Abstract
Background
Videofluoroscopic swallow study (VFSS) is the gold standard for diagnosis of dysphagia in veterinary medicine but lacks standardized protocols that emulate physiologic feeding practices. Age impacts swallow function in humans but has not been evaluated by VFSS in dogs.
Hypothesis/Objectives
To develop a protocol with custom kennels designed to allow free‐feeding of 3 optimized formulations of contrast media and diets that address limitations of current VFSS protocols. We hypothesized that dogs evaluated by a free‐feeding VFSS protocol would show differences in objective swallow metrics based on age.
Animals
Healthy juvenile, adult, and geriatric dogs (n = 24).
Methods
Prospective, experimental study. Custom kennels were developed to maintain natural feeding behaviors during VFSS. Three food consistencies (thin liquid, pureed food, and dry kibble) were formulated with either iohexol or barium to maximize palatability and voluntary prehension. Dogs were evaluated by 16 swallow metrics and compared across age groups.
Results
Development of a standardized VFSS protocol resulted in successful collection of swallow data in healthy dogs. No significant differences in swallow metrics were observed among age groups. Substantial variability was observed in healthy dogs when evaluated under these physiologic conditions. Features typically attributed to pathologic states, such as gastric reflux, were seen in healthy dogs.
Conclusions and Clinical Importance
Development of a VFSS protocol that reflects natural feeding practices may allow emulation of physiology resulting in clinical signs of dysphagia. Age did not result in significant changes in swallow metrics, but additional studies are needed, particularly in light of substantial normal variation.
Keywords: Iohexol, Barium, Free‐feeding, Aspiration, Reflux
Abbreviations
- AIR
air column reopening
- cP
centipoise
- ETT
esophageal transit time
- IQR
interquartile range
- ISI
interswallow interval
- MLE
time to maximum laryngeal excursion
- MPC
time to maximum pharyngeal constriction
- MRI
magnetic resonance imaging
- MU VHC
University of Missouri Veterinary Health Center
- PAS
penetration aspiration scale
- PCR
pharyngeal constriction ratio
- PESC
time to proximal esophageal sphincter closure
- PESO
time to proximal esophageal sphincter opening
- PES
proximal esophageal sphincter
- VFSS
videofluoroscopic swallow study
Dysphagia, defined as difficulty swallowing, is the clinical manifestation of defects in the oral, pharyngeal, or esophageal stages of swallowing, or some combination of these.1 Clinical features include prehension difficulty, gagging, retching, repetitive swallowing, or regurgitation or some combination of these. Although clinical features may be similar across location and swallowing stage, treatment is not, with correct localization required for targeted intervention. Incorrect diagnoses may result in morbidity and mortality as a result of malnutrition, aspiration, or both. Dysphagia is under‐recognized, under‐reported, and poorly characterized in dogs in part due to limitations in current diagnostic testing.2, 3, 4, 5
The preferred method for diagnosing dysphagia is by contrast videofluoroscopic swallow study (VFSS) to allow real‐time imaging.2, 6 Current veterinary protocols for VFSS are hampered by a lack of objective, standardized, and clinically relevant swallowing metrics, a reliance upon nonphysiologic feeding conditions, motion artifact, and operator radiation exposure. A limited number of VFSS metrics for dogs have been reported, all with feeding in lateral or sternal recumbency.2, 5 Nonphysiologic feeding positions impact pharyngeal motion and esophageal transit times (ETT), limiting the clinical utility of many existing objective metrics.3, 4, 5, 7 Furthermore, these positions require physical restraint and force feeding.2, 3, 5, 7 When physical restraint is utilized during feeding, struggling behaviors are common, with head and body motion substantially impacting image quality. Moreover, manual restraint results in increased radiation exposure to personnel. Restraint devices still are associated with radiation exposure because of dependence upon force‐ or hand‐feeding. Although measurable swallowing metrics can be obtained, the consequence is an examination of unnatural feeding activity rather than the dog's typical feeding behavior and swallow function.
Barium sulfate is the PO contrast agent commonly used for VFSS and typically is administered in 2 forms: thin liquid barium (60% w/v) and barium‐soaked kibble.4, 5 Self‐feeding (a hallmark of human VFSS), although encouraged, often is impossible because of a combination of taste and smell aversion and anxiety.8, 9 This necessitates force feeding, which impacts swallow metrics and limits the clinical utility of the data obtained.4 Furthermore, studies in humans have shown that barium alters the density of food and liquids, resulting in longer oral and pharyngeal bolus transit times.10 An alternative to barium is iohexol,1 which is used infrequently for VFSS in humans and animals and has not yet been standardized for this purpose.2, 11
To overcome the limitations of VFSS in dogs, we sought to develop an innovative protocol for assessment of dysphagia in freely behaving dogs which, in drawing from human practices, would allow for improved data collection and clinical decision‐making. The objectives of our study were 3‐fold. The first aim was to develop custom‐designed kennels to permit self‐feeding, limit motion artifact, and decrease radiation exposure to personnel. The second aim was to formulate recipes that decrease the need for force feeding techniques and reflect physiologically relevant consistency and viscosity. The final aim was to evaluate healthy dogs of various ages to develop a standardized data acquisition protocol and to use those data for assessment of objective metrics of swallowing. We hypothesized that optimizing VFSS with a free‐feeding observation kennel would show differences in swallowing metrics among juvenile, adult, and geriatric dogs.
Materials and Methods
Animals
All testing was performed in accordance with MU Institutional Animal Care and Use Committee (IACUC) protocol numbers 8,339 and 8,833. Healthy research dogs and companion animals were recruited for participation (n = 24). Companion dogs were recruited with informed owner consent. Dogs were determined to be healthy based on history (no evidence of dysphagia or other gastrointestinal signs) and physical examination. Dolichocephalic and mesaticephalic dogs were included in our study population. Exclusion criteria included brachycephalic breeds, and dogs with clinically relevant periodontal disease, orofacial malformations, abnormal neurologic examination findings, or a history of respiratory, esophageal, or gastrointestinal disease within the preceding 6 months. Control dogs were analyzed with respect to age, sex, food consistency, and body size for each swallow measurement (metric). Small and large breed dogs were investigated because they represented a clear difference in conformation. Age groups were as follows: juvenile (1 month to ≤1 year), adult (˃1 to ≤10 years in small breeds; ˃1 to ≤7 years in large breeds), and geriatric (˃ 10 years in small breeds; ˃ 7 years in large breeds).
