Abstract
Introduction
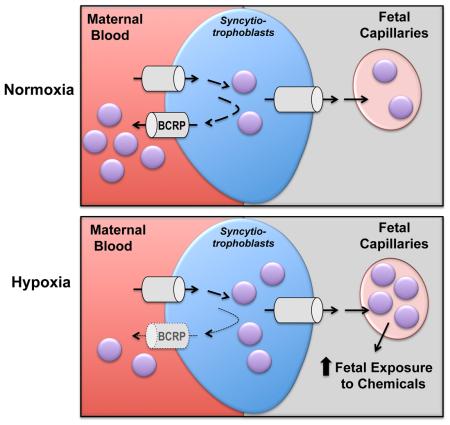
The BCRP/ABCG2 efflux transporter protects the developing fetus by limiting the transplacental transfer of drugs and chemicals and prevents the apoptosis of trophoblasts. The purpose of this study was to determine whether hypoxia-related signaling alters placental BCRP expression and function in vitro and in human pregnancies.
Methods
Human BeWo choriocarcinoma cells were treated with the hypoxia mimetic, cobalt chloride (CoCl2), or 3% oxygen for 24-48 h. Activation of HIF-1α signaling and regulation of BCRP was assessed using qPCR, ELISA, western blotting and a fluorescent substrate transport assay. In addition, healthy term placentas from high altitude pregnancies with chronic hypoxia were assessed for BCRP expression.
Results
CoCl2 and 3% oxygen increased HIF-1α protein signaling and decreased the mRNA and protein expression of BCRP by 30-75% in BeWo cells. Reduced BCRP expression corresponded with impaired efflux activity during hypoxia as evidenced by accumulation of the substrate Hoechst 33342. A number of transcription factors known to regulate BCRP, including AHR, NRF2 and PPARγ, were also coordinately down-regulated by 3% oxygen in BeWo cells. Moreover, women who gave birth at a high altitude (3100 m) exhibited signs of chronic placental hypoxia, including enhanced protein expression of the HIF-1α target GLUT1, and had reduced BCRP levels in microvillous membranes compared to women at a moderate altitude (1600 m).
Discussion
This study provides novel insight into the regulation of the placental BCRP transporter by hypoxia, which may be important for exposure of the fetus to chemicals during early development and in hypoxia-related pregnancy disorders.
Keywords: BCRP, ABCG2, placenta, transporter, hypoxia
Graphical abstract
Introduction
The breast cancer resistance protein (BCRP/ABCG2) is an efflux transporter that is highly expressed on the apical membrane of placenta syncytiotrophoblasts and fetal endothelial cells [1, 2]. BCRP actively transports xenobiotics and endogenous chemicals away from the fetus and into the maternal circulation [3]. BCRP substrates include many compounds that may be relevant during pregnancy such as the gestational diabetes drug glyburide, the antibiotic nitrofurantoin, the acid reflux medication cimetidine, and the dietary phytoestrogen genistein [4-7]. Mouse fetuses lacking Bcrp expression in the placenta exhibit elevated concentrations of glyburide, nitrofurantoin, and genistein [4-7]. In addition to serving as a key component of the blood-placental barrier, BCRP critically prevents cytokine-induced apoptosis and enhances syncytial formation in placental cells [12, 13]. Reductions in BCRP expression lead to greater cytokine-induced damage and lower expression of human chorionic gonadotropin beta and syncytin-1 [12, 13]. Due to the important role of BCRP in maintaining placental health and protecting the fetus from xenobiotic exposure, it is critical to understand factors and conditions that may compromise its function.
While the placental environment is maintained under low oxygen tension relative to the maternal circulation throughout gestation the influence of hypoxia on placental BCRP expression remains unclear. Importantly, oxygen tension is quite low in the first trimester and increases during the 2nd and 3rd trimesters (1st trimester: 2-3% O2, ~20 mmHg; 2nd trimester: 8-10% O2, ~60 mmHg, 3rd trimester 40-60 mmHg, 5-8% O2) [8]. A previous study has demonstrated an up-regulation of Bcrp protein expression in mouse hematopoietic stem cells under hypoxic conditions following direct transactivation by the hypoxia inducible factor-1 alpha (Hif-1α) transcription factor [9]. Furthermore, a direct relationship between Hif-1α and Bcrp mRNA levels has been reported in mouse placentas [10]. However, more recent evidence suggests that hypoxia may have the opposite effect in the human placenta. In fact, expression of BCRP mRNA was shown to be directly related to oxygen levels in human 1st trimester placental explants, such that lower BCRP expression was observed in explants exposed to 3% O2 as compared to greater expression at 20% O2 [11]. These divergent results suggest that additional mediators may be involved in the regulation of BCRP in the human placenta during hypoxia.
