Abstract
Background
Previous studies have identified hypoalbuminemia as a risk factor for negative outcome in dogs with chronic enteropathy (CE), but it has not been determined whether histopathology differs between CE dogs with and without hypoalbuminemia.
Objective
To compare histopathologic findings in dogs with biopsy‐diagnosed inflammatory CE with and without hypoalbuminemia.
Animals
83 dogs that had intestinal biopsy performed between January 2010–July 2015. Dogs had signs compatible with CE of at least 3‐weeks' duration and no evidence of clinically relevant extra‐gastrointestinal (GI) disease or potential non‐GI causes of hypoalbuminemia. Dogs had primary diagnosis of inflammatory enteritis based on histopathology.
Methods
Dogs were grouped into CE with normoalbuminemia (CEN; serum albumin concentration ≥3.0 g/dL, N = 46) or chronic enteropathy with hypoalbuminemia (CEH; serum albumin concentration <3.0 g/dL, N = 37). A pathologist (SLP) blinded to the groups reviewed biopsy samples and applied the World Small Animal Veterinary Association scoring system to all samples.
Results
Intestinal biopsy samples from dogs in the CEH group were significantly more likely to display villous stunting, epithelial injury, crypt distension, and lacteal dilatation, and were more likely to have intraepithelial lymphocytes and lamina propria neutrophils than biopsy samples from dogs in the CEN group. Additionally, higher scores for each of the above listed histopathologic criteria were associated with a lower serum albumin concentration.
Conclusions and Clinical Importance
Histopathologic features of chronic inflammatory enteropathy differ between dogs that are hypo‐ versus normoalbuminemic. Additional work is needed to elucidate the clinical relevance of these differences.
Keywords: Canine, Chronic enteropathy, Histopathology, Albumin
Abbreviations
- CE
chronic enteropathy
- CEH
chronic enteropathy with hypoalbuminemia
- CEN
chronic enteropathy with normoalbuminemia
- LPE
lymphoplasmacytic enteritis
- PLE
protein‐losing enteropathy
Chronic enteropathy (CE) is a term used to describe various inflammatory conditions of the intestinal tract.1 It is characterized by the presence of gastrointestinal (GI) signs such as weight loss, vomiting, diarrhea, and decreased appetite of at least several weeks’ duration, and is associated with histologic evidence of inflammation in the small intestine.1, 2, 3 In dogs, the type of CE often is determined by response to treatment and can include antibiotic‐responsive disease, food‐responsive disease, and idiopathic inflammatory bowel disease, which may be steroid responsive.1 The prognosis is reported to be highly variable1, 2, 3, 4, 5 and dependent on response to treatment.6
When a GI disorder results in hypoalbuminemia as a consequence of excessive loss of plasma proteins through the intestinal mucosa, it is commonly referred to as protein‐losing enteropathy (PLE).7 Causes of PLE are numerous, including diseases that result in infiltration, inflammation, hemorrhage, or edema of the GI tract,7 including intestinal lymphangiectasia, alimentary lymphoma, hookworm infestation, infection by Histoplasma capsulatum, and intestinal intussusception.8 Chronic enteropathies can result in PLE,8 and for the purposes of this study, we refer to this type of PLE as chronic enteropathy with hypoalbuminemia (CEH).
Although several previous studies have identified hypoalbuminemia as a risk factor for negative outcome in cases of CE, it is still unclear whether histopathology differs between CE dogs with and without hypoalbuminemia.1, 4, 5 Historically, substantial interobserver variation in histopathologic evaluation of intestinal tissue has compounded this problem,9 making it difficult to compare changes among dogs with different categories of intestinal disease. Recently, the GI Standardization Group developed guidelines for the interpretation of inflammatory change in the GI mucosa of the dog and cat. These standards, known as the World Small Animal Veterinary Association (WSAVA) scoring system, include classification and scoring of morphologic and inflammatory changes in the canine intestinal mucosa.10
The objective of our study was to compare histopathologic findings (as determined by the WSAVA scoring system) in dogs with biopsy‐diagnosed CE with and without hypoalbuminemia. Our hypothesis was that dogs with CE and hypoalbuminemia would have different histopathologic features than CE dogs without hypoalbuminemia.
