Abstract
Background
Chronic kidney disease (CKD) is a common comorbidity in cats with hypercalcemia, but whether CKD is a risk factor for hypercalcemia is unclear. Hypercalcemia often is diagnosed based on total calcium concentration (tCa), which tends to underestimate the ionized calcium concentration (iCa) in cats.
Objectives
Assessment of the performance of tCa for the diagnosis of ionized hypercalcemia, and exploration of factors influencing the relationship between iCa and tCa. Determination of risk factors for incident total hypercalcemia (ie, the development of hypercalcemia based on tCa during follow‐up).
Animals
Records of a cross‐section (n = 477) and observational cohort (n = 367) of client‐owned cats with and without azotemic CKD from first opinion practice.
Methods
Retrospective cross‐sectional and retrospective cohort study. The diagnostic accuracy of tCa as an index test for ionized hypercalcemia was evaluated, and risk factors for underestimation were explored by binary logistic and linear regression in a cross‐section of cats with and without azotemic CKD. Chronic kidney disease and clinicopathological variables were assessed as predictors of incident total hypercalcemia by both time‐invariant and time‐dependent Cox regression in a cohort of cats.
Results
Specificity of tCa for identification of ionized hypercalcemia was high (100%), but sensitivity was low. Underestimation was associated with lower venous bicarbonate concentrations. Cats with CKD had increased risk for incident total hypercalcemia (hazard ratio, 4.29; 95% confidence interval, 1.96–9.37; P < .001). Higher tCa predicted incident total hypercalcemia in both azotemic and nonazotemic cats (P < .001).
Conclusions and Clinical Importance
Chronic kidney disease is a risk factor for incident total hypercalcemia, and most cats with increased tCa had concurrent ionized hypercalcemia. Higher baseline tCa predicts incident total hypercalcemia. Prospective studies assessing changes in iCa are warranted.
Keywords: Azotemia, Calcium, Feline, Bicarbonate
Abbreviations
- AUROC
area under the receiver operating characteristic curve
- CI
confidence interval
- CKD
chronic kidney disease
- CKD‐MBD
chronic kidney disease‐mineral and bone disorder
- FGF‐23
fibroblast growth factor 23
- HCO3−
venous bicarbonate concentration
- HR
hazard ratio
- iCa
ionized calcium concentration
- IRIS
International Renal Interest Society
- OR
odds ratio
- PTH
parathyroid hormone
- ROC
receiver operating characteristic
- tCa
total calcium concentration
- USG
urine specific gravity
Hypercalcemia in cats is associated with chronic kidney disease (CKD), neoplasia, and rarely primary hyperparathyroidism,1, 2, 3 although hypercalcemia of idiopathic origin is considered the most common etiology.4 It is unclear whether renal dysfunction is truly a risk factor for the development of hypercalcemia, because CKD is a common comorbidity in older cats,5, 6 and hypercalcemia could decrease glomerular filtration rate4 by decreasing medullary tonicity7 and renal blood flow.8 Several mechanisms by which CKD may contribute to hypercalcemia have been proposed, including decreased glomerular filtration, increased tubular reabsorption, and decreased bone storage of calcium.9 Based on total calcium concentration (tCa), early studies report CKD in 25% of hypercalcemic cats,2 whereas hypercalcemia was found in 11.5–21% of cats with CKD.1, 3, 10 The prevalence appears to increase as renal function declines, from 8% in early stage to 32% in end‐stage disease.1 Approximately half of tCa consists of hydrated free calcium ions, and the other half consists of protein‐bound calcium and a small but variable portion of ionic complexes, such as calcium phosphate.9, 11 Hypercalcemia in CKD typically is represented by an increase in tCa, with a normal ionized calcium concentration (iCa).12, 13, 14 Ionized hypercalcemia has been reported in 10–30% of cats with CKD,1, 10 even though iCa tends to decrease in end‐stage disease.1
The ionized calcium fraction is biologically active and therefore superior to tCa in assessing calcium status.11 Due to practical implications, however, tCa is often relied on in practice.11 The sensitivity and specificity of tCa to predict ionized hypercalcemia in cats with CKD are reported to be 30 and 92%, respectively.10 Hypoalbuminemia, causing a decrease in the protein‐bound fraction, could result in underestimation of ionized calcium.10, 15 However, tCa and albumin concentrations correlate poorly, and the diagnostic accuracy of tCa could not be improved by adjustment for serum albumin concentration in cats.16, 17 In addition to hypoalbuminemia, metabolic acidosis appears to increase the risk for underestimation of iCa in human CKD,15 but this has not been reported in cats.
The aims of our study were as follows: first, to assess the performance of plasma tCa in the prediction of ionized hypercalcemia and explore factors influencing the relationship between iCa and tCa, and second, to determine whether azotemic CKD is a risk factor for the development of total hypercalcemia and to explore predictors of incident total hypercalcemia.
Materials and Methods
Case Selection
Cases were retrospectively selected from the records of geriatric cat clinics held at 2 London‐based first opinion practices (People's Dispensary for Sick Animals in Bow and Beaumont Sainsbury Animal Hospital in Camden), where apparently healthy cats (≥9 years) had blood samples collected every 6 months for general health screening, and cats with CKD or systemic hypertension were blood sampled every 4 months for disease management. Criteria for a diagnosis of azotemic CKD were plasma creatinine concentration ≥2 mg/dL in conjunction with a urine specific gravity (USG) <1.035, or plasma creatinine concentration ≥2 mg/dL on 2 consecutive occasions 2–4 weeks apart. Records of cats with clinical signs of hyperthyroidism, plasma total thyroxine concentration >40 nmol/L, medical treatment for hyperthyroidism, or diabetes mellitus, or treatment with corticosteroids were excluded from all analyses. Cats receiving amlodipine besylate for treatment of systemic hypertension were included.
Collection and storage of blood samples was performed with owner consent and approval of the Ethics and Welfare Committee of the Royal Veterinary College. Blood was obtained by jugular venipuncture, collected in a heparinized tube, and stored on ice for a maximum of 6 hours until centrifugation and separation. Heparinized plasma was sent to an external laboratory1 for routine biochemistry, including tCa. Ionized calcium concentration was measured in nonheparinized whole blood immediately after venipuncture using a point‐of‐care analyzer,2 which also reported venous bicarbonate concentration (HCO3 −). Venous HCO3 − is an accurate substitution for arterial HCO3 − in humans.18, 19 Time between iCa and tCa measurement was 24 hours. Results of intact fibroblast growth factor 23 (FGF‐23), intact parathyroid hormone (PTH), calcidiol, and calcitriol concentrations were available for some cats. Fibroblast growth factor 23 and PTH had been measured in EDTA plasma using validated20, 21 ELISA3 and immunoradiometric4 assays, respectively. Calcidiol and calcitriol had been measured at an external commercial laboratory.5
Evaluation of Diagnostic Accuracy of tCa as a Marker of Ionized Hypercalcemia
For the cross‐sectional analysis assessing the diagnostic performance of tCa for the detection of ionized hypercalcemia, patient records from January 1, 2000 to December 31, 2015 were reviewed for cats that had both iCa and tCa measured on the same blood sample. If cats had both tCa and iCa measured on multiple occasions, only the first visit on which this occurred was included. Hypercalcemia was diagnosed based on a 95% reference interval for iCa in older cats (4.76–5.48 mg/dL) previously derived using an identical analyzer.22 Ionized calcium status was compared to the matched total calcium status for which the laboratory reference interval (8.20–11.80 mg/dL) was used.
Evaluation of Risk of Incident Total Hypercalcemia in Cats with and Without Renal Azotemia
For the retrospective cohort, the records between January 1, 2000 and September 1, 2014 were reviewed to identify nonazotemic cats and cats with a diagnosis of azotemic CKD. Data extraction was performed on July 27, 2015. For nonazotemic cats, the first visit to the geriatric cat clinic was designated as baseline, whereas for CKD cases the date of diagnosis of azotemic CKD was used. All records were included from baseline to the visit when total hypercalcemia was noted (ie, incident total hypercalcemia; tCa >11.80 mg/dL). Animals that remained normocalcemic based on tCa had to have follow‐up of at least 330 days to be included. Cases with a diagnosis of total hypercalcemia before or at baseline were excluded. Cats were classified according to renal azotemia and development of total hypercalcemia, resulting in the following 4 groups: cats with a diagnosis of CKD that remained normocalcemic during follow‐up (CKD‐NCa group); cats with a diagnosis of CKD that developed total hypercalcemia (CKD‐HCa group); nonazotemic cats that remained normocalcemic (H‐NCa group); and nonazotemic cats that developed total hypercalcemia (H‐HCa group).
