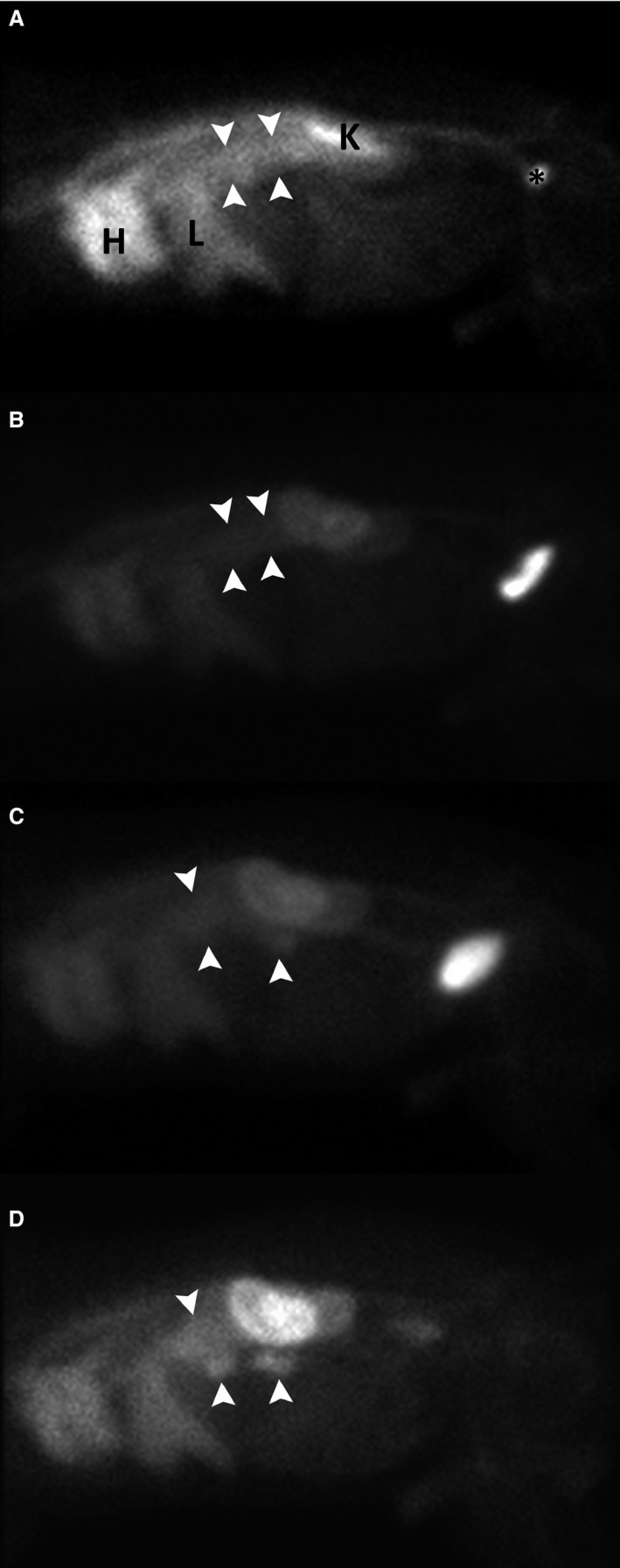
Right lateral static scintigrams of a dog (mixed breed, female spayed, 11 years old) with focal small intestinal radiopharmaceutical exudation 10 minutes (A), 1 hour (B), 2 hours (C), and 4 hours (D) after intravenous injection of 99mTc‐HSA. Two focal radiopharmaceutical exudations (white arrowheads) are evident on all views, but the focally increased intestinal activity level increases relative to the activity within the liver and heart over time and is best appreciated in D (4 hours). HSA, human serum albumin.
Discussion
The results of our study suggest that 99mTc‐HSA scintigraphy may be a useful method to diagnose protein loss into the gastrointestinal tract in dogs with hypoalbuminemia. Each dog with hypoalbuminemia showed radiopharmaceutical exudation into the intestinal tract, whereas only 1 control dog was positive; no free pertechnetate was detected. We could not only confirm enteric protein loss in all dogs with hypoalbuminemia, but also localize the site of protein loss within the gastrointestinal tract. Determination of the site of protein loss influenced our further procedures and treatment substantially: in the 2 dogs with localized radiopharmaceutical exudation, subsequent partial enterectomy was proposed, a combination of procedures already established in human medicine.25 Both dogs experienced a rapid increase in serum albumin concentration after surgery with the sole treatment comprising postoperative antibiotics and pain medications, and continuous use of a hypoallergenic diet. In the remaining 5 dogs, the site of protein loss could be limited to either small or large intestine. The dog with diffuse radiopharmaceutical exudation into the large intestine was diagnosed with marked T. vulpis infection. Treatment with fenbendazole (50 mg/kg once daily) on 3 consecutive days resolved clinical signs and hypoalbuminemia. The possibility of false‐positive results caused by intestinal bleeding was considered unlikely based on the absence of anemia, melena, and hematochezia; testing for occult blood was not performed.