Materials and Methods
Electronic medical records at Colorado State University were reviewed for dogs that had intestinal biopsy performed between January 2010 and July 2015. Included dogs had clinical signs compatible with CE of at least 3‐weeks' duration, including weight loss, diarrhea, vomiting, and decreased appetite, and intestinal biopsy samples that indicated variable types and degrees of inflammatory infiltrate. Dogs were included if their primary diagnosis was an inflammatory enteritis on their original histopathologic evaluation. Additionally, all dogs had an appropriate history of fecal testing or deworming, and most (67/83; 80%) had abdominal ultrasonography performed before intestinal biopsy to screen for intestinal or abdominal masses.
Dogs with clinically relevant concurrent extra GI disease, with potential non‐GI causes of hypoalbuminemia, with causes of GI disease other than inflammatory enteritis (eg, intestinal lymphoma), or for which a complete medical record could not be obtained, were excluded. Specifically, hypoalbuminemic dogs that were not screened for proteinuria, had substantial proteinuria (UPC >1.0), or had clinically abnormal serum bile acid concentrations (preprandial >20 μmol/L; postprandial >40 μmol/L) were excluded. Dogs were placed in either the CE with normoalbuminemia (serum albumin concentration ≥3.0 g/dL) group (chronic enteropathy with normoalbuminemia, CEN) or the CE with hypoalbuminemia (serum albumin concentration <3.0 g/dL) group (CEH). Serum albumin concentration <3.0 g/dL was defined as hypoalbuminemia based on the Colorado State University Diagnostic Laboratory's normal reference interval for serum albumin concentration in dogs (3.0–4.3 g/dL).
Recorded data included age, breed, sex, clinical signs, additional clinicopathologic abnormalities, tissue types available, and biopsy method. After cases were selected for study, histopathologic evaluation of previously obtained intestinal tissue was performed by a single pathologist blinded to clinical group (SLP). The pathologist established that the biopsy samples were adequate for evaluation, and then determined the presence of morphologic criteria (villous stunting, epithelial injury, crypt distension, lacteal dilatation, mucosal fibrosis) and inflammatory criteria (intraepithelial lymphocytes [IEL], lamina propria eosinophils, lamina propria lymphocytes or plasma cells, lamina propria neutrophils) and scored the degree of change based on WSAVA guidelines. For the degree of each change, the following scores were applied based on established criteria: 0 = normal, 1 = mild, 2 = moderate, 3 = marked. In cases where >1 tissue type was available, the tissue type with the highest total WSAVA score was used in the analysis.
Statistical Analysis
The proportion of each variable present was compared between the 2 groups (CEN versus CEH) using Fisher's exact test. A Spearman (rank‐based) correlation also was performed to evaluate for a correlation between score for each biopsy variable and serum albumin concentration. A multiple logistic regression analysis then was performed using those variables found to be significantly associated with hypoalbuminemia based on Fisher's exact test (crypt distention, IEL, lacteal dilatation, neutrophils, villous epithelial injury, and villous stunting). Specifically, hypoalbuminemia (yes or no) was the response variable and the histopathologic variables (presence/absence) were used as predictors. Backward elimination then was used to decrease the number of predictors in the logistic regression model. For Fisher's exact test and Spearman's correlation analyses, a Bonferroni adjustment was used to account for multiple testing.
Statistical significance for all analyses was defined as the probability of the null hypotheses (ie, no relationship or no difference) being true at <5.0% (P < .05).
Results
Medical records of 270 dogs that underwent intestinal biopsy at CSU (Colorado State University) from January 2010 to July 2015 were reviewed. Of the 270, 83 met the inclusion criteria. Ninety‐six of the excluded dogs had either evidence of clinically relevant concurrent extra‐GI disease, or clinically relevant concurrent extra‐GI disease could not be excluded. Sixty‐four of the excluded dogs had a primary diagnosis other than inflammatory enteritis as the cause of their chronic GI signs (eg, chronic foreign body and neoplasia). In 27 excluded cases, no intestinal tissue or previously prepared slide was available for evaluation.
Of the included dogs, 37/83 (45%) were hypoalbuminemic (CEH group; serum albumin concentration <3.0 g/dL), and 46/83 (55%) were normoalbuminemic (CEN group; serum albumin concentration ≥3.0 g/dL).