Statistical Analysis
All statistical analyses were performed using commercial software.6 Statistical significance was set as P < .05. Continuous clinical data are presented as mean (range) or median [25th and 75th percentiles]. Normality was assessed by visual inspection of histograms, and skewed variables were log‐transformed for normalization. Correlations were assessed by Pearson's correlation (r).
Evaluation of Diagnostic Accuracy of tCa as a Marker of Ionized Hypercalcemia
Simple linear regression was used to assess the association between tCa and iCa. Sensitivity and specificity were calculated for tCa as an index test of true ionized hypercalcemia. Measures of accuracy are presented as percentage (95% confidence interval [CI]). Receiver operating characteristic (ROC) curves were plotted to assess overall diagnostic accuracy of tCa for detecting ionized hypercalcemia. Age, sex, weight, creatinine, phosphate, albumin, cholesterol, sodium, potassium, chloride, HCO3 −, pH, and PCV were assessed as risk factors by binary logistic regression with underestimation of iCa status as the event of interest. Only ionized hypercalcemic cats were used in this analysis, classified as either underestimated (total normocalcemia with ionized hypercalcemia) or in agreement (both total and ionized hypercalcemia). Only variables from the univariable analysis with a type I error <10% were entered into multivariable logistic regression analysis. The final models were derived by backward elimination. Results are reported as odds ratio (OR) (95% CI).
In addition to dichotomization of cases as either underestimated or in agreement based on reference ranges, the amount of underestimation was quantified by standardizing tCa and iCa using the formula: z‐score = (measured value − reference range mean)/standard deviation of the reference range.15, 23, 24 Cats were classified as underestimated if the standard score of tCa was lower than the standard score of the matching iCa value (z‐score tCa − z‐score iCa < 0). Linear regression was used to explore what variables influence tCa in cats in which tCa underestimates iCa. Variables with a type I error <10% entered multivariable linear regression. The final models were derived by backward elimination. Results are reported as regression coefficient (β) (95% CI).
Evaluation of Risk of Incident Total Hypercalcemia in Cats with and Without Renal Azotemia
Baseline variables of the 4 groups were compared using 1‐way ANOVA with Bonferroni posthoc comparison or independent samples t‐test and proportions by Fisher's exact test. To assess whether azotemic CKD is a risk factor for incident total hypercalcemia, Kaplan–Meier curves of the nonazotemic and CKD group were compared using log‐rank test, and a hazard ratio (HR) was calculated with univariable Cox proportional hazard analysis. Incident total hypercalcemia was the event of interest, and cats that remained normocalcemic were censored when lost to follow‐up. Cats with CKD had blood samples collected more frequently (every 4 months) because of their condition than did nonazotemic cats (every 6 months). A subanalysis that only included visits at 6‐month intervals, ignoring other visits, was carried out to evaluate the above‐mentioned potential bias.
Baseline predictors of incident total hypercalcemia for the azotemic and nonazotemic groups were explored with time‐invariant Cox regression, and repeatedly measured predictors were assessed using time‐dependent Cox regression. No missing data imputation was performed. Age, weight, USG, iCa, and plasma creatinine, phosphate, tCa, FGF‐23, chloride, sodium, and potassium concentrations were entered as continuous variables, and hair length, sex, and plasma albumin and PTH concentrations based on terciles, as categorical variables. Variables with a type I error <10% entered multivariable analysis. The final time‐invariant and time‐dependent models were derived by backward elimination. Results are reported as HR (95% CI). Receiver operating characteristic curves were constructed to explore cutoff values of baseline predictors of incident total hypercalcemia.
Results
Evaluation of Diagnostic Accuracy of tCa as a Marker of Ionized Hypercalcemia
A total of 590 independent samples were identified with both tCa and iCa measured, of which 113 were excluded because of a diagnosis of hyperthyroidism (n = 85), or undetermined renal function (n = 28), allowing 477 cats for analysis, of which 168 were nonazotemic and 309 had CKD. Domestic shorthair was the most common breed (n = 368), followed by domestic longhair (n = 51), and purebreds or crosses of the following breeds: Burmese (n = 15), Persian (n = 15), Siamese (n = 8), Birman (n = 4), 3 each of British blue, British shorthair, Chinchilla, and Russian blue, 2 Maine Coons, 1 Abyssinian, and 1 Tiffany. In the CKD group, 222 cats had International Renal Interest Society (IRIS) (www.iris-kidney.com) stage 2; 66, IRIS stage 3; and 21, IRIS stage 4 CKD. Clinicopathologic variables are summarized in Table 1. Based on iCa, 58 cats were hypercalcemic, 31 hypocalcemic, and 388 normocalcemic. In ionized hypercalcemic cats, mean iCa was 5.91 (range, 5.52–7.40) mg/dL and mean tCa 11.20 (range, 9.48–14.84) mg/dL. No concurrent diseases other than CKD, hypertension, or bacterial urinary tract infections were diagnosed.
Table 1.
Clinicopathologic variables for cats included in the cross‐sectional study grouped according to renal status
| Variable (Reference Interval) | CKD (n = 309) | Nonazotemic (n = 168) |
|---|---|---|
| Median [25th, 75th Percentile] | Median [25th, 75th Percentile] | |
| Age (years) | 14.4 [11.4, 16.6] | 12.3 [11.0, 14.6] |
| Weight (kg) | 3.90 [3.20, 4.54] | 4.25 [3.43, 5.04] |
| % Male | 55.5 | 50.6 |
| Albumin (2.5–4.5 g/dL) | 3.2 [3.0, 3.3] | 3.2 [3.0, 3.4] |
| Chloride (100–124 mEq/L) | 119 [116, 122] | 120 [118, 122] |
| Creatinine (0.23–2.00 mg/dL) | 2.48 [2.17, 2.91] | 1.53 [1.38, 1.73] |
| HCO3 − (17–24 mEq/L) | 20 [18, 22] | 20 [19, 22] |
| iCa (4.76–5.48 mg/dL) | 5.16 [5.00, 5.36] | 5.12 [4.96, 5.27] |
| PCV (30–45%) | 34 [30, 38] | 37 [33, 40] |
| Venous pH (7.21–7.44) | 7.36 [7.31, 7.39] | 7.36 [7.32, 7.39] |
| Phosphate (2.79–6.81 mg/dL) | 3.93 [3.41, 5.05] | 3.60 [3.14, 4.11] |
| Potassium (3.5–5.5 mEq/L) | 3.7 [3.5, 4.0] | 3.7 [3.4, 3.9] |
| Sodium (145–157 mEq/L) | 151 [150, 153] | 151 [150, 152] |
| tCa (8.2–11.8 mg/dL) | 10.2 [9.7, 10.5] | 9.7 [9.4, 10.2] |
| Total protein (6.0–8.0 g/dL) | 7.6 [7.3, 8.0] | 7.7 [7.3, 8.1] |
| USG (≥1.035) | 1.018 [1.015, 1.021] | 1.043 [1.030, 1.050] |
CKD, chronic kidney disease; n, number of cats; HCO3 −, venous bicarbonate; iCa, ionized calcium; tCa, total calcium; USG, urine specific gravity.
The association between tCa and iCa was moderate in both groups (r = 0.62 for the nonazotemic group and r = 0.63 for the azotemic group, Fig 1). Total calcium concentration was significantly associated with iCa (P < .001), with 0.28 (95% CI, 0.22–0.33) mg/dL and 0.31 (95% CI, 0.27–0.36) mg/dL increase in iCa per 1 mg/dL increase in tCa in the nonazotemic and azotemic groups, respectively (R 2, 0.39 for both groups). Ionized hypercalcemia was correctly identified in 13 of 47 CKD cases and in 1 of 11 nonazotemic cases based on tCa. Sensitivity of tCa >11.80 mg/dL to detect ionized hypercalcemia was 27.7% (95% CI, 16.9–41.8) in CKD cats and 9.1% (95% CI, 1.6–37.7) in the nonazotemic group. Specificity was 99.6% (95% CI, 97.9–99.9) in azotemic and 100% (95% CI, 97.6–100) in nonazotemic cats. The area under the ROC curve (AUROC) was 0.80 (95% CI, 0.73–0.87) for CKD cats and 0.97 (95% CI, 0.93–1.00) for nonazotemic cats. The ROC curve analysis indicated that in the CKD group, sensitivity and specificity would be 72.3% (95% CI, 57.4–84.4) and 71.0% (95% CI, 65.1–76.4), respectively, if a lower tCa cutoff of 10.3 mg/dL had been used to diagnose ionized hypercalcemia. In the nonazotemic group, 90.9% (95% CI, 58.7–99.8) sensitivity and 94.9% (95% CI, 90.2–97.8) specificity were predicted for a cutoff of 10.5 mg/dL.
Figure 1.