In addition to HIF-1α, a number of transcription factors have been shown to directly bind to the promoter and transactivate the ABCG2 gene that encodes the BCRP protein leading to increased mRNA expression. Of these factors, those expressed in placenta include the aryl hydrocarbon receptor (AHR) [12, 13], estrogen receptor-α (ERα) [14, 15], nuclear factor (erythroid-derived 2)-like 2 (NRF2) [16, 17], peroxisome proliferator-activated receptors (PPAR) [18, 19], and retinoid X receptor-α (RXRα). More recent studies into the dynamic mechanisms regulating BCRP mRNA have demonstrated that levels can also be inversely related to the expression of certain miRNAs, including miR-519c, miR-520h, and miR-328, that target the ABCG2 gene [20, 21]. Specifically, methylation of the miR-328 5’-flanking region correlated negatively with miR-328 levels and positively with BCRP mRNA expression in human placentas [22]. These pathways provide potential mechanisms by which hypoxia could alter BCRP expression.
There are a number of experimental approaches to study hypoxia-related signaling. For example, the hypoxia mimetic cobalt chloride (CoCl2) has been shown to prevent the breakdown of HIF-1α protein and enhance the expression of target genes such as the glucose transporter 1 (GLUT1) in placental choriocarcinoma BeWo cells [23, 24]. Similar to CoCl2, exposure of BeWo cells to 1-5% O2 increases expression of GLUT1 protein [24]. Analogous results have been observed in vivo from placentas obtained from healthy pregnant women at different elevations. Interestingly, placentas from women living at high altitudes (3100m in Colorado) exhibited higher HIF-1α protein expression and the neonates had reduced birth weights compared to those at moderate altitudes (1600m in Colorado) [25]. Across both altitudes HIF-1α protein levels were strongly, negatively associated with the placental:birth weight ratio, indicating that greater HIF-1α stimulation is related to lower placental efficiency, i.e. a larger placenta is required to sustain a certain level of fetal growth where HIF-1α levels are elevated.
Using complementary models of placental hypoxia, the purpose of this study was to 1) characterize the effect of HIF-1α activation on BCRP expression and function in human placental BeWo cells, 2) identify potential regulatory factors controlling BCRP expression during hypoxia, and 3) quantify BCRP protein expression during chronic hypoxia using maternal-facing microvillus membranes from healthy, term human placentas from high altitude pregnancies.
Materials and Methods
Chemicals
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
BeWo cell culture
The BeWo choriocarcinoma cell line was maintained in DMEM: F-12 containing 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA) and 1% penicillin-streptomycin at 37°C and 5% CO2 (atmospheric O2, ~20%). CoCl2 Studies. BeWo cells were cultured in DMEM:F-12 with 0.5% bovine serum albumin, 5mM glucose and 1% penicillin-streptomycin 24 h prior to CoCl2 treatment [24]. Cells were then treated with CoCl2 (200 µM) for 24 to 48 h. Hypoxia Chamber Studies. BeWo cells in normal culture media were placed in the Modular Incubator Chamber (MIC-101) (Billups-Rothenberg, Inc, Del Mar, CA) and gas (3% O2, 5% CO2) was flushed through the chamber at a regulated flow rate of 25-50 liters/min for 6 min in order to obtain a 100% exchange of gases. After flushing was completed, the chamber was placed into the cell culture incubator at 37°C for 24 h. A separate set of BeWo cells were placed into the same cell culture incubator and incubated for 24 h under standard culture conditions (37°C, 20% O2, 5% CO2).
Placental microvillous membranes
Placenta microvillous membranes were prepared from frozen tissue as described [26]. The high altitude samples (3100 m, Leadville, CO) were previously characterized for markers of hypoxia compared to the moderate altitude samples (1600 m, Denver, CO) [26]. The current study was approved by the Rutgers Institutional Review Board (exempt protocol E15-729). Subjects included n=8 from the moderate altitude (n=2 male, n=6 female) and n=7 from the high altitude (n=2 male, n=5 female). The race of all mothers was Caucasian. The race of fathers was Caucasian with two fathers in the high altitude group who identified their ethnicity as Hispanic. Both groups were similar with regards to maternal age (range 18-36 y, p=0.783), pre-pregnancy BMI (range 19.4-25.7, p=0.530), weight gain during pregnancy (range 8.2-24 kg, p= 0.624), and gestational age at delivery (range 37.1-41.1 weeks, p=0.797).
Alamar Blue Assay
BeWo cell viability was assessed using the Alamar Blue Assay (Life Technologies) with fluorescence detection using a Spectramax M5 plate reader (Molecular Devices, Sunnyvale, CA).
