Abstract
Background
Radioiodine (131I) is effective treatment for hyperthyroidism in cats, but optimal dose to restore euthyroidism without inducing hypothyroidism is unclear. Treatment‐induced hypothyroidism can lead to azotemia and reduced duration of survival.
Objective
To compare efficacy and short‐term outcomes of low‐dose 131I versus higher, standard‐dose 131I as treatment for hyperthyroidism.
Animals
A total of 189 client‐owned cats undergoing 131I treatment for mild‐to‐moderate hyperthyroidism (serum T4 ≥ 4.0 μg/dL and <13.0 μg/dL).
Methods
Prospective, nonrandomized, cohort study comparing treatment with either low‐dose (2 mCi, n = 150) or standard‐dose (4 mCi, n = 39) 131I. Serum T4, thyroid‐stimulating hormone (TSH), and creatinine concentrations were measured after 1, 3, and 6 months to determine persistent hyperthyroidism, overt hypothyroidism (low T4, high TSH), subclinical hypothyroidism (normal T4, high TSH), and azotemia.
Results
There was no significant difference in prevalence of cats with persistent hyperthyroidism between standard‐ and low‐dose treatment groups at 3 (0% versus 5.3%; P = .34) and 6 (0% versus 3.3%; P = .51) months. Overt (18% versus 1%; P = .0005) or subclinical (46% versus 21%; P = .004) hypothyroidism was more common in cats at 6 months after standard‐dose 131I. No difference in incidence of azotemia existed between groups, but cats treated with standard‐dose 131I had higher creatinine concentrations (P < .05) and higher percent rises in creatinine (P < .0001).
Conclusions and Clinical Importance
Low‐dose 131I is safe and effective for cats with mild‐to‐moderate hyperthyroidism, as evidenced by a cure rate of >95% with reduced frequency of iatrogenic hypothyroidism and azotemia.
Keywords: 131I, Feline, Radioactive iodine, Radiotherapy, Thyroid gland
Abbreviations
- 131I
radioiodine
- AEC
Animal Endocrine Clinic
- C.I.
confidence interval
- CKD
chronic kidney disease
- CUHA
Cornell University Hospital for Animals
- GFR
glomerular filtration rate
- IQR
interquartile range
- L‐T4
levothyroxine
- mCi
millicurie
- T/S
thyroid‐to‐salivary gland
- T4
thyroxine
- TSH
thyroid‐stimulating hormone
Hyperthyroidism resulting from adenomas/adenomatous hyperplasia of the thyroid gland is the most common endocrine disorder of older cats.1, 2 Treatment options include antithyroid drugs such as methimazole or carbimazole, iodine‐restricted diet, thyroidectomy, or radioactive iodine.3, 4, 5, 6 Radioiodine (131I) is generally considered the optimal treatment for hyperthyroidism in cats because of its safety and efficacy.1, 5, 7, 8
Ideally, the dose of 131I used to treat hyperthyroidism in cats should restore euthyroidism without inducing hypothyroidism. In other words, the goal of radioiodine treatment is to irradiate and destroy all of the abnormal adenomatous thyroid gland while leaving normal thyroid tissue intact. Several schemes exist to determine the appropriate dose of 131I to administer to hyperthyroid cats.8 The most commonly employed approach is a fixed standard‐dose protocol in which 4–5 millicuries (mCi; 148–185 megabecquerel [mBq]) 131I is administered to cats regardless of severity of hyperthyroidism or size of thyroid tumor.9, 10, 11, 12, 13, 14 Less commonly, individualized or cat‐specific dosing protocols are used in which the 131I dose might be low (3 mCi; 110 mBq), medium (4 mCi; 150 mBq), or high (5 mCi; 185 mBq) dependent on severity of hyperthyroidism and size of thyroid goiter.5, 8 Recently, a preliminary study suggested that even the 3 mCi dose could be more radioiodine than many hyperthyroid cats need and that 2 mCi (75 mBq) 131I could successfully treat most cats with mild‐to‐moderate hyperthyroidism.1
The main disadvantage of lower doses of radioiodine is the potential for treatment failure. In hyperthyroid cats receiving the higher standard dose of 131I, persistent hyperthyroidism has been rarely reported (<5%).5, 11, 12, 15 Possible benefits of administering lower doses of 131I in the management of hyperthyroidism in cats include lower radiation exposure to cats and personnel, decreased cost of radioiodine, shorter quarantine times for cats after treatment, and likely reduced incidence of iatrogenic hypothyroidism.8 Importantly, hypothyroidism induced by treatment for hyperthyroidism appears to contribute to the development of azotemia and reduced duration of survival in cats.16 Iatrogenic hypothyroidism, based on the finding of subnormal serum thyroxine (T4) concentration 3 or more months after standard‐dose 131I treatment, occurs in as few as 3%10 and up to as many as 79%17 of all treated cats, with most studies reporting a prevalence of ≤9%.9, 11, 12 No study has examined the prevalence of iatrogenic hypothyroidism in cats with milder degrees of hyperthyroidism treated with standard‐dose 131I. Likewise, the frequency of iatrogenic hypothyroidism in hyperthyroid cats treated with low‐dose (2 mCi) 131I has not been well established.
The objective of this study was to compare the efficacy of 2 different doses of 131I (2 mCi and 4 mCi) in the treatment of cats with mild‐to‐moderate hyperthyroidism. Our hypothesis was that hyperthyroid cats treated with low‐dose 131I would develop iatrogenic hypothyroidism less frequently than cats treated with the higher standard dose, yet have a similarly low prevalence of persistent hyperthyroidism.
Materials and Methods
Selection of Animals
A prospective, non‐randomized, cohort study of client‐owned hyperthyroid cats presenting to either the Cornell University Hospital for Animals (CUHA) or the Animal Endocrine Clinic (AEC) for 131I treatment between October 2013 and December 2014 was performed. Cornell University College Veterinary Medicine Clinical Studies Committee approved the study after an ethical and scientific review. The project was reviewed by the IACUC office and exempted from IACUC review because the procedures to be performed were clinically required, represented standard accepted veterinary practice for the animals’ condition, and did not meet the regulatory and Cornell policy description for the use of animals for research, teaching, or testing. Informed client consent was obtained for all cats in the study.