Test Kennels
Four polycarbonate test kennels (US Patent 9,107,385) were developed to accommodate small or toy (<3.2 kg to ≤16 kg), medium (>16 kg to ≤29 kg), large (>29 kg to ≤39 kg), and giant breed (≥39 kg) dogs and permit free‐feeding behavior, direct patient visualization, and contrast videofluoroscopy (Fig 1A–C). The kennels were built by the University of Missouri Physics Machine Shop2 and R2Fact Product Development3 Polycarbonate was chosen because it is transparent and easy to clean. Additionally, polycarbonate is nonmagnetic and radiolucent, allowing for possible use in conjunction with magnetic resonance imaging (MRI) and static radiography. The funnel (trapezoidal) shape confines the head in proximity to a height‐adjustable ring designed to accommodate a height‐adjustable food bowl and allow rapid switching of test food consistencies. The 4 kennel sizes allow for adequate confinement to maintain body alignment in the lateral plane within the fluoroscopy machine and permit imaging in the lateral and dorso‐ventral planes. A floor lift within the kennel accommodates dogs of shorter stature, and sliding doors on the kennel sides and ceiling maximize patient accessibility and ease of use. All dogs in this study readily entered the kennel for VFSS testing.
Figure 1.
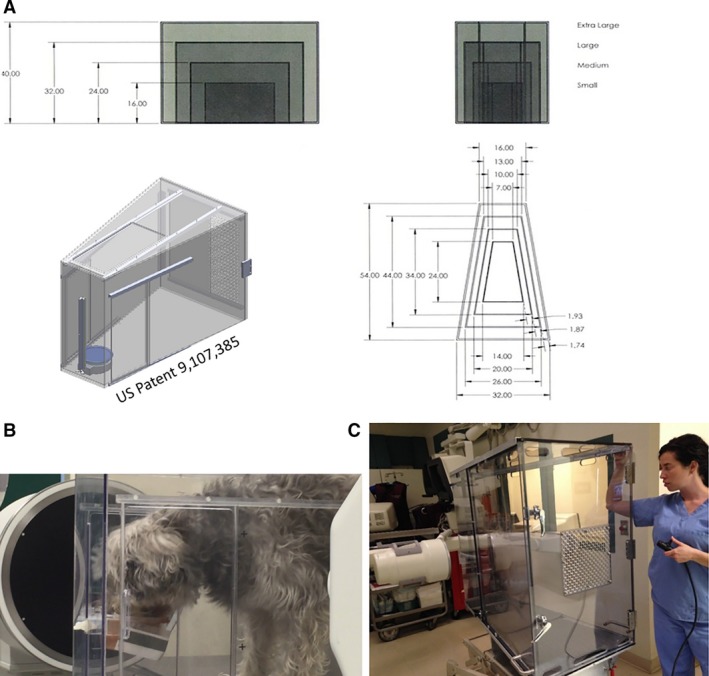
(A, B, C) Development of 4 sizes of observation kennels focused on the need to permit unrestrained, physiologic free‐feeding behavior during videofluoroscopic swallow study. (A) Small, medium, large, and giant breed dogs are accommodated by the kennel design; the trapezoidal shape helps confine the head to the level of the height‐adjustable food bowl to minimize motion artifact during data acquisition. (B) Chinese Crested and Beagle mix in the small observation kennel. (C) Extra‐large kennel shown on a hydraulic lift with remote control.
Recipe Formulation and Palatability Trials
Recipes were formulated to maintain the physical properties of the food while providing adequate radiopacity. For thin liquid, various powered or liquid palatants were mixed with water and contrast media, either barium sulfate (40–60% w/v) or iohexol (350 mg iodine per ml, diluted to a final concentration of 10–50% iohexol). Thin liquid viscosity was evaluated with a Haake RS‐100 RheoStress Cone and Plate Viscometer4 and was targeted to closely resemble water (1 centipoise, cP). For canned pureed food, barium or iohexol was added using the same concentration ranges as for thin liquid. For dry, crunchy kibble, 2 different preparation methods were evaluated: (1) soaking kibble in flavor‐enhanced barium or iohexol and then drying in a Nesco Pet Treat Maker,5 and (2) extruding barium through each piece of kibble during manufacturing by an industry partner, AFB International,6 followed by coating with various proprietary palatants. We chose barium for this extrusion process because it can withstand high baking temperatures.
Objective palatability testing was performed in cooperation with AFB International. This facility is audited annually by the USDA and meets or exceeds the standards set forth by the Animal Welfare Act. Palatability feeding trials were approved by the IACUC of AFB International, which includes a nonaffiliated member, a veterinarian, and several nonscientist members as required by the USDA. Healthy adult dogs of various breeds (n = 10) were included in feeding trials over a 9‐month period, and all trials were conducted without radiation exposure. Dogs were group‐housed in compatible social groups with daily outdoor access. During testing, dogs were individually tested in a clinical setting rather than in their normal environment to evaluate their acceptance of the free‐feeding protocol in a hospital setting. The volume of each test item was adjusted based on body weight. Diets meeting the following outcome measures subsequently were evaluated at the University of Missouri Veterinary Health Center (MU VHC): latency to begin consumption (≤15 seconds), time to finish (≤1.5 minute), number of head raises (≤3 head raises), and percent consumption (≥90%). Adverse events (eg, diarrhea) after VFSS testing were recorded for all dogs.
Videofluoroscopic Swallow Study and Objective Metrics
Contrast VFSS was performed at 30 frames/s with a GE Advantx or GE OEC 9900 Elite Mobile C‐Arm system7 at MU VHC. Dogs were size‐matched to the appropriate test kennel to allow assessment of swallow function. A radiopaque imaging marker was positioned near the mandible to correct for magnification. The food bowl was positioned at shoulder level to minimize the effect of gravity on swallow metrics, minimize raising of the head during prehension, and facilitate swift movement of the fluoroscope from the oral cavity and pharynx to evaluate esophageal swallow metrics. Images were calibrated to an additional marker on the bowl ring. During testing, each dog was offered 3 consistencies of food containing PO contrast, presented in the following order: pureed canned food, dry kibble, and thin liquid. The head and neck were centered in the fluoroscopy field of view by repositioning the table or C‐arm. Imaging of the oral and pharyngeal stages of swallowing was obtained from this position. An attempt was made to record at least 3 consecutive pairs of swallows at this position. The 6th swallow was followed to the stomach by moving the table or C‐arm to visualize the esophageal stage of swallowing. This process was repeated at least twice for each consistency of food that was readily consumed.
Videos were analyzed by Pinnacle Studio 148 and ImageJ software9 by 2 trained, independent investigators to quantify 16 swallow metrics (Table 1, Figs 2, 3, 4, 5). Penetration‐aspiration was assessed with the Penetration Aspiration Scale (PAS).12 Bolus area was calculated for pureed foods only, because this consistency forms a cohesive bolus that can be readily outlined for analysis.7 During development of our protocol, additional swallow metrics were added. Therefore, not every dog was evaluated for every metric. For all 3 consistencies, a minimum of 4 control dogs were evaluated for each swallow metric included in the final protocol (Table 2).
Table 1.
