Abstract
Background
Cardiovascular disease has been associated with oxidative stress, which has been suggested to contribute to myocardial remodeling in human patients. Little is known about the relationship between myxomatous mitral valve disease (MMVD) and oxidative stress in dogs.
Objective
To determine whether clinical stage of MMVD is associated with changes in the plasma concentrations of certain markers of oxidative stress in clinically healthy dogs and dogs with MMVD.
Animals
Seventy five privately owned dogs: 59 cavalier King Charles Spaniels (CKCS) with different severities of MMVD and 16 dogs of different breeds with clinical signs of congestive heart failure (CHF) caused by MMVD.
Methods
Markers of oxidative stress including malondialdehyde (MDA), oxidized low‐density lipoprotein (oxLDL), and vitamin E (α‐tocopherol and γ‐tocopherol) were measured in plasma and their association with clinical stage of MMVD was assessed by regression analyses.
Results
Plasma oxLDL concentration was significantly lower in female dogs compared with males (P = .01). Significantly higher plasma γ‐tocopherol concentrations were found in neutered (P = .003) dogs. Vitamin E (α‐tocopherol [P = .0004] and γ‐tocopherol [P = .003]) was associated with body condition score (BCS), but the association disappeared when cholesterol was included in the analyses. All markers of oxidative stress (MDA, oxLDL, and vitamin E) were positively associated with serum cholesterol concentration (P ≤ .04), but none were associated with clinical stage of MMVD.
Conclusions
In conclusion, markers of oxidative stress are associated with sex, BCS, neuter status, and cholesterol. The results cannot confirm a relationship between oxidative stress and clinical stage of the disease in dogs with MMVD.
Keywords: Malondialdehyde, Oxidized low‐density lipoprotein, Valvular disease, Vitamin E
Abbreviations
- ACVIM
American College of Veterinary Internal Medicine
- BCS
body condition score
- BW
body weight
- CHF
congestive heart failure
- CKCS
Cavalier King Charles Spaniel
- cTnI
cardiac troponin‐I
- CV
coefficient of variation
- DBP
diastolic blood pressure
- DCM
dilated cardiomyopathy
- FS
fractional shortening
- IVSDN
interventricular septal thickness in diastole normalized for body weight
- IVSSN
interventricular septal thickness in systole normalized for body weight
- LA/Ao
left atrial‐to‐aortic root ratio
- LVIDDN
left ventricular end‐diastolic diameter normalized for body weight
- LVIDSN
left ventricular end‐systolic diameter normalized for body weight
- LV
left ventricular
- LVPWDN
left ventricular free wall thickness in diastole normalized for body weight
- LVPWSN
left ventricular free wall thickness in systole normalized for body weight
- MBP
mean blood pressure
- MDA
malondialdehyde
- MMVD
myxomatous mitral valve disease
- MR
mitral regurgitation
- oxLDL
oxidized low‐density lipoprotein
- ROS
reactive oxygen species
- SBP
systolic blood pressure
Oxidative stress describes an imbalance between production of reactive oxygen species (ROS) and antioxidant defenses in the body.1 The ROS may cause tissue damage by oxidative modification of lipids, proteins, and DNA and, in addition, by the release of pro‐inflammatory cytokines.2, 3 Because it is difficult to measure ROS directly, indirect measures of circulating concentrations of oxidative products such as malondialdehyde (MDA) and oxidized low‐density lipoproteins (oxLDL) are used to estimate the degree of oxidative stress.3, 4 Other indirect markers of oxidative stress include measurement of circulating concentrations of antioxidants such as vitamin E.3
The pathogenesis of myxomatous mitral valve disease (MMVD) remains unresolved.5 It is characterized by progressive myxomatous degeneration of the mitral valves resulting in insufficient coaptation of the valve leaflets and mitral regurgitation (MR).6, 7, 8 Chronic MR causes left ventricular (LV) and atrial volume overload with subsequent risk of remodeling and myocardial alterations.9, 10, 11, 12, 13 Oxidative stress has been suggested to contribute to development of remodeling and dysfunction in human patients with MR.14, 15 In agreement, in vitro studies have demonstrated that overstretching and cyclic stretch of cardiomyocytes increase ROS production, and the process may be involved in mediating apoptosis and contractile dysfunction.16, 17 Interestingly, in a study of experimentally induced heart failure in guinea pigs, increased oxidative stress was reported in the transition phase from myocardial hypertrophy to heart failure.18 Few previous studies have suggested altered oxidative status in dogs with either naturally occurring or experimentally induced MMVD.19, 20, 21
The aim of our study was to investigate whether or not clinical stage of MMVD is associated with changes in plasma concentrations of biomarkers of oxidative stress in healthy dogs and dogs with MMVD.
Materials and Methods
Our study included client‐owned dogs ≥4 years of age with no MMVD or different clinical stages of MMVD. All owners agreed to participate by written informed consent and the study was approved by the Danish Animal Experiments Inspectorate (license no. 2012‐15‐2934‐00700). Exclusion criteria included medical treatment (except for cardiac treatment) and presence of cardiac disorders other than MMVD and noncardiac illnesses. All dogs were recruited at the University of Copenhagen, Faculty of Health and Medical Sciences, and Din Veterinär Animal Hospital, Helsingborg, Sweden, between October 2011 and February 2012 and examined by a standardized protocol in the following order: interview with the owner, collection of venous blood, physical examination, blood pressure measurement, and echocardiography. Owner interview included passive exposure to smoke, defined as dogs living in a home where the owner smoked indoors. Body condition score (BCS) was graded 1–9.22 Left apical systolic heart murmur intensity was graded 1–6.23 Blood pressure was determined by use of high definition oscillometry equipment1 , 24 on the proximal part of the tail and an average of 5 repetitive measurements was used as previously described.25 To verify the diagnosis of CHF and to rule out concomitant pulmonary disease, dogs showing signs of overt CHF had thoracic radiographs (laterolateral and dorsoventral) taken (except 4 dogs because of logistic reasons). All dogs were part of previous studies concerning echocardiography and biomarkers in MMVD.21, 26, 27
Blood Sampling
Blood was collected from the jugular vein with a vacutainer system connected to a 21‐G butterfly catheter. Dogs were fasted 6–18 hours before blood sampling. To assess health status, CBC and serum biochemistry analysis was performed on all dogs. Plasma was separated by centrifugation within 30 minutes of collection and stored in cryotubes at −80°C until batched analysis was performed. Plasma vitamin E (α‐tocopherol and γ‐tocopherol) was analyzed in duplicate by high‐performance liquid chromatography (HPLC) with electrochemical detection as previously described.28 Plasma MDA was determined in triplicate as its genuine MDA‐thiobarbituric acid adduct by HPLC with fluorescence detection as described previously.29 Plasma oxLDL was assayed in triplicate with a commercially available ELISA kit2 according to the manufacturer's instructions, with mouse monoclonal antibodies (mAb 4E6) against a conformational epitope at the oxidized apolipoprotein B100 of the oxLDL.30
One dog with an inexplicably high concentration of plasma MDA (3.99 μmol/L) and 1 dog with an inexplicably high concentration of plasma oxLDL (35.4 U/L) were excluded from statistical analysis of MDA and oxLDL, respectively, because they were considered to be outliers based on visual inspection of the residual plots.