Cats were eligible for enrollment if they had mild‐to‐moderate hyperthyroidism (serum T4 concentration ≥4 μg/dL but <13 μg/dL; reference range 0.9–3.9 μg/dL) together with compatible clinical features (e.g, weight loss despite a good appetite, palpable goiter).18 Cats with serum T4 ≥ 13 μg/dL were excluded to focus on cats with mild‐to‐moderate hyperthyroidism that might be expected to be cured with a low dose of 131I.1 Cats must not have been fed a prescription iodine‐restricted diet2 for at least 2 weeks before evaluation. If cats had been previously treated with methimazole, the drug must have been discontinued for at least 1 week before evaluation. A prior methimazole trial was not required for enrollment. Cats were excluded from the study if they were azotemic (serum creatinine ≥2 mg/dL) at the time of enrollment, had thyroid scintigraphic findings compatible with thyroid carcinoma (i.e, large heterogeneous areas of uptake with irregular or speculated margins, linear multifocal uptake patterns, or evidence of metastasis to regional lymph nodes or lungs),19, 20 or were previously treated by thyroidectomy or percutaneous thyroid ablation.
Study Protocol
For all cats, before‐treatment evaluation included complete history, physical examination, routine laboratory testing (CBC and serum biochemistry profile), serum T4 and TSH concentrations, and quantitative thyroid scintigraphy.19, 20, 21 All before‐treatment laboratory testing was performed at a single commercial veterinary diagnostic laboratory.3 Thyroid scintigraphy confirmed unilateral or bilateral thyroid lobe enlargement (i.e, “hot” thyroid nodules) together with a high thyroid‐to‐salivary gland (T/S) ratio (≥1.5) in all study cats. Cats were treated with a single subcutaneous dose of 131I (4 mCi at CUHA or 2 mCi at AEC) within 24 hours of scintigraphy, and then hospitalized and monitored in accordance with nuclear safety regulations.
Recheck examinations were performed at 1, 3, and 6 months after 131I treatment. In most cases, the referring veterinarian performed these follow‐up examinations. At each follow‐up visit, blood samples were collected to determine serum concentrations of T4, TSH, and creatinine. To improve case enrollment and compliance with follow‐up, the referring veterinarians had the option to submit these serum samples to 1 of 2 commercial veterinary diagnostic laboratories3 , 4 (almost all practices in the northeastern USA use one of these designated commercial laboratories). Approximately half of all referring veterinarians used 1 laboratory, whereas the remainder used the other laboratory. There was no significant difference (P = .74; chi‐square test) between the proportion of low‐ or standard‐dose 131I‐treated cats rechecked by either veterinary diagnostic laboratory (63% vs 59%3 and 37% vs 41%4).
In both laboratories, serum T4 concentration was measured by a homogenous enzyme immunoassay validated for use in cats (Data S1).19 In both laboratories, serum TSH was measured by a canine chemiluminescent immunoassay, also validated for use in cats (Data S1).19 As described in Data S1, when serum samples were divided into 2 aliquots and submitted to both laboratories for analysis, results showed good agreement for both T4 and TSH concentrations, especially within the ranges for clinical decision surrounding their reference intervals (0.9–3.9 μg/dL and 0.03–0.3 ng/mL, respectively).
Data and Statistical Analysis
All statistical analyses were performed by proprietary statistical software.5 , 6 Continuous data were assessed for normality by the Shapiro‐Wilk test and by visual inspection of graphical plots.22, 23 Data were not normally distributed; therefore, all analyses used nonparametric tests.23 Results are reported as median (interquartile range [IQR], 25th–75th percentile) and are represented graphically as box‐and‐whisker plots and bar graphs. For all analyses, statistical significance was defined as P ≤ .05.
For analysis, cats were classified into 1 of 4 thyroid categories based on the reference intervals established for serum T4 (0.9–3.9 μg/dL) and TSH (0.03–0.3 ng/mL) concentrations: persistent hyperthyroidism (T4 ≥ 4.0 μg/dL; TSH <0.03 ng/mL), euthyroidism (T4, 0.9–3.9 μg/dL; TSH ≤ 0.3 ng/mL), overt hypothyroidism (T4 ≤ 0.8 μg/dL; TSH > 0.3 ng/mL), and subclinical hypothyroidism (T4 0.9–3.9 μg/dL; TSH > 0.3 ng/mL). See Data S1 for details on how we determined the reference intervals for serum T4 and TSH concentrations. The thyroid status of each cat was assessed at 3 and 6 months after 131I treatment, but not at 1 month to prevent misclassification of cats that might still be recovering from TSH suppression secondary to the original hyperthyroid state.
Although dependent on the discretion of both owner and referring veterinarian, supplementation with a low dose (0.075 mg once daily) of levothyroxine (L‐T4) was recommended in any cat that had overt hypothyroidism 3 or more months after radioiodine treatment, or in any cat with subclinical hypothyroidism and worsening azotemia detected at the 3‐month examination. For cats supplemented with L‐T4 at 3 months, the T4 and TSH concentrations measured at 6 months were excluded from numerical statistical analysis. However, the categorical data still classified these cats as hypothyroid at the 6‐month recheck examination.
Continuous variables were compared between groups by the Mann‐Whitney U‐test; comparisons among 2 or more measurements within a group (before–after) were compared with the Wilcoxon signed ranks test. Categorical variables were compared among groups by the chi‐square test (or Fisher's exact test, where appropriate).
Before study enrollment, the sample size for the study was estimated based on an estimated incidence of overt hypothyroidism of approximately 25% for the standard‐dose treatment and 5% for the low‐dose treatment (effect size of ~20% difference) with twice the number of cats being enrolled in the low‐dose group. With an alpha error rate of 5% and a power of 80%,24 this yielded an estimate of approximately 68 cats in the low‐dose group and 34 cats in the standard‐dose group. During the enrollment period, cats receiving low‐dose 131I were enrolled at a ratio of about 3.8:1 cat treated with standard‐dose treatment. These cats were included to increase the power to detect an even smaller significant treatment difference should it be observed.
