Abstract
Background
Darbepoetin alfa (darbepoetin) is an erythropoiesis‐stimulating agent used for the treatment of anemia secondary to chronic kidney disease (CKD) in dogs, but reports describing response are lacking.
Hypothesis/Objectives
To evaluate the effectiveness of darbepoetin in dogs with anemia secondary to CKD, dosing protocols, and adverse events.
Animals
Thirty‐three client‐owned dogs with naturally occurring CKD, including 26 with comorbidities.
Methods
Multi‐institutional retrospective study.
Results
The median starting dosage and highest dosage of darbepoetin administered were 0.5 and 0.8 μg/kg SC once weekly, respectively. Response to treatment was defined as achieving a packed cell volume (PCV) ≥30% or an increase in PCV ≥10%. Twenty‐eight of 33 dogs (85%) achieved a PCV ≥30% and 22 of 33 (67%) dogs achieved an increase in PCV ≥10%. Median time to achieve a PCV ≥30% was 29 days. A higher starting dosage was associated with achieving an increase in PCV ≥10% (P = .01). No dog sustained a response at a dosing interval >q21d. Potential adverse events included increased blood pressure requiring treatment (n = 12), seizures (n = 5), vomiting (n = 3), diarrhea (n = 3), and possible pure red cell aplasia (PRCA) (n = 2).
Conclusions and Clinical Importance
Darbepoetin, when combined with treatment of comorbidities, is an effective treatment for anemia secondary to CKD in dogs. A dosing interval >q21d was ineffective at maintaining a response to treatment. PRCA was a possible adverse event in 2 of 33 dogs (6%).
Keywords: Erythropoietin, Iron, Pure red cell aplasia, Renal disease
Abbreviations
- AKI
acute kidney injury
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- ESA
erythropoiesis‐stimulating agent
- IRIS
International Renal Interest Society
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean corpuscular volume
- PCV
packed cell volume
- pRBC
packed red blood cells
- PRCA
pure red cell aplasia
Chronic kidney disease (CKD) is a metabolic disorder of companion animals, estimated to occur in 0.4–1.5% of dogs and represented in a higher percentage of older dogs and those evaluated at tertiary care facilities.1, 2 A progressive, normocytic, normochromic hypoproliferative anemia develops as a feature of CKD, and although there are no published data on the prevalence of anemia in dogs with CKD, it is expected to occur in most dogs that progress to end‐stage disease.3, 4 Although the pathogenesis of the anemia of CKD is multifactorial, decreased production of erythropoietin by the diseased kidneys is an important factor.3, 5 The severity of anemia correlates positively with serum creatinine concentrations,5 and clinically relevant anemia is a hallmark of patients with International Renal Interest Society (IRIS) CKD Stages 3–4. In addition to contributing to lethargy, anorexia, and weakness, severe anemia may exacerbate progression of CKD because of decreased oxygen delivery to the residual kidney.4, 6
Erythropoiesis‐stimulating agents (ESA) are administered to some dogs with anemia secondary to CKD. Epoetin alfa (epoetin) was the first ESA used in human and veterinary medicine, but it has largely been replaced by darbepoetin alfa (darbepoetin) due to a 3‐fold longer half‐life, allowing for less frequent dosing.7, 8 Darbepoetin is a hyperglycosylated synthetic recombinant human erythropoietin analog. The recommended treatment protocol in humans is 0.45–0.75 μg/kg/wk to achieve an optimal response based on an increase in hemoglobin concentration.9 A previous study investigated the efficacy of darbepoetin in cats with anemia secondary to CKD.10 Fourteen of 25 cats responded, with 13 of 14 responding cats receiving a dosage of ≥1 μg/kg/wk SC.10 Recommended dosing in dogs is extrapolated from human and feline medicine and ranges from 0.45 to 1.5 μg/kg/wk SC until a target packed cell volume (PCV) is achieved.4, 6 The therapeutic targets for PCV currently published range from 25 to 40%.4, 6 Once the target PCV is achieved, dosing is decreased in frequency to the longest interval that maintains the target.
Adverse events associated with ESA administration in humans include iron deficiency, hypertension, stroke, vomiting, diarrhea, polycythemia, hyperkalemia, cutaneous reactions, and pure red cell aplasia (PRCA).11, 12, 13, 14, 15, 16, 17, 18 In cats and dogs, reported adverse events included vomiting, hypertension, seizures, fever, and PRCA.,1 , 3, 10, 19, 20, 21 Anecdotally, darbepoetin is perceived to be less likely to elicit formation of anti‐erythropoietin antibodies resulting in PRCA than is epoetin in dogs.4, 22 Similarly in cats, the prevalence of PRCA is reported to be 25–30% in those treated with epoetin compared with 8% in those treated with darbepoetin.10 A single report exists describing the use of darbepoetin in a dialysis‐dependent dog with IRIS CKD Stage 4.23 Darbepoetin treatment (1 μg/kg SC q7d) was initiated when the PCV was 21%, and the dog responded by day 28, achieving a PCV of 40%. Despite continued darbepoetin treatment, the dog's PCV decreased to 16% on day 49. Bone marrow aspirate cytology was consistent with PRCA.
The purpose of our study was to retrospectively evaluate the effectiveness of darbepoetin to stimulate erythropoiesis and correct anemia in dogs secondary to CKD. Additional goals were to describe the dosage protocols used for administration of darbepoetin and to document the prevalence of associated adverse events.
Materials and Methods
The medical records of dogs receiving darbepoetin2 (non‐albumin‐containing formulation) between July 2005 and July 2015 at the University of Pennsylvania School of Veterinary Medicine, University of California‐Davis School of Veterinary Medicine, and Cummings School of Veterinary Medicine at Tufts University were reviewed in a retrospective manner. Dogs were included in the study if they received darbepoetin to correct anemia associated with IRIS CKD Stages 3–4 and had a minimum follow‐up of 28 days. Dogs were excluded if they were treated with darbepoetin for reasons other than CKD, were undergoing chronic dialysis for CKD, were concurrently receiving other medications that have the potential to suppress the bone marrow, or had received a packed red blood cell (pRBC) transfusion during treatment that precluded evaluation of response to darbepoetin. One dog was included that completed dialysis for suspected acute kidney injury (AKI) 1 week before the initiation of darbepoetin treatment that subsequently was determined to have CKD based on renal biopsy. The management of non‐dialysis‐dependent dogs with IRIS CKD Stages 3–4 did not change substantially over the duration of the study or vary considerably among institutions.
The following information was collected from each medical record: signalment, staging of CKD based on IRIS guidelines, concern for an AKI exacerbating CKD at the time of initiation of darbepoetin treatment, and all comorbidities. Data collected before and during darbepoetin treatment included CBC, reticulocyte count, serum biochemistry profile, serum iron parameters, systolic blood pressure, pRBC transfusion, iron supplementation, darbepoetin dosage and frequency of administration, comorbidities, adverse events, and survival.
