Abstract
Background
Urethral sphincter mechanism incompetence (USMI) is the most common cause of urinary incontinence in neutered bitches and is most common in dogs weighing >20 kg.
Objectives
To describe a population of neutered bitches with USMI and investigate their initial presentation, the relationship between weight and age at neuter, and treatment.
Animals
One hundred and sixty‐three female dogs with USMI (UI) diagnosed between January 2009 and December 2012, and 193 continent neutered control (C) bitches.
Methods
Retrospective data were collected from neutered female dogs with USMI and healthy, continent neutered females presented between January 2009 and December 2012.
Results
Urinary incontinent dogs weighed more than C dogs (P = .003), and there was no difference in age at neuter. The relationship between weight at diagnosis and age at neuter was found to impact the hazard of USMI. A decrease in the hazard of USMI was found in dogs weighing >25 kg for every month delay of neuter in the first year. The hazard did not change for dogs <15 kg. Median time from neuter to development of incontinence was 3.73 years. Phenylpropanolamine was prescribed in 75.5%, diethylstilbestrol in 21.5%, and both in 3.1% of dogs.
Conclusions and Clinical Importance
Neutering bitches expected to be >25 kg adult weight later in their first year may decrease the hazard of developing USMI, whereas age at neutering of bitches <25 kg may not impact continence. Heavier dogs have increased risk of USMI, and onset occurs within a few years of neuter.
Keywords: Incontinence, Ovariohysterectomy
Abbreviations
- C
control group of continent dogs
- CI
confidence interval
- DES
diethylstilbestrol
- HR
hazard ratio
- IQR
interquartile range
- OVH
ovariohysterectomy
- PPA
phenylpropanolamine
- SD
standard deviation
- UI
study group with USMI
- USMI
urethral sphincter mechanism incompetence
- UTI
urinary tract infection
Urethral sphincter mechanism incompetence (USMI) is the most common cause of acquired urinary incontinence in female neutered dogs. Historically referred to as “hormone‐responsive urinary incontinence,” it is now understood that its origins and pathophysiology are more complex than loss of estrogen and likely involve changes in tissue structure,1, 2 collagen content,3, 4 vasculature,5, 6 and estrogen receptors,7 as well as alterations in follicle‐stimulating hormone and luteinizing hormone concentrations.8, 9, 10 Conformation of the animal (e.g, pelvic bladder, recessed vulva), tail docking, and the position of the urogenital tract in the pelvis also may play roles in the development of USMI.11, 12, 13, 14
Previously published reports have indicated an increased risk of USMI in dogs weighing >20 kg, and overrepresentation of some breeds (e.g, German shepherd, Rottweiler, Doberman pinscher, Old English sheepdog, Boxer, English springer spaniel, Weimaraner, Irish setter).11, 15, 16 Urinary incontinence also has been reported to develop most often within 2–4 years of ovariohysterectomy (OVH).14, 15, 17 Several studies have investigated the relationship of age at OVH and development of USMI, and some of these studies have reported that dogs neutered before 3 months of age or after their first estrus are at increased risk of development of USMI.14, 15, 16, 17 However, a recent study of 566 dogs in the USA showed no relationship to age at OVH,18 and a systematic analysis of the available English language literature found no consistent evidence that supported a relationship between age at OVH and development of urinary incontinence in female dogs.19 No study has looked at the interaction of age at neuter and adult body weight as continuous variables, and only 1 has attempted to evaluate them together.18 The small number of age and weight categories in that study may have masked their impact.
Urethral sphincter mechanism incompetence in dogs often develops over time, and owners may delay seeking veterinary care until the frequency or severity of the incontinence reaches an intolerable point. One study noted that up to 39% of dogs had signs of urinary incontinence 1–2 years before presentation to a veterinarian for the problem.14
The objectives of our study were to describe a large population of spayed female dogs with USMI in the USA, to investigate their initial presentation and evaluation by veterinarians for the client complaint of urinary incontinence, to examine the impact of age at OVH and weight at presentation for incontinence on the hazard of USMI, and to identify the treatment most commonly chosen. We hypothesized that dogs with USMI would have been neutered at a younger age than normal dogs and that dogs of larger size at presentation for incontinence would have a lower hazard of USMI the later OVH was performed. We also hypothesized that dog owners would report clinical signs of USMI lasting at least 3 months before to seeking veterinary consultation.
Materials and Methods
Dogs
Five hundred randomly selected American Animal Hospital Association accredited hospitals located within the continental USA were contacted by mail inviting them to submit case data. Data consisted of case information for incontinent neutered female dogs at first presentation for the problem and healthy continent female dogs that presented for a wellness examination on the same day. Dogs with diagnosed urinary tract infection, diabetes mellitus, hyperadrenocorticism, renal disease, or those receiving corticosteroids or immunosuppressive medications were to be excluded. Cases were to be drawn from the period of January 2009 to December 2012. Dogs were labelled as incontinent group (UI) or control group (C).