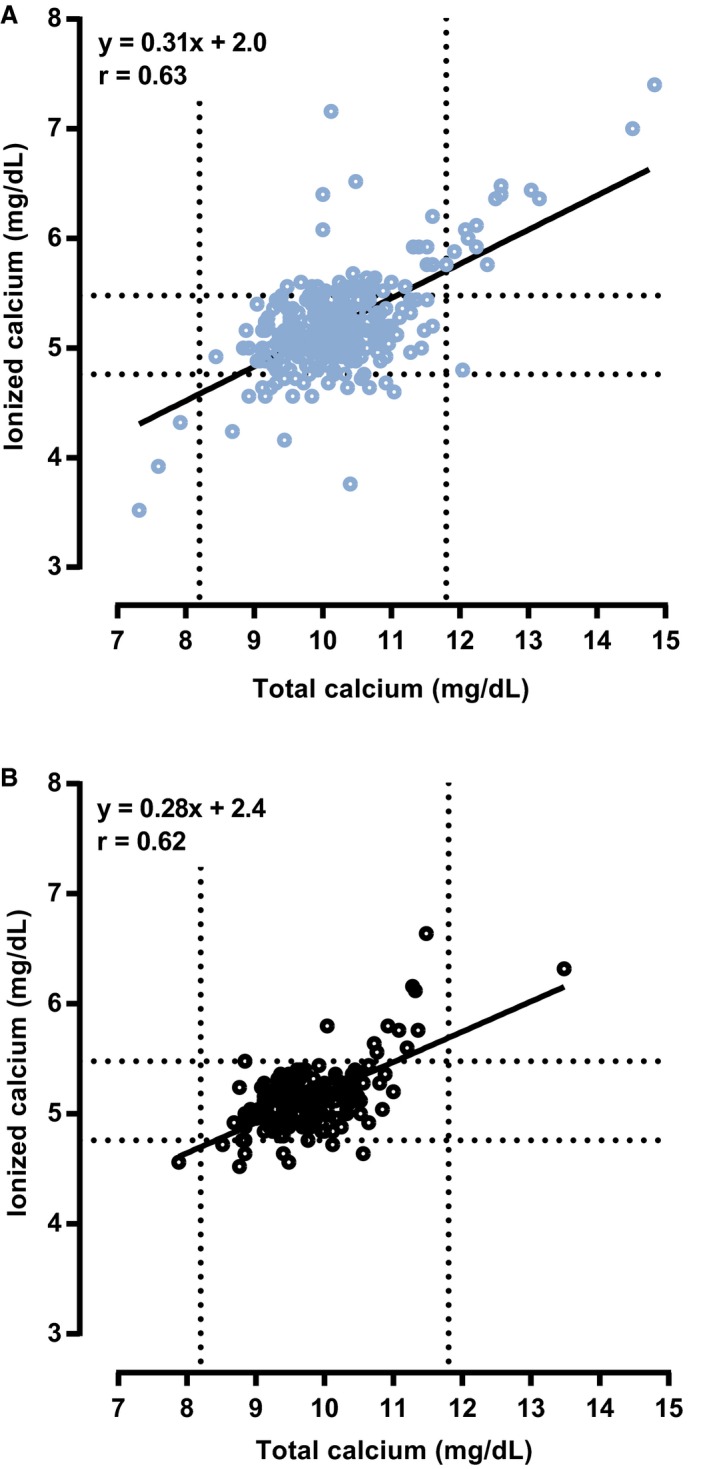
Scatter plots illustrating the relationship between plasma tCa and whole blood iCa in (A) azotemic (n = 309) and (B) nonazotemic (n = 168) cats. Diagonal black lines represent regression lines. Dashed lines represent the lower and upper limits of the reference intervals for plasma tCa (8.20–11.80 mg/dL) and whole blood iCa (4.76–5.48 mg/dL). r, Pearson's correlation coefficient.
With binary logistic regression analysis, lower HCO3 − was identified as a significant risk factor for underestimation of calcium status if the azotemic and nonazotemic ionized hypercalcemic cats were combined for analysis (OR, 0.74; 95% CI, 0.55–0.93; P = .007), but not when the CKD group was analyzed separately (n = 47; P = .062).
The standardized tCa was lower than the standardized iCa in 171 cats with CKD and 99 nonazotemic cats. Linear regression analysis was performed to assess what variables influenced plasma tCa in these underestimated cases. Venous HCO3 −, and plasma creatinine and albumin concentrations were associated with plasma tCa at the 10% level in both groups (Table 2). In the multivariable models adjusted for iCa, HCO3, creatinine, and albumin concentrations remained significant in the CKD group, and albumin and HCO3 − concentrations in the nonazotemic group.
Table 2.
Univariable and multivariable linear regression models to identify predictors of plasma total calcium concentration in azotemic and nonazotemic cats with underestimated ionized calcium concentration
| Univariable Results | Multivariable Results | |||
|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |
| CKD cats (n = 171) | ||||
| Ionized calcium (mg/dL) | 1.89 (1.65–2.13) | <.001 | 1.74 (1.50–1.97) | <.001 |
| HCO3 − (mEq/L) | 0.08 (0.03–0.13) | .002 | 0.06 (0.03–0.09) | <.001 |
| Creatinine (mg/dL) | 0.30 (0.09–0.51) | .005 | 0.23 (0.09–0.38) | .001 |
| Albumin (g/dL) | 0.62 (0.15–1.09) | .010 | 0.48 (0.17–0.78) | .002 |
| Nonazotemic cats (n = 99) | ||||
| Ionized calcium (mg/dL) | 2.03 (1.71–2.35) | <.001 | 1.92 (1.66–2.19) | <.001 |
| Albumin (g/dL) | 0.87 (0.49–1.26) | <.001 | 0.68 (0.47–0.89) | <.001 |
| HCO3 − (mEq/L) | 0.05 (0.00–0.11) | .065 | 0.04 (0.02–0.07) | .002 |
| Creatinine (mg/dL) | 0.52 (0.02–1.02) | .042 | ||
CKD, chronic kidney disease; β, regression coefficient; 95% CI, 95% confidence interval; HCO3 −, venous bicarbonate; P, significance.
Evaluation of Risk of Incident Total Hypercalcemia in Cats with and Without Renal Azotemia
For the observational cohort, 2,198 cats were identified, of which 1,818 were excluded from analysis because of insufficient follow‐up (n = 566), suspect or documented hyperthyroidism during follow‐up (n = 1251), and corticosteroid administration (n = 1). Thereafter, 13 cats were excluded for total hypercalcemia at baseline. In total, 367 cats were included: 176 nonazotemic cats, of which 10 developed total hypercalcemia, and 191 cats with a diagnosis of azotemic CKD, of which 60 developed total hypercalcemia. Most common breeds were domestic shorthair (n = 270) followed by domestic longhair (n = 41), and purebreds or crossbreeds of Burmese (n = 15), Persian (n = 14), Siamese (n = 6), British shorthair (n = 5), British blue (n = 3), 2 each of Russian blue, Tiffany, Bengal, and Exotic shorthair, and 1 each of Abyssinian, Maine Coon, American shorthair, Devon rex, and Ocicat. Of the 191 cats with CKD, 143 had IRIS stage 2, 46 IRIS stage 3, and 2 IRIS stage 4 CKD. Forty‐six of the cats that developed total hypercalcemia were part of the study population used for the cross‐sectional analysis, with 3 visits being identical. Baseline values of clinicopathologic variables can be found in Table 3. Baseline tCa was higher in both nonazotemic and azotemic cats that developed total hypercalcemia during follow‐up, and iCa was higher in the CKD‐HCa group. Baseline plasma intact PTH concentration was lower in cats that developed total hypercalcemia. Plasma creatinine concentration did not differ between cats that developed total hypercalcemia and cats that remained normocalcemic in each group.
Table 3.