The median age of dogs in the CEH group was 3 years (range, 1–12 years). The median age of dogs in CEN group was 2 years (range, 5 months–14 years). In the CEH group, 3 dogs were intact males, 15 dogs were castrated males, 1 dog was an intact female, and 18 dogs were spayed females. In the CEH group, 2 dogs were intact males, 30 dogs were castrated males, 2 dogs were intact females, and 12 dogs were spayed females. There was no significant difference in age or sex status between groups.
Breeds included mixed breed (15), Labrador Retriever (9), German Shepherd Dog (4), Boxer (3), Cocker Spaniel (3), Pomeranian (3), West Highland White Terrier (3), Australian Shepherd (2), Border Collie (2), English Bulldog (2), French Bulldog (2), Golden Retriever (2), Great Pyrenees (2), Newfoundland (2), Yorkshire Terrier (2), and 1 each of the following: American Eskimo, Airedale Terrier, Akita, Bernese Mountain Dog, Boston Terrier, Chesapeake Bay Retriever, Doberman, Miniature Dachshund, German Shorthaired Pointer, Giant Schnauzer, Irish Setter, Maltese, Norwich Terrier, Lundehund, Rottweiler, Soft Coated Wheaten Terrier, Samoyed, Shar Pei, Shetland Sheepdog, Shiba Inu, Shih Tzu, Siberian Husky, Saint Bernard, Standard Poodle, Tibetan Terrier, and Weimaraner.
In the CEH group, biopsy samples were obtained by endoscopy in 33/37 (89%) cases, with 24/33 (73%) having only gastroduodenoscopy performed and 9/33 (27%) having both gastroduodenoscopy and ileocolonoscopy performed. Three dogs in the CEH group had intestinal biopsy samples obtained celiotomy, and 1 dog by laparoscopy. In the CEN group, biopsy samples were obtained by endoscopy in 42/46 (91%) cases, with 22/42 (52%) having only gastroduodenoscopy performed and 20/42 (48%) having both gastroduodenoscopy and ileocolonoscopy performed. Four dogs in CEN group had intestinal biopsy samples obtained by celiotomy. Method of biopsy procurement was not different between groups. Median serum albumin concentration in the CEH group was 1.9 g/dL (range, 0.8–2.8 g/dL). Median serum albumin concentration in the CEN group was 3.7 g/dL (range, 3.0–4.4 g/dL).
A Fisher's exact test indicated that dogs in the CEH group had a greater proportion of villous stunting, epithelial injury, crypt distension, and lacteal dilatation than did dogs in the CEN group. Additionally, dogs in the CEH group had a greater proportion of IEL and lamina propria neutrophils than did dogs in the CEN group. Proportions of lamina propria eosinophils and lamina propria lymphocytes or plasma cells were not different between groups. The criterion of mucosal fibrosis was not represented often enough to be suitable for statistical analysis (Fig 1).
Figure 1.
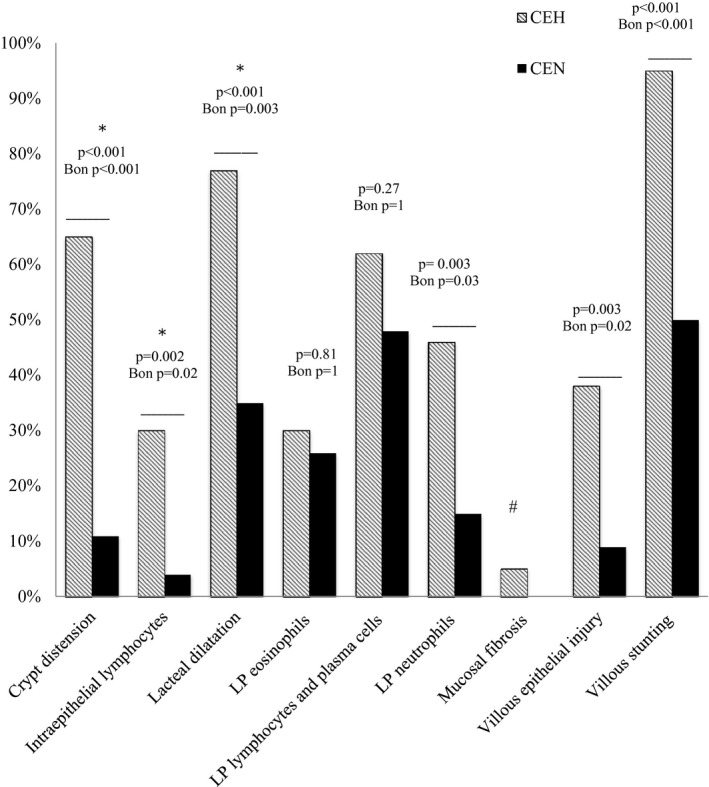
The presence of histopathologic variables in dogs with CEH versus dogs with CEN. P values (Fischer's exact test) and adjusted P values (after Bonferroni correction) shown for significant and nonsignificant variables. *Retained significance in multivariate analysis; #Statistical tests unreliable because of low expression of this trait; LP, lamina propria; CEH, chronic enteropathy and hypoalbuminemia; CEN, chronic enteropathy and normoalbuminemia.