Data Collection
Data were collected and entered into a spreadsheet1 provided by the investigators. Data collected for both groups of dogs included breed, weight, body condition score (BCS), date of birth, date of OVH, and date and age at presentation. Additional data collected for UI dogs included duration of clinical signs before presentation, initial diagnostic testing, initial diagnosis and treatment, date of USMI diagnosis, and incontinence treatment used. Participating clinics were compensated monetarily for their time as an incentive to contribute to the study.
Data Analysis
Continuous variables are presented as medians and interquartile range (IQR) with range across group (UI vs. C) and were compared by Wilcoxon rank‐sum test. The P‐values were adjusted by the Holm's procedure to conserve the overall type I error at .05 due to the multiple comparisons.
Urethral sphincter mechanism incompetence was the event of interest. It could occur anytime during the study period. There is always the possibility that it will not occur and in these situations USMI is censored at the end of the study period. Cox proportional hazard regression is the method of choice for analysis of time to event data. It was used to determine the hazard of incontinence when OVH was performed in the first year of the dog's life. The method of fractional polynomials was used to determine whether the relationship between continuous variables (OVH age and weight at presentation for incontinence) and time to incontinence was other than linear. After model convergence, linear contrast statements were used to find the hazard of incontinence for various combinations of OVH age, and weight at presentation. This information was used to generate the hazard ratios for specific changes in weight at set OVH ages and specific changes in OVH age at set weights. In addition, a contour plot of the hazard of incontinence by OVH age and weight was produced to visualize the relationship. All analyses were performed by a statistical software package.2
Results
Signalment
Sixteen veterinary practices contributed a mean (SD) of 12 (8) dogs from each group to the study (range, 1–37). One hundred and sixty‐three UI and 193 C dogs were enrolled in the study. All dogs in both groups were neutered females. Demographical information is provided in Table 1 and includes cases submitted from California, Colorado, Florida, Indiana, Massachusetts, Nevada, North Dakota, Ohio, Oregon, Utah, Virginia, Washington, Wisconsin, and Wyoming. Median age (IQR) of the UI dogs at presentation for USMI was 72 months (36–108 months) with a range of 2–315 months, and median age at wellness visit was 65 months (36–108 months) with a range of 2–297 months for C dogs (Table 1). There was no significant difference in age at presentation between the 2 groups.
Table 1.
Descriptive statistics for control and UI groups
| N | Median | IQR | P‐value | |||
|---|---|---|---|---|---|---|
| 25th | 75th | |||||
| Control | Age (months) | 193 | 65 | 36 | 108 | |
| OVH age (months) | 154 | 8.5 | 5.6 | 18.1 | ||
| Weight (kg) | 193 | 17.7 | 8.2 | 30.4 | ||
| BCS | 179 | 5.0 | 5.0 | 7.0 | ||
| UI | Age (months) | 163 | 72 | 36 | 108 | .927 |
| OVH age (months) | 137 | 8.5 | 5.0 | 24.8 | .999 | |
| Weight (kg) | 163 | 25.3 | 16.8 | 32.5 | .003 | |
| BCS | 148 | 5.0 | 5.0 | 6.0 | .078 | |
IQR, interquartile range; N, number of dogs; OVH, ovariohysterectomy; BCS, body condition score. P < .05 is considered significant. P‐values have been adjusted by the Holm's procedure to conserve the overall type I error at .05.
Median (IQR) weight of UI dogs was 25.3 kg (16.8–32.5 kg) with a range of 0.5–66.4 kg and of C dogs was 17.7 kg (8.2–30.4 kg) with a range of 2.0–71.6 kg. The UI dogs weighed significantly more than C dogs (P = .003). Body condition score (BCS) was available for 148 dogs in the UI group and for 179 in the C group. Median BCS (IQR) was 5 (5 to 6) with a range of 4–8 and 5 (5 to 7) with a range of 3–9 for UI and C dogs, respectively (P = .078).
Breeds represented by ≥10 dogs across both groups were Labrador retriever or Labrador cross (69), German shepherd or German shepherd cross (21), Boxer (18), Beagle (15), Golden Retriever or Golden Retriever cross (14), and Chihuahua (13).
Relationship to OVH
Ninety‐nine C dogs and 79 UI dogs were ≤ 12 months of age at the time of OVH. There was no significant difference in median age at OVH between UI dogs (8.5 months) and C dogs (8.5 months). A trend of increased hazard of incontinence with higher adult body weight and earlier OVH was found (Fig 1, Table 2). This increased hazard was found for an increase in adult body weight at a set OVH age, earlier age at OVH for a set adult body weight, as well as changes in both age and body weight. This trend became statistically significant at 25‐kg body weight (hazard ratio [HR], 0.89; 95% confidence interval [CI], 0.82–0.97; P = .006).