For the purposes of our study, PCV and hematocrit (HCT) were regarded as similar values. Starting PCV is the PCV at the time treatment with darbepoetin was initiated. Baseline PCV reflects any initial increase in PCV associated with a concurrent pRBC transfusion. Dogs were classified as responders or nonresponders, with a response to treatment defined in 1 of 2 ways: achieving a PCV ≥30% or a maximum increase in PCV from baseline (hereafter referred to as ΔPCV) ≥10% during the course of treatment. Days to response was the number of days from initiation of treatment until the first recorded PCV ≥30% or a ΔPCV ≥10%.
Statistical analyses were performed by standard statistical software.3 Descriptive statistics were calculated. Continuous variables were described with means and standard deviation (SD) if normally distributed and median values and ranges if not. Categorical variables were described as proportions and frequencies. Due to non‐normality of the data, the Mann–Whitney U‐test was used to determine whether there was a difference in starting and maximum dosages of darbepoetin administered between responders and nonresponders. Student's t‐test was used to determine whether there was a difference in canine age, pretreatment PCV, serum creatinine concentration, BUN concentration, and average number of comorbidities per dog between responders and nonresponders. Fisher's exact test was used to determine whether the occurrence of iron supplementation and concurrent pRBC transfusion was significantly different between responders and nonresponders. The Wilcoxon signed‐rank test was used to determine whether blood pressure increased significantly during treatment. The paired t‐test was used to determine whether serum potassium concentration increased significantly during treatment. Survival curves were generated by the Kaplan–Meier product limit method, and log‐rank analysis was used to determine whether there was a significant difference in survival time between responders and nonresponders as well as between CKD Stages 3 and 4. A P value of ≤.05 was considered significant for all statistical tests.
Results
Baseline Patient Characteristics
A total of 102 dogs with CKD receiving darbepoetin were identified. Of those, 33 dogs met the inclusion criteria and were treated at the University of California‐Davis (n = 22), University of Pennsylvania (n = 9), and Tufts University (n = 2). Reasons for exclusion were as follows: 23 dogs died or were euthanized before day 28 of treatment, 23 dogs were lost to follow‐up before day 28 of treatment, 12 dogs were dialysis dependent, 6 medical records were incomplete, 2 owners were noncompliant, in 2 cases darbepoetin was used transiently, and 1 dog received a pRBC transfusion 13 days after starting darbepoetin, precluding evaluation of response to darbepoetin.
Of the 33 dogs that met the inclusion criteria, the mean (±SD) age at the time of initiation of treatment was 7.4 (±4.5) years. Twenty‐five breeds were represented. There were 19 castrated males, 11 spayed females, 3 intact males, and 1 intact female. The cause of CKD was unknown for 29 dogs. Renal dysplasia in 1 dog and renal amyloidosis in 2 dogs were confirmed with histopathology. One dog developed CKD as a sequela to previous AKI that occurred >3 months before initiation of darbepoetin treatment. At the initiation of darbepoetin treatment, 10 of 33 dogs were hospitalized for acute decompensation of CKD, and thorough diagnostic evaluation, including abdominal ultrasound examination, urine culture, and infectious disease testing (including leptospirosis) failed to identify a cause of potential AKI.
Over the course of treatment, 45 comorbidities were reported in 26 of 33 dogs. Comorbidities included gastrointestinal disease (n = 15; lymphoplasmacytic inflammatory bowel disease [1], unclassified chronic enteropathy [4], acute gastroenteritis [5], gastrointestinal hemorrhage [3], and Trichuris vulpis infection [1]), cardiac disease (n = 12; chronic degenerative valvular disease [10] and unclassified heart murmur [2]), pancreatitis (n = 6), hepatobiliary disease (n = 5; increased liver enzyme activities [4] and liver mass of unknown etiology [1]), endocrine disease (n = 4; hypothyroidism controlled with thyroxine [2] and renal secondary hyperparathyroidism [2]), immune‐mediated disease (n = 3; polyarthritis [2] and familial shar pei fever [1]), and respiratory disease (n = 1; unclassified pleural effusion). Concurrent immune‐mediated disease was not reported in any dog suspected of developing PRCA.
At the start of darbepoetin treatment, the degree of anemia varied from mild to severe (Table 1). Fifteen of 33 dogs had a starting PCV ≤20%, 15 of 33 had a starting PCV between 20 and 25%, and 3 of 33 had a starting PCV ≥25% but <30%. Two of 33 dogs had reticulocyte counts >60,000/μL. Seven dogs received pRBC transfusions concurrently with initiation of treatment. After transfusions, baseline PCV was only slightly higher than the starting PCV (Table 1). Serum iron concentrations were measured in only 3 of 33 dogs. In 2 of those dogs, serum iron concentration was decreased, and total iron‐binding capacity (TIBC) and serum ferritin concentration were normal. In the third dog, serum iron and ferritin concentrations were normal, and TIBC was not evaluated.
Table 1.
Hematologic and biochemical parameters before initiation of darbepoetin treatment
| N | Mean | SD | |
|---|---|---|---|
| Starting packed cell volume (PCV) (%) | 33 | 20 | 4.1 |
| Baseline PCV (%) | 33 | 21.8 | 3.1 |
| MCV (fL) | 31 | 67.8 | 7.0 |
| MCHC (g/dL) | 31 | 33.9 | 1.6 |
| Absolute reticulocyte count (×103/μL)a | 26 | 20.7 | 0–88.5 |
| Platelet count (×103/μL) | 30 | 440 | 242 |
| BUN (mg/dL) | 33 | 110 | 49.7 |
| Creatinine (mg/dL) | 33 | 6 | 2.5 |
| Phosphorous (mg/dL) | 33 | 9.4 | 3.8 |
| Potassium (mmol/L) | 33 | 4.9 | 0.8 |
Starting PCV is the PCV at the time treatment with darbepoetin was initiated. Baseline PCV reflects an initial increase in PCV due to a concurrent packed red blood cells transfusion.
Absolute reticulocyte count was not normally distributed; the median and range are reported.
Darbepoetin Administration—Dose and Frequency
The median starting dosage of darbepoetin was 0.5 μg/kg (range, 0.4–2.1 μg/kg). Seven of 33 dogs were started at a dosage <0.45 μg/kg, and 1 was started at a dosage >1.0 μg/kg. The initial dosing frequency was 7 days in 30 of 33 dogs; 1 dog each was started with dosing every 14 days, 21 days, and as needed based on clinician preference. Darbepoetin was administered SC in all dogs.
