The human gastrointestinal tract is predominantly a bacterial ecosystem (microbiome) that harbors >100 trillion microbial cells, with the highest microbe densities found in the colon. Gut microbes are for the most part co-dependent, both on one another and on their host, requiring metabolic support from additional members of the community for survival and a symbiotic relationship with the host. For example, gut microbes help with the digestion of nutrients, prevent significant colonization of pathogens and promote gut immunity, while the host provides a favorable environment for microbial survival.
Gut microbiome changes (so-called “dysbiosis”) leading to increased long-term susceptibility to disease can originate early in life, similar to that of traditional risk factors. There is a growing awareness that microbial inhabitants within the host often contribute to global metabolism within the host, and dysbiosis can fuel enhanced susceptibility for metabolic and immunologic diseases, sometimes emerging decades later. Indeed, alterations in the composition of the human gut-associated microbiome and accompanying functional changes in metabolism have been implicated in the etiology of several chronic conditions ranging from atherosclerosis and thrombosis to obesity and insulin resistance.
Gut Microbial Involvement in Cardiovascular Disease Pathogenesis
It is increasingly appreciated that gut microbes represent a filter of our greatest environmental exposure – what we eat. It is now clear that we each experience a given meal differently, based on our distinct gut microbial communities. Microbial metabolites such as short-chain fatty acids (SCFA) are fermentation byproducts of carbohydrates and proteins that escape absorption in the small intestine during digestion. A microbial origin for SCFA is perhaps best observed by the demonstration that plasma levels of SCFA in germ free mice are nearly undetectable. SCFA help maintain gut barrier function by reducing luminal pH and inhibiting some pathogenic microorganisms. In addition, in another clear example of symbiosis, gut microbe-derived SCFA are used as a source of energy by colonocytes and bacterial communities. Further, they may have distinct physiological effects, including host-signaling mechanisms (such as G-protein coupled receptors, Gpr41 and Olfr78) that can directly and indirectly modulate blood pressure control.
Meanwhile, gut microbe-derived metabolites that are biologically active, such as trimethylamine N-oxide (TMAO), are now recognized as contributors to atherogenesis (Figure). Using untargeted metabolomics as a discovery platform, we identified TMAO as a strong predictor of CAD risk, and then through animal studies revealed TMAO’s causal link to atherogenesis.1 Our mechanistic studies show an obligatory role for gut microbes in TMAO generation from trimethylamine (TMA) -containing nutrients such as phosphatidylcholine, choline and L-carnitine, in both mice and humans.1-3 In humans, circulating TMAO levels increase 4-8 hours after ingestion of phosphatidylcholine and/or L-carnitine, and are largely normalized within 24 hours in the setting of preserved renal clearance. Consistent with the effects of dietary exposure impacting microbial function, vegetarians and vegans (without L-carnitine dietary intake) produce less TMAO as compared with omnivorous subjects, with correspondingly distinct microbiota composition.2 We have also confirmed a mechanistic role for both gut microbes and TMA/TMAO generation in atherogenesis, tissue cholesterol balance, and thrombosis risks.1-4 In mice, diet-induced increases in systemic TMAO levels decrease reverse cholesterol transport and bile acid transport, as well as altering bile acid composition and pool size.2
Figure.