HIF-1α ELISA
The HIF-1alpha Human SimpleStep ELISA Kit (Abcam, Cambridge, MA) was used.
RNA isolation and real-time quantitative PCR
RNA was isolated and qPCR performed as previously described [27]. Stem-loop reverse transcription real-time qPCR for microRNA (miRNA) was performed using the primers and methods as reported [20]. Primer sequences are listed in Supplemental Table 1. Cycle threshold (Ct) values were first converted to delta Ct values by comparing to the reference genes, RPL13A (for mRNA) and U6 (for miRNA) and then to delta delta Ct values by comparing to the respective control-treated cells [28].
Western blot analysis
Proteins from cell homogenates (10µg) or placental microvillous membranes (15µg) were separated on SDS-polyacrylamide 4-12% Bis-Tris gels (Life Technologies) by electrophoresis [27]. The following antibodies were used: BCRP (BXP-53 Enzo LifeSciences, Farmingdale, NY, 1:5000), GLUT1 (Ab652 Abcam, Cambridge, MA, 1:1000) and β-ACTIN (Ab8227 Abcam, 1:2000). BCRP and GLUT1 protein levels in BeWo cells were normalized to β-Actin levels. Due to changes in protein loading controls in the high altitude placentas, raw luminescence values for BCRP and GLUT1 staining were presented after confirming equal protein loading with Coomassie blue dye.
Hoechst 33342 retention assay
Transporter function was quantified using a Nexcelom Cellometer fluorescent cell counter (Lawrence, MA) and the fluorescent substrate Hoechst 33342 (5 μM) as previously described [29].
Statistical Analysis
Data are expressed as mean ± SE and analyzed using Graphpad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA). One-way ANOVA followed by a Newman-Keuls multiple comparison post hoc test was used when comparing 3 or more groups, while an unpaired Student’s t-test was used when comparing 2 groups. Statistical significance was set at p<0.05.
Results
Regulation of HIF-1α signaling and BCRP expression by the hypoxia mimetic CoCl2 in BeWo cells
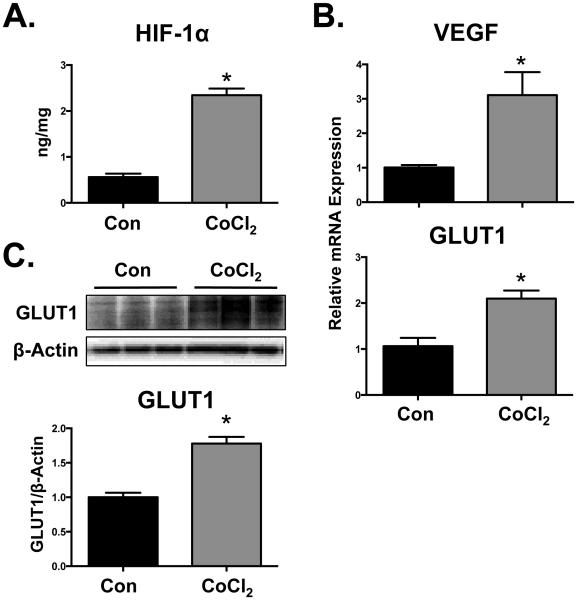
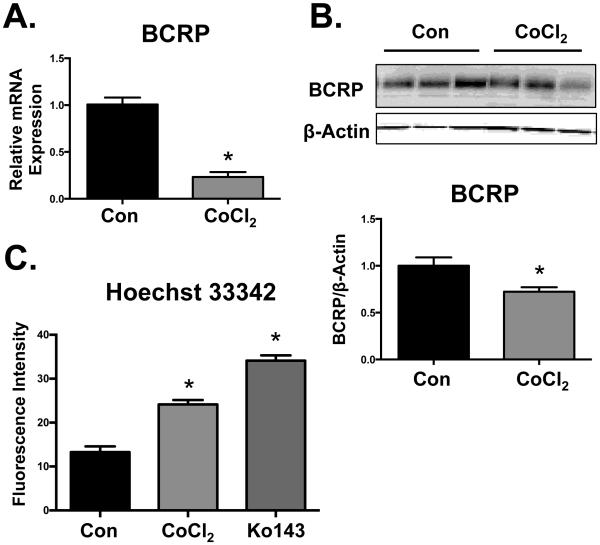
Treatment of BeWo cells with CoCl2 (200 μM) increased total cellular HIF-1α protein at 24 h (4-fold) (Fig. 1A) compared to control cells. Prototypical HIF-1α target genes, VEGF and GLUT1, as well as GLUT1 protein were also induced by CoCl2 at 48 h (Fig. 1B and 1 C). Conversely, CoCl2 treatment reduced BCRP mRNA and protein expression at 48 h to 25% and 70% of control levels, respectively (Fig. 2A and B). Reduced BCRP protein expression in BeWo cells corresponded with impaired transport activity as evidenced by a 2-fold increase in the cellular retention of the fluorescent BCRP substrate, Hoechst 33342 (Fig. 2C). The BCRP inhibitor Ko143 (1 µM) was used as a positive control and increased Hoechst 33342 retention 3-fold. It should be noted that the concentration of CoCl2 used in these studies did not alter cell viability at 48 h as determined by the Alamar Blue Assay (data not shown).