Results
Study Populations
During the study period, 189 cats were successfully enrolled: 39 cats were treated with standard‐dose 131I and 150 cats were treated with low‐dose 131I. All cats completed the study, but 1 cat treated with the standard dose missed the 1‐month recheck appointment. Cats treated with standard‐dose 131I included 31 domestic shorthair, 6 domestic longhair, 1 Maine Coon, and 1 Siamese. Median age of these cats was 13 years (range, 5–18 years), with a sex distribution of 20 (51%) spayed females and 19 (49%) neutered males. Cats treated with low‐dose 131I included 114 domestic shorthair, 16 domestic medium or longhair, 7 Maine Coon, 5 Siamese, 2 Burmese, 2 Norwegian Forest, and 1 each Birman, Ragdoll, Russian Blue, and Tonkinese. Median age of these cats was 13 years (range, 7–19 years), with 74 (49%) spayed females and 76 (51%) neutered males.
There were no significant differences in breed (P = .49), age (P = .62), or sex (P = .86) between the 2 study populations. Similarly, no significant differences in before‐treatment serum concentrations of T4, TSH, or creatinine were found among cats treated with low‐ or standard‐dose 131I (Figs 1, 2, 3). Thyroid scintigraphy also failed to reveal any significant differences in median T/S ratio (3.7 vs 4.4; P = .255) or bilateral lobe involvement (102/150 [68%] vs 28/39 [72%]; P = .702) between the low‐ or standard‐dose treatment groups, respectively.
Figure 1.
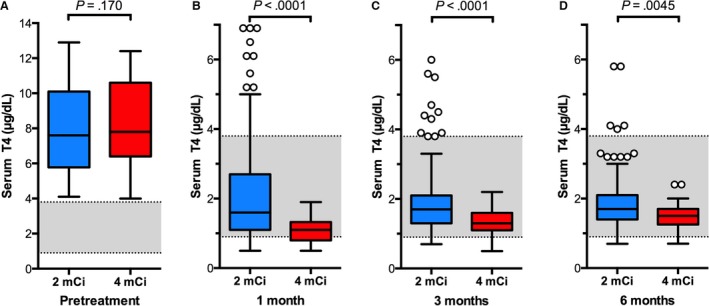
Box plots of serum T4 concentrations in 189 cats before and after treatment with low‐ (2 mCi; n = 150) or standard‐dose (4 mCi; n = 39) 131I. (A) Before treatment; (B) 1 month after 131I treatment; (C) 3 months after 131I treatment; and (D) 6 months after 131I treatment. The significant differences between the 2 treatment groups are indicated for each time period. Boxes represent the interquartile range (IQR) from the 25th to 75th percentile. The horizontal bar in each box represents the median value. The T‐bars represent the main body of data, which in most instances is equal to the range. Open circles represent outlying data points.
Figure 2.
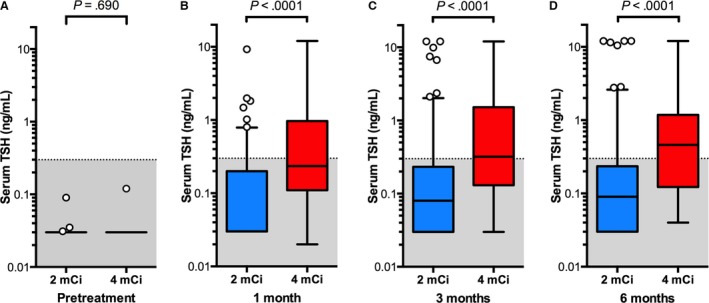
Box plots of serum TSH concentrations in 189 cats before and after treatment with low‐dose (2 mCi; n = 150) or standard‐dose (4 mCi; n = 39) 131I. Data are plotted as in Fig. 1.
Figure 3.
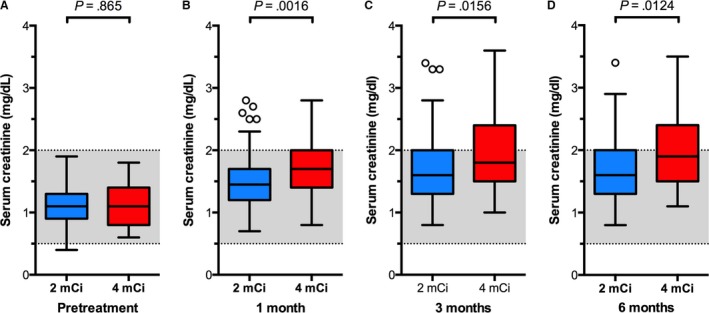
Box plots of serum creatinine concentrations in 189 cats before and after treatment with low‐ (2 mCi; n = 150) or standard‐dose (4 mCi; n = 39) 131I. Data are plotted as in Fig. 1.
After‐Treatment Thyroid Function
At 1, 3, and 6 months after treatment, cats treated with standard‐dose 131I had significantly lower serum T4 concentrations (P < .005) and higher TSH concentrations (P < .001) than did the cats treated with low‐dose 131I (Figs 1 and 2).
At the 3‐month recheck, 5 cats (3 with overt and 2 with subclinical hypothyroidism) that received standard‐dose 131I and 1 cat (with subclinical hypothyroidism) that received low‐dose 131I were started on low‐dose L‐T4 supplementation. Therefore, the serum T4 and TSH concentrations measured at 6 months from these 6 cats were not included in the data analysis shown in Figs. 1 and 2. After L‐T4 replacement, the median serum T4 increased from 0.8 μg/dL (range, 0.5–1.5 μg/dL) at 3 months to 1.6 μg/dL (range, 1.3–3.1 μg/dL) at 6 months in the 5 cats of the standard‐dose group (P = .063) and from 1.1 μg/dL to 2.4 μg/dL in the 1 cat of the low‐dose group. Median serum TSH concentration decreased from 10.7 ng/mL (range, 0.4–12.0 ng/mL) to 2.9 ng/mL (P = .25) but remained high (range, 0.6–12.0 ng/mL) in all 5 of the standard‐dose cats when tested at 6 months. Serum TSH fell from 2.4 ng/mL to 1.0 ng/mL in the 1 cat of the low‐dose group.