Canine videofluoroscopic swallow study (VFSS) swallow metrics
| VFSS Metrics | Description | |
|---|---|---|
| Oral stage | Interswallow interval (ISI) | The time (s) between 2 successive, uninterrupted swallows, from the onset frame of 1 swallow to the onset frame of the subsequent swallow. The swallow onset frame is the first frame of perpendicular movement of the bolus from the valleculae into the pharynx (Fig 2A) |
| Swallow rate | The number of swallows per 3‐second intervals of uninterrupted prehension | |
| Jaw cycles per swallow ratio | The number of licks, from maximum jaw excursion to the following maximum jaw excursion, per swallow | |
| Pharyngeal stage | Time to proximal esophageal sphincter opening (PESO) | The time (s) from the swallow onset until the head of the bolus has entered the esophagus (Fig 2B) |
| Time to maximum laryngeal excursion (MLE) | The time (s) from the swallow onset until the maximal rostral displacement of the trachea (Fig 2C) | |
| Time to maximum pharyngeal constriction (MPC) | The time (s) from swallow onset until the tail of the bolus has been maximally constricted in the pharynx, pushing the bolus tail through the proximal esophageal sphincter (Fig 2D) | |
| Time to air column reopening (AIR) | The time (s) from swallow onset until the first frame of the pharyngeal air column reopening (Fig 2E) | |
| Time to proximal esophageal sphincter closure (PESC) | The time (s) from swallow onset until the tail of the bolus has entered the esophagus (Fig 2F). This is the pharyngeal transit time (PTT) | |
| Pharyngeal constriction ratio | Pharyngeal area (centimeters squared) when the pharynx is at rest (the rest frame) divided by the pharyngeal area at maximum pharyngeal constriction during swallowing (Fig 3). Measured during consumption of pureed food | |
| Esophageal stage | Esophageal transit time (ETT) | The time (s) from the bolus entering the esophagus (PESC) until the tail of the bolus has completely entered the stomach |
| Proximal esophageal bolus area | Area of the bolus (centimeters squared) when the bolus is in the proximal esophagus. Measured during consumption of pureed food (Fig 4A) | |
| Proximal esophageal retention | The presence of bolus retention in the proximal esophagus, rostral to the thoracic inlet (Fig 4B) | |
| Distal esophageal retention | The presence of bolus retention in the distal esophagus, caudal to the thoracic inlet (Fig 4B) | |
| Gastric reflux | The presence of reflux, when a bolus passes the lower esophageal sphincter into the stomach, followed by part or all of the bolus flowing out of the lower esophageal sphincter back into the esophagus (Fig 4C) | |
| Retrograde flow | The presence of bolus flow toward the upper esophageal sphincter before reaching the stomach (Fig 4D) | |
| Bolus discohesion | The breaking apart of the bolus within the esophagus as it moves toward the stomach (Fig 4E) | |
Objective swallow metrics.
Figure 2.
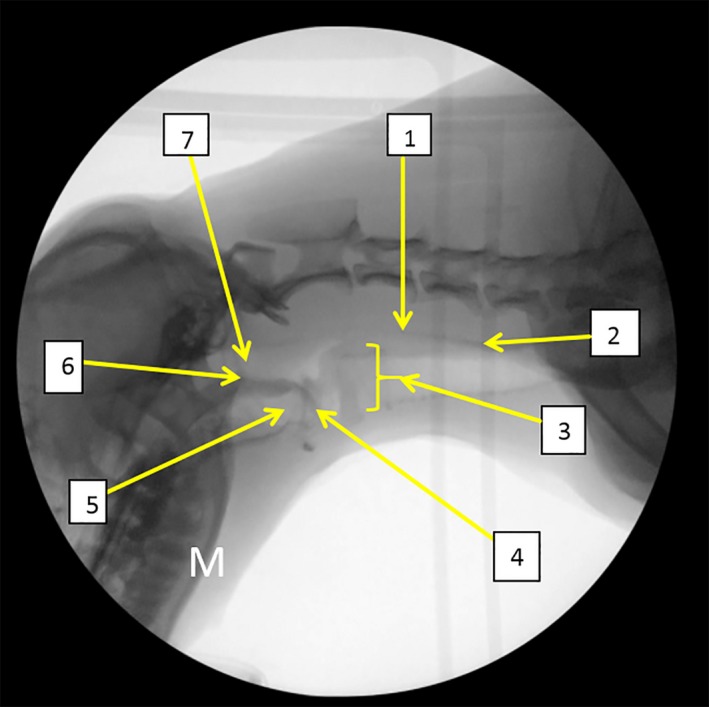
Normal anatomy (1) proximal esophageal sphincter (PES), (2) esophagus, (3) larynx, (4) epiglottis, (5) valleculae, (6) soft palate, (7) nasopharynx. M: mandible.
Figure 3.
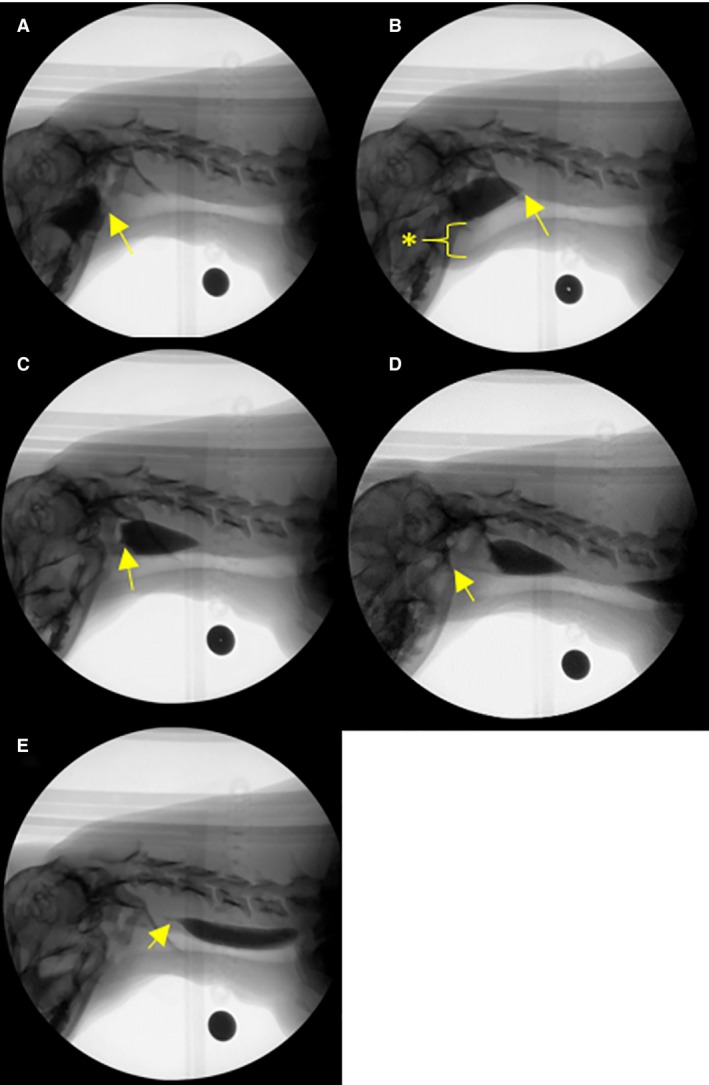
(A–E) Representative examples of pharyngeal stage swallow metrics (transit measures of bolus flow) during videofluoroscopic swallow study in the healthy dog. (A) Swallow onset frame indicated by the first frame of perpendicular movement of the bolus from the valleculae into the pharynx. (B) proximal esophageal sphincter (PES) opening indicated by the first frame of movement of the head of the bolus into the proximal esophagus. Maximum laryngeal excursion indicated by the maximal rostral displacement of the trachea (asterisk). (C) Maximum pharyngeal constriction indicated by the tail of the bolus being maximally constricted by the pharynx, pushing the bolus head through the proximal esophageal sphincter. (D) Air column reopening indicated by the air column beginning to re‐appear after pharyngeal constriction. (E) PES closing indicated by the tail of the bolus completely entering the esophagus. Black circle: calibration marker.