Figure 1.
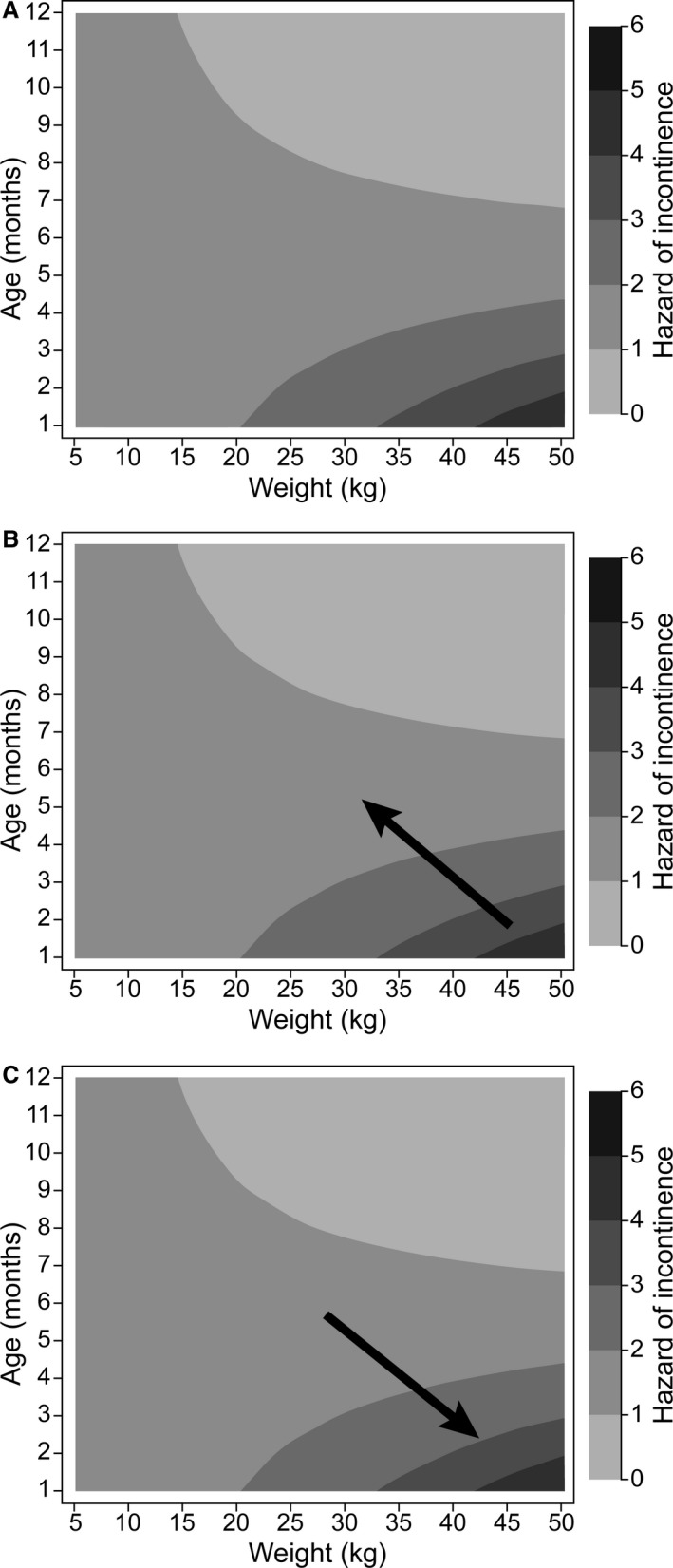
Contour plot of the hazard of incontinence by OVH age and weight at presentation. This figure is based on 178 observations where the OVH age was ≤12 months. A hazard ratio is visualized in (A) as an arrow anywhere on the figure. The hazard at the tip of the arrow relative to the hazard at the tail of the arrow is the hazard ratio. A protective hazard ratio would be an arrow that points from a darker to a lighter region in (B), whereas an increasing hazard ratio would point from lighter to a darker region, (C). The hazard of incontinence is highest in the lower right corner, whereas it is lowest in the upper right corner. OVH, ovariohysterectomy.
Table 2.