Plasma cardiac troponin‐I (cTnI) concentration was analyzed in duplicate with an ELISA kit3 as described elsewhere.31
Echocardiography
Echocardiographic examination was performed and evaluated by a single operator (LHO). During the offline echocardiographic analysis4 , the operator was blinded to the identity and clinical status of the dog. A standardized transthoracic echocardiographic examination from parasternal and apical windows32 was performed and digitally stored on an ultrasonographic unit5 with 3S and 5S ultrasound transducers and continuous electrocardiographic (ECG) monitoring.
Severity of MR was assessed from the left apical 4‐chamber view by 2D color Doppler flow mapping and was classified as minimal (<20%), mild (20–50%), or moderate‐severe (>50%) based on regurgitant jet area relative to left atrial area.33, 34 Left atrial‐to‐aortic root ratio (LA/Ao) at the level of the aortic root was determined from the 2D right parasternal short‐axis view.35 The LV dimensions and fractional shortening (FS) were obtained from M‐mode short‐axis images.36 All LV dimensions were normalized to body weight (BW).37
Classification of MMVD
Clinical stage of MMVD was determined according to American College of Veterinary Internal Medicine (ACVIM) consensus statement guidelines as follows: group A (Cavalier King Charles Spaniels [CKCS] with no auscultatory heart murmur and normal echocardiogram [MR < 20%]), group B1 (CKCS with auscultatory heart murmur or MR ≥ 20% and LA/Ao ≤ 1.5), group B2 (CKCS with auscultatory heart murmur or MR ≥ 20% and LA/Ao > 1.5), and group C (dogs of various breeds with CHF).35, 38 The inclusion of dog breeds other than CKCS in group C was carried out to increase the number of dogs. Classification of CHF included dogs with previous or current clinical signs of CHF (eg, cough, dyspnea, tachypnea, nocturnal restlessness, exercise intolerance), echocardiographic and radiographic changes compatible with CHF caused by MMVD, and response to treatment with diuretics.
Statistical Analysis
Data were analyzed by statistical software6 and the level of significance was set at P < .05.
Group associations were investigated by a nonparametric Kruskal‐Wallis test because many groups did not follow a normal distribution. Exploratory differences among groups were assessed by the Wilcoxon rank sum test with Bonferroni adjustment (except for LA/Ao because this variable was used to allocate dogs into disease groups). Fisher's exact test was performed on categorical data to investigate group differences (except for MR severity because this variable was used to allocate dogs into disease groups).
Intra‐assay coefficients of variation (CV) were calculated for all markers of oxidative stress.
Univariable regression analyses were performed to evaluate associations among the markers of oxidative stress, dog characteristics (age, sex, BCS, passive smoking, neuter status), disease group, cTnI, and serum cholesterol concentration. Logarithmic transformation was performed when necessary to ensure normal distribution of data. For the purpose of statistical analysis, BCS group 3 and 4 and groups 6 and 7 were merged as only 1 dog was found with a BCS 3 and 4 dogs with a BCS 7.
Multivariable regression analysis models initially were performed including all explanatory variables with P < .2 in the univariable regression analysis; subsequently, analyses were repeated excluding serum cholesterol concentration as an explanatory variable. Response variable residuals were tested for homogeneity of variation based on visual inspection of residual and QQ plots and the Shapiro‐Wilks test. All response variables (oxidative stress markers) were logarithmically transformed based on Box‐Cox analysis. Multivariable regression analyses were performed in a backward stepwise manner based on P values. Differences among groups in class variables were investigated by performing posthoc testing by Tukey‐Kramer adjustment when appropriate for multiple testing.
Results
Three dogs were excluded according to exclusion criteria. The final study population consisted of 75 dogs allocated in disease groups as follows: group A (n = 14), group B1 (n = 27), group B2 (n = 18), and group C (n = 16). Dog breeds in group C included 10 CKCS and 1 of each of the following breeds Crossbreed, Springer Spaniel, Dachshund, Bull terrier, Shetland Sheepdog, and Yorkshire Terrier. Baseline characteristics, conventional echocardiographic variables, cTnI, and concentrations of the different markers of oxidative stress of the final study population are shown in Table 1. All dogs in group C received cardiac medication as follows: diuretics (n = 15), pimobendan (n = 12), angiotensin converting enzyme inhibitor (n = 12), digoxin (n = 3), and hydralazine (n = 2). One dog did not receive diuretics at the time of examination, but subsequently responded well to diuretic treatment initiated on the day of examination.
Table 1.