The dosage of darbepoetin was increased over the course of treatment in 9 of 33 dogs. Five of these 9 dogs did not achieve a PCV ≥30% at the initial dosage, but with dosage increases, 4 of these 5 dogs achieved a PCV ≥30%. The other 4 dogs initially were responders but required dosage increases later in the course of treatment. One of these dogs had a dosage increase when moderate anemia (PCV 26%) recurred with progression of CKD. The second dog initially responded to 0.5 μg/kg SC once weekly and was transitioned to 0.23 μg/kg SC once weekly after this initial response; as the response waned at the lower dosage, the dosage was subsequently increased to 0.8 μg/kg SC once weekly. The darbepoetin dosage for the third dog was increased from 0.45 μg/kg every 14 days to 0.6 μg/kg once weekly on day 178 when anemia recurred concurrently with Trichuris vulpis infection. The darbepoetin dosage for the fourth dog was increased from 0.6 μg/kg administered every 4–6 weeks to 1.25 μg/kg every 14 days on day 169 when PCV decreased from 26 to 19%; the PCV subsequently rebounded to 25%. After dosage increases, the median highest dosage of darbepoetin administered over the course of treatment was 0.8 μg/kg (range, 0.4–2.17 μg/kg).
The dosing interval was prolonged beyond once weekly in 22 of 33 dogs, including in 12 dogs with the longest survival times (125–403 days). In 10 dogs, the dosing interval was extended to every 14 days. Of those, 4 dogs required subsequent shortening of the dosing interval to once weekly, 3 dogs maintained PCV ≥30% at a dosing interval of 14 days, and 3 dogs were lost to follow‐up after the extension of the dosing interval. In 1 dog, the dosing interval was extended to every 21 days, but this dog subsequently required reinstitution of once weekly dosing because of a decreasing PCV. In 11 dogs, the dosing interval was extended to ≥28 days, but all 11 dogs failed to maintain PCV ≥30%, and subsequent dosing interval reductions were recommended. Overall, 15 of 22 dogs required a subsequent shortening of the dosing interval due to failure to maintain target PCV at the prolonged dosing interval.
Of the 33 dogs starting darbepoetin treatment, 7 dogs received a pRBC transfusion for stabilization concurrent with initiation of treatment, and 23 dogs were already receiving iron supplementation (iron dextran4 10 mg/kg IM [n = 1] and unspecified dose IM [n = 1]; ferrous sulfate5 unspecified dose PO [n = 2]) or received iron supplementation concurrently with the start of darbepoetin treatment (iron dextran4 10 mg/kg IM [n = 13], and 1 dog each received iron dextran at dosages of 2, 8, 11, 15, 24 mg/kg, and an unspecified dose IM). Four dogs receiving iron supplementation also received a pRBC transfusion at the start of darbepoetin treatment.
Response to Darbepoetin
All dogs experienced an increase in PCV during treatment with darbepoetin. After dosage adjustments, the initial response was deemed adequate by all clinicians based on resolution of clinical signs associated with anemia, including improved energy and appetite. For the purposes of analysis, the magnitude of response to darbepoetin was quantified in 2 ways. In the first analysis, dogs were considered responders if a PCV ≥30% was achieved. Twenty‐four of 33 dogs achieved a PCV ≥30% at the initial starting dosage. After dosage adjustments, 28 of 33 dogs ultimately achieved a PCV ≥30% and were considered responders. The median time to response was 29 days (range, 6–106 days; Fig 1). In this analysis, there were no differences between responders and nonresponders in baseline PCV, iron supplementation in any form (eg, pRBC transfusion, iron dextran, ferrous sulfate) at the start of or throughout treatment, initial dosage of darbepoetin, maximum dosage of darbepoetin, severity of azotemia at the start of treatment, age, or comorbidities (Table 2). A lower starting PCV and receiving a pRBC transfusion at the onset of treatment were associated with being a nonresponder (P = .041 and .004, respectively).
Figure 1.
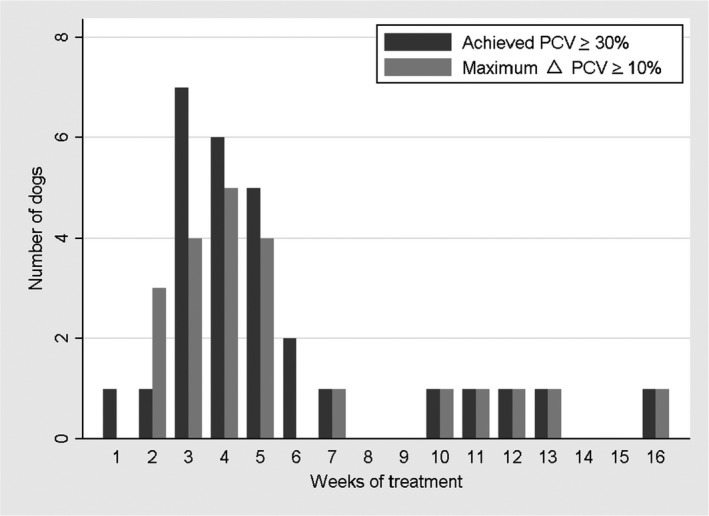
Number of weeks following initiation of darbepoetin for dogs to achieve a target packed cell volume (PCV) ≥30% and a ΔPCV ≥10%.
Table 2.
Comparison of disease and treatment variables between responders and nonresponders as defined by achieving a target PCV ≥30%
| Responders (n = 28) | Nonresponders (n = 5) | P value | |
|---|---|---|---|
| Starting packed cell volume (PCV) (%) | 20.6 (4.04) | 16.6 (4.03) | .041 |
| Baseline PCV (%) | 21.8 (3.1) | 21.8 (3.7) | .989 |
| Starting BUN (mg/dL) | 111.4 (53.2) | 102.8 (24.9) | .728 |
| Starting creatinine (mg/dL) | 6.0 (2.6) | 6.42 (2.2) | .731 |
| Initial darbepoetin dose (μg/kg)a | 0.52 (0.4–2.08) | 0.45 (0.4–0.7) | .092 |
| Maximum darbepoetin dose (μg/kg)a | 0.82 (0.4–2.17) | 0.51 (0.41–0.9) | .152 |
| Age (years) | 7.3 (4.5) | 8 (5.1) | .762 |
| Packed red blood cells (pRBC) with start of treatment | 3/28 | 4/5 | .004 |
| pRBC during treatment | 6/28 | 2/5 | .574 |
| Iron (pRBC or parenteral/oral) supplementation given at initiation of treatment | 21/28 | 5/5 | .559 |
| Iron (parenteral/oral) supplementation given throughout treatment | 13/28 | 3/5 | .656 |
| Comorbidities (average number per dog) | 1.36 | 1.4 | .736 |
P values <.05 are in bold.
Variables are presented as mean (standard deviation).
Initial and maximum darbepoetin doses were not normally distributed; the median and range are reported.