Hazard of USMI based on age at OVH and weight at presentation of USMI and are based on a Cox proportional hazard regression
| Change in OVH age or change in weight | HR | 95% CI | P‐value | |
|---|---|---|---|---|
| 1 month increase in OVH age at 5 kg | 1.02 | 0.87 | 1.19 | .841 |
| 1 month increase in OVH age at 10 kg | 0.98 | 0.87 | 1.12 | .788 |
| 1 month increase in OVH age at 15 kg | 0.95 | 0.86 | 1.05 | .340 |
| 1 month increase in OVH age at 20 kg | 0.92 | 0.84 | 1.00 | .063 |
| 1 month increase in OVH age at 25 kg | 0.89 | 0.82 | 0.97 | .006 |
| 1 month increase in OVH age at 30 kg | 0.86 | 0.78 | 0.94 | .001 |
| 1 month increase in OVH age at 35 kg | 0.83 | 0.74 | 0.93 | .001 |
| 1 month increase in OVH age at 40 kg | 0.81 | 0.70 | 0.92 | .002 |
| 1 month increase in OVH age at 45 kg | 0.78 | 0.66 | 0.92 | .003 |
| 1 month increase in OVH age at 50 kg | 0.75 | 0.62 | 0.92 | .004 |
Hazard ratios are based on a Cox proportional hazard regression. OVH, ovariohysterectomy; HR, hazard ratio; CI, confidence interval.
Clinical Presentation
Among 136 incontinent dogs for which the information was available, the median (IQR) reported time from OVH to first clinical signs of USMI was 1,363 days (536.5–2,737.5 days) or 3.73 years with a range of 5–5417 days. Based on 155 dogs for which the information was available, median (IQR) time from initial clinical signs to presentation to a veterinarian with a complaint of incontinence was 21 days (10–45 days) with a range of 0–2464 days.
Diagnostic Evaluation
Diagnostic testing performed at presentation for urinary incontinence was reported for 137 UI dogs. Of these, 133 (97%) had a urinalysis performed, and 15 (10.9%) had a urine culture performed. A complete blood count (CBC), serum biochemistry profile, or both were performed in 37 (27.0%) and 41 (29.9%) of the UI and C dogs, respectively. Four dogs had abdominal radiography performed and 4 dogs had total serum thyroxine concentration evaluated. Two dogs had vaginal cytology performed. One dog each had the following diagnostic tests performed: fecal flotation, urine cortisol‐to‐creatinine ratio, water deprivation testing, and systolic blood pressure.
Treatment
Initial USMI treatment data were available for 163 UI dogs. The synthetic, nonsteroidal estrogenic compound diethylstilbestrol (DES) was prescribed as a single agent in 35 dogs (21.5%). Phenylpropanolamine (PPA), an α‐adrenergic agonist, was prescribed as a single agent in 123 dogs (75.5%). Combination treatment with DES and PPA was prescribed in 5 dogs (3.1%).
Discussion
No significant difference in age at OVH was found between UI and C dogs, failing to support our first hypothesis. However, a significant interaction was found between the weight of the dog at presentation for incontinence and the age at OVH in the Cox proportional hazard regression. The hazard is defined as the instantaneous rate at which an event (USMI) occurs any time during the study period. The value of the hazard depends of both the age at neuter and the adult weight. The HR is determined by dividing the hazard for a specific age at neuter and adult weight (the condition of interest) by the hazard at a different age and adult weight (the referent condition). The consequences of this interaction mean the body weight HR depends on the age of OVH and the HR of OVH age depends on a specific body weight. Thus, the HR for a 1‐month decrease in OVH depends on the body weight of the dog. For example, for a 10‐kg dog, a 1‐month delay in OVH age decreases the hazard of incontinence by 2% (HR = 0.98, 95% CI: 0.87–1.12, P = .788), whereas if the dog's weight was 30 kg, then a 1‐month delay in OVH age decreased the hazard of incontinence by 24% (HR, 0.86; 95% CI, 0.78–0.94; P = .001). This held true for a 1‐month delay between any 2 consecutive months. It has been widely demonstrated that larger dogs are at higher risk of development of USMI than smaller dogs, but ours is the first study to demonstrate the interaction of OVH age and size on this risk. Previous studies have been contradictory in their findings of the risk of USMI in dogs neutered “early” or “late.” The definition of these time periods has varied among studies as either specific ages14, 18 (i.e, < or >6 months of age) or reproductive status14, 15 (i.e, before or after first estrus). The data reported here may partially explain the lack of consensus by directly incorporating the impact of body weight with age at OVH on the hazard of USMI.