Dog characteristics, echocardiographic variables, cardiac troponin‐I, and concentrations of plasma oxidative stress markers in 75 dogs with no or different severities of myxomatous mitral valve disease
| Disease group | N | A | B1 | B2 | C |
|---|---|---|---|---|---|
| Total number | 75 | 14 | 27 | 18 | 16 |
| Sex (female/male)a | 75 | 7/7 | 18/9 | 9/9 | 2/14 |
| Age (years) | 75 | 4.8 [4.2;5.9]B1,C | 6.5 [6.0;7.8]A,C | 7.5 [5.3;8.4]C | 11.0 [9.6;12.9]A,B1,B2 |
| BCS (3 + 4/5/6 + 7) | 72 | 4/7/3 | 5/10/11 | 4/8/6 | 2/4/8 |
| BW | 74 | 8.3 [7.6;8.8]C | 9.4 [7.9;10.5] | 9.1 [8.4;10.4] | 11.0 [9.9;13.1]A |
| Passive smoking (y/n) | 75 | 6/8 | 6/21 | 4/14 | 3/13 |
| Neutered (y/n) | 75 | 2/12 | 4/23 | 3/15 | 3/13 |
| SBP (mmHg) | 61 | 151 [140;164] | 154 [143;162] | 147 [138;155] | 150 [143;162] |
| DBP (mmHg) | 61 | 81 [69;87] | 79 [74;88] | 76 [74;79] | 86 [79;92] |
| MBP (mmHg) | 61 | 102 [100;109] | 105 [99;112] | 100 [98;107] | 108 [102;116] |
| MR severity (mini/mi/mo or se) | 75 | 14/0/0 | 2/19/6 | 0/7/11 | 0/0/16 |
| LA/Ao | 75 | 1.3 [1.2;1.4] | 1.4 [1.4;1.5] | 1.6 [1.6;1.8] | 2.2 [2.0;2.5] |
| LVIDDN | 74 | 1.5 [1.4;1.5]B2,C | 1.6 [1.4;1.6]C | 1.6 [1.5;1.8]A,C | 2.1 [1.9;2.3]A,B1,B2 |
| LVIDSN | 74 | 1.0 [1.0;1.1] | 1.0 [1.0;1.1] | 1.1 [1.0;1.2] | 1.2 [1.1;1.4] |
| FS (%) | 75 | 26 [24;32]C | 29 [23;33]C | 31 [24;38] | 41 [32;45]A, B1 |
| LVPWDN | 74 | 0.5 [0.4;0.5] | 0.5 [0.4;0.5] | 0.5 [0.4;0.5] | 0.5 [0.4;0.5] |
| LVPWSN | 74 | 0.6 [0.5;0.6] | 0.6 [0.5;0.6] | 0.6 [0.6;0.7] | 0.6 [0.5;0.6] |
| IVSDN | 74 | 0.4 [0.3;0.4] | 0.4 [0.4;0.5] | 0.4 [0.4;0.5] | 0.4 [0.4;0.4] |
| IVSSN | 74 | 0.5 [0.4;0.5]C | 0.5 [0.5;0.6]C | 0.5 [0.4;0.6] | 0.6 [0.6;0.6]A,B1 |
| Plasma cTnI (μg/L) | 75 | 0.01 [0.01;0.02]C | 0.03 [0.01;0.03]C | 0.03 [0.01;0.04] | 0.04 [0.03;0.08]A,B1 |
| Serum cholesterol (mmol/L) | 75 | 5.3 [5.0;7.4] | 6.4 [5.3;8.0] | 6.2 [4.7;6.8]C | 7.4 [6.4;8.8]B2 |
| Plasma MDA (μmol/L) | 74 | 1.10 [0.83;1.29] | 1.05 [0.84;1.30] | 0.92 [0.72;1.24] | 1.04 [0.86;1.12] |
| Plasma OxLDL (U/L) | 74 | 5.65 [4.92;6.45] | 5.73 [5.10;6.82] | 6.27 [5.50;6.55] | 6.07 [5.43;7.16] |
| Plasma α‐tocopherol (μmol/L) | 75 | 45.36 [38.22;52.83] | 58.48 [46.69;76.30] | 54.92 [47.34;61.13] | 59.63 [45.27;73.18] |
| Plasma γ‐tocopherol (μmol/L) | 75 | 0.98 [0.72;1.16] | 0.94 [0.69;1.03] | 0.81 [0.61;0.95] | 0.94 [0.74;1.32] |
BCS, body condition score; BW, body weight; cTnI, cardiac troponin‐I; DBP, diastolic blood pressure; FS, fractional shortening; IVSDN, interventricular septal thickness in diastole normalized for BW; IVSSN, interventricular septal thickness in systole normalized for BW; LA/Ao, ratio of left atrium to aortic root; LVIDDN, left ventricular end‐diastolic diameter normalized for BW; LVIDSN, left ventricular end‐systolic diameter normalized for BW; LVPWDN, left ventricular free wall thickness in diastole normalized for BW; LVPWSN, left ventricular free wall thickness in systole normalized for BW; MBP, mean blood pressure; MR, mitral regurgitation by jet area method where mini=minimal, mi=mild, mo=moderate, se=severe (mini: <20%, mi:20–50%, mo or se: >50%); SBP, systolic blood pressure. Values reported are median and interquartiles. Within each row, superscripts A,B1,B2,Crepresent the group from which there is statistically significant difference.
Sex (P = .008) differed significantly among disease groups.
The following intra‐assay CV% were obtained: MDA: 13.0%, oxLDL 9.6%, α‐tocopherol: 2.5%, and γ‐tocopherol: 5.6%.
Regression Analyses
Results from the univariable regression analyses are provided in Table 2.
Table 2.
P values of the univariable analyses in 75 dogs with no or different severities of myxomatous mitral valve disease
| Response Variables | ||||
|---|---|---|---|---|
| MDAa | OxLDLa | α‐tocopherol | γ‐tocopherol | |
| Explanatory variables | ||||
| Disease group | 0.73 | 0.56 | 0.51 | 0.42 |
| Sex | 0.099 | 0.012 | 0.92 | 0.52 |
| Age | 0.88 | 0.53 | 0.20 | 0.52 |
| BCS | 0.068 | 0.31 | 0.00045 | 0.0053 |
| Passive smoking (y/n) | 0.85 | 0.25 | 0.28 | 0.27 |
| Neutered (y/n) | 0.52 | 0.28 | 0.24 | 0.0038 |
| cTnI | 0.75 | 0.90 | 0.12 | 0.90 |
| Cholesterol | 0.00045 | 0.050 | <0.0001 | <0.0001 |
BCS, body condition score; cTnI, cardiac troponin‐I; MDA=malondialdehyde; oxLDL=oxidized low‐density lipoprotein; bold values represent variables with P < .2 that are included in multivariable regression analysis.
The statistical analysis of MDA and oxLDL included 74 dogs as 1 dog was excluded (outlier).
The final multivariable analyses resulted in the following associations: Only serum cholesterol concentration (β = 0.07, adjusted R 2 = 0.2, P = .0003) was associated with plasma MDA concentration.