Figure 4.
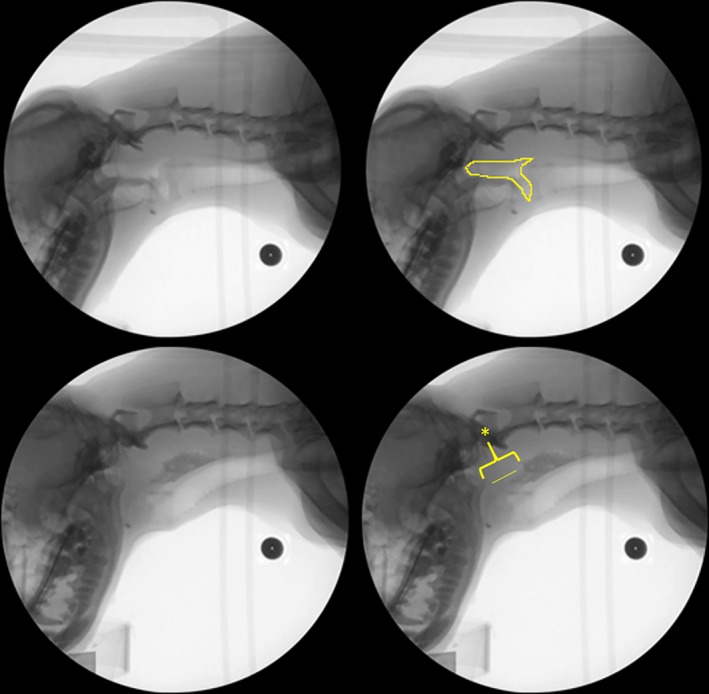
Representative example of pharyngeal constriction ratio (pharyngeal stage swallow metric) during videofluoroscopic swallow study in the healthy dog. Top Left: Hold frame. Top Right: Hold frame outlined to indicate pharyngeal area. Bottom Left: Maximum pharyngeal constriction. Bottom Right: Maximum pharyngeal constriction (line indicates constricted pharynx, marked by asterisk). Black circle: calibration marker.
Figure 5.
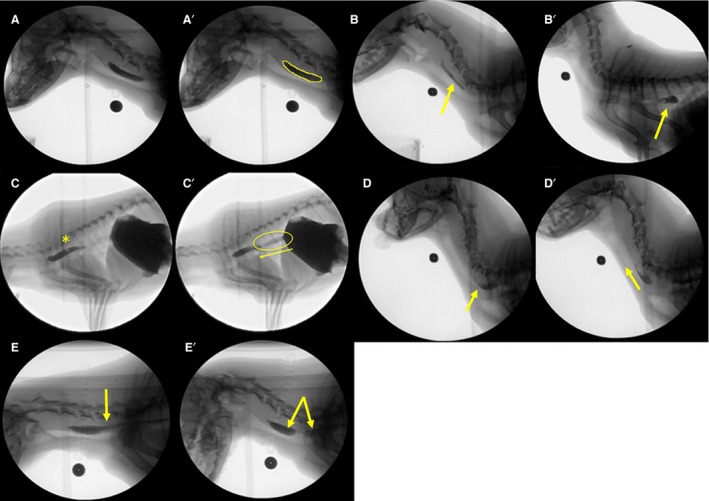
(A–E) Representative examples of esophageal stage swallow metrics during videofluoroscopic swallow study in healthy dogs. (A) Bolus in the proximal esophagus. (A′) Pureed food bolus is outlined to calculate bolus area. (B) Retention of a portion of a liquid bolus in the proximal esophagus after the swallow (arrow). (B′) Retention of the liquid bolus in the distal esophagus (arrow). (C) Complete clearance of the liquid bolus into the stomach is followed by another swallow (asterisk). (C′) Reflux of gastric contents back into the distal esophagus to join the bolus from the subsequent swallow (asterisk). (D) Pureed food bolus in the mid esophagus. (D′) Retrograde flow of a pureed food bolus toward the proximal esophageal sphincter (arrow). (E) Intact pureed food bolus moving down the esophagus. (E′) Breaking apart (bolus discohesion) of the pureed food bolus within the esophagus as it moves toward the stomach (arrows).
Table 2.
Number of complete data sets during and through optimization of the VFSS protocol
| Control: n = 24 dogs | Oral Stage Metrics | Pharyngeal Stage Metrics | Esophageal Stage Metrics |
|---|---|---|---|
| Pureed | 12 | 12 | 9 |
| Kibble | 15 | 15 | 6 |
| Thin | 10 | 10 | 4 |
Number of dogs with complete data sets for each stage of swallowing per consistency.
Statistics
Statistical analysis was performed by SigmaPlot data analysis software10 (version 12.3). Data were stratified by study group. Descriptive statistics were performed where appropriate. Nonparametric analysis was performed on swallow metrics because of the small sample size. A Kruskal‐Wallis ANOVA on ranks or Wilcoxon signed‐rank test was performed to evaluate for differences within each consistency relating to age, sex, breed, and body size. Post hoc analysis (Dunn's method for multiple comparisons) was performed where appropriate. Data are presented as median ± interquartile range (IQR). A P value of ≤.05 was considered significant.
Results
Animals
Twenty‐four healthy research and companion dogs were enrolled in our study. Ages ranged from 1 month to 14 years, with a mean ± SD age of 6.4 ± 4.7 years. The population consisted of juvenile (n = 5), adult (n = 12), and geriatric (n = 7) dogs. Mean age ± SD (years) for each age group was 0.36 ± 0.35 (juvenile), 5.51 ± 2.22 (adult), and 12.14 ± 2.22 (geriatric). Fourteen dogs were spayed females and 10 dogs were castrated males. Breeds represented included Pembroke Welsh Corgis (n = 8), long‐haired Dachshunds (n = 5), Jack Russell Terrier (n = 1), Chinese Crested and Beagle mix (n = 6), German Shepherd (n = 1), and large mixed breed dogs (n = 3). Twenty dogs were considered small breed, and 4 were considered large breed.