The 5 dogs deemed nonresponders based on failure to achieve a PCV ≥30% during treatment received darbepoetin for 100, 105, 192, 383, and 485 days. Starting PCVs of these nonresponders were 17, 10, 21, 18, and 17%. Four of 5 nonresponders received blood transfusions before initiation of darbepoetin, which increased baseline PCVs to 23, 16, 21, 23, and 26%, respectively. The maximum PCVs achieved by the nonresponder dogs were 28, 25, 28, 29, and 29%, respectively.
In the second analysis, dogs were considered responders if a ΔPCV ≥10% was achieved in response to darbepoetin. In this analysis, 22 of 33 dogs were deemed responders. The median time to achieve a ΔPCV ≥10% was 26 days (range, 9–106 days; Fig 1). When comparing responders to nonresponders based on ΔPCV, there were no differences in starting PCV, iron supplementation in any form at the start of or throughout treatment, severity of azotemia at the start of treatment, age, or comorbidities (Table 3). Higher initial and maximum dosages of darbepoetin administered were associated with being a responder (P = .013 and .031, respectively). Higher baseline PCV and receiving a pRBC transfusion at the start of treatment were associated with being a nonresponder (P = .043 and .027, respectively).
Table 3.
Comparison of disease and treatment variables between responders and nonresponders as defined by a maximum delta PCV ≥10%
| Responders (n = 22) | Nonresponders (n = 11) | P value | |
|---|---|---|---|
| Starting packed cell volume (PCV) (%) | 20.3 (3.3) | 19.5 (5.5) | .64 |
| Baseline PCV (%) | 21 (2.6) | 23.3 (3.6) | .043 |
| Starting BUN (mg/dL) | 121 (52.8) | 88.4 (35.7) | .077 |
| Starting creatinine (mg/dL) | 6.5 (2.6) | 5.2 (2.0) | .187 |
| Initial darbepoetin dose (μg/kg)a | 0.6 (0.41–2.08) | 0.45 (0.4–0.7) | .013 |
| Maximum darbepoetin dose (μg/kg)a | 0.82 (0.43–2.17) | 0.55 (0.4–0.98) | .031 |
| Age (years) | 6.8 (4.4) | 8.6 (4.6) | .263 |
| Packed red blood cells (pRBC) with start of treatment | 2/22 | 5/11 | .027 |
| pRBC during treatment | 5/22 | 3/11 | 1.000 |
| Iron (pRBC or parenteral/oral) supplementation given at initiation of treatment | 16/22 | 10/11 | .378 |
| Iron (parenteral/oral) supplementation given throughout treatment | 10/22 | 6/11 | .721 |
| Comorbidities (average number per dog) | 1.23 | 1.63 | .156 |
P values <.05 are in bold.
Variables are presented as mean (standard deviation).
Initial and maximum darbepoetin doses were not normally distributed; the median and range are reported.
Given the potential for resolution of AKI to be associated with improvement in anemia independently of darbepoetin, response also was evaluated after exclusion of 10 dogs that might have experienced AKI. The response rates were similar to those reported above, with 21 of 23 dogs (91%) achieving a target PCV ≥30% and 16 of 23 dogs (69%) having a ΔPCV ≥10%.
Transfusion treatment and Iron Supplementation After Initiation of Darbepoetin treatment
After initiation of treatment and pRBC transfusions given concurrently with the initial dosage of darbepoetin, 8 dogs received 12 additional pRBC transfusions over the course of treatment after determination of their response to darbepoetin. Of these 8 dogs, 6 initially were deemed responders based on achieving a PCV ≥30% and 5 initially were considered responders based on achieving a ΔPCV ≥10%. Three dogs required pRBC transfusions at times of known or suspected gastrointestinal bleeding occurring at 42, 105, and 112 days into darbepoetin treatment. Two dogs required pRBC transfusions before general anesthesia for various procedures. One dog required a pRBC transfusion to correct recurrence of severe anemia after prolongation of the darbepoetin dosing interval to >28 days. One dog required a pRBC transfusion to correct recurrence of anemia on day 169, without a specific cause of recurrent anemia identified. One dog developed biopsy‐confirmed myelofibrosis.
Over the course of treatment, 16 of 33 dogs received iron supplementation on a regular basis (iron dextran4 10 mg/kg IM monthly [n = 13], 17 mg/kg IM monthly [n = 1], 3 mg/kg IM monthly [n = 1]; ferrous sulfate5 10 mg/kg PO q24h [n = 1]), and 4 additional dogs received iron supplementation sporadically (iron dextran4 10 mg/kg IM [n = 2] and 15–16 mg/kg IM [n = 2]).
Survival After Initiation of Darbepoetin treatment
An estimated 439 doses of darbepoetin were administered over the course of the study. The median number of doses administered per dog was 12 (range, 2–61). Twenty‐five of 33 dogs were followed until death or euthanasia, and 8 of 33 dogs were lost to follow‐up. The median survival time for all dogs was 162 days (95% confidence interval [CI], 118–404; Fig 2). There was no significant difference (P = .671) in survival time between IRIS CKD Stage 3 (162 days) and Stage 4 (182 days) dogs (Fig 3). There was no significant difference (P = .172) in survival time between responders (n = 28; median, 144 days) and nonresponders (n = 5; median, 469 days) based on achieving a PCV ≥30% (Fig 4). There was also no significant difference (P = .442) in survival time between responders (n = 22; median, 162 days) and nonresponders (n = 11; median, 121 days) based on achieving a ΔPCV ≥10% (Fig 5).
Figure 2.
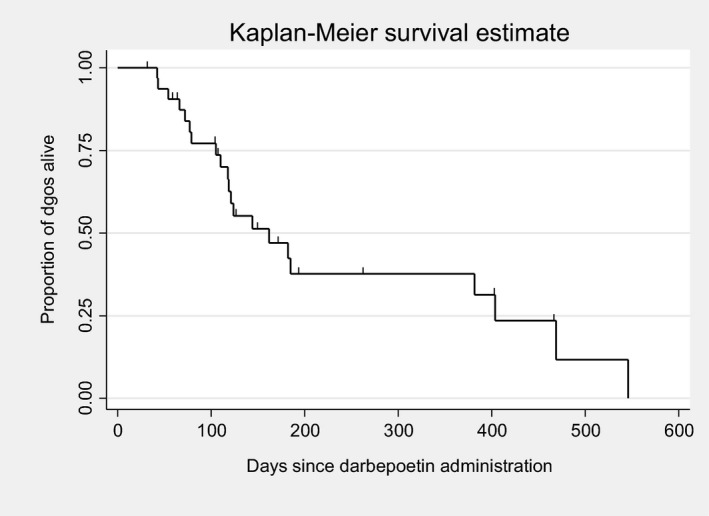
Kaplan–Meier overall survival estimates for dogs treated with darbepoetin.
Figure 3.