Plasma oxLDL was associated with sex (P = .01) and serum cholesterol concentration (β = 0.03, P = .04). The model had an adjusted R 2 of 0.1. Females had significantly lower plasma oxLDL concentration (Fig 1). When excluding serum cholesterol concentration from the analysis, sex (P = .01) remained significant and the adjusted R 2 of this model was 0.07.
Figure 1.
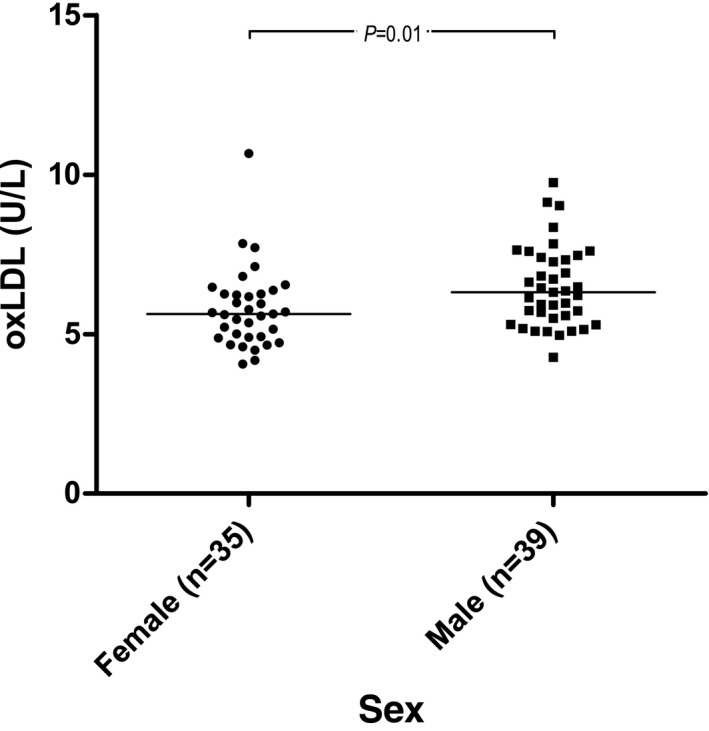
Raw data plot of plasma oxidized low‐density lipoprotein (oxLDL) concentrations in males and females. Medians are indicated with horizontal lines. Horizontal bars indicate statistically significant comparisons and their P values from multivariable regression analysis. n = 74.
Only a positive association with serum cholesterol concentration (β = 0.15, adjusted R 2 = 0.5, P < .0001) was found for plasma α‐tocopherol concentration. When excluding serum cholesterol from the multivariable analysis, BCS (adjusted R 2 = 0.2, P = .0004) was significantly associated with plasma α‐tocopherol concentrations and dogs with BCS ≤ 4 and BCS 5 had significantly lower plasma α‐tocopherol concentrations compared to dogs with BCS ≥ 6 (Fig 2).
Figure 2.
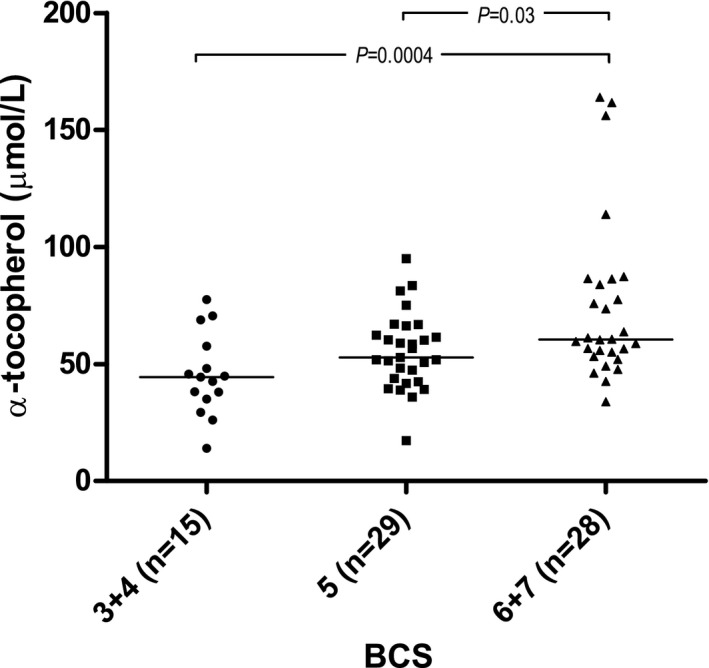
Raw data plot of plasma α‐tocopherol concentrations in the different body condition score (BCS) groups. Medians are indicated with horizontal lines. Horizontal bars indicate statistically significant comparisons and their P values from multivariable regression analysis. n = 72.
Regarding γ‐tocopherol concentration, a positive association with serum cholesterol concentration (β = 0.11, P < .0001) was found. Significantly increased plasma γ‐tocopherol concentrations in neutered (P = .003) dogs also were found (Fig 3). The adjusted R 2 of the model was 0.4. When excluding serum cholesterol concentration from the analysis, BCS (P = .003) also was associated with plasma γ‐tocopherol concentration (in addition to neuter status [P = .003]). Dogs with BCS 5 had significantly lower plasma γ‐tocopherol concentrations compared to dogs with BCS ≥6 (Fig 4). The model had an adjusted R 2 of 0.2.
Figure 3.
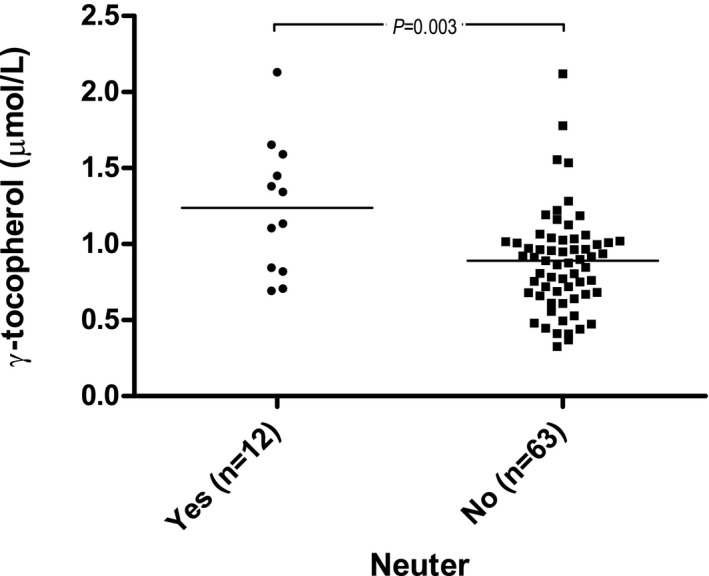
Raw data plot of plasma γ‐tocopherol concentrations and neuter status. Medians are indicated with horizontal lines. Horizontal bars indicate statistically significant comparisons and their P values from multivariable regression analysis. n = 75.