All paired serum T4 and TSH concentrations allowed categorization of cats into 1 of 4 outcome categories (euthyroidism, persistent hyperthyroidism, overt hypothyroidism, or subclinical hypothyroidism). There were no discordant results.
Euthyroidism
(T4 0.9–3.9 μg/dL; TSH ≤ 0.3 ng/mL): At 3 months, 17 (44%) of the 39 cats that received standard‐dose 131I and 111 (74%) of the 150 cats that received low‐dose 131I were euthyroid (Fig 4). At 6 months, 14 (36%) of the 39 cats that received standard‐dose 131I and 113 (75%) of the 150 cats that received low‐dose 131I were euthyroid (Fig 4). Cats treated with standard‐dose 131I were significantly less likely to be euthyroid than those treated with low‐dose 131I at both 3 (P = .0014) and 6 (P = .0001) months.
Figure 4.
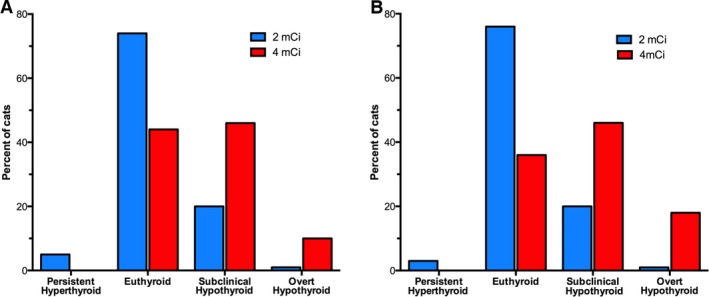
Bar graphs depicting the thyroid status in 189 cats treated with low‐dose (2 mCi; n = 150) or standard‐dose (4 mCi; n = 39) 131I. (A) Percentage of cats with persistent hyperthyroidism, euthyroidism, subclinical hypothyroidism, or overt hypothyroidism at 3 months after 131I treatment. (B) Percentage of cats with persistent hyperthyroidism, euthyroidism, subclinical hypothyroidism, or overt hypothyroidism at 6 months after 131I treatment. The prevalence of euthyroidism at 6 months is twice as high in cats treated with low‐dose 131I, whereas the prevalence of overt and subclinical hypothyroidism is 3‐fold higher in cats treated with standard‐dose 131I.
Persistent Hyperthyroidism
(T4 ≥ 4 μg/dL; TSH <0.03 ng/mL): Persistent hyperthyroidism was not observed in any of the cats treated with standard‐dose 131I. Of the 150 cats treated with low‐dose 131I, persistent hyperthyroidism was detected in 8 (5.3%; 95% C.I. 2.7–10.2%) cats at 3 months and 5 (3.3%; 95% C.I. 1.4–7.6%) cats at 6 months (Fig 4). However, there was no significant difference in the percentage of cats with persistent hyperthyroidism between treatment groups at either 3 (0% vs 5.3%; P = .34) or 6 (0% vs 3.3%; P = .51) months. Generally, our target T4 concentration for cats with hyperthyroidism after radioiodine treatment is 1.5–3.0 μg/dL. Based on that desired range of 1.5–3.0 μg/dL, 7 cats in the low‐dose group had T4 >3.0 μg/dL but ≤3.9 μg/dL (higher than considered ideal, yet still within the reference interval).
Overt Hypothyroidism
(T4 ≤ 0.8 μg/dL; TSH >0.3 ng/mL): At 3 months, 4 (10%) of the 39 cats that received standard‐dose 131I had overt hypothyroidism (Fig 4). In contrast, only 1 (1%) of the 150 cats that received low‐dose 131I had overt hypothyroidism (Fig 4). Cats receiving standard‐dose 131I had a significantly (P = .014) higher incidence of overt hypothyroidism than did cats treated with low‐dose 131I. At 6 months, 7 (18%) of the 39 cats that received standard‐dose 131I had overt hypothyroidism (Fig 4). In contrast, only 1 (1%) of the 150 cats that received low‐dose 131I had overt hypothyroidism (Fig 4). Cats receiving standard‐dose 131I had a significantly (P = .0005) higher incidence of overt hypothyroidism than did cats treated with low‐dose 131I.
Subclinical Hypothyroidism
(T4, 0.9–3.9 μg/dL; TSH> 0.3 ng/mL): At 3 and 6 months, 18 (46%) of the 39 cats treated with standard‐dose 131I had subclinical hypothyroidism (Fig 4). In comparison, only 30 (20%) and 31 (21%) of the 150 cats treated with low‐dose 131 I had subclinical hypothyroidism at 3 and 6 months, respectively (Fig 4). Cats treated with standard‐dose 131I were significantly (P = .004) more likely to develop subclinical hypothyroidism at 3 and 6 months.
Incidence of Overt or Subclinical Hypothyroidism
Either overt or subclinical hypothyroidism was observed in 22 (56%) of the 39 cats receiving standard‐dose 131I and 32 (21%) of the 150 cats receiving low‐dose 131I at 3 months. By 6 months, 25 (64%) of the 39 cats treated with standard‐dose 131I and 31 (21%) of the 150 cats treated with low‐dose 131I had overt or subclinical hypothyroidism. Cats receiving standard‐dose 131I had significantly (P < .0001) higher incidence of hypothyroidism (either overt or subclinical) at 3 and 6 months after radioiodine treatment.
Incidence of Azotemia
Both groups of hyperthyroid cats experienced increases in median serum creatinine concentrations after 131I treatment (Fig 3). At 3 months, azotemia (creatinine ≥2 mg/dL) was found in 15 (38%) of the 39 cats treated with standard‐dose 131I and in 44 (29%) of the 150 cats treated with low‐dose 131I. At 6 months, azotemia was detected in 18 (46%) of cats treated with standard‐dose 131I and 44 (29%) of cats treated with low‐dose 131I. There was no difference in the incidence in azotemia between treatment groups at 3 (38% vs 29%; P = .27) or 6 (46% vs 29%; P = .06) months.