Subsequent monitoring of radiopharmaceutical exudation over time is essential to define the site of protein loss because bowel transit of excreted radiopharmaceutical may be confounding. This applies not only to identification of the site of protein loss in localized radiopharmaceutical exudation but also to the differentiation between localized and diffuse radiopharmaceutical loss. The 2 discrepant results were caused by variation in assessment of a diffuse or localized problem. Diffuse protein loss may go unnoticed and be mistaken for focal disease without progressive monitoring as shown in Figure 2. Moreover, the variable position and size of the spleen may be confounding when not monitored subsequently.
Accumulation of 99mTc‐HSA in inflamed intestines so far has not been reported. To decrease the possibility of false‐positive results caused by transit of activity rather than a second site of protein loss and to increase the detection rate for PLE, serial images were performed over a 4‐hour time period. The finding of marked activity in the kidneys and urinary bladder (which are visible in every image) is well known when using 99mTc‐HSA26 but does not occur when other radiopharmaceuticals such as 111In‐transferrin are used.26 Proteinuria or free radiopharmaceutical activity as a cause has been ruled out as described.
Possible pitfalls of 99mTc‐HSA scintigraphy for diagnosing PLE are (1) false‐positive findings due to gastrointestinal bleeding, (2) in vivo breakdown of the radiopharmaceutical producing a false‐positive result mimicking radiopharmaceutical exudation, and (3) bowel transit of the radiopharmaceutical which may result in either incorrect localization or false‐negative results.22 These pitfalls were excluded as much as possible by (1) having no dog with melena or anemia, (2) obtaining scintigraphic images of thyroid glands, and (3) obtaining multiple images over a 4‐hour period.
A potential limitation of this study is the use of HSA. Infusion of supraphysiological doses of HSA (250 g/L) was fatal or produced life‐threatening adverse reactions in healthy dogs. Furthermore, all healthy dogs given supraphysiological doses of HSA produced anti‐HSA antibodies which might preclude future use of HSA.27, 28 However, infusion of similar supraphysiological doses of HSA to 185 critically ill or hypoalbuminemic dogs resulted in no adverse reactions.29, 30, 31, 32 In our study, only 30 mg HSA per dog was used and no reaction was seen, neither in hypo‐ nor in normo‐albuminemic dogs. A low dose of HSA or binding to 99mTc rendering the sites for unwanted reaction unavailable might have been the reason. No measurement of anti‐HSA antibodies was carried out, but their production later is possible and thus repeated scintigraphy for re‐examination with 99mTc‐HSA was not performed. Other radiopharmaceuticals might circumvent this problem, such as 99mTc‐dextran or 111In‐transferrin which have been used in human medicine.22 Species‐specific canine albumin is newly available for therapeutic use and could be used as radiopharmaceutical in the future.33 Finally, in human medicine, 99mTc‐labeled to autologous serum albumin has been used, thus avoiding the production of antibodies and therefore opening the possibility for scintigraphic re‐examinations.34 Other limitations of the study are the use of control dogs not age, sex, or breed matched to the dogs with PLE. This might have given some few false‐positive results considering that specificities of 51–72% are reported in humans when patients with other forms of hypoproteinemia are used as controls. In 1 control dog, discrepant findings between both diplomates were seen, and having 1 examiner not blinded to the status of the dog might have added some bias. Finally, low case numbers precluded calculating inter‐ and intra‐observer variability.
In conclusion, noninvasive 99mTc‐HSA scintigraphy can be used to detect gastrointestinal protein loss in dogs with hypoalbuminemia and potentially localize the site of protein loss within the gastrointestinal tract. Future studies are needed to see whether early PLE or other intestinal diseases without marked hypoproteinemia can be detected using this technique.
Supporting information
Table S1. Signalment, clinical signs, histology and scintigraphic findings in 7 dogs with PLE undergoing 99mTc‐HSA scintigraphy.
Acknowledgments
The authors thank Tina Günther for technical assistance and Dr. Manfred Henrich, Diplomate European College of Veterinary Pathology for histopathological analyses.