Figure 4.
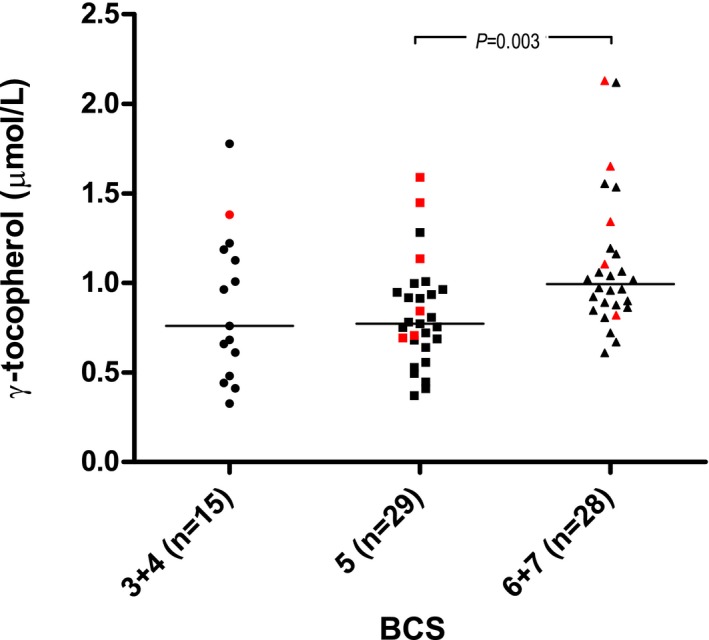
Raw data plot of plasma γ‐tocopherol concentrations in the different body condition score (BCS) groups. Red symbols indicate dogs that are neutered. Medians are indicated with horizontal lines. Horizontal bars indicate statistically significant comparisons and their P values from multivariable regression analysis. n = 72.
Discussion
Our study suggests that sex differences exist for plasma oxLDL concentrations because males generally had higher plasma oxLDL concentrations. Plasma vitamin E (α‐tocopherol and γ‐tocopherol) concentrations were associated with BCS, but the association did not remain when including serum cholesterol concentration in the multivariable regression analysis. Additionally, significantly increased plasma γ‐tocopherol concentration was found in neutered dogs. Serum cholesterol concentrations were positively associated with all of the evaluated markers of oxidative stress. An association between plasma concentrations of the selected biomarkers of oxidative stress and clinical stage of MMVD in dogs was not found.
Malondialdehyde is a secondary product formed during lipid peroxidation (ie, a reaction of oxygen with unsaturated lipids).39 In a previous study in dogs with MMVD, no association was found between plasma MDA and clinical stage of MMVD.20 This finding is in accordance with our study. In contrast, recent preliminary findings have suggested a significant relationship between plasma MDA concentrations and heart disease in a cohort of control dogs and dogs with different stages of heart disease of various causes.7 Plasma MDA concentrations in the studies differ however, because median MDA concentrations in the previous study were approximately 4 μM20 but approximately 1 μM in the preliminary study7 and in our study. Breed or assay differences might account for these differences. Interestingly, the recent preliminary findings indicate higher plasma MDA concentrations in dogs with untreated CHF compared to dogs with CHF that were receiving cardiac treatment.7 In our study, this phenomenon could not be investigated because all dogs with CHF had received cardiac treatment. Furthermore, although we found no association between plasma MDA concentration and clinical stage of MMVD, a possible role for MDA in the volume overloaded heart may exist as a previous study found increased MDA concentrations in the cardiac tissue of dogs with volume overload‐mediated CHF due to experimentally induced MR.19 These findings might indicate that changes in tissue MDA concentration in MMVD do exist but may not be reflected in plasma MDA concentrations. Similar plasma MDA concentrations as found in dogs have been reported in people40, but to the best of our knowledge, circulating concentrations of MDA have not been evaluated in human patients with MR.
Low‐density lipoprotein is involved in cholesterol transport in the body.41 Oxidation of LDL has been described as part of the atherosclerotic process in humans,42 and several studies have identified increased concentrations of oxLDL in human patients with coronary artery disease. Furthermore, oxLDL has been suggested to mediate apoptosis in human coronary smooth muscle cells.43, 44 In addition, plasma oxLDL concentration has been suggested as a useful predictor of coronary artery disease and of mortality in human patients.45, 46 In our study, plasma concentrations of oxLDL were significantly higher in male dogs compared with females. To the best of our knowledge, plasma oxLDL has not been measured previously in dogs with MMVD. However, in accordance with our data, human male patients appear to have higher circulating oxLDL concentrations compared with women.46 Furthermore, in humans, oxLDL has been determined to be increased in patients with MR if complicated by atrial fibrillation compared with clinically healthy controls.47
Dogs may have lower plasma oxLDL concentrations compared with humans.30, 48 This might be due to species differences and is in accordance with differences in lipid profiles found in humans and dogs.49, 50
In our study, plasma vitamin E (α‐tocopherol and γ‐tocopherol) concentrations were not associated with clinical stage of MMVD. Accordingly, preliminary findings from another recent study showed no association between CHF of various causes and plasma vitamin E concentrations in dogs.7 Furthermore, a study in guinea pigs with experimentally induced heart failure reported no difference in myocardial vitamin E (α‐tocopherol) content between sham‐operated controls and sham‐operated animals.18 Interestingly, vitamin E treatment in the guinea pigs did improve hemodynamic function (as assessed by LV function and blood pressure). The benefit of vitamin E supplementation in cardiac function also has been reported in experimentally induced MR in dogs.19 In the previously mentioned study, plasma vitamin E (α‐tocopherol) was significantly lower in dogs with CHF (caused by MMVD or DCM), suggesting a depletion that could be caused by increased ROS scavenging.20 The discrepancy between vitamin E status in the present study and the previously mentioned study might be a result of the higher number of dogs included in our study, breed differences, or both. Although the reported associations differ, vitamin E concentrations in our study and previous studies on dogs with MMVD were similar.20, 51 , 8 Because vitamin C is capable of regenerating vitamin E in vitro 52, it would have been interesting to know the plasma vitamin C concentrations in the dogs to determine whether vitamin C had been utilized to restore vitamin E concentration in dogs with severe disease or whether neither vitamin E nor vitamin C concentrations were decreased, suggesting no alterations in antioxidant status. Surprisingly, the previous study reported a significant increase in plasma vitamin C concentrations in dogs with CHF compared with healthy controls.20 However, because dogs in contrast to humans are capable of synthesizing vitamin C, this may be explained as a compensatory response.