The most compelling clinical impact of the results of our study is the need to make age of neuter recommendations based on the projected adult weight of the bitch. In the past, recommendations to neuter female dogs before the first estrus were based on reduction in unwanted litters and decreased risk of mammary neoplasia.20 This recommendation has been challenged by the increased risk of USMI, particularly in cases of early (<3 months) OVH.21 The data presented here show that the risk of USMI is not significantly decreased in smaller breed dogs by delaying OVH, thus making the decision to neuter before initiation of estrus more appropriate. However, in dogs with projected adult weights >25 kg, the timing of neuter and its associated risks and benefits should be carefully considered and discussed with pet owners. Special consideration should be taken when making recommendations for females of breeds that have been identified as at increased risk of USMI such as Boxers, Old English sheep dogs, and Doberman pinschers. Larger female dogs may benefit from a delay in neuter by a reduction in USMI risk.
Previous studies have shown an increased risk of developing USMI in dogs in which OVH was performed after the pubertal estrus.15, 17, 22 We performed our regression model on dogs neutered within the first 12 months of life because the majority of OVH procedures in the USA occur during that time, and most bitches experience pubertal estrus during the first year. After the dog reaches sexual maturity, it could be assumed that the impact of estrogen cessation on adult tissues (and risk of USMI) has a different physiology than its lack during the developmental stages. Information on whether the dogs had OVH performed before or after pubertal estrus was not available in our study. The wide variability in age of first estrus among (and even within) breeds23 prevents us from making a recommendation for neutering related to sexual maturity.
Our data on the age and body weight of dogs with USMI are similar to that reported in other studies13, 18, 24 and are supportive of previous reports of the increased frequency of the disease in larger dogs. Previously published data suggest that dogs with higher BCS had greater risk of USMI; however, our data do not support this conclusion. Body condition score is a very subjective assessment and is not conducted or recorded as part of the medical record by all veterinarians. Although there was no difference detected in BCS between the groups, we must be cautious in drawing conclusions from these unstandardized data. The question of obesity contributing to the development of USMI in dogs requires more standardized assessment and remains open at this time. Additional studies that assess a range of BCSs across breeds of similar size and the relationship to USMI are needed to draw more concrete conclusions.
Dogs in the UI group weighed more than those in the C group. Given the lack of significant difference in BCS between groups, it might be concluded that larger breed dogs were more likely to develop USMI. This conclusion also would be consistent with previously reported information. To more definitively evaluate breed size‐related risk, more rigorous case–control matching across a variety of breeds of variable sizes must be performed. This level of control was not possible in our study and likely would require prospective evaluation.
Similarly, we are unable to draw many conclusions from the breed data presented here. Without knowing the breed distribution within the participating hospitals, we cannot fully assess the risk of development of USMI among breeds. Some breeds had more representatives in our USMI group than others, but this may simply represent national trends in breed popularity.
The data presented here support the previously published timing of development of USMI after OVH of 2–4 years.18 The information in our study did not support our hypothesis that owners would wait several months to seek veterinary care for signs of USMI in their dogs. Our study showed variability in the time of presentation, but 75% of owners consulted veterinarians approximately 1 month after the onset of clinical signs. There were a few owners who reported urinary incontinence several years before presentation, but these were outliers. One previous study reported a mean duration of clinical signs before presentation to a veterinarian for 48 dogs with USMI between 8.6 and 14.8 months.25 Factors that may influence the owner of a USMI dog to seek veterinary care are likely numerous and individualized. Attentiveness and time spent with the dog, whether the dog spends the majority of its time inside or outside of the house, and the fastidiousness of the dog itself in cleaning the perineal region may influence the owner's awareness of the problem. Additional factors such as financial considerations and suspicion that inappropriate urination is a behavior problem also may delay presentation of the animal to a veterinarian. The previous study was performed nearly 30 years ago and changes in pet ownership trends, such as spending more time indoors or in close contact with the owner, may explain the difference seen here. A prospective investigation of client factors leading to veterinary consultation for signs of USMI would be needed to determine trends among dog owners.
Diagnostic evaluation of UI dogs in our study varied widely, although some trends were identified. The performance of a urinalysis in a dog with urinary incontinence was nearly universal in those dogs with diagnostic testing reported. Identification of dogs with dilute urine may be of use in determining the causes and treatment of urinary incontinence. Dogs with polyuria may develop urinary incontinence as the volume of urine produced and stored in the bladder increases. Increased pressure within the urinary bladder may overcome urethral resistance, particularly if it is already suboptimal. In patients with urinary incontinence and polyuria, the treatment of underlying causes of increased urine production may decrease the severity of the incontinence, particularly if the onset of both coincides.
Although no studies specifically evaluating the prevalence of urinary tract infection (UTI) in dogs with USMI have been reported, the two may occur together. This is particularly true in patients with severe incontinence and a persistently wet perineum, which may allow for a larger peri‐vulvar bacterial load and wicking of bacteria up through the urethra. Despite this, urine culture was performed in only 10.9% of UI dogs in our study. A number of factors may have led to this finding including cost or difficulty in obtaining an uncontaminated urine sample by cystocentesis. Case selection bias may have decreased the number of cases with urine cultures, by the exclusion of dogs with suspected UTI and clinical signs related to it. The presence of UTI may make the clinical signs of USMI worse, and may decrease treatment response.