Clinicopathological variables for cats included in the retrospective cohort study grouped according to renal status and development of total hypercalcemia
| Variable (Reference Interval) | H‐NCa (n = 166) | H‐HCa (n = 10) | CKD‐NCa (n = 131) | CKD‐HCa (n = 60) | ||||
|---|---|---|---|---|---|---|---|---|
| Median [25th, 75th Percentile] | n | Median [25th, 75th Percentile] | n | Median [25th, 75th Percentile] | n | Median [25th, 75th Percentile] | n | |
| Age (years) | 12.0 [10.4, 14.0]a | 161 | 13.9 [10.6, 14.7] | 10 | 14.6 [12.0, 16.1]b | 117 | 13.8 [12.0, 15.7]b | 56 |
| Weight (kg) | 4.43 [3.53, 5.29]a | 161 | 4.29 [3.71, 5.76] | 10 | 3.94 [3.39, 4.71]b | 128 | 4.16 [3.21, 4.94] | 60 |
| % Male | 49 | 166 | 30 | 10 | 52 | 131 | 43 | 60 |
| % Longhair | 18 | 166 | 20 | 10 | 15 | 131 | 12 | 60 |
| Albumin (2.5–4.5 g/dL) | 3.2 [3.1, 3.4] | 166 | 3.4 [3.2, 3.5] | 10 | 3.1 [3.0, 3.3] | 131 | 3.1 [2.9, 3.4] | 60 |
| Calcidiol (65–170 nmol/L) | – | 0 | – | 0 | 140.5 [128.3, 171.5] | 14 | 144.0 [110.0, 211.0] | 11 |
| Calcitriol (90–342 pmol/L) | – | 0 | – | 0 | 418.0 [366.0, 447.0] | 15 | 320.0 [116.0, 475.0] | 11 |
| Chloride (100–124 mEq/L) | 119 [117, 120] | 166 | 118 [117, 120] | 10 | 118 [116, 121] | 131 | 118 [116, 120] | 60 |
| Creatinine (0.23–2.00 mg/dL) | 1.47 [1.34, 1.70]a | 166 | 1.59 [1.34, 2.04]a | 10 | 2.37 [2.13, 2.68]b | 131 | 2.42 [2.19, 2.90]b | 60 |
| FGF‐23 (56–700 pg/mL) | 164 [108, 243]a | 57 | 261 [221, 604] | 6 | 491 [283, 1035]b | 62 | 518 [342, 1701]b | 29 |
| iCa (4.76–5.48 mg/dL) | 5.08 [5.00, 5.24] | 35 | 5.16 [–] | 3 | 5.16 [5.02, 5.32]a | 37 | 5.34 [5.20, 5.44]b | 24 |
| PCV (30–45%) | 38 [34, 40]a | 162 | 39 [36, 42] | 10 | 35 [31, 38]b | 129 | 35 [30, 39] | 59 |
| Venous pH (7.21–7.44) | 7.35 [7.32, 7.38] | 35 | 7.35 [–] | 3 | 7.36 [7.31, 7.39] | 33 | 7.35 [7.31, 7.37] | 22 |
| Phosphate (2.79–6.81 mg/dL) | 3.84 [3.35, 4.37] | 166 | 3.53 [3.18, 4.46] | 10 | 4.03 [3.50, 4.84] | 131 | 4.12 [3.43, 4.95] | 60 |
| Potassium (3.5–5.5 mEq/L) | 3.9 [3.7, 4.3] | 164 | 4.0 [3.8, 4.2] | 10 | 4.0 [3.7, 4.3] | 130 | 4.1 [3.8, 4.3] | 60 |
| PTH (2.6–17.6 pg/mL) | 7.0 [2.6, 12.0]a | 31 | 4.6 [2.6, 7.1]a | 4 | 15.0 [8.7, 27.7]b | 62 | 7.9 [2.6, 22.0]a | 30 |
| SBP (<160 mmHg) | 136 [122, 152] | 165 | 134 [121, 159] | 10 | 142 [126,158] | 131 | 138 [123, 156] | 60 |
| Sodium (145–157 mEq/L) | 152.3 [150.9, 153.8] | 165 | 151.9 [150.5, 153.9] | 10 | 152.5 [150.8, 154.6] | 130 | 152.9 [150.0, 154.6] | 60 |
| tCa (8.2–11.8 mg/dL) | 9.8 [9.5, 10.2]a | 166 | 10.6 [9.7, 11.3]b | 10 | 10.1 [9.7, 10.4]b,c | 131 | 10.4 [10.0, 11.0]b,d | 60 |
| Total protein (6.0–8.0 g/dL) | 7.6 [7.3, 8.1] | 166 | 7.5 [7.1, 7.8] | 10 | 7.8 [7.4, 8.1] | 131 | 7.7 [7.4, 8.1] | 60 |
| Urea (7.0–27.7 mg/dL) | 29.1 [24.9, 32.8]a | 166 | 27.8 [24.3, 33.8]a | 10 | 47.3 [39.9, 55.7]b | 131 | 45.4 [37.7, 55.6]b | 60 |
| USG (≥1.035) | 1.044 [1.031, 1.056]a | 108 | 1.060 [1.041, 1.070]a | 5 | 1.020 [1.016, 1.022]b | 101 | 1.020 [1.016, 1.024]b | 44 |
| Follow‐up (days) | 732 [516, 1023]a | 166 | 386 [166, 542]b | 10 | 679 [490, 1022]a | 131 | 175 [63, 403]b | 60 |
H‐NCa, apparently healthy‐normocalcemic; H‐HCa, apparently healthy‐developed hypercalcemia; CKD‐NCa, CKD‐normocalcemic; CKD‐HCa, CKD‐developed hypercalcemia; n, number of cats; FGF‐23, fibroblast growth factor 23; PTH, parathyroid hormone; SBP, systolic blood pressure; USG, urine specific gravity; P, significance.
Rows bearing a different superscript letter are significantly different from one another.
At the hypercalcemic visit, mean tCa was 12.74 (range, 11.92–14.16) mg/dL for nonazotemic cats and 12.72 (range, 11.84–19.08) mg/dL for CKD cats. Data on iCa were available for 2 nonazotemic cats (6.16 and 6.60 mg/dL) and 18 CKD cats (mean, 6.31; range, 5.48–7.40 mg/dL). All but 1 had concurrent ionized hypercalcemia. Median phosphate concentration was 3.52 [2.57, 4.09] mg/dL in nonazotemic and 4.23 [3.58, 5.19] mg/dL in azotemic cats. In CKD cats, median plasma creatinine concentration was 2.44 [2.23, 3.07] mg/dL, and a >25% increase compared to baseline was observed in 8 cats. Parathyroid hormone concentration was <2.6 pg/mL in 7 and 8.0 pg/mL in 1 cat, and decreased compared to baseline in 5 cats (baseline median, 21.5 [9.0, 47.8] pg/mL). Five cats were treated PO with aluminum hydroxide. Concurrent findings with total hypercalcemia were abdominal masses (n = 2), calcium oxalate crystalluria (n = 2), and ureterolithiasis (n = 1).
The incidence rate of total hypercalcemia was 0.03 per patient‐year for the nonazotemic group and 0.18 per patient‐year for the CKD group. Risk of total hypercalcemia within the first year from baseline was 2.3% for nonazotemic cats and 23.4% for CKD cats. The Kaplan‐Meier curves (Fig 2A) were significantly different between the 2 groups (P < .001), and cats with azotemic CKD were at significantly higher risk of developing total hypercalcemia (HR, 6.66; 95% CI, 3.41–13.02; P < .001). When adjusted for frequency of sampling, the study population included 174 nonazotemic cats, of which 8 developed total hypercalcemia, and 172 CKD cases, of which 30 cats developed hypercalcemia. The incidence rate of total hypercalcemia was 0.02 per patient‐year for the nonazotemic group and 0.08 per patient‐year for the CKD group. The Kaplan–Meier curves (Fig 2B) remained significantly different (P < .001) as did the hazards for incident total hypercalcemia (HR, 4.29; 95% CI, 1.96–9.37; P < .001).
Figure 2.
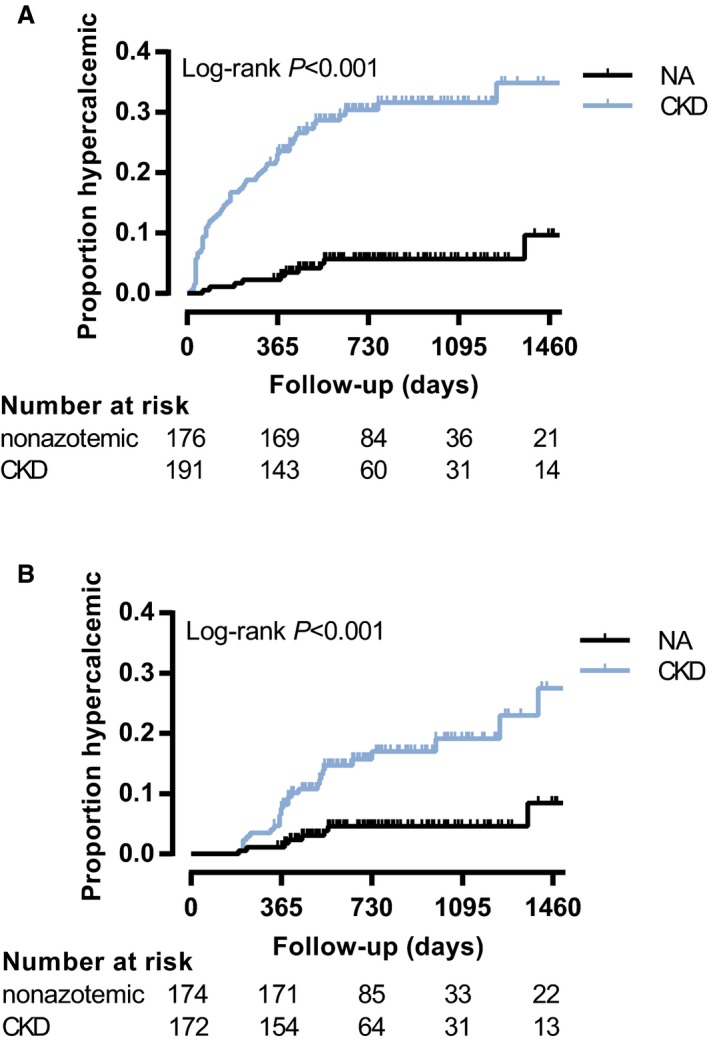
Kaplan–Meier curves illustrating the development of total hypercalcemia in nonazotemic cats and cats with azotemic chronic kidney disease (CKD). (A) Nonadjusted model. CKD cats (blue curve) were at increased risk of developing total hypercalcemia (n = 191: 60 developed total hypercalcemia, 131 censored) compared to nonazotemic cats (NA, black curve, n = 176: 10 developed total hypercalcemia, 166 censored) with a hazard ratio (HR) of 6.66 (95% confidence interval [CI], 3.41–13.02; P < .001). (B) Model adjusted for frequency of blood sampling. CKD cases (blue curve, n = 172: 30 developed total hypercalcemia, 142 censored) remain at increased risk of total hypercalcemia compared to nonazotemic cats (NA, black curve, n = 174: 8 developed total hypercalcemia, 166 censored) with a HR of 4.29 (95% CI, 1.96–9.37; P < .001).