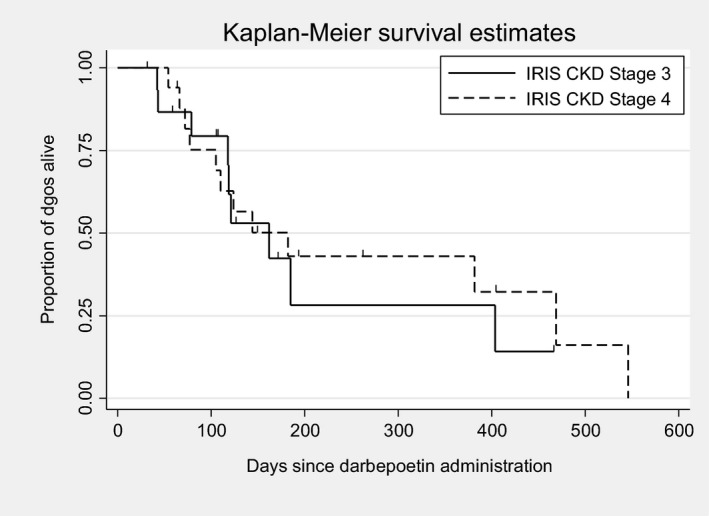
Kaplan–Meier survival estimates for dogs based on chronic kidney disease (CKD) stage at the beginning of treatment.
Figure 4.
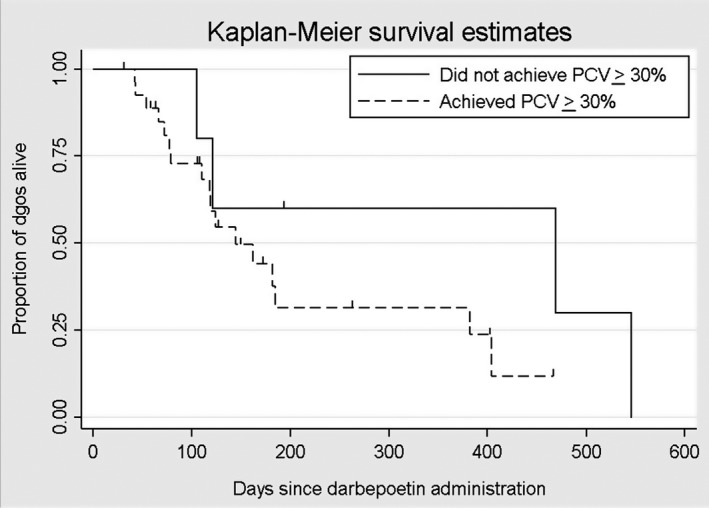
Kaplan–Meier survival estimates for dogs based on response to darbepoetin, with a responder defined as achieving a target packed cell volume (PCV) ≥30%.
Figure 5.
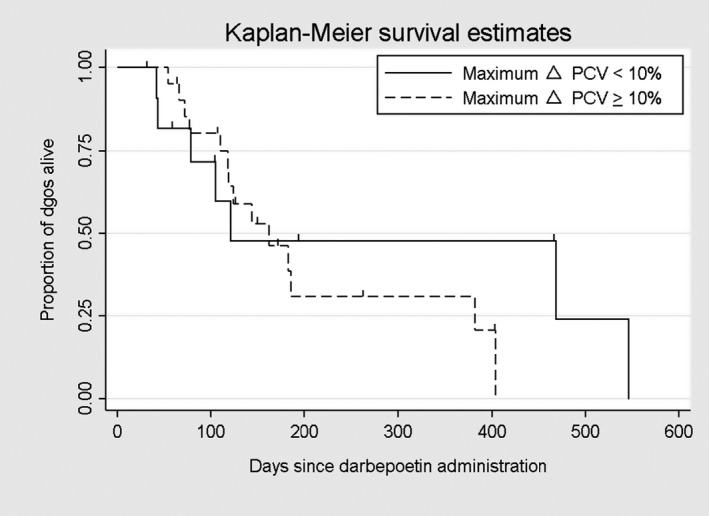
Kaplan–Meier survival estimates for dogs based on response to darbepoetin, with a responder defined as achieving an increase in Δpacked cell volume (PCV) ≥10%.
Potential Adverse Events Associated with Darbepoetin
Potential adverse events associated with darbepoetin treatment were noted in all 33 dogs, but discontinuation of darbepoetin was deemed necessary in only 2 dogs (Table 4). The most common adverse event observed was an increase in systolic blood pressure noted in 24 of 25 dogs in which blood pressure was monitored. The mean change in systolic blood pressure was 18 (±16) mmHg. When comparing responders to nonresponders, there was no difference in the mean change in systolic blood pressure between groups. The highest systolic blood pressure for each dog recorded over the course of treatment ranged from 150 to 222 mmHg (median, 170 mmHg) and was significantly higher than the systolic blood pressure at the start of treatment, which ranged from 120 to 176 mmHg (median, 153 mmHg; P < .001). There was no discernable pattern to the changes in blood pressure related to the institution of darbepoetin treatment.
Table 4.
Frequency of adverse events noted over the course of treatment with darbepoetin
| Adverse event | Number of dogs monitored | Adverse event occurred during darbepoetin treatment | Intervention for adverse event deemed warranted | Darbepoetin discontinued |
|---|---|---|---|---|
| Increase in systolic blood pressure | 25 | 24 | 13 | 0 |
| Hyperkalemia (>5.2 mmol/L) | 33 | 14 | 0 | 0 |
| Thrombocytosis (>530,000/μL) | 23 | 4 | 0 | 0 |
| Polycythemia (packed cell volume >60%) | 33 | 0 | 0 | 0 |
| Seizures | 33 | 5 | 2 | 0 |
| Diarrhea | 33 | 3 | 0 | 0 |
| Vomiting | 33 | 3 | 0 | 0 |
| Cutaneous reaction | 33 | 0 | 0 | 0 |
| Hypersensitivity reaction to darbepoetin | 33 | 0 | 0 | 0 |
| Hypersensitivity reaction to iron dextran | 33 | 1 | 1 | 0 |
| Pure red cell aplasia suspected | 33 | 2 | 2 | 2 |
Serum potassium concentration was increased in 13 of 33 dogs before administration of darbepoetin (mean, 4.93 ± 0.77 mmol/L). There was a mean change in serum potassium concentration of 0.92 ± 0.71 mmol/L (range, 0–2.5 mmol/L) during the course of treatment, with no difference between responders and nonresponders. The highest recorded serum potassium concentration (mean, 5.85 ± 0.6 mmol/L) after initiation of darbepoetin treatment was significantly higher than at baseline (P < .001). No dog developed clinical signs associated with hyperkalemia, and darbepoetin treatment was not discontinued.