However, compared with the low‐dose 131I group, cats treated with standard‐dose 131I had significantly (P < .05) higher serum creatinine concentrations at 1, 3, and 6 months (Fig 3B–D). Additionally, the median percent rise of serum creatinine in cats treated with standard‐dose 131I (52% at 1 month, 70% at 3 months, and 77% at 6 months) was higher than the median percent rise in cats treated with low‐dose 131I (31% at 1 month, 46% at 3 months, and 50% at 6 months). Cats treated with standard‐dose 131I had significantly (P < .0001) greater increases in the median percent rise of serum creatinine over baseline concentration at all after‐treatment time points.
In the 6 cats in which L‐T4 supplementation was initiated after the 3‐month recheck, the median serum creatinine concentration at 6 months (2.60 mg/dL; range, 2.1–3.4 mg/dL) did not differ (P = .63) from that measured at 3 months (2.65 mg/dL; range, 2.3–3.6 mg/dL). Therefore, the 6‐month serum creatinine concentrations from the L‐T4‐treated cats were included in the data analysis (shown in Fig 3). However, if these 6 cats with severe azotemia were excluded from the data analysis (5 in the standard‐dose and 1 in the low‐dose groups), then there was no longer a difference in creatinine concentration between treatment groups (P = .06).
Discussion
Results of the present study confirm that low‐dose (2 mCi) 131I is safe and effective for treating cats with mild‐to‐moderate hyperthyroidism. Cats with mild‐to‐moderate hyperthyroidism treated with 2 mCi 131I were significantly less likely to develop iatrogenic hypothyroidism compared with cats receiving the higher, standard dose of 4 mCi. Furthermore, persistent hyperthyroidism in cats dosed with 2 mCi 131I was rare (3.3%) and comparable to published rates for cats treated with conventional doses of radioiodine.5, 11, 12
It is well known that treatment of hyperthyroidism in cats results in a reduction in glomerular filtration rate (GFR), potentially unmasking underlying chronic kidney disease (CKD).17, 25, 26 A previous retrospective study of hyperthyroid cats found that treatment‐induced hypothyroidism was associated with an increased risk of azotemia compared with cats that became euthyroid.16 Furthermore, hypothyroid cats that developed azotemia had significantly reduced survival times.16 Another study found that after 131I treatment in hyperthyroid cats, emergent renal disease was a predictor of negative outcome and the most common clinical complaint at death.27 Because hypothyroidism likely plays a role in progression of renal disease in cats after treatment for hyperthyroidism,16, 28 it seems logical that a major goal of 131I treatment should be to minimize development of iatrogenic hypothyroidism. In the present study, treatment with low‐dose 131I was associated with a significant reduction in the development of treatment‐induced hypothyroidism compared with standard dosing. This finding refutes the idea that the 4 mCi “one‐size‐fits‐all” 131I‐dosing regimen is best for all hyperthyroid cats.
Six months after 131I treatment, 29% of cats receiving low‐dose and 46% of cats given standard‐dose 131I became azotemic (serum creatinine ≥2.0 mg/dL). Although this difference in incidence of azotemia was not significant (P = .06), cats treated with standard‐dose 131I developed both higher serum creatinine concentrations (P < .05) and higher percent rises in creatinine over before‐treatment concentrations (P < .0001) than did cats treated with low‐dose 131I. Although the importance of these findings is unknown, the pattern is of clinical concern. The degree of azotemia that develops after treatment of hyperthyroidism is, at least in part, related to the absolute serum T4 concentration, and cats that become overtly hypothyroid are more likely to develop lower GFRs (and thereby more severe azotemia) than cats that maintain normal serum T4 concentrations (Data S2).16, 17, 28 In other words, severe overt iatrogenic hypothyroidism is likely to both reduce GFR and increase serum creatinine concentrations to a greater extent than does subclinical hypothyroidism, in which serum T4 concentrations remain within the lower end of the reference interval. The different resultant thyroid status after 131I treatment in treated cats might explain the varied incidence of azotemia (15–49%) reported after treatment.7, 17, 29
Supplementing cats with iatrogenic overt hypothyroidism with L‐T4 to achieve a euthyroid state is expected to increase GFR and improve renal function.28, 30, 31 In this study, no improvement in serum creatinine was seen in 6 cats with either subclinical or overt hypothyroidism treated with L‐T4 for 3 months. However, these cats were given a low replacement dose (0.075 mg/d) that was suboptimum for most older hypothyroid cats according to recent reports.31 , 7 In support of that premise, serum TSH concentrations remained very high, and peak, after pill serum T4 concentrations remained in the lower half of the reference interval (below the ideal target range of 2.5–3.5 μg/dL)31 despite L‐T4 supplementation. Because of the rise and fall of circulating T4 concentration that occurs after each L‐T4 dose, the peak, after pill value should be in the high‐normal range to keep circulating T4 concentrations within the euthyroid range as concentrations fall throughout the rest of the day (until the next L‐T4 dose is administered). Increasing the total daily L‐T4 dose to 0.1–0.2 mg divided twice daily generally helps restore euthyroidism and lowers serum creatinine concentrations in these cats.31, 5
All 131I‐treated cats improved clinically, as evidenced by weight gain and reduced hyperactivity and no classic clinical signs of hypothyroidism (other than azotemia). However, cats with overt hypothyroidism (i.e, low T4 and high TSH concentrations) generally manifest few, if any, obvious or classic clinical signs (i.e, hair loss, severe lethargy) during the first few months of 131I treatment.31 Noticeable clinical features of hypothyroidism in cats usually take many months or years to develop.31 Therefore, we would not expect obvious or classic clinical signs of hypothyroidism to develop within 6 months of 131I treatment in these cats (in either the overt or subclinical categories) and that is exactly what we found.