A CBC, serum biochemistry profile, or both were performed in almost 30% of UI dogs. It is not known whether these diagnostic tests were performed as part of a routine wellness visit, during which the owner first complained of urinary incontinence, as follow‐up for an unrelated disease process, or in direct relationship to the diagnostic evaluation of urinary incontinence. One reason to perform a CBC in the evaluation of urinary incontinence would be in preparation for treatment with an estrogenic compound. It has been well documented that high doses of some estrogen compounds, such as DES and estradiol cypionate, can lead to myelodysplasia and associated anemia26, but it has not been reported at dosages most commonly used in management of USMI in veterinary patients, nor has it been reported for compounded DES at once‐weekly dosing.25, 27, 28 Of the 35 dogs treated with DES, 10 (28.6%) of them had a CBC performed at initial examination. It is not known whether any of these dogs had reevaluation of their CBC after starting treatment with DES, nor is it known whether any developed bone marrow dyscrasias during treatment. More recently, estriol3, the only FDA‐approved estrogenic compound for treatment of USMI in dogs, has become available. Bone marrow suppression is not listed as an expected adverse event of its daily administration.25, 28
Measurement of systolic blood pressure in only 1 UI dog is concerning, given the high frequency of PPA use in the patients reported here. The dog in which a blood pressure was taken was placed on PPA, but no other dog treated with this medication was reported to be evaluated in this manner. Hypertension is a widely recognized adverse effect of α‐agonist use in humans. It has been reported with PPA treatment in dogs at a much lower rate,29, 30 but preexisting hypertension or comorbidities that predispose to hypertension such as kidney disease or hyperthyroidism are considered contraindications for its use.31 Reevaluation of blood pressure during treatment with PPA is recommended to monitor for hypertension, particularly because USMI patients are likely to be on lifelong treatment, often with dose escalation over time.
The remaining reported diagnostics such as abdominal radiography, serum total thyroxine concentration, and vaginal cytology may have been performed to assess for causes of changes in urination behaviors, or for reasons unrelated to the assessment of urinary incontinence. Based on the information provided in the study, no conclusions may be drawn as to their clinical relevance.
Phenylpropanolamine was used to treat USMI more frequently than DES. This may be due to the reported lower efficacy of DES when compared to the α‐agonist, pseudoephedrine in a study published in the late 1980's.25 This trend also may reflect the availability of a veterinary‐specific PPA preparation4 and the lack of an equivalent estrogenic product. Compounding pharmacies have been the primary source of DES in the USA since the early 1970s, increasing the cost and difficulty in obtaining it for use in dogs. Since then, alternative estrogenic compounds such as estriol3, conjugated equine estrogen5, and the α‐adrenergic agonist, PPA, which have better efficacy than pseudoephedrine or DES, have been used for treatment of USMI in female dogs.28, 30 Diethylstilbestrol was the only estrogenic compound reported in this study. Conjugated equine estrogen was not reported to be used in any of the UI dogs, although it was widely available during the study period. Estriol3 was not available in the USA until near the end of the period covered by this study, although it has been available and widely used in Europe and other markets for over 14 years. Although no direct comparisons have been made, estriol has been shown to have better response rates in treating USMI than previously reported for DES.28, 32 No dosing data were reported here for treatment of USMI because of the wide variability in concentration as well as administration frequency of both DES and PPA in the UI dogs.
There are several limitations with the data presented here. The reliance upon the medical records of a large number of clinics and their choice of cases to submit may have introduced bias into the study. The data were drawn solely from the dog's medical record, which required reliance on the completeness and interpretation of the clinician or staff member who took the history from the owner. An attempt was made to perform a random‐effects Cox proportional hazard analysis looking for inter‐clinic bias, but this was not possible because some clinics contributed only 1 case (data not shown). Another limitation of our study is the lack of a case–control population for breed, age, and other factors. This makes the comparison between the UI and C groups a challenge and limits the conclusions that may be drawn from the data. An attempt was made to age‐match the cases, but we found that compliance with this request was not easy to obtain from the participating clinics. In addition, client input was not sought in our investigation of duration of clinical signs or timing of veterinary intervention.
Previous studies have evaluated the age of neuter and its relationship to time of onset of incontinence.24 We did attempt to fit a nonlinear exponential regression model of this relationship, but we found no significant relationship with our data (model not shown). Based on this model, no predictions of time to onset of incontinence can be made based on age at OVH.