In cats with azotemic CKD, univariable Cox regression identified higher baseline plasma tCa and creatinine concentrations, and lower baseline plasma chloride and PTH concentrations associated with incident total hypercalcemia at the 10% level (Table 4). Only higher baseline tCa was an independent predictor in the final multivariable model (HR, 2.55; 95% CI, 1.68–3.87; P < .001). Both higher plasma tCa and higher creatinine concentrations were independent predictors of incident total hypercalcemia in the multivariable time‐dependent Cox regression model (Table 5).
Table 4.
Univariable time‐invariant Cox regression results identifying baseline predictors of total hypercalcemia in cats stratified by renal status
| n | HR (95% CI) | P | |
|---|---|---|---|
| CKD cats | |||
| Total calcium (mg/dL) | 191 | 2.55 (1.68–3.87) | <.001 |
| PTH (<9.0 pg/mL) | 32 | .025 | |
| 9.0–25.0 pg/mL | 32 | 0.38 (0.16–0.89) | .026 |
| >25.0 pg/mL | 28 | 0.36 (0.14–0.92) | .033 |
| Creatinine (mg/dL) | 191 | 1.42 (0.96–2.10) | .076 |
| Chloride (mEq/L) | 191 | 0.93 (0.86–1.01) | .089 |
| Nonazotemic cats | |||
| Total calcium (mg/dL) | 176 | 5.92 (2.21–15.83) | <.001 |
| Creatinine (mg/dL) | 176 | 5.95 (0.99–35.61) | .051 |
CKD, chronic kidney disease; n, number of cats; HR, hazard ratio; 95% CI, 95% confidence interval; PTH, parathyroid hormone; P, significance.
Table 5.
Univariable and multivariable time‐dependent Cox regression results identifying risk factors for incident total hypercalcemia in cats with azotemic CKD (n = 191)
| HR (95% CI) | P | |
|---|---|---|
| Univariable results | ||
| Total calcium (mg/dL) | 2.40 (2.05–2.80) | <.001 |
| Creatinine (mg/dL) | 1.28 (1.05–1.55) | .015 |
| Multivariable results | ||
| Total calcium (mg/dL) | 2.42 (2.07–2.84) | <.001 |
| Creatinine (mg/dL) | 1.28 (1.05–1.56) | .017 |
n, number of cats; CKD, chronic kidney disease; HR, hazard ratio; 95% CI, 95% confidence interval; P, significance.
In the nonazotemic group, higher baseline plasma tCa and creatinine concentrations were associated with incident total hypercalcemia (Table 4). In multivariable analysis, only tCa (HR, 5.92; 95% CI, 2.21–15.83; P < .001) was a significant independent predictor. A time‐dependent Cox regression model was not constructed because no variable was significant with a type I error <10% in univariable analysis (data not shown).
Based on ROC curve analysis, the diagnostic accuracy for baseline tCa to predict incident total hypercalcemia was fair at best with AUROC of 0.69 (95% CI, 0.62–0.77) for the CKD group and 0.75 (95% CI, 0.58–0.93) for the nonazotemic group (Fig 3). In the CKD group, a baseline tCa cutoff of 9.7 mg/dL had a sensitivity of 96.7% (95% CI, 88.5–99.6; specificity 26.7%), whereas a cutoff of 11.2 mg/dL had a specificity of 94.7% (95% CI, 89.3–97.8; sensitivity 16.7%). In nonazotemic cases, a baseline tCa cutoff of 10.1 mg/dL had a sensitivity of 70.0% (95% CI, 34.8–93.3) and a specificity of 71.7% (95% CI, 64.2–78.4) to predict incident total hypercalcemia.
Figure 3.
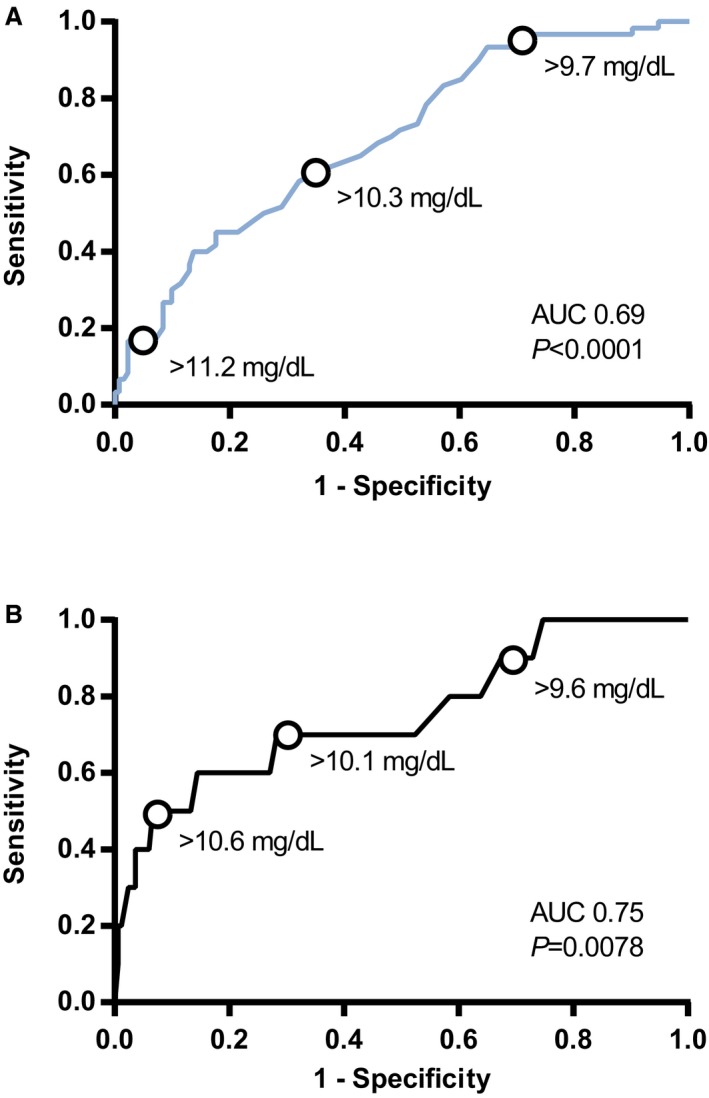
Receiver operating characteristic (ROC) curves illustrating the predictive ability of different baseline plasma tCa for development of total hypercalcemia in (A) chronic kidney disease (CKD) and (B) nonazotemic cats. Diagnostic accuracy of the model was poor in CKD cases (area under the ROC curve [AUROC], 0.69; 95% confidence interval [CI], 0.62–0.77; P < .0001) and fair in nonazotemic cases (AUROC, 0.75; 95% CI, 0.58–0.93; P = .0078). Points on the graphs illustrate decision thresholds of baseline tCa for the prediction of incident total hypercalcemia during follow‐up (tCa reference interval: 8.20–11.80 mg/dL). AUC, area under the curve; P, significance.
Discussion
Results of our retrospective study show that cats with azotemic CKD were more likely to develop total hypercalcemia than nonazotemic cats. Higher baseline tCa predicted incident total hypercalcemia in both azotemic and nonazotemic animals. At any time, higher plasma tCa and creatinine concentrations were associated with incident total hypercalcemia in cats with azotemic CKD. The majority of cats with total hypercalcemia had concomitant ionized hypercalcemia. Conversely, underestimation of iCa by tCa was common and associated with lower venous HCO3 −, and plasma albumin and creatinine concentrations.