A rank‐based Spearman's correlation indicated that a higher score for villous stunting, epithelial injury, crypt distension, lacteal dilatation, IEL, or lamina propria neutrophils was moderately associated with a lower serum albumin concentration (Table 1).
Table 1.
Scores for histopathologic variables in dogs with chronic enteropathy and correlation with hypoalbuminemia (Spearman rank‐based)
| Variable | Group | Med | Min | Max | Spearman's Correlation Score | P Value | Adjusted P Valuea |
|---|---|---|---|---|---|---|---|
| Crypt distension | CEH | 1 | 0 | 3 | −0.587 | <.001 | <.001 |
| CEN | 0 | 0 | 2 | ||||
| Intraepithelial lymphocytes | CEH | 0 | 0 | 3 | −0.347 | .001 | .01 |
| CEN | 0 | 0 | 1 | ||||
| Lacteal dilatation | CEH | 1 | 0 | 3 | −0.422 | <.001 | <.001 |
| CEN | 0 | 0 | 3 | ||||
| Eosinophils | CEH | 0 | 0 | 2 | −0.09 | .42 | 1 |
| CEN | 0 | 0 | 3 | ||||
| Lymphocytes and plasma cells | CEH | 1 | 0 | 2 | −0.157 | .16 | 1 |
| CEN | 0 | 0 | 2 | ||||
| Neutrophils | CEH | 0 | 0 | 3 | −0.308 | .005 | .04 |
| CEN | 0 | 0 | 2 | ||||
| Mucosal fibrosis | CEH | 0 | 0 | 1 | NA | NA | NA |
| CEN | 0 | 0 | 0 | ||||
| Villous epithelial injury | CEH | 0 | 0 | 3 | −0.372 | <.001 | .005 |
| CEN | 0 | 0 | 1 | ||||
| Villous stunting | CEH | 1 | 0 | 3 | −0.551 | <.001 | <.001 |
| CEN | 0.5 | 0 | 2 |
CEH, chronic enteropathy with hypoalbuminemia; CEN, chronic enteropathy with normoalbuminemia; LP, lamina propria; NA, not applicable: statistical tests unreliable due to low expression of this trait.
After Bonferroni correction.
After backward elimination (using alpha = 0.05), multiple logistic regression analysis indicated that the following variables remained significant: crypt distension (odds ratio [OR], 12.487; P < .001), intraepithelial lymphocytes (OR, 10.060; P = .013), and lacteal dilatation (OR, 5.037; P = .009). The OR represents the odds of hypoalbuminemia comparing the presence versus the absence of each histopathologic variable, with other variables held constant.
Discussion
Lymphoplasmacytic or eosinophilic intestinal infiltrates or both are considered the hallmark of idiopathic CE. In our population of dogs with CE, the proportions of lamina propria lymphoplasmacytic or eosinophilic infiltrates were not different between the hypoalbuminemic and normoalbuminemic groups. Additionally, the proportions and severity of these infiltrates were not correlated with serum albumin concentration. This finding was not surprising, and it is in agreement with previous studies, in which the histologic scores of infiltrates of eosinophils, lymphocytes, or plasma cells were not correlated with the patient's serum albumin concentration or outcome.1, 5 Interestingly, the cellular infiltrates that were different between CEH and CEN dogs included IEL and lamina propria neutrophils.
Intraepithelial lymphocytes are a component of the first line of defense in the GI immune system and have both pro‐ and anti‐inflammatory roles.11, 12 We found that IEL were more likely to be present (P < .001) in dogs with hypoalbuminemia versus normoalbuminemic dogs with CE, but as a consequence of the retrospective nature of our study, we were not able to classify these cells further. Additional study is needed to determine the role of these cells in dogs with CE.