A recent study in humans reported increased concentrations of biomarkers of oxidative stress and decreased circulating concentrations of antioxidants (including both α‐tocopherol and γ‐tocopherol) in candidates for cardiac surgery (including patients undergoing mitral valve surgery) compared with controls.53 Interestingly, among different etiologies of heart disease, mitral patients had the highest levels of oxidative stress and more prominent impairment of factors involved in nitric oxide generation.53 Compared with humans, the plasma concentrations of α‐tocopherol appear to be higher, whereas the concentrations of γ‐tocopherol seem to be lower, in dogs.53, 54
Our study indicates a tendency toward increased vitamin E concentrations in overweight dogs. However, it is worth noticing that no severely obese dogs participated. Vitamin E is a fat‐soluble vitamin stored in adipose tissue, and previous studies in humans have shown an association between vitamin E concentrations and circulating cholesterol concentrations.54, 55, 56 When serum cholesterol concentration was taken into account in our study, the association with BCS disappeared. This finding is in accordance with a study in humans reporting a positive relationship among plasma α‐tocopherol, percentage fat mass, and age that disappeared when adjusting for plasma cholesterol concentration.56 However, results regarding the relationship between vitamin E and overweight in humans are conflicting. Another study found a positive association between body mass index and serum vitamin E (α‐tocopherol) even when taking serum cholesterol concentrations into account.55 Vitamin E might be associated with cholesterol in dogs in a similar manner as in humans, although lipoprotein composition in dogs and humans differs.49, 50 In dogs, high‐density lipoprotein (suggested to protect against atherosclerosis) is the most abundant plasma lipoprotein, whereas low‐density lipoprotein (suggested to contribute to atherosclerosis) dominates human plasma.49, 50
Discrepancies in markers of oxidative stress between humans and dogs may be explained by the high number of patients with ischemic heart disease included in the studies of humans whereas MMVD in dogs is considered to be of nonischemic origin, and both etiology and duration of the heart disease may be of importance for these markers.57, 58 However, very few studies have assessed circulating markers of oxidative stress in people with MR.15, 47, 53
Although circulating biomarkers of oxidative stress were not associated with MMVD in our study and the associations reported were relatively weak, oxidative stress may play a role in the pathogenesis of MMVD. Studies in humans and animals with experimentally induced and spontaneously occurring MR and MMVD indicate the presence of oxidative stress in the myocardium.14, 18, 59, 60 Tissue analysis may be necessary to identify these changes or circulating concentrations of these specific oxidative stress markers may not represent the myocardial oxidative stress levels and other circulating markers of oxidative stress may have been more informative. Previous studies in dogs have found alterations in other circulating markers of oxidative stress in dogs with MMVD and in humans with MR.15, 20, 21
Limitations of our study include differences in dietary regimens and supplementation which may have influenced antioxidant concentrations and markers of oxidative stress. Although the dogs were fasted for at a minimum of 6 hours before blood sampling, daily variations (partly due to variation in dietary intake) in blood concentration of vitamins may have influenced the results.61
The oxLDL assay used in our study has not been formally validated in dogs (this was not possible because the assay kit is no longer commercially available) and thus actual numbers reported should be interpreted with caution. Breed distribution among groups differed because various breeds with CHF were included (group C) to increase the number of dogs in this group. It would have been preferable if all dogs with CHF had been CKCS.
Additionally, minor deviations from reference values in CBC and serum biochemistry results were allowed considering the high number of geriatric dogs included in the study. These deviations may have influenced results because they could be a sign of early or mild disease. Another limitation of our study is the different cardiac medications given to all dogs with CHF. Because individual dogs respond differently to cardiac treatment, it was not considered ethically justifiable to standardize cardiac medications. However, several types of cardiac medication have been suggested to have antioxidant properties.62 , 7
Conclusions
Our results did not identify an association between clinical stage of MMVD and biomarkers of oxidative stress in dogs. However, serum cholesterol concentration was associated with all markers of oxidative stress, and oxLDL was associated with sex. Vitamin E was associated with neuter status and BCS. Circulating concentrations of MDA, oxLDL, and vitamin E may not be optimal markers for assessing the degree of oxidative stress in dogs with MMVD.
Acknowledgments
We thank Joan Elisabeth Frandsen and Christina Tirsdal Kjempff at Department of Veterinary Disease Biology and Dennis Jensen at Department of Veterinary Clinical and Animal Sciences, University of Copenhagen, Denmark, for their technical assistance.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Department of Veterinary Disease Biology, University of Copenhagen, Denmark, and Din Veterinär Animal Hospital, Helsingborg, Sweden.
The study was supported financially by a PhD study grant from the Novo Nordisk—LIFE In Vivo Pharmacology Centre (LIFEPHARM) to MJR and grants from the Danish National Research Council (Project no. 271‐08‐0998) and Agria and the Swedish Kennel Club Research Foundation for Pets (Reg no. N2013‐0017).
Results were presented as an abstract presentation at the 2016 American College of Veterinary Internal Medicine (ACVIM) Forum, Denver, Colorado.