Although the biologically active ionized calcium fraction most accurately reflects true calcium status, tCa usually is relied on in practice. Total calcium concentration only shows moderate association with iCa in cats. The correlation of plasma tCa to whole blood iCa found in this study (r = 0.63) is similar to the previously reported correlation for cats with CKD (r = 0.68).10 This association was found to be higher in the general population (r = 0.87),10 but here the association in nonazotemic cats (r = 0.62) was similar to that in cats with renal azotemia. Differences in age and sample handling could have accounted for this observation. Age was not a selection criterion in the previous study,10 whereas nonazotemic cats in our study were ≥9 years. Both analytes were measured in serum in the previous study, whereas in our study iCa was measured in whole blood directly after sampling, and tCa was measured in heparinized plasma.
In contrast to the concept that hypercalcemia in cats with CKD generally represents an increase in tCa only,12, 13, 14 the majority of cats with hypercalcemia based on tCa in our study had a coexistent increase in iCa. Specificity for tCa to detect ionized hypercalcemia was close to 100%, which is in agreement with high specificity values reported previously for cats10 and humans15 with CKD, but dissimilar to the situation in dogs with CKD, in which tCa tends to overestimate iCa.25
A large proportion of ionized hypercalcemic cats had a normal tCa in the cross‐sectional study. The ROC curve analysis showed that the possibility of ionized hypercalcemia should be considered if tCa is within the upper half of the reference interval. Low sensitivity of tCa to detect ionized hypercalcemia has been reported previously in humans15 and cats10 with CKD, and was associated with lower total CO2 and albumin concentrations in human CKD patients.15 Our study identified lower HCO3 − as a risk factor for failure of tCa to detect ionized hypercalcemia in cats. Noncorrected tCa previously was shown to be influenced by HCO3 − and albumin concentrations in human CKD patients with underestimated iCa.15 In agreement with this finding, HCO3 −, albumin, and creatinine were identified as predictors of tCa in cats with CKD. These associations were independent of iCa, and lower concentrations of these analytes could therefore increase the risk for underestimation of iCa.
Bicarbonate concentration, reflecting acid‐base status, affects plasma calcium concentration by influencing bone storage,26, 27, 28 protein binding,29 and tubular reabsorption of calcium.30 Metabolic acidosis stimulates osteoclastic bone resorption,26, 27, 28 but decreases the protein‐bound calcium fraction as calcium and hydrogen ions compete for albumin binding sites.29, 31 Free and complexed calcium concentrations could possibly increase as a result, but these ultrafilterable fractions are under control of renal regulation.32 Indeed, metabolic acidosis was associated with increased urinary calcium excretion in dogs,30, 33 whereas iCa did not appear affected.34 However, if renal calcium excretion were to be impaired, ionized hypercalcemia could occur in conjunction with total normocalcemia. The association between creatinine and tCa supports a role for metabolic acidosis, because its prevalence increases with a decrease in kidney function.35 Conversely, a higher HCO3 − is expected to increase tCa by stimulation of tubular calcium reabsorption30 and increased protein binding,29 as reflected by the iCa‐independent association between HCO3 − and tCa found in the cross‐sectional analysis.
A lower albumin concentration would decrease the protein‐bound calcium fraction36 and was found to increase risk of underestimation. Historically, correction formulas were developed in an attempt to adjust tCa for albumin binding in humans37 and dogs,38 but not for cats because of the particularly weak association between calcium and albumin in this species.16, 17 Correction formulas currently are considered obsolete due to poor performance.15, 25
The finding that creatinine was independently associated with tCa in CKD cats could be indicative not only of a role for metabolic acidosis, but also for increased availability of calcium‐binding anions. In dogs with CKD, tCa is mainly influenced by increases in the complexed calcium fraction, which underlies the tendency of tCa to overestimate the prevalence of ionized hypercalcemia in that species.39 Data on the plasma concentrations of most calcium‐binding anions were not available for our study, except for phosphate, which showed no association with risk of underestimation (P = .802).
Results from our retrospective cohort study show that cats with CKD are at risk for the development of total hypercalcemia. Sixty of 191 cats (31.4%) developed total hypercalcemia at a median of 175 [63, 403] days after diagnosis of azotemic CKD. Total hypercalcemia previously was reported with a prevalence of 10–32% of cats with CKD,1, 3, 5, 10 and incident hypercalcemia, with both tCa and iCa increased, in 2 of 15 cats within 6 months after diagnosis of CKD.40
The 3 most common causes of hypercalcemia in human CKD patients are neoplasia, hyperparathyroidism, and milk‐alkali syndrome.41 In a previous publication on total hypercalcemia in cats, 6 of 33 azotemic cats had concurrent neoplastic disease, and 9 had concurrent urolithiasis,2 and in our study, abdominal masses and calcium oxalate crystalluria were identified in 2 cases each. Ionized hypercalcemia in cats with CKD is thought to occur secondary to severe renal hyperparathyroidism, or as a result of administration of vitamin D metabolites (eg, calcitriol).42 Hypervitaminosis D appears to be an unlikely cause of total hypercalcemia in our study,43 because none of the cats were known to have been exposed to or had been treated with vitamin D analogues or metabolites. Furthermore, plasma phosphate concentrations were generally within the reference interval. Severe hyperparathyroidism cannot be ruled out. Plasma PTH concentrations were not available in most cats at the hypercalcemic visit. In cats with total hypercalcemia that had PTH measured, concentrations were low and generally had decreased compared to baseline. In a previous study, plasma PTH concentrations of cats that developed hypercalcemia after diagnosis of CKD decreased to below the lowest limit of detection of the assay,40 which also is a common finding in cats with idiopathic hypercalcemia.7 , 4 Idiopathic hypercalcemia could have been the underlying cause of total hypercalcemia in our study in the nonazotemic group as well as possibly in the cats with concurrent CKD.7 , 4 However, in our study, other causes of hypercalcemia were not completely excluded because investigations such as diagnostic imaging were not performed, and therefore, a confirmed diagnosis of idiopathic hypercalcemia in the nonazotemic cats cannot be made. It also remains unclear what role pathophysiological mechanisms resulting in idiopathic hypercalcemia might play in the development of total hypercalcemia in azotemic cats.
Phosphate restriction potentially could contribute to development of hypercalcemia in CKD, because withdrawal of dietary phosphate restriction restored normocalcemia in 1 study.40 A randomized placebo‐controlled clinical trial in human CKD patients not on dialysis showed that phosphate‐binding treatment led to progressive vascular calcification,44 even with calcium‐free binders. The authors hypothesized that intestinal calcium would be more readily absorbed with phosphate restriction. Higher PO intake of calcium has been shown to result in hypercalcemia in children with CKD.45 Other dietary factors, such as potassium and any accompanying bicarbonate‐generating organic anion, also are involved in calcium homeostasis.46
Baseline tCa was significantly higher in cats that developed total hypercalcemia and predicted incident total hypercalcemia in both nonazotemic cats and cats with azotemic CKD. In cats with renal azotemia, a higher tCa at any time point was associated with incident total hypercalcemia independent of severity of azotemia. Higher plasma calcium concentrations, and ultimately hypercalcemia, could have been caused by several mechanisms. Polymorphisms in the calcium‐sensing receptor have been identified in cats.8 , 47 and were associated with higher serum tCa in healthy women48, 49 and in human end‐stage CKD patients.50 Decreased bone formation is a known consequence of CKD in humans, leading to inability of bone to buffer changes in serum calcium concentration.51, 52 Five cats received aluminum‐containing phosphate binders, the use of which has been associated with low bone turnover and hypercalcemia in human CKD patients.53
Higher plasma creatinine concentrations at any time point were associated with incident total hypercalcemia independent of tCa in cats with CKD. The prevalence of total hypercalcemia was shown to increase with severity of CKD previously, from 8% in early stage kidney disease to 32% in end‐stage CKD.1 As discussed above, a decline in kidney function could contribute to an increase in calcium‐containing ionic complexes, as has been shown in dogs with CKD.39 Metabolic acidosis is found with increasing frequency in cats as renal function declines.35 In rodent models, metabolic acidosis has been documented to stimulate osteoclastic bone resorption,54 releasing calcium carbonate into the circulation.27 Increased bone resorption and decreased bone mineral density are known consequences of CKD in cats.55 Interestingly, previous studies of cats report that hypochloremia develops before manifestation of metabolic acidosis in cats with CKD,56 possibly in an attempt to maintain HCO3 − concentrations within normal limits.57 In our study, lower baseline plasma chloride concentrations appeared associated with incident total hypercalcemia with a type I error <10% in univariable analysis (P = .089). Therefore, an increased requirement for skeletal buffering of acid could be related to total hypercalcemia in cats with CKD. Further work is warranted to test this hypothesis.