Lamina propria neutrophils were more likely to be present (P = .003) in dogs with hypoalbuminemia (17/37; 46%) compared to dogs with normoalbuminemia (7/46; 15%). Whether the neutrophils are representative of an underlying etiology, are present because of adhesion and possible translocation of microbial flora or pathogens, or are simply part of the inflammatory response is not well understood and deserves further study.
Crypt distension also was found more often in dogs with CEH versus dogs with CEN (P < .001). Previous studies have reported on crypt lesions, sometimes referred to as crypt abscesses, in dogs with PLE.13 In a case series of 6 dogs with PLE,14 only 2/6 had inflammatory infiltrates or lymphangiectasia histologically. Interestingly, both dogs that had inflammatory infiltrates had neutrophils noted in the lamina propria, and 5/6 dogs had neutrophils present in distended crypts. In another series of 58 dogs with CE, dogs with crypt abscesses were found to have more severe intestinal protein loss and shorter survival times than dogs with crypt distension or no crypt lesions.15 The clinical relevance of crypt lesions in dogs with CE also deserves further study.
Dilated lacteals were present in a high proportion of dogs overall (44/83; 53%), but were significantly more likely (P < .001) to be present in dogs with hypoalbuminemia (28/37; 76%) compared to dogs with normal serum albumin concentration (16/46; 35%). A previous retrospective analysis of full‐thickness intestinal biopsy samples in dogs with chronic GI disease showed that, in 38/64 dogs (59%), the major histopathologic abnormality was intestinal lymphangiectasia (dilated lacteals). Lymphoplasmacytic enteritis (LPE) was identified in only 5/64 dogs (8%).16 These findings are in contrast to a study that evaluated histologic findings of endoscopic biopsy samples in 368 dogs with chronic diarrhea, where LPE was identified in approximately 25% of cases, and lymphangiectasia was not common.17 The serum albumin concentration of dogs in these studies was not compared. A more recent study evaluated the histologic features of 136 dogs with chronic GI signs, 94 with LPE, and 42 with GI disease not caused by inflammatory bowel disease. All 94 dogs with LPE had lacteal dilatation graded as moderate to severe by WSAVA standards. Additionally, lacteal height, width, and height/width ratio were inversely correlated with serum albumin concentration in this group of dogs.18 The reason for the discrepancies among the studies likely is multifactorial, but could include the fact that intestinal lymphangiectasia may be segmental or multifocal and biopsies may miss these lesions if the affected area is not sampled. Most of the biopsy samples in our study were obtained endoscopically with the potential to miss lesions deep within the submucosal and muscularis layers of the intestinal wall.19 Nonetheless, the high prevalence of intestinal lymphangiectasia in dogs with a primary diagnosis of inflammatory enteritis may be important. Although secondary lymphangiectasia is thought to resolve with treatment targeting idiopathic inflammatory bowel disease, it may be that dogs not responding to this treatment would benefit from fat restriction because of persistent intestinal lymphangiectasia. Also, lymphatic dysfunction and lymphangiogenesis have long been suspected as a component of the pathology of inflammatory bowel diseases in humans, and therapeutic interventions to stimulate lymphatic function have shown some promise in recent experimental murine models of inflammatory bowel disease.20, 21, 22, 23
In a previous study of dogs with diet‐responsive enteropathy, WSAVA histopathologic scoring identified more villous stunting in the enteropathy dogs versus controls.24 Ultrastructural lesions of the mitochondria and brush border also were evaluated and in those dogs clinically responsive to food, and the microvillus height and additional ultrastructural lesions improved. The authors postulated that the recovery of enterocyte health after clinical response to diet suggests that architectural changes may be at least as important as more standard measures of pathology in the intestine, namely inflammatory infiltrates. It was suggested that this might explain why previous studies have failed to identify improvement in the inflammatory infiltrates of dogs with CE, despite clinical improvement.24 Our study is in agreement that the morphologic lesions appear to be important in dogs with CE. Additionally, because these lesions were more common in hypoalbuminemic dogs, we suspect they may indicate a more severe form of CE. Whether the presence or relative severity of these lesions indicates a possible alternative etiology or the need for alternative treatment approaches is unknown. A prospective study in which clinical data and response to treatment can be monitored based on the histologic criteria is warranted.