Footnotes
Vet HDO monitor (Memodiagnostic), S+B medVET GmbH, Babenhausen, Germany
Mercodia AB, Uppsala, Sweden
Access Systems AccuTnI Assay, Beckman Coulter Inc, Fullerton, CA
EchoPAC PC. Version 112, GE Vingmed Ultrasound AS, Horten, Norway
Vivid® i echocardiograph, GE‐medical, Milwaukee, Wisconsin
R studio, version 0.98.1091, © 2009‐2014 RStudio, Inc, Boston, Massachusetts
Petric AD, Verk B, Salobir J, et al. Plasma Vitamin E, Plasma Malondialdehyde and Serum NT‐ProBNP in Dogs with various Stages of Heart Failure (abstract). J Vet Intern Med 2014;28:1008–1008
In the study by freeman et al.51, a typographical error in the vitamin E concentration units (α‐tocopherol and γ‐tocopherol) have been confirmed by the author. The reported unit μg/dL should have been μg/mL
References
- 1. Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994;344:721–724. [DOI] [PubMed] [Google Scholar]
- 2. Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol 1997;24:287–296. [DOI] [PubMed] [Google Scholar]
- 3. Betteridge DJ. What is oxidative stress? Metabolism 2000;49:3–8. [DOI] [PubMed] [Google Scholar]
- 4. Braunwald E. Biomarkers in heart failure. N Engl J Med 2008;358:2148–2159. [DOI] [PubMed] [Google Scholar]
- 5. Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet Clin N Am ‐Small Anim Pract 2010;40:651–663. [DOI] [PubMed] [Google Scholar]
- 6. Whitney JC. Observations on the effect of age on the severity of heart valve lesions in the dog. J Small Anim Pract 1974;15:511–522. [DOI] [PubMed] [Google Scholar]
- 7. Kogure K. Pathology of chronic mitral valvular disease in the dog. Nihon Juigaku Zasshi 1980;42:323–335. [DOI] [PubMed] [Google Scholar]
- 8. Buchanan JW. Chronic valvular disease (Endocardiosis) in dogs. Adv Vet Sci Comp Med 1977;21:75–106. [PubMed] [Google Scholar]
- 9. Urabe Y, Mann DL, Kent RL, et al. Cellular and ventricular contractile dysfunction in experimental canine mitral regurgitation. Circ Res 1992;70:131–147. [DOI] [PubMed] [Google Scholar]
- 10. Tibayan FA, Yun KL, Fann JI, et al. Torsion dynamics in the evolution from acute to chronic mitral regurgitation. J Heart Valve Dis 2002;11:39–46 discussion 46. [PubMed] [Google Scholar]
- 11. McGinley JC, Berretta RM, Chaudhary K, et al. Impaired contractile reserve in severe mitral valve regurgitation with a preserved ejection fraction. Eur J Heart Fail 2007;9:857–864. [DOI] [PubMed] [Google Scholar]
- 12. Falk T, Jonsson L, Olsen LH, Pedersen HD. Arteriosclerotic changes in the myocardium, lung, and kidney in dogs with chronic congestive heart failure and myxomatous mitral valve disease. Cardiovasc Pathol 2006;15:185–193. [DOI] [PubMed] [Google Scholar]
- 13. Borgarelli M, Tarducci A, Zanatta R, Haggstrom J. Decreased systolic function and inadequate hypertrophy in large and small breed dogs with chronic mitral valve insufficiency. J Vet Intern Med 2007;21:61–67. [DOI] [PubMed] [Google Scholar]
- 14. Ahmed MI, Gladden JD, Litovsky SH, et al. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60%. J Am Coll Cardiol 2010;55:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen MC, Chang JP, Liu WH, et al. Increased serum oxidative stress in patients with severe mitral regurgitation: A new finding and potential mechanism for atrial enlargement. Clin Biochem 2009;42:943–948. [DOI] [PubMed] [Google Scholar]
- 16. Cheng W, Li B, Kajstura J, et al. Stretch‐induced programmed myocyte cell death. J Clin Invest 1995;96:2247–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pimentel DR, Amin JK, Xiao L, et al. Reactive oxygen species mediate amplitude‐dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res 2001;89:453–460. [DOI] [PubMed] [Google Scholar]
- 18. Dhalla A, Hill M, Singal P. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol 1996;28:506–514. [DOI] [PubMed] [Google Scholar]
- 19. Prasad K, Gupta J, Kalra J, et al. Oxidative stress as a mechanism of cardiac failure in chronic volume overload in canine model. J Mol Cell Cardiol 1996;28:375–385. [DOI] [PubMed] [Google Scholar]
- 20. Freeman LM, Rush JE, Milbury PE, Blumberg JB. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J Vet Intern Med 2005;19:537–541. [DOI] [PubMed] [Google Scholar]
- 21. Reimann MJ, Haggstrom J, Mortensen A, et al. Biopterin status in dogs with myxomatous mitral valve disease is associated with disease severity and cardiovascular risk factors. J Vet Intern Med 2014;28:1520–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ettinger SJ. The physical examination of the dog and cat In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat. 7th ed St. Louis, Mo: Saunders Elsevier; 2010:1–9. [Google Scholar]
- 23. Gompf RE. The clinical approach to heart disease: History and physical examination In: Fox PR, ed. Canine and Feline Cardiology. New York: Churchill Livingstone; 1988:29–42. [Google Scholar]
- 24. Hanzlicek AS, Baumwart RD, Payton ME. Systolic arterial blood pressure estimated by mitral regurgitation velocity, high definition oscillometry, and doppler ultrasonography in dogs with naturally occurring degenerative mitral valve disease. J Vet Cardiol 2016;18:226–233. [DOI] [PubMed] [Google Scholar]
- 25. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 26. Reimann MJ, Moller JE, Haggstrom J, et al. R‐R interval variations influence the degree of mitral regurgitation in dogs with myxomatous mitral valve disease. Vet J 2014;199:348–354. [DOI] [PubMed] [Google Scholar]
- 27. Reimann MJ, Ljungvall I, Hillstrom A, et al. Increased serum C‐reactive protein concentrations in dogs with congestive heart failure due to myxomatous mitral valve disease. Vet J 2016;209:113–118. [DOI] [PubMed] [Google Scholar]
- 28. Sattler W, Mohr D, Stocker R. Rapid Isolation of lipoproteins and assessment of their peroxidation by high‐performance liquid chromatography postcolumn chemiluminescence. Methods Enzymol 1994;233:469–489. [DOI] [PubMed] [Google Scholar]
- 29. Lykkesfeldt J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: Comparison with ultraviolet‐visible spectrophotometry. Clin Chem 2001;47:1725–1727. [PubMed] [Google Scholar]