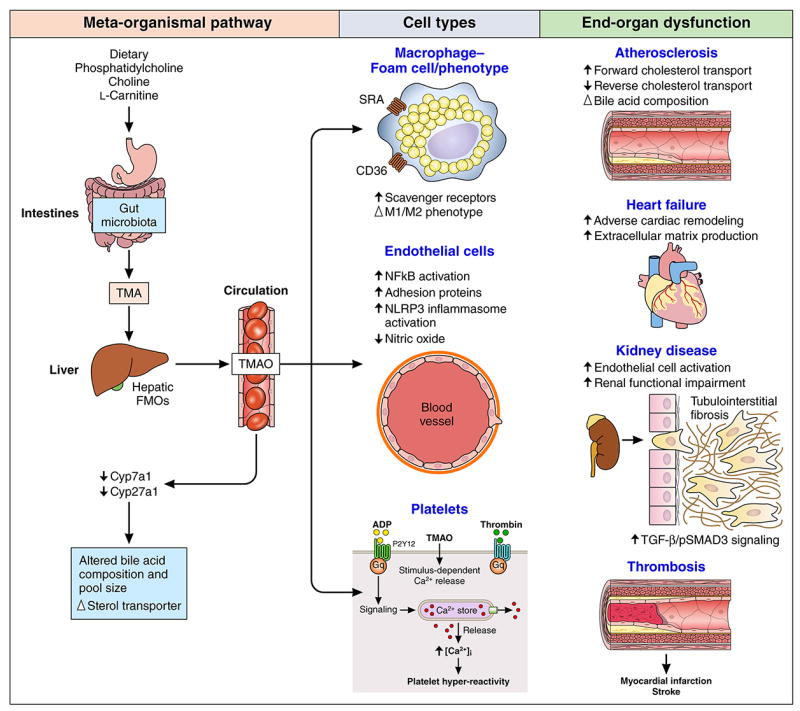
Examples of Metabolic Pathway Linking Gut Microbiota to Cardiovascular Diseases
Fulfilling one of the essential Koch’s postulates, microbial transplantation studies have confirmed gut microbe-dependent TMA/TMAO involvement in atherosclerosis plaque development, and more recently, TMAO-dependent enhanced susceptibility for thrombosis.4 This latter finding followed the discovery that TMAO modulates stimulus-dependent calcium mobilization in platelets, enhancing platelet responsiveness and thrombosis potential in vivo.4 In recent proof-of-concept studies, small molecule inhibitors of microbial TMA and TMAO production have been used to directly inhibit diet-induced atherosclerosis in animal models without altering microbial survival (in contrast to antibiotic therapy),5 which brings a therapeutic strategy of “drugging” the microbiome closer to reality.
Challenges and Potential Pitfalls
Many studies explore the gut microbiome’s role in cardiovascular diseases by characterizing traditional ecological indicators of microbial community composition and diversity between those with versus without cardiovascular disease. However, these approaches often preclude investigations into the functional alterations of specific organisms and their adverse consequences despite broad availability of vast genomic sequencing data. Systems biology approaches that combine genomic with proteomic/ metabolomic data hold promise for developing a more integrated understanding of the relationship between microbes and their host, yet available data are often static (i.e. at a single timepoint), largely associative in nature, and primarily hypothesis-generating. Even pathogenic pathways with proof-of-concept demonstrations in animal models that fulfill Koch’s postulates and have mechanism biomarkers (such as TMA/TMAO) will still require prospective validation with clinical studies testing specific interventions targeting these pathways to lower major adverse cardiac events.
Conclusion
The TMA/TMAO pathways likely represent only one of many microbe-dependent pathways that will ultimately be linked to cardiovascular disease pathogenesis and prove to be an important diagnostic and therapeutic target for cardiovascular diseases. Key to the discovery of this pathway was untargeted metabolomics studies in large patient cohorts to demonstrate reproducibility of associations, and then more important, performance of animal model studies to test for causal connections beyond associations. Such approaches will be critical to understanding new microbial participants and pathways linked to development of atherosclerosis and thrombosis. Importantly, these studies also help to better understand how nutrition is linked to host health and disease susceptibility, requiring a global examination and view of nutrition, microbe community composition and function, and host genetics. It is not only conceivable but probable that multiple distinct microbial pathways contribute to and protect against cardiovascular and other metabolic disorders. Their identification and discovery of the mechanisms through which they participate in cardiovascular disease susceptibility is an exciting new and important field of investigation. Once revealed, novel diagnostic, therapeutic, and preventive strategies that leverage their identification may become part of our arsenal for halting and reversing cardiovascular diseases.
Acknowledgments
Sources of Funding
Drs. Tang and Hazen are supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000, R01HL126827) related to the content of this paper. Dr. Hazen was partially supported by a gift from the Leonard Krieger endowment.
Footnotes
Disclosures
Dr. Hazen is named as inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen is a paid consultant for Esperion and P&G. Dr. Hazen has received research funds from Abbott, P&G, Pfizer Inc., Roche Diagnostics, and Takeda. Dr. Hazen has received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, Siemens, Esperion, and Frantz Biomarkers, LLC. Dr. Tang has no relationships relevant to the contents of this paper to disclose.
References
- 1.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]