Grant support: None.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was conducted at the Justus‐Liebig University, Giessen, Germany.
Previous presentation: Abstract presented at the 2014 ACVIM Forum, Nashville, Tennessee.
Footnotes
Albumon HSA Tc‐99 labelling kit, Medi‐Radiopharma Ltd., Érd, Hungary
Gamma Diagnost Tomo, Siemens LEM, Siemens AG, Erlangen, Germany
Siemens ICON, software version 7.5, Erlangen, Germany
References
- 1. Dossin O, Lavoue R. Protein‐losing enteropathies in dogs. Vet Clin North Am Small Anim Pract 2011;41:399–418. [DOI] [PubMed] [Google Scholar]
- 2. Maclachlan NJ, Breitschwerdt EB, Chambers JM, et al. Gastroenteritis of Basenji dogs. Vet Pathol 1988;25:36–41. [DOI] [PubMed] [Google Scholar]
- 3. Littman MP, Dambach DM, Vaden SL, Giger U. Familial protein‐losing enteropathy and protein‐losing nephropathy in Soft Coated Wheaten Terriers: 222 cases (1983–1997). J Vet Intern Med 2000;14:68–80. [DOI] [PubMed] [Google Scholar]
- 4. Berghoff N, Ruaux CG, Steiner JM, Williams DA. Gastroenteropathy in Norwegian Lundehunds. Compend Contin Educ Vet 2007;29:456–465. [PubMed] [Google Scholar]
- 5. Bush WW, Kimmel SE, Wosar MA, Jackson MW. Secondary hypoparathyroidism attributed to hypomagnesemia in a dog with protein‐losing enteropathy. J Am Vet Med Assoc 2001;219:1732–1734. [DOI] [PubMed] [Google Scholar]
- 6. Kimmel SE, Waddell LS, Michel KE. Hypomagnesemia and hypocalcemia associated with protein‐losing enteropathy in Yorkshire Terriers: Five cases (1992–1998). J Am Vet Med Assoc 2000;217:703–706. [DOI] [PubMed] [Google Scholar]
- 7. Breitschwerdt EB, Waltman C, Hagstad HV, et al. Clinical and epidemiologic characterization of a diarrheal syndrome in Basenji dogs. J Am Vet Med Assoc 1982;180:914–920. [PubMed] [Google Scholar]
- 8. Flesja K, Yri T. Protein‐losing enteropathy in the Lundehund. J Small Anim Pract 1977;18:11–23. [DOI] [PubMed] [Google Scholar]
- 9. Kull PA, Hess RS, Craig LE, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996–1998). J Am Vet Med Assoc 2001;219:197–202. [DOI] [PubMed] [Google Scholar]
- 10. Willard MD, Helman G, Fradkin JM, et al. Intestinal crypt lesions associated with protein‐losing enteropathy in the dog. J Vet Intern Med 2000;14:298–307. [DOI] [PubMed] [Google Scholar]
- 11. Heilmann RM, Paddock CG, Ruhnke I, et al. Development and analytical validation of a radioimmunoassay for the measurement of alpha(1)‐proteinase inhibitor concentrations in feces from healthy puppies and adult dogs. J Vet Diagn Invest 2011;23:476–485. [DOI] [PubMed] [Google Scholar]
- 12. Frias R, Collado MC, Spillmann T, et al. The 51‐chromium‐labeled ethylenediamine tetra‐acetic acid intestinal permeability blood test in healthy Beagle dogs: A comparison between serum and plasma levels. J Vet Diagn Invest 2010;22:949–953. [DOI] [PubMed] [Google Scholar]
- 13. Murphy KF, German AJ, Ruaux CG, et al. Fecal alpha(1)‐proteinase inhibitor concentration in dogs with chronic gastrointestinal disease. Vet Clin Pathol 2003;32:67–72. [DOI] [PubMed] [Google Scholar]
- 14. Chau TN, Mok MY, Chan EYT, et al. Evaluation of performance of measurement of faecal alpha(1)‐antitrypsin clearance and technetium‐99m human serum albumin scintigraphy in protein‐losing enteropathy. Digestion 2011;84:199–206. [DOI] [PubMed] [Google Scholar]
- 15. Sutherland‐Smith J, Penninck DG, Keating JH, Webster CRL. Ultrasonographic intestinal hyperechoic mucosal striations in dogs are associated with lacteal dilatation. Vet Radiol Ultrasound 2007;48:51–57. [DOI] [PubMed] [Google Scholar]
- 16. Gaschen L, Kircher P, Lang J, et al. Pattern recognition and feature extraction of canine celiac and cranial mesenteric arterial waveforms: Normal versus chronic enteropathy – A pilot study. Vet J 2005;169:242–250. [DOI] [PubMed] [Google Scholar]