Finally, our study did not address the efficacy of various treatments for USMI, or adverse effects associated with medical treatment. These topics have been investigated in several other studies in both prospective and retrospective manners and were deemed out of scope for this investigation.25, 28, 30, 32, 33, 34
Acknowledgment
The authors thank the participating veterinary hospitals for contributing case material to this study.
Conflict of Interest Declaration: Dr. Madeleine Stahl is an employee of Merck Animal Health.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Work was performed at The Ohio State University College of Veterinary Medicine, Columbus, Ohio.
Supported in part by Merck Animal Health.
Presented in part as an oral abstract at the 2015 American College of Veterinary Internal Medicine Forum, Indianapolis, Indiana.
Footnotes
Microsoft Excel, Microsoft, Redmond, WA, 2010
Stata, version 14.1, StataCorp, College Station, TX
Incurin, Estriol, Merck Animal Health, Madison, NJ
Proin, Phenylpropanolamine, PRN Pharmaceutical, Pensacola, FL
Premarin, conjugated equine estrogen, Pfizer, New York, NY
References
- 1. Gregory SP, Parkinson TJ, Holt PE. Urethral conformation and position in relation to urinary incontinence in the bitch. Vet Rec 1992;131:167–170. [DOI] [PubMed] [Google Scholar]
- 2. Gregory SP, Holt PE, Parkinson TJ, et al. Vaginal position and length in the bitch: Relationship to spaying and urinary incontinence. J Small Anim Pract 1999;40:180–184. [DOI] [PubMed] [Google Scholar]
- 3. Augsburger HR, Oswald M. Immunohistochemical analysis of collagen types I, III, IV and alpha‐actin in the urethra of sexually intact and ovariectomized beagles. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:1071–1075. [DOI] [PubMed] [Google Scholar]
- 4. Byron JK, Graves TK, Becker MD, et al. Evaluation of the ratio of collagen type III to collagen type I in periurethral tissues of sexually intact and neutered female dogs. Am J Vet Res 2010;71:697–700. [DOI] [PubMed] [Google Scholar]
- 5. Rud T, Andersson KE, Asmussen M, et al. Factors maintaining the intraurethral pressure in women. Invest Urol 1980;17:343–347. [PubMed] [Google Scholar]
- 6. Plzak L 3rd, Staskin D. Genuine stress incontinence theories of etiology and surgical correction. Urol Clin North Am 2002;29:527–535. [DOI] [PubMed] [Google Scholar]
- 7. Chen GD, Oliver RH, Leung BS, et al. Estrogen receptor alpha and beta expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil Steril 1999;71:1099–1102. [DOI] [PubMed] [Google Scholar]
- 8. Reichler IM, Hung E, Jochle W, et al. FSH and LH plasma levels in bitches with differences in risk for urinary incontinence. Theriogenology 2005;63:2164–2180. [DOI] [PubMed] [Google Scholar]
- 9. Reichler IM, Jochle W, Piche CA, et al. Effect of a long acting GnRH analogue or placebo on plasma LH/FSH, urethral pressure profiles and clinical signs of urinary incontinence due to Sphincter mechanism incompetence in bitches. Theriogenology 2006;66:1227–1236. [DOI] [PubMed] [Google Scholar]
- 10. Donovan CE, Gordon JM, Kutzler MA. Gonadotropin‐releasing hormone immunization for the treatment of urethral sphincter mechanism incompetence in ovariectomized bitches. Theriogenology 2014;81:196–202. [DOI] [PubMed] [Google Scholar]
- 11. Holt PE, Thrusfield MV. Association in bitches between breed, size, neutering and docking, and acquired urinary incontinence due to incompetence of the urethral sphincter mechanism. Vet Rec 1993;133:177–180. [DOI] [PubMed] [Google Scholar]
- 12. Adams WM, DiBartola SP. Radiographic and clinical features of pelvic bladder in the dog. J Am Vet Med Assoc 1983;182:1212–1217. [PubMed] [Google Scholar]
- 13. Atalan G, Holt PE, Barr FJ. Relationships between urethrovesical angles and urinary incontinence due to urethral sphincter mechanism incompetence in bitches. J Small Anim Pract 1997;38:551–553. [DOI] [PubMed] [Google Scholar]
- 14. de Bleser B, Brodbelt DC, Gregory NG, et al. The association between acquired urinary sphincter mechanism incompetence in bitches and early spaying: A case‐control study. Vet J 2011;187:42–47. [DOI] [PubMed] [Google Scholar]