Recipes and Palatability Trials
Formulations for all recipes were evaluated in vitro under fluoroscopy to ensure sufficient radiopacity during subsequent VFSS trials (data not shown). We determined that 25% iohexol (350 mg iodine/mL) for pureed food and thin liquid, and 40% w/v barium sulfate for dry kibble (ie, the industry standard for barium in VFSS in humans), resulted in sufficient radiopacity during in vitro testing. Therefore, these standards were used for subsequent recipe formulations and VFSS testing.
Pureed canned food mixed with either barium or iohexol was readily consumed by the majority (87%) of dogs and therefore was not subjected to further recipe formulation or palatability testing. Thin liquid trials with barium resulted in refusal by all dogs. Therefore, we focused on optimizing an iohexol‐based thin liquid recipe for our VFSS protocol. Despite trials with numerous commercially available flavor enhancers, palatability of think liquids during VFSS remained poor, with only 53.9% of dogs meeting or exceeding our standards of palatability (acceptance). Soaking kibble in these same thin liquid recipes (and subsequently drying to a crunchy consistency) resulted in only 51.6% acceptance during VFSS, whereas barium‐soaked kibble was refused by all dogs. Based on these suboptimal results, we subjected thin liquid and kibble to additional recipe formulation and taste testing by AFB international (Table 3).
Table 3.
Palatability testing for thin liquid and kibble
| Formulation: Thin liquid | Time to start | Time to finish | No. of head raises | Percent consumed |
| Median ± IQR | 0.0 ± 0.025 | 0.48 ± 0.38 | 0 ± 1 | 100 ± 3.5% |
| Formulation: Kibble | Time to start | Time to finish | No. of head raises | Percent consumed |
| Median ± IQR | 0.07 ± 0.08 | 1:41 ± 0.79 | 2 ± 2.75 | 100 ± 0.00% |
IQR, interquartile range.
Results (median ± IQR) for palatability testing performed with a cohort of heathy dogs (n = 10) at AFB international (St. Charles, MO) for thin liquid and kibble. Results reflect the final formulations, which were subsequently tested at the MU VHC.
The most promising iohexol‐based thin liquid recipe developed by AFB International was eagerly consumed by >90% of dogs during initial taste tests, but it was markedly less reliably consumed when offered to dogs during VFSS (approximately 27%). Optimization ultimately was achieved with broth extracted from canned chicken, which substantially improved palatability to 89.4%. This chicken‐flavored thin liquid recipe remained within the target viscosity range of water and is considered our final thin liquid recipe for future use during VFSS. For kibble, barium powder (40% w/v) was added during an extrusion process at AFB International and was subsequently coated with a proprietary palatant. This kibble recipe resulted in 81% acceptance by dogs during VFSS; dogs that refused were deemed picky eaters or highly anxious by their owners. Therefore, further efforts for recipe improvement were deemed unnecessary.
After taste‐testing trials or VFSS, self‐limiting diarrhea was reported in some dogs approximately 12 hours after feeding, which resolved within 24 hours. No other adverse effects were reported. Diarrhea is a reported adverse effect of iohexol ingestion13 and was thus thought to be the main contributing factor in our study. However, both thin liquid and pureed food were fed during the same testing period. Therefore, the contribution of either the thin liquid or pureed food could not be determined. Additionally, the research dogs were group‐housed and the exact number of affected dogs per group could not be determined.
Videofluoroscopic Swallow Protocol and Objective Metrics
The initial test protocol was developed in an early cohort of control dogs and progressively modified to its final form (Table 4), the data sets were incomplete because the protocol underwent revision during the optimization process. However, at least 4 control dogs had complete data sets for each metric and for each consistency. Evaluating within each consistency, there was no significant difference between any of the age groups for any swallow metric (Table 5). Therefore, the median ± IQR values of each metric from 24 dogs were combined and reported (Table 6). Female dogs (n = 10) were significantly (P = .025) more likely to exhibit reverse peristalsis than males (n = 3). Males had a significantly (P = .036) longer time to proximal esophageal sphincter opening (PESO) compared with females when consuming thin liquid. Small dogs had a significantly shorter time to proximal esophageal opening when consuming pureed food and significantly greater esophageal retention than large dogs for all consistencies (P = .018). No other statistically significant differences were identified for any other metric with respect to sex, breed, or body size within liquid, kibble, and pureed consistencies.
Table 4.
Videofluoroscopic swallow study (VFSS) test protocol for dogs
| 1. | Overnight food restriction to maximize food motivation/participation |
| 2. | Prepare test items with oral contrast agent immediately before use. Split each serving of each test item into 2 bowls for testing oral/oropharyngeal versus esophageal stages of swallowing |
| 3. | Adjust bowl ring to shoulder height, then position bowl ring in lower left corner of fluoroscopy field of view (FOV) |
| 4. | Adjust frame rate to record at 30 fps. Record calibration marker (cross) on bowl for 5 seconds |
| 5. | Place first test item in bowl ring. Place radiopaque food label on bowl ring |
| 6. | Allow animal to freely enter kennel to consume test item from bowl |
| 7. | Record swallows with pharynx and larynx centered in FOV until animal finishes eating/drinking |
| 8. | Place second half of first test item in bowl ring. Center FOV over upper esophagus (should still see pharyngeal swallows) until bolus moves, then follow bolus to the stomach |
| 9. | Repeat step 8 at least once until animal is finished eating/drinking |
| 10. | Proceed to next test consistency and place radiopaque label corresponding to food consistency on bowl ring |
| 11. | Allow animal to freely exit kennel |
| 12. | Clean kennel surfaces, floor mat, and bowl ring with Simple Green11. a |
Protocol for conducting freely behaving canine VFSS.
Nontoxic cleaner appropriate for polycarbonate.
Table 5.