Our study had several limitations. The median age of dogs in our CEH and CEN groups was younger (CEH, 3 years; CEN, 2 years) when compared to other studies of dogs with CE.1, 25, 26, 27, 28 Therefore, our population of dogs with CE may not reflect those seen at other institutions. Additionally, the retrospective design of our study did not allow us to determine the dogs that had received proper food or antibiotic trials before intestinal biopsy so that we could better classify them. Therefore, we applied the term CE to these cases, rather than idiopathic inflammatory bowel disease. However, at our institution, it is common for food and antibiotic trials to be completed before the recommendation of biopsy, provided the patient is stable. Response to treatment and prognosis as it relates to the above histopathologic features could not be determined because of incomplete follow‐up of many of the cases. Most biopsy samples were obtained endoscopically, which is the standard of practice at our institution but does have limitations, as described above.19 Also, ileal biopsy samples (via ileocolonoscopy, celiotomy, or laparoscopy) were obtained in just 35% of dogs with CEH and 57% of dogs with CEN. The method of biopsy sample procurement was not different between groups, but a larger proportion of the CEN dogs had ileal biopsy samples obtained, which may have affected the results. Pathology can differ among sections of small intestine,26, 29 and ideally, ileal biopsy samples would have been available for evaluation in all dogs. Finally, despite the use of the WSAVA guidelines for histopathologic scoring of endoscopic intestinal biopsy samples, substantial interobserver variability occurs, controversy still exists, and scoring is still considered relatively subjective.30
In conclusion, many histopathologic features of chronic inflammatory enteropathy differ between dogs that are hypo‐ versus normoalbuminemic. The classic features of CE, lymphoplasmacytic cellular infiltrate and eosinophilic infiltrates, were not different between groups, whereas other inflammatory infiltrates and many of the morphologic features were different. These morphologic lesions may simply indicate a more severe disease process occurring in the intestine. Additional work is needed to elucidate the clinical relevance of these differences and to determine whether the presence or relative severity of these lesions indicates a possible alternative etiology or the need for alternative or supplemental treatment strategy in some cases of CE in dogs.
Acknowledgments
The authors acknowledge Dr. Ann Hess’ contribution to this manuscript. They also acknowledge the support of the Naniboujou Research Legacy Fund.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
References
- 1. Allenspach K, Wieland B, Gröne A, et al. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 2. Jergens AE, Moore FM, Haynes JS, et al. Idiopathic inflammatory bowel disease in dogs and cats: 84 Cases (1987–1990). J Am Vet Med Assoc 1992;201:1603–1608. [PubMed] [Google Scholar]
- 3. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med 2003;17:8–20. [DOI] [PubMed] [Google Scholar]
- 4. Simpson KW, Jergens AE. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract 2011;41:381–398. [DOI] [PubMed] [Google Scholar]
- 5. Craven M, Simpson JW, Ridyard AE, et al. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J Small Anim Pract 2004;45:336–342. [DOI] [PubMed] [Google Scholar]
- 6. Nakashima K, Hiyoshi S, Ohno K, et al. Prognostic factors in dogs with protein‐losing enteropathy. Vet J 2015;205:28–32. [DOI] [PubMed] [Google Scholar]
- 7. Equilino M, Théodoloz V, Gorgas D, et al. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein‐losing enteropathy. J Am Vet Med Assoc 2015;246:91–99. [DOI] [PubMed] [Google Scholar]
- 8. Dossin O, Lavoué R. Protein‐losing enteropathies in dogs. Vet Clin North Am Small Anim Pract 2011;41:399–418. [DOI] [PubMed] [Google Scholar]
- 9. Willard MD, Jergens AE, Duncan RB, et al. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc 2002;220:1177–1182. [DOI] [PubMed] [Google Scholar]