- 17. Divgi CR, Lisann NM, Yeh SDJ, Benua RS. TC‐99m albumin scintigraphy in the diagnosis of protein‐losing enteropathy. J Nucl Med 1986;27:1710–1712. [PubMed] [Google Scholar]
- 18. Berry CR, Guilford WG, Koblik PD, et al. Scintigraphic evaluation of four dogs with protein‐losing enteropathy using (111)Indium‐labeled transferrin. Vet Radiol Ultrasound 1997;38:221–225. [DOI] [PubMed] [Google Scholar]
- 19. Takeda H, Takahashi T, Ajitsu S, et al. Protein‐losing gastroenteropathy detected by Tc‐99m‐labeled human serum albumin. Am J Gastroenterol 1991;86:450–453. [PubMed] [Google Scholar]
- 20. Halaby H, Bakheet SM, Shabib S, et al. Tc‐99m‐human serum albumin scans in children with protein‐losing enteropathy. J Nucl Med 2000;41:215–219. [PubMed] [Google Scholar]
- 21. Hildebrand P, Henze E, Lietzenmayer R, Schoetensack M. Localization of enteral protein loss by 99m‐technetium‐albumin‐scintigraphy. Eur J Nucl Med 1989;15:217–218. [DOI] [PubMed] [Google Scholar]
- 22. Khalesi M, Nakhaei AA, Seyed AJ, et al. Diagnostic accuracy of nuclear medicine imaging in protein losing enteropathy : Systematic review and meta‐analysis of the literature. Acta Gastroenterol Belg 2013;76:413–422. [PubMed] [Google Scholar]
- 23. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 24. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 25. Tseng KC, Sheu BS, Lee JC, et al. Application of technetium‐99m‐labeled human serum albumin scan to assist surgical treatment of protein‐losing enteropathy in Cronkhite‐Canada syndrome: Report of a case. Dis Colon Rectum 2005;48:870–873. [DOI] [PubMed] [Google Scholar]
- 26. Aburano T, Yokoyama K, Kinuya S, et al. Indium‐111 transferrin imaging for the diagnosis of protein‐losing enteropathy. Clin Nucl Med 1989;14:681–685. [DOI] [PubMed] [Google Scholar]
- 27. Cohn LA, Kerl ME, Lenox CE, et al. Response of healthy dogs to infusions of human serum albumin. Am J Vet Res 2007;68:657–663. [DOI] [PubMed] [Google Scholar]
- 28. Francis AH, Martin LG, Haldorson GJ, et al. Adverse reactions suggestive of type III hypersensitivity in six healthy dogs given human albumin. J Am Vet Med Assoc 2007;230:873–879. [DOI] [PubMed] [Google Scholar]
- 29. Trow AV, Rozanski EA, deLaforcade AM, Chan DL. Evaluation of use of human albumin in critically ill dogs: 73 cases (2003–2006). J Am Vet Med Assoc 2008;233:607–612. [DOI] [PubMed] [Google Scholar]
- 30. Viganó F, Perissinotto L, Bosco VR. Administration of 5% human serum albumin in critically ill small animal patients with hypoalbuminemia: 418 dogs and 170 cats (1994–2008). J Vet Emerg Crit Care (San Antonio) 2010;20:237–243. [DOI] [PubMed] [Google Scholar]
- 31. Mathews KA, Barry M. The use of 25% human serum albumin: Outcome and efficacy in raising serum albumin and systemic blood pressure in critically ill dogs and cats. J Vet Emerg Crit Care (San Antonio) 2005;15:110–118. [Google Scholar]
- 32. Mathews KA. The therapeutic use of 25% human serum albumin in critically ill dogs and cats. Vet Clin North Am Small Anim Pract 2008;38:595–605. [DOI] [PubMed] [Google Scholar]
- 33. Craft EM, Powell LL. The use of canine‐specific albumin in dogs with septic peritonitis. J Vet Emerg Crit Care (San Antonio) 2012;22:631–639. [DOI] [PubMed] [Google Scholar]
- 34. Wang YF, Chen YC, Li DK, Chuang MH. Technetium‐99m‐labeled autologous serum albumin: A personal‐exclusive source of serum component. J Biomed Biotechnol 2011;2011:413802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Signalment, clinical signs, histology and scintigraphic findings in 7 dogs with PLE undergoing 99mTc‐HSA scintigraphy.