An increase in platelet count over the course of treatment was noted in 19 of 22 dogs in which platelet monitoring was available. Of those, 4 developed thrombocytosis (platelet count >530 × 103/μL) during treatment. The increase in platelet count was observed at the first re‐evaluation in 3 dogs, with platelet counts increasing from 363, 488, and 414 × 103/μL at the start of treatment to 638, 587, and 972 × 103/μL, respectively. The fourth dog began treatment with a platelet count of 234 × 103/μL and developed a thrombocytosis of 539 × 103/μL on day 356 when gastrointestinal bleeding was suspected. None of these 4 dogs developed thromboembolic complications.
Seizure activity was reported in 5 dogs during treatment with darbepoetin. One dog had seizure activity observed on days 116 and 131 (PCV, 29%; platelet count, 416 × 103/μL; serum creatinine concentration, 7.7 mg/dL; systolic blood pressure, 171 mmHg); magnetic resonance imaging of the brain and cerebrospinal fluid analysis were inconclusive. A second dog was reported to have a seizure on day 335 and was subsequently evaluated on day 370 for teeth chattering and tremors (PCV, 27%; platelet count 287 × 103/μL; serum creatinine concentration, 2.6 mg/dL; systolic blood pressure, not recorded). A third dog was suspected to have had a seizure on day 125 and presented in shock, with acute anemia and aspiration pneumonia, experienced cardiac arrest, and died the same day (PCV, 14%; platelet count, 1044 × 103/μL; serum creatinine concentration, 3.2 mg/dL; systolic blood pressure, not recorded). This dog had persistently documented marked thrombocytosis before and throughout treatment with darbepoetin (baseline platelet count, 1043 × 103/μL). A fourth dog was presented on day 119 for euthanasia after having a grand mal seizure (PCV, 26%; platelet count, 233 × 103/μL; serum creatinine concentration, 4.7 mg/dL; systolic blood pressure, 140 mmHg). The fifth dog developed seizures on day 108 of treatment (PCV, 24%; platelet count, not determined; serum creatinine concentration, 7.1 mg/dL; systolic blood pressure, 175 mmHg). A precise cause of the seizures was unknown in all 5 dogs.
Gastrointestinal adverse effects were directly associated with darbepoetin administration in 1 dog that experienced self‐limiting diarrhea consistently within 24 hours of darbepoetin administration. All other gastrointestinal adverse effects were presumed to be associated with underlying kidney disease. One dog experienced an acute anaphylactic reaction 5 minutes after the first iron dextran3 injection (2 mg/kg IM). The dog was resuscitated, and iron supplementation was discontinued.
Pure red cell aplasia was considered as a differential diagnosis by the attending veterinarian in 5 dogs. In 2 dogs, the diagnosis of PRCA was discarded and darbepoetin was continued based on an alternative diagnosis for worsened anemia (gastrointestinal bleeding and gastrointestinal parasites in combination with decreased dosing frequency). One dog developed progressive anemia (PCV 14%) by day 125 of treatment and required pRBC transfusions in the face of weekly administration of darbepoetin. A diagnosis of PRCA was suspected, and the dog was euthanized without further diagnostic evaluation. In 2 dogs, treatment with darbepoetin was discontinued because of concern for PRCA. Based on complete review of the medical records, PRCA was considered likely in 1 dog in which the PCV decreased from >30% to 20% on day 54 while the dog was receiving darbepoetin at 1 μg/kg SC once weekly. Bone marrow cytology showed marked erythroid hypoplasia, with an M:E ratio of 30 : 1 and an abundance of iron‐laden macrophages; the dog was lost to follow‐up. The other dog developed pancytopenia, and a bone marrow biopsy showed generalized marrow hypoplasia and myelofibrosis. Pending bone marrow biopsy results, immunosuppressive treatment with prednisone was initiated, after which the dog was lost to follow‐up.
Discussion
Our study demonstrates that darbepoetin, when combined with treatment of comorbidities, is an effective treatment for the anemia of CKD in dogs, with 85 and 67% of dogs achieving a target PCV ≥30% and a ΔPCV ≥10%, respectively, after a median treatment time of 3–4 weeks. Although it is not possible to determine the optimal darbepoetin dosage based on this study, dogs receiving 0.8 μg/kg rather than 0.5 μg/kg SC once weekly were more likely to respond. The estimated cost of treating a 20 kg dog with darbepoetin at a dosage of 0.8 μg/kg once weekly for 4 weeks is $700. Although it is difficult to attribute most adverse events observed in this study to darbepoetin administration rather than a complication of CKD, the most serious adverse event directly associated with darbepoetin administration, PRCA, was documented in 1 of 33 dogs and considered possible in another dog.
Three dogs with PCV >25% and 2 dogs with evidence of RBC regeneration (absolute reticulocyte count >60,000/μL) were included in this study. Moderate anemia (PCV >25%) associated with CKD typically is not treated with darbepoetin, but, in these 3 dogs, intervention was deemed appropriate by the attending veterinarian, because the dogs had clinical signs attributable to anemia and advanced CKD, and a diagnostic evaluation failed to identify other causes of anemia. Although a regenerative response is not a feature of the anemia of CKD, multiple physiologic factors may be involved in the onset of anemia. In both dogs, the mildly regenerative response (absolute reticulocyte counts of 78,300/μL and 88,500/μL) was inadequate to correct the persistent anemia (PCV 24 and 21%, respectively). Loss of production of endogenous erythropoietin is progressive over the course of advancing CKD5 and insufficient RBC regeneration may precede nonregenerative anemia as the disease develops.
Because there is no universally accepted definition for the response to ESAs in dogs, we defined the response to treatment in 2 ways. When defining dogs as responders based on achieving a PCV ≥30%, there was no difference in the initial and highest dosages of darbepoetin administered between responders and nonresponders. However, response was positively associated with the dosage of darbepoetin used when defined as achieving a ΔPCV ≥10%. Importantly, 5 dogs that initially were classified as nonresponders based on failure to achieve a target PCV ≥30% subsequently underwent a dosage increase, after which 4 of 5 dogs became responders. Based on these results, and an understanding of the mechanism of action of ESAs, a dose‐dependent response to darbepoetin is supported. Although a singular effective starting dosage could not be determined from our study, dosage escalation should be considered if the initial dosage fails to achieve the therapeutic target.
Current recommendations are to initiate darbepoetin treatment weekly and then decrease the dosing frequency after initial target PCV has been achieved in order to reach the longest interval that maintains the desired maintenance target.4, 22 In our study, 11 of 33 dogs had the dosing interval extended to ≥21 days. Subsequent shortening of that dosing interval was recommended in all 11 dogs because of failure to maintain the desired PCV. Based on these findings, we recommend that the maintenance dosing interval not exceed 21 days without careful monitoring for relapse of anemia.