Normal swallow metrics by age (median ± IQR)
| Juvenile (0.1 ± 0.6 years) | Adult (4.9 ± 0.93 years) | Geriatric (11.3 ± 3.55 years) | P value | |
|---|---|---|---|---|
| Liquid | ||||
| Interswallow interval (ISI) | 1.51 ± 1.97 | 1.14 ± 0.53 | 1.19 ± 0.69 | .215 |
| Swallow rate | 1.65 ± 1.20 | 2.65 ± 0.7 | 2.35 ± 0.65 | .670 |
| Jaw cycles per swallow ratio | 2.70 ± 2.68 | 2.85 ± 1.15 | 3.00 ± 1.25 | .847 |
| Time to proximal esophageal sphincter opening (PESO) | 0.04 ± 0.02 | 0.05 ± 0.03 | 0.03 ± 0.02 | .463 |
| Time to maximum laryngeal excursion (MLE) | 0.07 ± 0.03 | 0.08 ± 0.04 | 0.10 ± 0.06 | .254 |
| Time to maximum pharyngeal constriction (MPC) | 0.11 ± 0.06 | 0.12 ± 0.04 | 0.11 ± 0.02 | .834 |
| Time to air column reopening (AIR) | 0.28 ± 0.03 | 0.26 ± 0.06 | 0.30 ± 0.05 | .389 |
| Time to proximal esophageal sphincter closure (PESC) | 0.33 ± 0.03 | 0.31 ± 0.06 | 0.35 ± 0.06 | .743 |
| Pharyngeal constriction ratio | – | – | – | – |
| Esophageal transit time (ETT) | 3.1 ± 6.0 | 4.64 ± 1.13 | 4.5 ± 0.1 | .969 |
| Proximal esophageal bolus area | – | – | – | – |
| Puree | ||||
| Interswallow interval (ISI) | 2.53 ± 0.4 | 3.14 ± 1.49 | 4.04 ± 1.75 | .193 |
| Swallow rate | 2.0 ± 0.08 | 1.50 ± 1.0 | 1.25 ± 0.65 | .211 |
| Jaw cycles per swallow ratio | 7.7 ± 2.3 | 8.0 ± 5.7 | 9.35 ± 5.68 | .861 |
| Time to proximal esophageal sphincter opening (PESO) | 0.03 ± 0.02 | 0.06 ± 0.03 | 0.09 ± 0.04 | .385 |
| Time to maximum laryngeal excursion (MLE) | 0.07 ± 0.02 | 0.14 ± 0.04 | 0.09 ± 0.03 | .206 |
| Time to maximum pharyngeal constriction (MPC) | 0.11 ± 0.03 | 0.12 ± 0.02 | 0.12 ± 0.02 | .609 |
| Time to air column reopening (AIR) | 0.27 ± 0.04 | 0.30 ± 0.08 | 0.28 ± 0.08 | .732 |
| Time to proximal esophageal sphincter closure (PESC) | 0.34 ± 0.02 | 0.39 ± 0.05 | 0.37 ± 0.05 | .088 |
| Pharyngeal constriction ratio | 0.04 ± 0.01 | 0.05 ± 0.04 | 0.02 ± 0.01 | .083 |
| Esophageal transit time (ETT) | 4.74 ± 0.02 | 4.6 ± 0.09 | 4.62 ± 1.04 | .965 |
| Proximal esophageal bolus area | 2.96 ± 0.55 | 4.44 ± 5.69 | 3.44 ± 1.08 | .875 |
| Kibble | ||||
| Interswallow interval (ISI) | 6.61 ± 3.44 | 5.25 ± 2.90 | 6.43 ± 3.65 | .345 |
| Swallow rate | 1.25 ± 0.63 | 1.30 ± 0.70 | 1.0 ± 0.0 | .140 |
| Jaw cycles per swallow ratio | 15.5 ± 6.5 | 12.0 ± 9.3 | 10.0 ± 4.5 | .513 |
| Time to proximal esophageal sphincter opening (PESO) | 0.08 ± 0.05 | 0.12 ± 0.11 | 0.09 ± 0.04 | .265 |
| Time to maximum laryngeal excursion (MLE) | 0.14 ± 0.12 | 0.24 ± 0.05 | 0.14 ± 0.12 | .145 |
| Time to maximum pharyngeal constriction (MPC) | N/A | N/A | N/A | – |
| Time to air column reopening (AIR) | 0.37 ± 0.17 | 0.56 ± 0.27 | 0.53 ± 0.10 | .122 |
| Time to proximal esophageal sphincter closure (PESC) | 0.43 ± 0.44 | 1.02 ± 0.62 | 0.65 ± 0.12 | .116 |
| Pharyngeal constriction ratio | – | – | – | – |
| Esophageal transit time (ETT) | 4.95 ± 0.02 | 5.84 ± 3.78 | 5.9 ± 0.53 | .101 |
| Proximal esophageal bolus area | – | – | – | – |
| Other | ||||
| Proximal esophageal retention | 1/5 | 0/12 | 2/7 | .282 |
| Distal esophageal retention | 0/5 | 2/12 | 1/7 | |
| Distal and proximal esophageal retention | 3/5 | 10/12 | 4/7 | |
| Gastric reflux | 3/5 | 4/12 | 4/7 | .709 |
| Retrograde flow | 2/5 | 8/12 | 3/7 | .579 |
| Bolus discohesion | 4/5 | 11/12 | 5/7 | .675 |
| Aspiration | 0/5 | 0/12 | 0/7 | 1.00 |
Median ± IQR for objective swallow metrics for liquid, kibble, and puree for dogs stratified by age. Bolus area was calculated for pureed food only. Animals were evaluated for the presence of proximal and distal esophageal retention, gastric reflux, retrograde flow, and bolus discohesion and aspiration regardless of consistency. N/A indicates that sufficient data could not be collected for this metric due to movement out of frame or instrument limitations leading to delay in video capture and data clipping.
Table 6.
Normal swallow metrics (median ± IQR)
| Liquid | Puree | Kibble | |
|---|---|---|---|
| Interswallow interval (ISI) | 1.22 ± 0.50 | 3.05 ± 1.62 | 5.75 ± 2.36 |
| Swallow rate | 2.30 ± 1.00 | 1.7 ± 1.00 | 1.00 ± 0.50 |
| Jaw cycles per swallow ratio | 2.70 ± 1.30 | 7.85 ± 5.03 | 12.0 ± 9.10 |
| Time to proximal esophageal sphincter opening (PESO) | 0.04 ± 0.03 | 0.06 ± 0.04 | 0.09 ± 0.09 |
| Time to maximum laryngeal excursion (MLE) | 0.08 ± 0.04 | 0.08 ± 0.03 | 0.21 ± 0.15 |
| Time to maximum pharyngeal constriction (MPC) | 0.11 ± 0.04 | 0.13 ± 0.04 | N/A |
| Time to air column reopening (AIR) | 0.28 ± 0.07 | 0.30 ± 0.07 | 0.52 ± 0.13 |
| Time to proximal esophageal sphincter closure (PESC) | 0.31 ± 0.04 | 0.37 ± 0.06 | 0.63 ± 0.56 |
| Pharyngeal constriction ratio | N/A | 0.03 ± 0.03 | N/A |
| Esophageal transit time (ETT) | 4.5 ± 0.93 | 4.6 ± 1.20 | 5.83 ± 1.00 |
| Proximal esophageal bolus area | – | 3.13 ± 2.71 | – |
| Proximal esophageal retention only | 3/24 | ||
| Distal esophageal retention only | 3/24 | ||
| Distal and proximal esophageal retention | 17/24 | ||
| Gastric reflux | 10/24 | ||
| Retrograde flow | 13/24 | ||
| Bolus discohesion | 20/24 | ||
| Aspiration | 0/24 |
Median ± IQR for objective swallow metrics for liquid, kibble, and puree for dogs of all ages combined. Bolus area was calculated for pureed food only. Animals were evaluated for the presence of proximal and distal esophageal retention, gastric reflux, retrograde flow, and bolus discohesion regardless of consistency. N/A indicates that sufficient data could not be collected for this metric due to movement out of frame or instrument limitations leading to delay in video capture and data clipping.