By contrast, milk‐alkali syndrome is a form of hypercalcemia in CKD associated with metabolic alkalosis, often caused by ingestion of calcium carbonate.41 Alkali ingestion, tubular reabsorption of HCO3 −, and suppression of PTH contribute to metabolic alkalosis,41 which in turn leads to increased tubular calcium reabsorption.30, 33 Cats in the retrospective cohort were not treated with calcium carbonate, and classic milk‐alkali syndrome is unlikely to have caused total hypercalcemia. However, HCO3 − was positively associated with tCa in the cross‐sectional analysis, and it could be hypothesized that higher tCa could in part be due to bicarbonate‐induced increases in tubular calcium reabsorption.
Our study had a number of limitations because of its retrospective design. Cats ate a wide variety of commercial cat foods at baseline, and all CKD cats were offered a phosphate‐restricted renal diet for management of their disease, but precise data on what proportion of the renal diet was fed and other dietary contributions often were unavailable. Data on dietary calcium and vitamin D concentrations were not available. Therefore, no conclusions could be drawn on any possible dietary interaction.
Although no diagnosis other than azotemic CKD was made in the majority of primary care cases presented, concurrent conditions, including occult neoplasia and hyperparathyroidism, cannot be ruled out because diagnostic tests other than biochemistry and urinalysis were not consistently performed. Lack of blood gas data prohibited evaluation of the effect of acid‐base disturbances on development of total hypercalcemia.
Classification of cohort cases was based purely on tCa. Because of low sensitivity of tCa to detect ionized hypercalcemia, ionized hypercalcemic cats likely were misclassified in the normocalcemic groups. Although our study focused on total hypercalcemia, factors affecting iCa could be expected to influence tCa, and differences between cats that remained normocalcemic and cats that developed total hypercalcemia could have been missed due to misclassification.
Our study highlights the importance of hypercalcemia in cats with CKD because total hypercalcemia developed in 60 of 191 cats (31.4%). The supposition that hypercalcemia in cats with CKD represents total hypercalcemia only, with the biologically active ionized calcium fraction adequately regulated and within its reference interval, should be questioned. Supported by the high specificity reported here and previously,10 most cats with total hypercalcemia are suspected to have ionized hypercalcemia. Sensitivity of tCa to detect ionized hypercalcemia is low, and therefore measurement of iCa is a necessity for accurate assessment of calcium status in cats. In human medicine, increased serum calcium concentrations as part of chronic kidney disease‐mineral and bone disorder (CKD‐MBD) have been associated with vascular calcification,58, 59, 60 cardiovascular morbidity, and increased mortality.61, 62 Similarly, in cats, previous case reports have identified calcification of the aorta, gastric wall, paws, and kidneys associated with a high calcium‐phosphorus product, although the full extent of soft tissue mineralization as a component of CKD‐MBD in cats remains to be fully elucidated.3, 63, 64, 65, 66, 67 Higher baseline tCa did not predict all‐cause mortality in cats with CKD,68 but underdiagnosis of ionized hypercalcemia may have impacted these results and therefore the full effect of hypercalcemia on prognosis in cats with CKD remains to be determined. Prospective studies that longitudinally assess iCa throughout the course of CKD are warranted to further understand both the biochemical and clinical consequences of calcium derangements in cats with CKD.
Acknowledgment
The Renal Research Clinic at the Royal Veterinary College acknowledges support from Royal Canin for its research on CKD‐mineral and bone disorder in cats.
Conflict of Interest Declaration: For transparency and completeness, the study is reported according to STARD and STROBE guidelines. HB received PhD studentship funded by Royal Canin. JE received funding from Consultancies: Elanco Ltd, CEVA Animal Health Ltd, Boehringer Ingelheim Ltd, Bayer Animal Health, Orion Incorp, Idexx Ltd, Nextvet Ltd, Waltham Centre for Pet Nutrition; grant funding from Elanco Ltd, Waltham Centre for Pet Nutrition, Royal Canin Ltd, Zoetis Ltd, CEVA Animal Health, Member of the International Renal Interest Society which receives a grant from Elanco Ltd. RJ received funding from PetPlan, Feline Foundation for Renal Research, RVC Internal Grant, PetSavers, and consultancy agreements: Boehringer Ingelheim, Merial. Speaking honoraria: Boehringer Ingelheim, Hills Pet Nutrition. YMC declared no conflicts of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Royal Veterinary College, London, UK, and supported by a grant from Royal Canin SAS, Aimargues, France.
Footnotes
Idexx laboratories, Wetherby, UK
i‐STAT 1 point‐of‐care analyzer, Abbott Point of Care Inc., Princeton, NJ
FGF‐23 ELISA Kit, Kainos Laboratories, Tokyo, Japan
Total intact PTH immunoradiometric assay—coated bead version, 3KG600, Scantibodies, Santee, CA
Michigan State University Diagnostic Center for Population and Animal Health, Lansing, MI
R i386 3.1.3, R Foundation for Statistical Computing, Vienna, Austria, and GraphPad Prism 6, GraphPad Software, La Jolla, CA
Schenck PA, Chew DJ, Refsal K, Nachreiner R, Rick M. Calcium metabolic hormones in feline idiopathic hypercalcemia. J Vet Intern Med 2004; 18: 442 (abstract)
Geddes RF, Forcada Y, Catchpole B, Elliott J, Syme HM. Eight novel polymorphisms identified in the feline calcium sensing receptor in cats with varying plasma ionised calcium concentrations. J Vet Intern Med 2013; 27: 687 (abstract)
References
- 1. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998;39:108–116. [DOI] [PubMed] [Google Scholar]
- 2. Savary KC, Price GS, Vaden SL. Hypercalcemia in cats: a retrospective study of 71 cases (1991–1997). J Vet Intern Med 2000;14:184–189. [DOI] [PubMed] [Google Scholar]
- 3. DiBartola SP, Rutgers HC, Zack PM, et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987;190:1196–1202. [PubMed] [Google Scholar]
- 4. Midkiff AM, Chew DJ, Randolph JF, et al. Idiopathic hypercalcemia in cats. J Vet Intern Med 2000;14:619–626. [DOI] [PubMed] [Google Scholar]
- 5. Lulich JP, Osborne CA, O'Brien TD, et al. Feline renal‐failure ‐ questions, answers, questions. Compend Contin Educ Vet 1992;14:127. [Google Scholar]
- 6. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014;16:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levi M, Peterson L, Berl T. Mechanism of concentrating defect in hypercalcemia. Role of polydipsia and prostaglandins. Kidney Int 1983;23:489–497. [DOI] [PubMed] [Google Scholar]
- 8. Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin‐angiotensin system, and calcium. J Clin Invest 1983;71:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol 2010;5(Suppl 1):S23–S30. [DOI] [PubMed] [Google Scholar]
- 10. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res 2010;74:209–213. [PMC free article] [PubMed] [Google Scholar]
- 11. Schenck PA, Chew DJ. Calcium: total or ionized? Vet Clin North Am Small Anim Pract 2008;38:497–502, ix. [DOI] [PubMed] [Google Scholar]
- 12. Schenck PA, Chew DJ. Hypercalcemia: a quick reference. Vet Clin North Am Small Anim Pract 2008;38:449–453, viii. [DOI] [PubMed] [Google Scholar]
- 13. Schenck PA. Electrolyte disorders: Ca‐P and Mg In: Ettinger SJ, Feldman EC. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat. St. Louis, MO, USA: Saunders Elsevier; 2010:308. [Google Scholar]
- 14. Finch NC. Hypercalcaemia in cats: The complexities of calcium regulation and associated clinical challenges. J Feline Med Surg 2016;18:387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gauci C, Moranne O, Fouqueray B, et al. Pitfalls of measuring total blood calcium in patients with CKD. J Am Soc Nephrol 2008;19:1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flanders JA, Scarlett JM, Blue JT, et al. Adjustment of total serum calcium concentration for binding to albumin and protein in cats: 291 cases (1986–1987). J Am Vet Med Assoc 1989;194:1609–1611. [PubMed] [Google Scholar]
- 17. Bienzle D, Jacobs RM, Lumsden JH. Relationship of serum total calcium to serum albumin in dogs, cats, horses and cattle. Can Vet J 1993;34:360–364. [PMC free article] [PubMed] [Google Scholar]
- 18. Malatesha G, Singh NK, Bharija A, et al. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J 2007;24:569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelly AM, McAlpine R, Kyle E. Agreement between bicarbonate measured on arterial and venous blood gases. Emerg Med Aust 2004;16:407–409. [DOI] [PubMed] [Google Scholar]