Iron supplementation and monitoring of serum iron parameters currently is recommended in veterinary patients receiving darbepoetin at the initiation of treatment and monthly thereafter.4, 22 Serum iron parameters were not consistently evaluated in our dogs. In human medicine, a deficiency in available iron for erythropoiesis is recognized as the leading cause of treatment failure in humans on ESA treatment.24, 25 Clinically, this deficiency may be most relevant after initiation of darbepoetin treatment because of consumption of iron stores in the therapeutically induced regenerative response. Based on the physiologic need for iron to support adequate erythropoiesis and data documenting the importance of human patients being iron replete for a positive response to ESAs, it is possible iron deficiency contributed to the lack of response in some dogs or the failure to maintain the targeted PCV. However, iron supplementation, whether in the form of pRBCs, considered the most bioavailable source of iron, parenteral iron dextran, or PO ferrous sulfate, did not have an apparent effect on response to darbepoetin in the dogs of our study. Nevertheless, we recommend documentation of an iron‐replete state before starting ESA treatment or if ESA resistance or decreased responsiveness to treatment is noted.
In our study, the known survival of 25 of 33 dogs receiving darbepoetin ranged from 42 to 546 days (median, 124 days). A Kaplan–Meier survival curve estimated a median survival time of 162 days for all dogs treated with darbepoetin. Survival was not positively associated with being a responder by either definition used to classify response in our study. In examining each case, we believe that dogs deemed nonresponders by our definitions likely would have been considered “adequate responders” rather than nonresponders by the attending veterinarians based on improvement in their clinical signs and survival time. For example, 2 of the 5 dogs that survived the longest failed to achieve a PCV ≥30% (maximum PCV 29% for both dogs) or a ΔPCV ≥10%, but both IRIS CKD Stage 4 dogs maintained a PCV in the range of 21–29% for >400 days while being treated with darbepoetin. For dogs with advanced CKD, maintenance of a PCV ≥21% for more than a year with darbepoetin treatment is clinically relevant, particularly in light of the reported survival time of 110–200 days and 14–80 days for dogs with IRIS CKD Stages 3 and 4, respectively.1, 26 A prospective controlled study comparing survival of anemic dogs with IRIS CKD Stages 3 and 4 treated with darbepoetin and those not receiving darbepoetin would be necessary to document the effect of darbepoetin on survival, but such a study would raise ethical concerns by withholding a seemingly effective treatment for the anemia of CKD. Although the definitions of response we used were an objective means to evaluate the effectiveness of darbepoetin treatment, they may underestimate the number of responders and help to explain the counterintuitive findings of higher baseline PCV being associated with nonresponder status. Alternatively, this association could be a type I error owing to small sample size.
Evaluating the adverse event profile for darbepoetin is challenging because there are a number of complications that are expected secondary to the progression of CKD itself, most importantly hypertension and seizures. In our study, 32% of dogs required initiation of or an increase in antihypertensive medication while being treated with darbepoetin, but in no case was darbepoetin discontinued because of hypertension. Development of hyperkalemia (range, 5.3–7.5 mmol/L) was observed in 42% of dogs and could be associated with CKD, administration of angiotensin‐converting enzyme (ACE) inhibitors (14/33 dogs), a combination of ACE inhibitor and angiotensin‐receptor blocker (3/33 dogs), thrombocytosis (ie, pseudohyperkalemia), improved dietary intake,27 or ESA administration.3 Development of thrombocytosis after initiation of darbepoetin treatment was noted in 4 dogs and could be associated with iron deficiency secondary to gastrointestinal bleeding, a common complication of advanced CKD, as well as increased bone marrow production due to permissive stimulation from darbepoetin. Fourteen percent of dogs developed seizures while being treated with darbepoetin. Differential diagnoses in all cases included progression of azotemia or hypertension, vascular events, primary central neurological disease, polycythemia, and hypoxemia. None of the dogs were polycythemic at the time of the seizures. Darbepoetin‐associated seizure was not a primary differential diagnosis for any dog, and, therefore the drug was not discontinued in any dog.
Pure red cell aplasia is the most serious adverse event associated with administration of ESAs. Published criteria for the diagnosis of ESA‐induced PRCA in humans require exclusion of the most frequent causes of PRCA, bone marrow examination showing <5% erythroid precursors, and the presence of EPO‐binding antibodies confirmed by a validated assay.28 Although assays for anti‐darbepoetin antibodies have been developed for humans,29 similar assays are not currently available for dogs. Therefore, a potential diagnosis of ESA‐induced PRCA in dogs is considered when there is a sudden decrease in PCV that cannot be attributed to another cause, such as gastrointestinal bleeding, and supported by the finding of marked erythroid hypoplasia on bone marrow cytology or histopathology, with normal megakaryocytic and myeloid series. Based on our retrospective review, darbepoetin‐induced PRCA is considered likely in only 1 dog and possible in 2 of 33 cases and, thus, a possible adverse event in 6% of the dogs. However, given that the follow‐up time for 4 dogs was <8 weeks, it is possible that more dogs would have developed PRCA if treated and monitored for a longer period of time. Although the incidence of darbepoetin‐induced PRCA appears to be low, clients should be informed of this potentially life‐threatening complication.
The limitations of our study include its retrospective nature, small sample size, and nonstandardized protocol for darbepoetin administration. In addition, because PCV and HCT can differ by several percentage points, use of PCV and HCT interchangeably could have overestimated the response to darbepoetin. Furthermore, unrecognized AKI complicating CKD could have influenced our results, because resolution of AKI could have resulted in improvement of anemia independently of darbepoetin. Similarly, some comorbidities could have exacerbated the anemia of CKD by blood loss or chronic disease, making it difficult to attribute increases in PCV observed during treatment solely to darbepoetin treatment. Finally, some dogs with CKD could have had some degree of iron deficiency, and thus a pRBC transfusion, iron supplementation, or both could have contributed to an increase in PCV.
In conclusion, darbepoetin, when combined with treatment of comorbidities, is an effective treatment for the anemia of CKD in dogs. Further studies are needed to define the most effective dosing protocol, but a dosage of 0.8 μg/kg SC weekly appears to be more effective than 0.5 μg/kg SC weekly. Dosing frequency should be decreased after the initial dosing target is achieved to the longest interval that maintains a stable maintenance target for PCV. A dosing interval of >21 days is unlikely to maintain an effective target PCV long term.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was not supported by a grant or otherwise.
Previously presented as a research abstract at the 2016 ACVIM Forum, Denver, CO.