Evaluating between each consistency, several significant differences were identified. The interswallow interval (ISI) was significantly longer for dry kibble compared with both liquid and pureed food (P < .05). The ISI for pureed food was significantly longer than for thin liquid (P < .05). Swallow rate was significantly faster and the number of jaw excursions was significantly decreased for liquids compared with kibble and pureed food (P < .05). The PESO, time to proximal esophageal sphincter closure (PESC), time to air column reopening (AIR), and time to maximal laryngeal excursion (MLE) were significantly longer for kibble compared with pureed food and thin liquid (P < .05). No significant differences were identified for ETT among liquid, kibble, or pureed food.
Discussion
This multidisciplinary study resulted in the successful development of a VFSS protocol for use in dogs that permitted normal feeding behavior. Specially developed kennels allowed feeding without physical restraint, thus removing the influence of nonphysiologic feeding positions on swallow metrics and decreasing operator radiation exposure. This was aided by the development of highly palatable recipes developed to facilitate adequate visualization during VFSS while emulating the physical characteristics of food and water consumed in the home environment. Objective swallow metrics allowed detailed evaluation of the oral, pharyngeal, and esophageal stages of swallowing in a cohort of healthy dogs.
In contrast to our hypothesis, with development of an optimized observation kennel and VFSS protocol, no significant differences in any oral, pharyngeal, or esophageal stage swallow metrics were detected among healthy juvenile, adult, and geriatric dogs. Additionally, substantial normal variation was observed when using physiologic feeding practices in conjunction with recipes designed to closely emulate water, canned pureed food, and dry kibble. Further investigation of these findings in normal dogs is needed to determine pathologic relevance in a clinical population of dogs with dysphagia. The consistency of the diet consumed also was shown to substantially impact swallow metrics, highlighting the need for standardization in the formulation of food or liquid intended for VFSS.
The VFSS can be further optimized by the use of a standardized protocol to evaluate clinically relevant swallow metrics. This diagnostic test currently is hampered by reliance on nonphysiologic feeding positions, subjective metrics, and force feeding, which do not reflect home feeding practices.2, 3, 4, 5, 6, 7 By deviating from the circumstances in which clinical signs are observed, veterinarians risk misidentifying and incorrectly classifying both normal and dysphagic animals. Additionally, motion artifact and radiation exposure complicate the utility of these studies in clinical practice.2
Free‐feeding is a key feature in VFSS in humans and is critical to developing optimal standardized protocols for VFSS in veterinary medicine. Comparing the findings of this study of free‐feeding dogs to previously published reports by physical restraint techniques, we identified objective swallow metrics in the pharyngeal stage that were different depending on body position. For example, the median ± IQR time to proximal esophageal sphincter (PES) closure in our study was 0.63 ± 0.55 seconds during free‐feeding, whereas the mean and SD in another study of 15 dogs were reported to be much faster for lateral (0.33 ± 0.07 seconds) and sternal (0.28 ± 0.05 seconds) recumbency.5 Additionally, we observed gastric reflux, esophageal retention, retrograde flow, and bolus discohesion in clinically normal dogs when free‐fed, which is counter to previous clinical thinking and cautions against over‐interpretation of these findings in clinical cases. It also raises concern that previous methodologies may not accurately reflect physiologic feeding conditions or capture normal variation in swallowing function. In addition to body position, changes in objective metrics may reflect changes in bolus sizes during self‐feeding.4 Consistency of the food also was shown to have significant impact on objective metrics.4 Therefore, a standardized diet for VFSS may be ideal to allow for direct comparisons of swallow metrics before and after intervention, as well as across patient populations and clinical sites.
In people, age‐related deterioration in swallow function affects approximately 20% of adults ˃ 65 years old and results in increased risk of malnutrition and aspiration.14 The effect of age on swallow function had not been investigated previously in dogs. Interestingly, in our population of healthy dogs, no significant differences in swallow metrics were observed with respect to age. This finding may reflect species‐specific differences in aging, the age range of our sample population, or small sample size leading to type 2 error and additional studies are needed. Because animal disease models are used to investigate a number of conditions causing dysphagia in humans, an understanding of age‐related changes in swallow function in dogs has relevance in both veterinary research and veterinary and human medical clinical practice. In contrast to age, significant differences were observed in our study among certain swallow metrics with respect to size and sex. Although statistically different, the clinical relevance of these findings is unknown. Thus, larger cohort studies addressing differences in age, conformation, breed, and sex are needed.
Aspiration of liquid, food, and contrast agents represents a real risk in dysphagic patients during VFSS. Although no aspiration events were detected for any food or liquid consistency in our study of healthy dogs, aspiration commonly is reported in dogs with dysphagia.1, 15, 16 We speculate that aspiration events in many cases may be caused or exacerbated by force feeding techniques that are typically used during VFSS. Therefore, our free‐feeding VFSS protocol may decrease the number and severity of aspiration episodes and result in a safer assessment that more closely reflects the severity of dysphagia and better informs clinical treatment decisions. In addition, the order of feeding (ie, thin liquid last) in our VFSS protocol initially was designed to minimize aspiration, because some clinically affected patients subjectively have increased difficulty swallowing thin liquids. However, additional studies are needed to assess the prevalence of aspiration in dysphagic patients before assertions can be made regarding the comparative incidence of macro‐aspiration in healthy dogs by this protocol. Starting with pureed food versus thin liquid during VFSS will likely need to be considered on a case‐by‐case basis.
We also recognize the choice of contrast agent is highly dependent on patient‐specific objectives and comorbidities.11 For our study, iohexol was selected as a contrast agent for thin liquid and pureed food trials because its physical properties are similar to those of water. Previous studies have cited pulmonary edema as a consequence of iohexol aspiration, given the osmolality of iohexol relative to plasma (541 mOsm/kg versus 300 mOsm/kg). More recent studies, however, have shown iohexol can be safely used as a contrast agent in gastrointestinal studies in medically fragile human infants.11 Significant intraluminal fluid shifting was not observed when iohexol was used as a contrast medium in gastrointestinal studies in dogs.17 Conversely, barium is not readily cleared from pulmonary tissues and results in a foreign body response in patients that aspirate. Moreover, barium is widely considered to be poorly palatable, and its physical characteristics are known to impact swallow metrics.9, 10 For our study, barium was selected as the contrast agent in the final dry kibble recipe because we were able to mitigate many of these concerns by our manufacturing process. Barium is stable at high temperatures, allowing it to be incorporated before extrusion and thus maintain the consistency of dry kibble. The use of a proprietary palatant sufficiently increased acceptance to allow clinical use. Barium also has a long shelf‐life and is cost‐effective compared with iohexol. Collectively, these factors increase ease of clinical use and potential for mass production of barium‐extruded kibble for use in standardized VFSS protocols.