- 20. Geddes RF, Finch NC, Elliott J, et al. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med 2013;27:234–241. [DOI] [PubMed] [Google Scholar]
- 21. Williams TL, Elliott J, Syme HM. Calcium and phosphate homeostasis in hyperthyroid cats: associations with development of azotaemia and survival time. J Small Anim Pract 2012;53:561–571. [DOI] [PubMed] [Google Scholar]
- 22. Geddes RF. Calcium‐Phosphate Homeostasis in Feline Chronic Kidney Disease. London: Royal Veterinary College, University of London; 2014. PhD Thesis. [Google Scholar]
- 23. Must A, Anderson SE. Body mass index in children and adolescents: considerations for population‐based applications. Int J Obes 2006;30:590–594. [DOI] [PubMed] [Google Scholar]
- 24. Abdi H. Z‐scores. Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- 25. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by use of serum total calcium concentration in dogs. Am J Vet Res 2005;66:1330–1336. [DOI] [PubMed] [Google Scholar]
- 26. Bushinsky DA. Stimulated osteoclastic and suppressed osteoblastic activity in metabolic but not respiratory acidosis. Am J Physiol 1995;268:C80–C88. [DOI] [PubMed] [Google Scholar]
- 27. Lemann J Jr, Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 1966;45:1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burnell JM. Changes in bone sodium and carbonate in metabolic acidosis and alkalosis in the dog. J Clin Invest 1971;50:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fogh‐Andersen N. Albumin/calcium association at different pH, as determined by potentiometry. Clin Chem 1977;23:2122–2126. [PubMed] [Google Scholar]
- 30. Marone CC, Wong NL, Sutton RA, et al. Effects of metabolic alkalosis on calcium excretion in the conscious dog. J Lab Clin Med 1983;101:264–273. [PubMed] [Google Scholar]
- 31. Costanzo LS. Regulation of calcium and phosphate homeostasis. Adv Physiol Educ 1998;20:S206–S216. [Google Scholar]
- 32. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 2015;10:1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sutton RA, Wong NL, Dirks JH. Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int 1979;15:520–533. [DOI] [PubMed] [Google Scholar]
- 34. Kogika MM, Lustoza MD, Notomi MK, et al. Serum ionized calcium in dogs with chronic renal failure and metabolic acidosis. Vet Clin Pathol 2006;35:441–445. [DOI] [PubMed] [Google Scholar]
- 35. Elliott J, Syme HM, Reubens E, et al. Assessment of acid‐base status of cats with naturally occurring chronic renal failure. J Small Anim Pract 2003;44:65–70. [DOI] [PubMed] [Google Scholar]
- 36. Berry EM, Gupta MM, Turner SJ, et al. Variation in plasma calcium with induced changes in plasma specific gravity, total protein, and albumin. Br Med J 1973;4:640–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Payne RB, Little AJ, Williams RB, et al. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J 1973;4:643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meuten DJ, Chew DJ, Capen CC, et al. Relationship of serum total calcium to albumin and total protein in dogs. J Am Vet Med Assoc 1982;180:63–67. [PubMed] [Google Scholar]
- 39. Schenck PA, Chew DJ. Determination of calcium fractionation in dogs with chronic renal failure. Am J Vet Res 2003;64:1181–1184. [DOI] [PubMed] [Google Scholar]
- 40. Barber PJ, Rawlings JM, Markwell PJ, et al. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract 1999;40:62–70. [DOI] [PubMed] [Google Scholar]
- 41. Picolos MK, Lavis VR, Orlander PR. Milk‐alkali syndrome is a major cause of hypercalcaemia among non‐end‐stage renal disease (non‐ESRD) inpatients. Clin Endocrinol 2005;63:566–576. [DOI] [PubMed] [Google Scholar]
- 42. Polzin DJ. Chronic kidney disease In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 7th ed St. Louis, MO, USA: Saunders; 2010:1990–2021. [Google Scholar]
- 43. Schenck PA, Chew DJ, Nagode LA, et al. Disorders of calcium: hypercalcemia and hypocalcemia In: DiBartola SP. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice, 4th edn St. Louis, MO, USA: Saunders; 2012:120–194. [Google Scholar]
- 44. Block GA, Wheeler DC, Persky MS, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 2012;23:1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pieper AK, Haffner D, Hoppe B, et al. A randomized crossover trial comparing sevelamer with calcium acetate in children with CKD. Am J Kidney Dis 2006;47:625–635. [DOI] [PubMed] [Google Scholar]
- 46. Rafferty K, Heaney RP. Nutrient effects on the calcium economy: emphasizing the potassium controversy. J Nutr 2008;138:166S–171S. [DOI] [PubMed] [Google Scholar]
- 47. Gal A, Ridge TK, Graves TK. Cloning and sequencing of the calcium‐sensing receptor from the feline parathyroid gland. Domest Anim Endocrinol 2010;38:57–61. [DOI] [PubMed] [Google Scholar]
- 48. Cole DE, Vieth R, Trang HM, et al. Association between total serum calcium and the A986S polymorphism of the calcium‐sensing receptor gene. Mol Genet Metab 2001;72:168–174. [DOI] [PubMed] [Google Scholar]
- 49. He Y, Han L, Li W, et al. Effects of the calcium‐sensing receptor A986S polymorphism on serum calcium and parathyroid hormone levels in healthy individuals: a meta‐analysis. Gene 2012;491:110–115. [DOI] [PubMed] [Google Scholar]
- 50. Eren PA, Turan K, Berber I, et al. The clinical significance of parathyroid tissue calcium sensing receptor gene polymorphisms and expression levels in end‐stage renal disease patients. Clin Nephrol 2009;72:114–121. [PubMed] [Google Scholar]
- 51. Andress DL. Adynamic bone in patients with chronic kidney disease. Kidney Int 2008;73:1345–1354. [DOI] [PubMed] [Google Scholar]
- 52. Kurz P, Monierfaugere MC, Bognar B, et al. Evidence for abnormal calcium homeostasis in patients with adynamic bone‐disease. Kidney Int 1994;46:855–861. [DOI] [PubMed] [Google Scholar]
- 53. Piraino B, Chen T, Puschett JB. Elevated bone aluminum and suppressed parathyroid hormone levels in hypercalcemic dialysis patients. Am J Nephrol 1989;9:190–197. [DOI] [PubMed] [Google Scholar]
- 54. Meghji S, Morrison MS, Henderson B, et al. pH dependence of bone resorption: mouse calvarial osteoclasts are activated by acidosis. Am J Physiol Endocrinol Metab 2001;280:E112–E119. [DOI] [PubMed] [Google Scholar]
- 55. Shipov A, Segev G, Meltzer H, et al. The effect of naturally occurring chronic kidney disease on the micro‐structural and mechanical properties of bone. PLoS ONE 2014;9:e110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elliott J, Syme HM, Markwell PJ. Acid‐base balance of cats with chronic renal failure: effect of deterioration in renal function. J Small Anim Pract 2003;44:261–268. [DOI] [PubMed] [Google Scholar]
- 57. Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med 2012;23:203–211. [DOI] [PubMed] [Google Scholar]
- 58. Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int 2004;66:2293–2299. [DOI] [PubMed] [Google Scholar]
- 59. Yamada K, Fujimoto S, Nishiura R, et al. Risk factors of the progression of abdominal aortic calcification in patients on chronic haemodialysis. Nephrol Dial Transplant 2007;22:2032–2037. [DOI] [PubMed] [Google Scholar]
- 60. Shanahan CM, Crouthamel MH, Kapustin A, et al. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res 2011;109:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kovesdy CP, Kuchmak O, Lu JL, et al. Outcomes associated with serum calcium level in men with non‐dialysis‐dependent chronic kidney disease. Clin J Am Soc Nephrol 2010;5:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008;52:519–530. [DOI] [PubMed] [Google Scholar]
- 63. Barber PJ. Parathyroid Gland Function in the Ageing Cat. London: Royal Veterinary College, University of London; 1998:289. PhD Thesis. [Google Scholar]
- 64. McLeland SM, Lunn KF, Duncan CG, et al. Relationship among serum creatinine, serum gastrin, calcium‐phosphorus product, and uremic gastropathy in cats with chronic kidney disease. J Vet Intern Med 2014;28:827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013;50:147–155. [DOI] [PubMed] [Google Scholar]
- 66. Bertazzolo W, Toscani L, Calcaterra S, et al. Clinicopathological findings in five cats with paw calcification. J Feline Med Surg 2003;5:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jackson HA, Barber PJ. Resolution of metastatic calcification in the paws of a cat with successful dietary management of renal hyperparathyroidism. J Small Anim Pract 1998;39:495–497. [DOI] [PubMed] [Google Scholar]
- 68. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor‐23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med 2015;29:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]