- 10. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 11. Haas E, Rütgen BC, Gerner W, et al. Phenotypic characterization of canine intestinal intraepithelial lymphocytes in dogs with inflammatory bowel disease. J Vet Intern Med 2014;28:1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawaguchi‐Miyashita M, Shimada S, Kurosu H, et al. An accessory role of TCRcd+ cells in the exacerbation of inflammatory bowel disease in TCRa mutant mice. Eur J Immunol 2001;31:980–988. [DOI] [PubMed] [Google Scholar]
- 13. Simmerson SM, Armstrong PJ, Wünschmann A, et al. Clinical features, intestinal histopathology, and outcome in protein‐losing enteropathy in yorkshire terrier dogs. J Vet Intern Med 2014;28:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willard MD, Helman G, Fradkin JM, et al. Intestinal crypt lesions associated with protein‐losing enteropathy in the dog. J Vet Intern Med 2000;14:298–307. [DOI] [PubMed] [Google Scholar]
- 15. Stroda K, Wakamatsu N, Gaschen L, et al. Histopathological, clinical, endoscopic, and ultrasound features of dogs with chronic enteropathies and small intestinal crypt lesions. J Vet Intern Med 2012;26:767–768. [Google Scholar]
- 16. Kleinschmidt S, Meneses F, Noltr I, et al. Retrospective study on the diagnostic value of full‐thickness biopsies from the stomach and intestine of dogs with chronic gastrointestinal disease symptoms. Vet Pathol 2006;43:1000–1003. [DOI] [PubMed] [Google Scholar]
- 17. Van der Gaag I, Happé RP. The histological appearance of peroral small intestinal biopsies in clinically healthy dogs and dogs with chronic diarrhea. Zentralbl Veterinarmed A 1990;37:401–416. [PubMed] [Google Scholar]
- 18. Rossi G, Cerquetella M, Antonelli E, et al. The importance of histologic parameters of lacteal involvement in cases of canine lymphoplasmacytic enteritis. Gastroenterol Hepatol Bed Bench 2015;8:33. [PMC free article] [PubMed] [Google Scholar]
- 19. Larson RN, Ginn JA, Bell CM, et al. Duodenal endoscopic findings and histopathologic confirmation of intestinal lymphangiectasia in dogs. J Vet Intern Med 2012;26:1087–1092. [DOI] [PubMed] [Google Scholar]
- 20. Alexander JS, Chaitanya GV, Grisham MB, et al. Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci 2010;1207:E75–E85. [DOI] [PubMed] [Google Scholar]
- 21. Tonelli F, Giudici F, Liscia G. Is lymphatic status related to regression of inflammation in Crohn's disease? World J Gastrointest Surg 2012;4:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Alessio S, Correale C, Arena V, et al. Stimulation of lymphatic function via VEGFR‐3 as a novel therapy for chronic experimental intestinal inflammation. Gastroenterology 2012;142:S84–S85. [Google Scholar]
- 23. D'Alessio S, Tacconi C, Fiocchi C, et al. Advances in therapeutic interventions targeting the vascular and lymphatic endothelium in inflammatory bowel disease. Curr Opin Gastroenterol 2013;29:608–613. [DOI] [PubMed] [Google Scholar]
- 24. Walker D, Knuchel‐Takano A, McCutchan A, et al. A comprehensive pathological survey of duodenal biopsies from dogs with diet‐responsive chronic enteropathy. J Vet Intern Med 2013;27:862–874. [DOI] [PubMed] [Google Scholar]
- 25. Toresson L, Steiner JM, Suchodolski JS, et al. Oral cobalamin supplementation in dogs with chronic enteropathies and hypocobalaminemia. J Vet Intern Med 2016;30:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Procoli F, Mõtsküla PF, Keyte SV, et al. Comparison of histopathologic findings in duodenal and ileal endoscopic biopsies in dogs with chronic small intestinal enteropathies. J Vet Intern Med 2013;27:268–274. [DOI] [PubMed] [Google Scholar]
- 27. Titmarsh HF, Gow AG, Kilpatrick S, et al. Low vitamin D status is associated with systemic and gastrointestinal inflammation in dogs with a chronic enteropathy. PLoS ONE 2015;10:e0137377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heilmann RM, Volkmann M, Otoni CC, et al. Fecal S100A12 concentration predicts a lack of response to treatment in dogs affected with chronic enteropathy. Vet J 2016;215:96–100. [DOI] [PubMed] [Google Scholar]
- 29. Casamian‐Sorrosal D, Willard MD, Murray JK, et al. Comparison of histopathologic findings in biopsies from the duodenum and ileum of dogs with enteropathy. J Vet Intern Med 2010;24:80–83. [DOI] [PubMed] [Google Scholar]
- 30. Jeergens AE, Evans RB, Ackermann M, et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet Pathol 2014;51:946–950. [DOI] [PubMed] [Google Scholar]