Limitations of our study include a relatively small sample size and incomplete data sets for each diet consistency and swallow metric. Additionally, the fluoroscopy unit has a small field of view and requires expertise to operate for VFSS, particularly with large dogs in which head lifting after intake of liquid or food leads to movement of structures out of the window of data collection. Feeding also was performed in a neutral position with the bowl slightly upraised (compared with a bowl on the floor). Although this position may not entirely emulate feeding in the home environment, it decreases the occurrence of head raises out of the fluoroscopy field of view, allows easier evaluation of the esophageal stage of swallowing, decreases the impact of gravity on feeding metrics, and most importantly allows investigation of intervention strategies (eg, upright feeding) at the time of diagnosis. These are substantial advantages over prior VFSS protocols. A final limitation of our study is the labor required for data analysis of swallowing metrics, which would benefit from automated analysis for widespread clinical adoption.
Despite dysphagia being an important cause of morbidity and mortality in dogs, accurate disease localization and thus therapeutic intervention are hampered by current methods of diagnosis. Our study addresses the limitations of current VFSS protocols by developing a novel, feeding protocol with specially designed kennels, highly palatable test items, and standardized objective swallow metrics. This protocol helps to maintain physiologic relevancy and to standardize data collection. Use of this novel VFSS protocol has the potential to expand our understanding of swallowing disorders in companion dogs. We have adapted the methods and metrics of VFSS in humans to VFSS in dogs, thereby providing opportunity for bidirectional advancements in dysphagia research. By bridging the gap between VFSS in humans and animals, there is potential to improve our understanding of normal physiology, discriminate among disease states, and expand the translational capacity of animal models of dysphagia.
Acknowledgments
The authors thank Andries Ferreira, Jan Kunkel, Kate Robbins, Vanessa Gaiser, Kaitlin Bishop, Sarah Weiss, Yaira Rivera, Elena Rodriquez, Lisa Anderson, Laila Al‐Khashti, Kelsey Pitcher, and Dr Martin Katz for their contributions to this project. Dr Grobman's salary is supported by the Boehringer Ingelheim Resident‐Postdoctoral Scholar Program.
Grant support: This study was supported by several internal funding mechanisms: MU CVM Faculty Research Award, Mizzou Advantage Undergraduate Research Team Award, Mizzou Advantage Round 4 Seed Grant, and MU Veterinary Research Scholars Program.
Conflict of Interest Declaration: Bridget Bennett and Julie Ledyayev are employees of AFB International. Nancy Rawson is a former employee of AFB International and a current employee of Monell Chemical Senses Center, Philadelphia, PA. United States Patent No. 9,107,385 for the free‐feeding kennels is held by the Curators of the University of Missouri, listing as inventors Teresa Lever, Joan Coates, Mitchell Allen, and Laila Al‐Khashti.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Harris and Grobman contributed equally to this manuscript.
This work was performed at the University of Missouri in cooperation with AFB International (St. Charles, MO) and R2Fact Product Development (Kansas City, MO).
Footnotes
Omnipaque, GE Healthcare, Salt Lake City, UT
Columbia, MO
Kansas City, MO
Thermo Scientific, Waltham, MA
The Metal Ware Corporation, Two Rivers, WI
St. Charles, MO
GE Healthcare
Pinnacle Systems, Mountain View, CA
Image J version 1.51 g, National Institutes of Health, Bathesda, MD
Systat Software, San Jose, CA
Sunshine Makers, Inc. Huntington Beach, CA
References
- 1. Ettinger SJ, Feldman EC, Edward C. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat. St. Louis, MO: Elsevier Saunders; 2010. [Google Scholar]
- 2. Pollard RE. Imaging evaluation of dogs and cats with dysphagia. ISRN Vet Sci 2012;2012:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pollard RE, Marks SL, Davidson A, et al. Quantitative videofluoroscopic evaluation of pharyngeal function in the dog. Vet Radiol Ultrasound 2000;41:409–412. [DOI] [PubMed] [Google Scholar]
- 4. Cheney DM, Marks SL, Pollard RE. Effect of bolus size on deglutition and esophageal transit in healthy dogs. Vet Radiol Ultrasound 2016;57:359–365. [DOI] [PubMed] [Google Scholar]
- 5. Bonadio C, Pollard RE, Dayton PA, et al. Effects of body positioning on swallowing and esophageal transit in healthy dogs. J Vet Intern Med 2009;23:801–805. [DOI] [PubMed] [Google Scholar]
- 6. Jaffer NM, Edmund FW‐FA, Steele CM. Fluoroscopic evaluation of oro‐pharyngeal dysphagia: Anatomy, technique, and common etiologies. AJR. Am J Roentgenol 2015;204:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pollard RE, Marks SL, Leonard R, et al. Preliminary evaluation of the pharyngeal constriction ration (PCR) for fluoroscopic determination of pharyngeal constriction in dysphagic dogs. Vet Radiol Ultrasound 2007;48:221–226. [DOI] [PubMed] [Google Scholar]
- 8. Association AS‐L‐H . Guidelines for Speech‐Language Pathologists Performing Videofluoroscopic Swallowing Studies. ASHA: Association AS‐L‐H; 2004. [Google Scholar]
- 9. Dietsch AM, Solomon NP, Steele CM, et al. The effect of barium on perceptions of taste intensity and palatability. Dysphagia 2014;29:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stokely SL, Molfenter SM, Steele CM. Effects of barium concentration on oropharyngeal swallow timing measures. Dysphagia 2014;29:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris JA, Bartelt D, Campion M, et al. The use of low‐osmolar water‐soluble contrast in videofluoroscopic swallowing exams. Dysphagia 2013;28:520–527. [DOI] [PubMed] [Google Scholar]
- 12. Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration‐aspiration scale. Dysphagia 1996;11:93–98. [DOI] [PubMed] [Google Scholar]
- 13. Cohen MD, Towbin R, Baker S, et al. Comparison of iohexol with barium in gastrointestinal studies of infants and children. Am J Roentgenol 1991;156:345–350. [DOI] [PubMed] [Google Scholar]
- 14. Wirth R, Dziewas R, Beck AM, et al. Oropharyngeal dysphagia in older persons–from pathophysiology to adequate intervention: A review and summary of an international expert meeting. Clin Interv Aging 2016;11:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niles J, Williams J, Sullivan M, et al. Resolution of dysphagia following cricopharyngeal myectomy in six young dogs. J Small Anim Pract 2001;42:32–35. [DOI] [PubMed] [Google Scholar]
- 16. Kogan DA, Johnson LR, Jandrey KE, et al. Clinical, clinicopathologic, and radiographic findings in dogs with aspiration pneumonia: 88 cases (2004–2006). J Am Vet Med Assoc 2008;233:1742–1747. [DOI] [PubMed] [Google Scholar]
- 17. Agut A, Sanchez‐Valverde M, Lasaosa J, et al. Use of iohexol as a gastrointestinal contrast medium in the dog. Vet Radiol Ultrasound 1993;34:171–177. [Google Scholar]