In human patients, the term subclinical hypothyroidism is used to describe the finding of a high serum TSH concentration together with a normal (usually low‐normal) T4 concentration.32, 33 By definition, subclinical hypothyroidism is a biochemical diagnosis because few, if any, clinical features of thyroid failure are present.34, 35, 36, 37 Subclinical hypothyroidism is a common finding after radioiodine treatment for human patients with Graves’ disease and toxic nodular goiter.34, 35, 36, 37 In the present study, 1 in 5 cats treated with low‐dose 131I and nearly 50% of cats treated with traditional 4 mCi 131I were categorized as having subclinical hypothyroidism 6 months after treatment. In contrast, overt hypothyroidism (high TSH with subnormal T4 concentrations) was much less common, developing in only 1% of the 2 mCi group and 18% of cats treated with 4 mCi 131I.
No study has published data on the test sensitivity and specificity of the serum TSH concentration as a diagnostic test for hypothyroidism in cats. In this study, all cats categorized with overt (low T4 concentration) or subclinical (low‐normal T4 concentration) hypothyroidism had serum TSH concentrations clearly higher than the upper limit of the reference interval. The reference interval was determined from data collected from a large number of older (≥7 years) clinically normal cats (see Data S1). Of those clinically normal cats, all had serum TSH concentrations <0.4 ng/mL; in contrast, all of these hypothyroid cats had values >0.4 ng/mL, suggesting that the cTSH test has high sensitivity as a diagnostic test for hypothyroidism in cats. In a previous study of cats with non‐thyroidal illness,8 serum TSH concentrations remained within the reference interval in 45 of 46 cats (98%), suggesting that the cTSH test is also highly specific for the diagnosis of hypothyroidism in cats. Although additional studies are certainly needed to further investigate and validate the use of the cTSH test as a diagnostic test for hypothyroidism in cats, high TSH in combination with low or low‐normal T4 appears to be a useful and accurate means to make that diagnosis.
In humans, hypothyroidism occurring within the first 6 months after 131I treatment can be transient or permanent;38, 39 permanent hypothyroidism is likely if the serum TSH concentration is high and the T4 concentration is low.40, 41 Permanent hypothyroidism develops by 1 year in at least 50% of patients given high doses42 and by 25 years in at least 50% of those given lower doses.41, 43 Furthermore, approximately 2–6% of humans with subclinical hypothyroidism will progress to overt hypothyroidism with each year after radioiodine treatment.34, 37, 41 It is currently unknown if a similar progression from subclinical to overt hypothyroidism occurs in cats, or if some cats with subclinical or overt hypothyroidism will recover normal thyroid function (with normalization of serum TSH and T4 concentrations) months to years after radioiodine treatment. Long‐term follow‐up studies are needed to classify the progression or resolution of iatrogenic thyroid damage in cats after radioiodine treatment.
The clinical consequences of subclinical hypothyroidism in the cat are unknown. In humans, subclinical hypothyroidism is associated with cardiac dysfunction, atherosclerosis, and hypercholesterolemia.36, 37, 44 Interestingly, there also appears to be an increased prevalence of subclinical hypothyroidism in people with CKD.45 A recent study of subclinical hypothyroidism and CKD found that human patients receiving L‐T4 supplementation had slower declines in GFR and were less likely to develop end‐stage CKD than those receiving a placebo.46 Another study demonstrated that GFR was decreased in female patients with subclinical hypothyroidism and that L‐T4 supplementation normalized the GFR.47 Although we do not know the effects of subclinical hypothyroidism in cats, it seems prudent to consider its impact in cats after 131I treatment, especially in those cats developing azotemia.
There are several limitations to this study. As 131I dose differed by hospital and was not randomized, it is possible that a hospital‐related variable contributed to the different outcomes. However, both hospitals standardized their diagnostic testing and treatment protocols to minimize dissimilarities. No significant differences were found in signalment or baseline thyroid and renal variables between study populations managed at each facility. Although it would have been ideal to have all after‐treatment T4, TSH, and creatinine concentrations determined at the same veterinary laboratory, that situation was not possible because the referring veterinarians collected most of the follow‐up samples and not all practices used the same commercial laboratory. To improve enrollment of cases and compliance with follow‐up in this study, we therefore allowed after‐treatment samples to be submitted to 2 commonly used laboratories in the northeastern USA. Both laboratories measured T4 and TSH by the same assay methods (see Data S1).
In this study, response to radioiodine treatment was based on serial evaluations of T4 and TSH concentrations rather than resolution of specific clinical signs. Although all cats showed clinical improvement (e.g, weight gain), no attempt was made to compare the time for resolution of any particular clinical sign between treatment groups. Therefore, it is impossible to know if cats treated with low‐dose 131I had a more protracted recovery phase than cats treated with the standard dose. Likewise, the outcome categories for hypothyroidism were based solely on serum T4 and TSH concentrations and not on any specific clinical findings because hypothyroid cats do not generally develop any noticeable clinical signs within 6 months of 131I treatment.31 Future longer‐term studies to evaluate potential clinical differences between cats with iatrogenic overt and subclinical hypothyroidism are needed. The long‐term outcome (> 6 months) of cats treated with low‐dose 131I remains unclear, and it is possible that these cats may experience higher rates of relapse than the cats treated with standard‐dose 131I. As noted earlier, 7 cats in the low‐dose group had T4 >3.0 μg/dL but ≤3.9 μg/dL (higher than considered ideal, yet still within the reference interval) 6 months after radioiodine treatment. Although not part of the study herein, these cats were followed to determine outcome. Five of the 7 cats had the serum T4 drop further (range 2.2–2.9 μg/dL) at 12 months. The remaining 2 cats still had T4 > 3.0 μg/dL and ≤3.9 μg/dL at 12 months, but free T4 was normal in both cats. One of these 2 cats did show clinical and biochemical relapse of hyperthyroidism at 24 months and was successfully retreated with 131I.