- 30. Holvoet P, Macy E, Landeloos M, et al. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin Chem 2006;52:760–764. [DOI] [PubMed] [Google Scholar]
- 31. Ljungvall I, Hoglund K, Tidholm A, et al. Cardiac Troponin I is associated with severity of myxomatous mitral valve disease, age, and C‐reactive protein in dogs. J Vet Intern Med 2010;24:153–159. [DOI] [PubMed] [Google Scholar]
- 32. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography committee of the specialty of cardiology, american college of veterinary internal medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 33. Pedersen HD, Schutt T, Sondergaard R, et al. Decreased plasma concentration of nitric oxide metabolites in dogs with untreated mitral regurgitation. J Vet Intern Med 2003;17:178–184. [DOI] [PubMed] [Google Scholar]
- 34. Pedersen HD, Haggstrom J, Falk T, et al. Auscultation in mild mitral regurgitation in dogs: Observer variation, effects of physical maneuvers, and agreement with color doppler echocardiography and phonocardiography. J Vet Intern Med 1999;13:56–64. [PubMed] [Google Scholar]
- 35. Haggstrom J, Hansson K, Karlberg BE, et al. Plasma concentration of atrial natriuretic peptide in relation to severity of mitral regurgitation in cavalier king Charles spaniels. Am J Vet Res 1994;55:698–703. [PubMed] [Google Scholar]
- 36. Lombard CW. Normal values of the canine M‐mode echocardiogram. Am J Vet Res 1984;45:2015–2018. [PubMed] [Google Scholar]
- 37. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐Mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 38. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 39. Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4‐Hydroxy‐2‐Nonenal. Oxid Med Cell Longev 2014;2014:360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nielsen F, Mikkelsen BB, Nielsen JB, et al. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life‐style factors. Clin Chem 1997;43:1209–1214. [PubMed] [Google Scholar]
- 41. Goldstein JL, Brown MS. The low‐density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem 1977;46:897–930. [DOI] [PubMed] [Google Scholar]
- 42. Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: Does it hold for humans? Trends Cardiovasc Med 2001;11:93–102. [DOI] [PubMed] [Google Scholar]
- 43. Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 2011;51:978–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Nigris F, Franconi F, Maida I, et al. Modulation by alpha‐ and gamma‐tocopherol and oxidized low‐density lipoprotein of apoptotic signaling in human coronary smooth muscle cells. Biochem Pharmacol 2000;59:1477–1487. [DOI] [PubMed] [Google Scholar]
- 45. Tsutsui T, Tsutamoto T, Wada A, et al. Plasma oxidized low‐density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol 2002;39:957–962. [DOI] [PubMed] [Google Scholar]
- 46. Wu T, Willett WC, Rifai N, et al. Is plasma oxidized low‐density lipoprotein, measured with the widely used antibody 4E6, an independent predictor of coronary heart disease among U.S. men and women? J Am Coll Cardiol 2006;48:973–979. [DOI] [PubMed] [Google Scholar]
- 47. Idriss NK, Blann AD, Sayed DM, et al. Circulating endothelial cells and platelet microparticles in mitral valve disease with and without atrial fibrillation. Angiology 2015;66:631–637. [DOI] [PubMed] [Google Scholar]
- 48. Holvoet P, Mertens A, Verhamme P, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2001;21:844–848. [DOI] [PubMed] [Google Scholar]
- 49. Maldonado EN, Romero JR, Ochoa B, Aveldano MI. Lipid and fatty acid composition of canine lipoproteins. Comp Biochem Physiol B Biochem Mol Biol 2001;128:719–729. [DOI] [PubMed] [Google Scholar]
- 50. Verkest KR. Is the metabolic syndrome a useful clinical concept in dogs? a review of the evidence. Vet J 2014;199:24–30. [DOI] [PubMed] [Google Scholar]
- 51. Freeman LM, Rush JE, Markwell PJ. Effects of dietary modification in dogs with early chronic valvular disease. J Vet Intern Med 2006;20:1116–1126. [DOI] [PubMed] [Google Scholar]
- 52. Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between Vitamin E and Vitamin C. Nature 1979;278:737–738. [DOI] [PubMed] [Google Scholar]
- 53. Cavalca V, Tremoli E, Porro B, et al. Oxidative stress and nitric oxide pathway in adult patients who are candidates for cardiac surgery: Patterns and differences. Interact Cardiovasc Thorac Surg 2013;17:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vogel S, Contois JH, Tucker KL, et al. Plasma retinol and plasma and lipoprotein tocopherol and carotenoid concentrations in healthy elderly participants of the framingham heart study. Am J Clin Nutr 1997;66:950–958. [DOI] [PubMed] [Google Scholar]
- 55. Wallstrom P, Wirfalt E, Lahmann PH, et al. Serum concentrations of beta‐carotene and alpha‐tocopherol are associated with diet, smoking, and general and central adiposity. Am J Clin Nutr 2001;73:777–785. [DOI] [PubMed] [Google Scholar]
- 56. Grolier P, Boirie Y, Levadoux E, et al. Age‐related changes in plasma lycopene concentrations, but not in Vitamin E, are associated with fat mass. Br J Nutr 2000;84:711–716. [PubMed] [Google Scholar]
- 57. Detweiler DK, Patterson DF. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci 1965;127:481–516. [DOI] [PubMed] [Google Scholar]
- 58. Jonsson L. Coronary arterial lesions and myocardial infarcts in the dog. Acta Vet Scand 1972;38:1–80. [PubMed] [Google Scholar]
- 59. Gladden JD, Ahmed MI, Litovsky SH, et al. Oxidative stress and myocardial remodeling in chronic mitral regurgitation. Am J Med Sci 2011;342:114–119. [DOI] [PubMed] [Google Scholar]
- 60. Oyama MA, Chittur SV. Genomic expression patterns of mitral valve tissues from dogs with degenerative mitral valve disease. Am J Vet Res 2006;67:1307–1318. [DOI] [PubMed] [Google Scholar]
- 61. Tangney CC, Shekelle RB, Raynor W, et al. Intra‐ and interindividual variation in measurements of beta‐carotene, retinol, and tocopherols in diet and plasma. Am J Clin Nutr 1987;45:764–769. [DOI] [PubMed] [Google Scholar]
- 62. Weglicki WB, Mak IT, Simic MG. Mechanisms of cardiovascular drugs as antioxidants. J Mol Cell Cardiol 1990;22:1199–1208. [DOI] [PubMed] [Google Scholar]