- 15. Stocklin‐Gautschi NM, Hassig M, Reichler IM, et al. The relationship of urinary incontinence to early spaying in bitches. J Reprod Fertil Suppl 2001;57:233–236. [PubMed] [Google Scholar]
- 16. Arnold S, Arnold P, Hubler M, et al. Urinary incontinence in spayed female dogs: Frequency and breed disposition. Schweiz Arch Tierheilkd 1989;131:259–263. [PubMed] [Google Scholar]
- 17. Thrusfield MV, Holt PE, Muirhead RH. Acquired urinary incontinence in bitches: Its incidence and relationship to neutering practices. J Small Anim Pract 1998;39:559–566. [DOI] [PubMed] [Google Scholar]
- 18. Forsee KM, Davis GJ, Mouat EE, et al. Evaluation of the prevalence of urinary incontinence in spayed female dogs: 566 cases (2003‐2008). J Am Vet Med Assoc 2013;242:959–962. [DOI] [PubMed] [Google Scholar]
- 19. Beauvais W, Cardwell JM, Brodbelt DC. The effect of neutering on the risk of urinary incontinence in bitches ‐ a systematic review. J Small Anim Pract 2012;53:198–204. [DOI] [PubMed] [Google Scholar]
- 20. Schneider R, Dorn CR, Taylor DON. Factors influencing canine mammary cancer development and postsurgical survival. J Natl Cancer Inst 1968;40:307–318. [PubMed] [Google Scholar]
- 21. Veronesi MC, Rota A, Battocchio M, et al. Spaying‐related urinary incontinence and oestrogen therapy in the bitch. Acta Vet Hung 2009;57:171–182. [DOI] [PubMed] [Google Scholar]
- 22. Spain CV, Scarlett JM, Houpt KA. Long‐term risks and benefits of early‐age gonadectomy in dogs. J Am Vet Med Assoc 2004;224:380–387. [DOI] [PubMed] [Google Scholar]
- 23. Arnold S, Jager P, DiBartola SP, et al. Treatment of urinary incontinence in dogs by endoscopic injection of Teflon. J Am Vet Med Assoc 1989;195:1369–1374. [PubMed] [Google Scholar]
- 24. Schaefers‐Okkens A. Estrous cycle and breeding management of the healthy bitch In: Ettinger SF, Feldman EC, ed. Textbook of Veterinary Internal Medicine, 7 ed. St. Louis, MO: Saunders Elsevier; 2010:1873–1883. [Google Scholar]
- 25. Nendick PA, Clark WT. Medical therapy of urinary incontinence in ovariectomised bitches: A comparison of the effectiveness of diethylstilboestrol and pseudoephedrine. Aust Vet J 1987;64:117–118. [DOI] [PubMed] [Google Scholar]
- 26. Sontas HB, Dokuzeylu B, Turna O, et al. Estrogen‐induced myelotoxicity in dogs: A review. Can Vet J 2009;50:1054–1058. [PMC free article] [PubMed] [Google Scholar]
- 27. Angioletti A, De Francesco I, Vergottini M, et al. Urinary incontinence after spaying in the bitch: Incidence and oestrogen‐therapy. Vet Res Commun 2004;28(Suppl 1):153–155. [DOI] [PubMed] [Google Scholar]
- 28. Mandigers RJ, Nell T. Treatment of bitches with acquired urinary incontinence with oestriol. Vet Rec 2001;149:764–767. [PubMed] [Google Scholar]
- 29. Peterson KL, Lee JA, Hovda LR. Phenylpropanolamine toxicosis in dogs: 170 cases (2004–2009). J Am Vet Med Assoc 2011;239:1463–1469. [DOI] [PubMed] [Google Scholar]
- 30. Byron JK, March PA, Chew DJ, et al. Effect of phenylpropanolamine and pseudoephedrine on the urethral pressure profile and continence scores of incontinent female dogs. J Vet Intern Med 2007;21:47–53. [DOI] [PubMed] [Google Scholar]
- 31. Plumb DC. Plumb's Veterinary Drug Handbook, 7th ed Stockholm, Wis: Ames, Iowa: PharmaVet; Distributed by Wiley; 2011: 1187 p. [Google Scholar]
- 32. Hamaide AJ, Grand JG, Farnir F, et al. Urodynamic and morphologic changes in the lower portion of the urogenital tract after administration of estriol alone and in combination with phenylpropanolamine in sexually intact and spayed female dogs. Am J Vet Res 2006;67:901–908. [DOI] [PubMed] [Google Scholar]
- 33. Kanca H, Karakas K, Yagci IP, et al. Evaluation of once daily dose of phenylpropanolamine in the treatment of urethral sphincter mechanism incompetence in spayed bitches. Ankara Univ Vet Fak 2012;59:203–210. [Google Scholar]
- 34. Hill K, Jordan D, Ray J, et al. Medical therapy for acquired urinary incontinence in dogs. Int J Pharm Compd 2012;16:369–375. [PubMed] [Google Scholar]