In conclusion, low‐dose (2 mCi) radioiodine treatment is a safe and effective treatment for cats with mild‐to‐moderate hyperthyroidism, as evidenced by cure in 97% of treated cats monitored for 6 months. Moreover, cats treated with low‐dose 131I had lower serum creatinine concentrations and developed hypothyroidism less often than cats treated with standard‐dose (4 mCi) 131I. Additional benefits of lower dose 131I for treating hyperthyroid cats may include decreased cost of 131I, reduced radiation exposure to cats and veterinary personnel, and decreased quarantine time. This study also revealed that the finding of a high serum TSH concentration coupled with a normal (usually low‐normal) serum T4 concentration (subclinical hypothyroidism) is relatively common in hyperthyroid cats after 131I treatment. Further work is needed to determine the consequences of subclinical hypothyroidism in cats and clarify any benefits of L‐T4 replacement on renal function in these cats.
Supporting information
Data S1. Hormone validation information and comparison of results for serum T4 and TSH concentrations between 2 commercial laboratories.
Data S2. Comparison of serum creatinine concentrations in cats that develop overt hypothyroidism, subclinical hypothyroidism, or euthyroidism after 131‐I treatment.
Acknowledgment
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Where Work was Performed: Cornell University Hospital for Animals and Animal Endocrine Clinic.
Presentation: The study was presented as an oral abstract at the 2015 ACVIM Forum, Indianapolis, IN.
Footnotes
Peterson ME, Broome MR. Ultra‐low doses of radioiodine are highly effective in restoring euthyroidism without inducing hypothyroidism in most cats with milder forms of hyperthyroidism: 131 cases (abstract). J Vet Intern Med 2014;28:1031
Hill's Prescription Diet —y/d Feline Thyroid Health; Hills Pet Nutrition, Topeka, KS
Antech Diagnostics, Lake Success, NY
Idexx Reference Laboratories, Westbrook, ME
Statistix. Version 10.0; Analytical Software, Tallahassee, FL
GraphPad Prism, version 6.0; GraphPad Software, La Jolla, CA
Davignon DL, Lucy JM, Randolph JF, Scarlett JM, Peterson ME. Effect of non‐thyroidal illness on serum concentrations of T4, free T4, and thyroid stimulating hormone in cats (abstract). J Vet Intern Med 2015;29:1174‐1175
Peterson ME, Guterl JN. Iatrogenic feline hypothyroidism: challenges and complexities of thyroid hormone replacement in cats (abstract). J Vet Intern Med 2015;29:448
References
- 1. Mooney CT, Peterson ME. Feline hyperthyroidism In: Mooney CT, Peterson ME, eds. Manual of Canine and Feline Endocrinology, 4th ed Quedgeley, Gloucester: British Small Animal Veterinary Association; 2012:199–203. [Google Scholar]
- 2. Peterson ME. Animal models of disease: Feline hyperthyroidism: An animal model for toxic nodular goiter. J Endocrinol 2014;223:T97–T114. [DOI] [PubMed] [Google Scholar]
- 3. Daminet S, Kooistra HS, Fracassi F, et al. Best practice for the pharmacological management of hyperthyroid cats with antithyroid drugs. J Small Anim Pract 2014;55:4–13. [DOI] [PubMed] [Google Scholar]
- 4. Scott‐Moncrieff JC, Heng HG, Weng HY, et al. Effect of a limited iodine diet on iodine uptake by thyroid glands in hyperthyroid cats. J Vet Intern Med 2015;29:1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterson ME, Becker DV. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc 1995;207:1422–1428. [PubMed] [Google Scholar]
- 6. Naan EC, Kirpensteijn J, Kooistra HS, et al. Results of thyroidectomy in 101 cats with hyperthyroidism. Vet Surg 2006;35:287–293. [DOI] [PubMed] [Google Scholar]
- 7. Milner RJ, Channell CD, Levy JK, et al. Survival times for cats with hyperthyroidism treated with iodine 131, methimazole, or both: 167 cases (1996–2003). J Am Vet Med Assoc 2006;228:559–563. [DOI] [PubMed] [Google Scholar]
- 8. Peterson ME, Broome MR. Radioiodine for feline hyperthyroidism In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy XV. Philadelphia: Saunders Elsevier; 2014:e112–e122. [Google Scholar]
- 9. Klausner JS. Results of radioactive iodine therapy in 23 cats with hyperthyroidism. Minn Vet J 1987;27:28–32. [Google Scholar]
- 10. Meric SM, Rubin SI. Serum thyroxine concentrations following fixed‐dose radioactive iodine treatment in hyperthyroid cats: 62 cases (1986–1989). J Am Vet Med Assoc 1990;197:621–623. [PubMed] [Google Scholar]
- 11. Craig A. A prospective study of 66 cases of feline hyperthyroidism treated with a fixed dose of intravenous 131‐I. Aust Vet Practit 1993;23:2–6. [Google Scholar]
- 12. Chun R, Garrett LD, Sargeant J, et al. Predictors of response to radioiodine therapy in hyperthyroid cats. Vet Radiol Ultrasound 2002;43:587–591. [DOI] [PubMed] [Google Scholar]
- 13. Puille M, Knietsch M, Spillmann T, et al. Radioiodine treatment of feline hyperthyroidism in Germany. Nuklearmedizin 2002;41:245–251. [PubMed] [Google Scholar]
- 14. van Dijl IC, Hof AJ. Treatment of feline hyperthyroidism with radioactive iodine‐131. Tijdschr Diergeneeskd 2008;133:54–62. [PubMed] [Google Scholar]
- 15. Slater MR, Komkov A, Robinson LE, et al. Long‐term follow‐up of hyperthyroid cats treated with iodine‐131. Vet Rad Ultrasound 1994;35:204–209. [Google Scholar]
- 16. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010;24:1086–1092. [DOI] [PubMed] [Google Scholar]
- 17. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007;161:711–715. [DOI] [PubMed] [Google Scholar]