Footnotes
Cowgill LD, Feldman B, Levy K, James, K. Efficacy of recombinant human erythropoietin for anemia in dogs and cats with renal failure. J Vet Intern Med 1990;4:126
Aranesp, Amgen, Thousand Oaks, CA
STATA 13.1, Stata Corporation, College Station, TX
Iron hydrogenated dextran injection, Henry Schein Animal Health, Dublin, OH
Ferrous sulfate, Rugby Laboratories, Livonia, MI
References
- 1. O'Neil DJ, Elliott J, Church DB, et al. Chronic kidney disease in dogs in UK veterinary practices: Prevalence, risk factors, and survival. J Vet Intern Med 2013;27:814–821. [DOI] [PubMed] [Google Scholar]
- 2. Sosnar M. Retrospective study of renal failure in dogs and cats admitted to University of Veterinary and Pharmaceutical Sciences Brno during 1999–2001. Acta Vet Brno 2003;72:593–598. [Google Scholar]
- 3. Cowgill LD, James KM, Levy JK, et al. Use of recombinant human erythropoietin for management of anemia in dogs and cats with renal failure. J Am Vet Med Assoc 1998;212:521–528. [PubMed] [Google Scholar]
- 4. Bartges JW. Chronic kidney disease in dogs and cats. Vet Clin North Am Small Anim Pract 2012;42:669–692. [DOI] [PubMed] [Google Scholar]
- 5. King LG, Giger U, Diserens D, Nagode LA. Anemia of chronic renal failure in dogs. J Vet Intern Med 1992;6:264–270. [DOI] [PubMed] [Google Scholar]
- 6. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012;26:275–281. [DOI] [PubMed] [Google Scholar]
- 7. Egrie JC, Dwyer E, Browne JK, et al. Darbepoetin alfa has a longer circulating half‐life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol 2003;31:290–299. [DOI] [PubMed] [Google Scholar]
- 8. Elliott S, Pham E, Macdougall I. Erythropoietins: A common mechanism of action. Exp Hematol 2008;36:1573–1584. [DOI] [PubMed] [Google Scholar]
- 9. Macdougall I. Novel erythropoiesis‐stimulating agents: A new era in anemia management. Clin J Am Soc Nephrol 2008;3:200–207. [DOI] [PubMed] [Google Scholar]
- 10. Chalboub S, Langston C, Farrelly J. The use of darbepoetin to stimulate erythropoiesis in anemia of chronic kidney disease in cats: 25 cases. J Vet Intern Med 2012;26:363–369. [DOI] [PubMed] [Google Scholar]
- 11. Schaefer F, Hoppe B, Jungraithmayr T, et al. Safety and usage of darbepoetin alfa in children with chronic kidney disease: Prospective registry study. Pediatr Nephrol 2016;31:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spinowitz B, Germain M, Benz R, et al. A randomized study of extended dosing regimens for initiation of epoetin alfa treatment for anemia of chronic kidney disease. Clin J Am Soc Nephrol 2008;3:1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mel JR, Salar A, Rodríguez CA, et al. A prospective observational study of the effectiveness, safety, and effect on fatigue of darbepoetin alfa for the treatment of chemotherapy‐induced anaemia. Curr Med Res Opin 2008;24:2931–2942. [DOI] [PubMed] [Google Scholar]
- 14. Frei U, Kwan JTC, Spinowitz BS, The Epoetin Delta 3002 Study Group . Anaemia management with subcutaneous epoetin delta in patients with chronic kidney disease (predialysis, haemodialysis, peritoneal dialysis): Results of an open‐label, 1‐year study. BMC Nephrol 2009;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pergola PE, Gartenberg G, Fu M, et al. A randomized controlled study of weekly and biweekly dosing of epoetin alfa in CKD patients with anemia. Clin J Am Soc Nephrol 2009;4:1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfeffer MA, Burdmann EA, Chen C‐Y, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019–2032. [DOI] [PubMed] [Google Scholar]
- 17. Macdougall IC, Casadevall N, Locatelli F, et al. Incidence of erythropoietin antibody‐ mediated pure red cell aplasia: The Prospective Immunogenicity Surveillance Registry (PRIMS). Nephrol Dial Transplant 2015;30:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eckardt K, Casadevall N. Pure red‐cell aplasia due to anti‐erythropoietin antibodies. Nephrol Dial Transplant 2003;18:865–869. [DOI] [PubMed] [Google Scholar]
- 19. Randolph J, Scarlett J, Stokol T, et al. Expression, bioactivity, and clinical assessment of recombinant feline erythropoietin. Am J Vet Res 2004;63:1355–1366. [DOI] [PubMed] [Google Scholar]
- 20. Randolph J, Scarlett J, Stokol T, et al. Clinical efficacy and safety of recombinant canine erythropoietin in dogs with anemia of chronic renal failure and dogs with recombinant human erythropoietin‐induced red cell aplasia. J Vet Intern Med 2004;18:81–91. [DOI] [PubMed] [Google Scholar]
- 21. Langston CA, Reine NJ, Kittrell D. The use of erythropoietin. Vet Clin North Am Small Animal Pract 2003;33:1245–1260. [DOI] [PubMed] [Google Scholar]
- 22. Polzin D. Evidence‐based step‐wise approach to managing chronic kidney disease in dogs and cats. J Vet Emerg Crit Care 2013;23:205–215. [DOI] [PubMed] [Google Scholar]
- 23. Blais M, Berman L, Oakley D, Giger U. Canine Dal blood type: A red cell antigen lacking in some Dalmatians. J Vet Intern Med 2007;21:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Macdougall IC. Strategies for iron supplementation: Oral versus intravenous. Kidney Int 1999;55(Suppl 69):S61–S66. [DOI] [PubMed] [Google Scholar]
- 25. Schiesser D, Binet I, Tsinalis D, et al. Weekly low‐dose treatment with intravenous iron sucrose maintains iron status and decreases epoetin requirement in iron‐replete haemodialysis patients. Nephrol Dial Transplant 2006;21:2841–2845. [DOI] [PubMed] [Google Scholar]
- 26. Parker VJ, Freeman LM. Association between body condition and survival in dogs with acquired chronic kidney disease. J Vet Intern Med 2011;25:1306–1311. [DOI] [PubMed] [Google Scholar]
- 27. Segev G, Fascetti AJ, Weeth LP, Cowgill LD. Correction of hyperkalemia in dogs with chronic kidney disease consuming commercial renal therapeutic diets by a potassium‐reduced home‐prepared diet. J Vet Intern Med 2010;24:546–550. [DOI] [PubMed] [Google Scholar]
- 28. Rossert J. Pure red cell aplasia global scientific advisory board (GSAB). Erythropoietin‐induced, antibody‐mediated pure red cell aplasia. Eur J Clin Invest 2005;35(Suppl 3):95–99. [DOI] [PubMed] [Google Scholar]
- 29. Mytycha DT, Lab S, Barger T, et al. The development and validation of a sensitive, dual‐flow cell, SPR‐based biosensor immunoassay for the detection, semi‐quantitation, and characterization of antibodies to darbepoetin alfa and epoetin alfa in human serum. J Pharm Biomed Anal 2009;49:415–426. [DOI] [PubMed] [Google Scholar]