- 18. Peterson ME, Guterl JN, Nichols R, et al. Evaluation of serum thyroid‐stimulating hormone concentration as a diagnostic test for hyperthyroidism in cats. J Vet Intern Med 2015;29:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson ME, Broome MR. Thyroid scintigraphy findings in 2,096 cats with hyperthyroidism. Vet Radiol Ultrasound 2015;56:84–95. [DOI] [PubMed] [Google Scholar]
- 20. Daniel GB, Neelis DA. Thyroid scintigraphy in veterinary medicine. Semin Nucl Med 2014;44:24–34. [DOI] [PubMed] [Google Scholar]
- 21. Peterson ME, Guterl JN, Rishniw M, et al. Evaluation of quantitative thyroid scintigraphy for diagnosis and staging of disease severity in cats with hyperthyroidism: Comparison of the percent thyroidal uptake of pertechnetate to the thyroid‐to‐salivary ratio and thyroid‐to‐background ratios. Vet Radiol Ultrasound 2016;57:427–440. [DOI] [PubMed] [Google Scholar]
- 22. D'Agostino RB. Tests for normal distribution In: D'Agostino RB, Stephens MA, eds. Goodness‐of‐Fit Techniques. New York: Macel Dekker; 1986:367–420. [Google Scholar]
- 23. Conover WJ. Practical Nonparametric Statistics, 3rd ed New York: John Wiley & Sons; 1999. [Google Scholar]
- 24. Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials 1981;2:93–113. [DOI] [PubMed] [Google Scholar]
- 25. Graves TK, Olivier NB, Nachreiner RF, et al. Changes in renal function associated with treatment of hyperthyroidism in cats. Am J Vet Res 1994;55:1745–1749. [PubMed] [Google Scholar]
- 26. Syme HM. Cardiovascular and renal manifestations of hyperthyroidism. Vet Clin North Am Small Anim Pract 2007;37:723–743. [DOI] [PubMed] [Google Scholar]
- 27. Slater MR, Geller S, Rogers K. Long‐term health and predictors of survival for hyperthyroid cats treated with iodine 131. J Vet Intern Med 2001;15:47–51. [DOI] [PubMed] [Google Scholar]
- 28. Vaske HH, Schermerhorn T, Grauer GF. Effects of feline hyperthyroidism on kidney function: A review. J Feline Med Surg 2016;18:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams WH, Daniel GB, Legendre AM, et al. Changes in renal function in cats following treatment of hyperthyroidism using 131‐I. Vet Radiol Ultrasound 1997;38:231–238. [DOI] [PubMed] [Google Scholar]
- 30. Williams TL, Elliott J, Syme HM. Effect on renal function of restoration of euthyroidism in hyperthyroid cats with iatrogenic hypothyroidism. J Vet Intern Med 2014;28:1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterson ME. Diagnosis and management of iatrogenic hypothyroidism In: Little SE, ed. August's Consultations in Feline Internal Medicine. St. Louis: Elsevier; 2016:260–269. [Google Scholar]
- 32. Evered DC, Ormston BJ, Smith PA, et al. Grades of hypothyroidism. Br Med J 1973;1:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cooper DS, Halpern R, Wood LC, et al. L‐Thyroxine therapy in subclinical hypothyroidism. A double‐blind, placebo‐controlled trial. Ann Intern Med 1984;101:18–24. [DOI] [PubMed] [Google Scholar]
- 34. Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid 2002;12:839–847. [DOI] [PubMed] [Google Scholar]
- 35. Cooper DS. Approach to the patient with subclinical hyperthyroidism. J Clin Endocrinol Metab 2007;92:3–9. [DOI] [PubMed] [Google Scholar]
- 36. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008;29:76–131. [DOI] [PubMed] [Google Scholar]
- 37. Garg A, Vanderpump MP. Subclinical thyroid disease. Br Med Bull 2013;107:101–116. [DOI] [PubMed] [Google Scholar]
- 38. MacFarlane IA, Shalet SM, Beardwell CG, et al. Transient hypothyroidism after iodine‐131 treatment for thyrotoxicosis. Br Med J 1979;2:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Connell JM, Hilditch TE, McCruden DC, et al. Transient hypothyroidism following radioiodine therapy for thyrotoxicosis. Br J Radiol 1983;56:309–313. [DOI] [PubMed] [Google Scholar]
- 40. Davies PH, Franklyn JA, Daykin J, et al. The significance of TSH values measured in a sensitive assay in the follow‐up of hyperthyroid patients treated with radioiodine. J Clin Endocrinol Metab 1992;74:1189–1194. [DOI] [PubMed] [Google Scholar]
- 41. Franklyn JA. Thyroid disease and its treatment: Short‐ and long‐term consequences. J R Coll Physicians Lond 1999;33:564–567. [PMC free article] [PubMed] [Google Scholar]
- 42. Kendall‐Taylor P, Keir MJ, Ross WM. Ablative radioiodine therapy for hyperthyroidism: Long term follow up study. Br Med J (Clin Res Ed) 1984;289:361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Franklyn JA, Daykin J, Drolc Z, et al. Long‐term follow‐up of treatment of thyrotoxicosis by three different methods. Clin Endocrinol (Oxf) 1991;34:71–76. [DOI] [PubMed] [Google Scholar]
- 44. Selmer C, Olesen JB, Hansen ML, et al. Subclinical and overt thyroid dysfunction and risk of all‐cause mortality and cardiovascular events: A large population study. J Clin Endocrinol Metab 2014;99:2372–2382. [DOI] [PubMed] [Google Scholar]
- 45. Lo JC, Chertow GM, Go AS, et al. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int 2005;67:1047–1052. [DOI] [PubMed] [Google Scholar]
- 46. Shin DH, Lee MJ, Kim SJ, et al. Preservation of renal function by thyroid hormone replacement therapy in chronic kidney disease patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2012;97:2732–2740. [DOI] [PubMed] [Google Scholar]
- 47. Adrees M, Gibney J, El‐Saeity N, et al. Effects of 18 months of L‐T4 replacement in women with subclinical hypothyroidism. Clin Endocrinol (Oxf) 2009;71:298–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Hormone validation information and comparison of results for serum T4 and TSH concentrations between 2 commercial laboratories.
Data S2. Comparison of serum creatinine concentrations in cats that develop overt hypothyroidism, subclinical hypothyroidism, or euthyroidism after 131‐I treatment.