Fig. 1. Activation of HIF-1α signaling in BeWo cells by CoCl2.
Following treatment of BeWo cells with CoCl2 (200 µM) (A) HIF-1α protein levels were quantified by ELISA at 24 h. (B) HIF-1α target gene (VEGF and GLUT1) expression was assessed at 48 h by qPCR and normalized to RPL13A. (C) GLUT1 protein expression was determined by western blot and β-ACTIN was used as a loading control. Data are presented as mean ± SE (n=3-4). Asterisks (*) represent statistically significant differences (p<0.05) compared to control cells (Con).
Fig. 2. Down-regulation of BCRP in BeWo cells by CoCl2.
BeWo cells were treated with CoCl2 (200 µM) for 48 h, following which they were processed for (A) BCRP and RPL13A mRNA expression by qPCR. (B) Protein expression of BCRP was determined by western blot and β-ACTIN was used as a loading control. (C) BCRP function was assessed by measuring the cellular retention of Hoechst 33342 (5 µM) in the presence or absence of the BCRP-specific inhibitor (1 μM Ko143). Intracellular fluorescence was quantified by a Cellometer Vision automated cell counter. Data are presented as mean ± SE (n=3-4). Asterisks (*) represent statistically significant differences (p< 0.05) compared to control cells (Con).
Regulation of HIF-1α signaling and BCRP expression by low oxygen content (3% O2) in BeWo cells
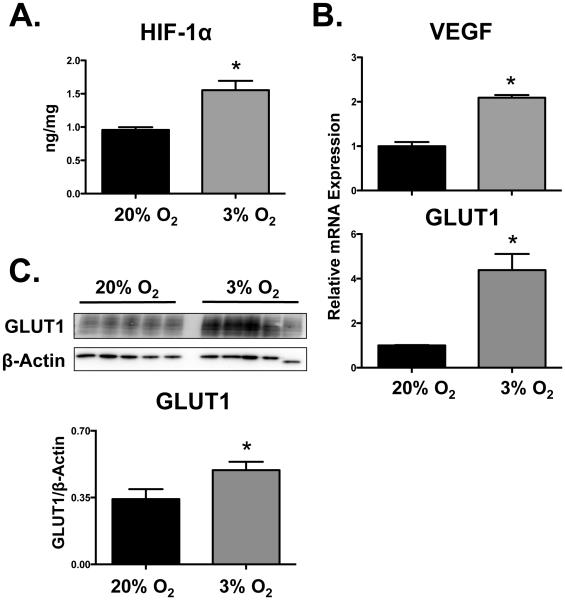
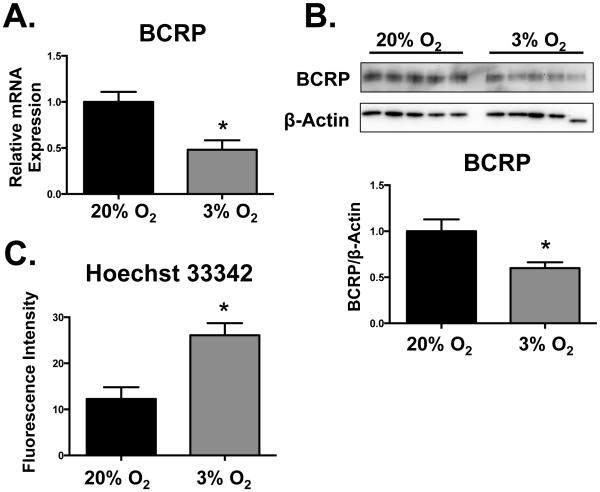
Compared to standard culture conditions (20% O2), exposure of BeWo cells to low oxygen levels (3% O2) for 24 h increased HIF-1α protein levels by 50% (Fig. 3A). Corresponding increases in VEGF and GLUT1 mRNAs (2- and 4-fold, respectively) as well as GLUT1 protein were observed in cells exposed to 3% O2 (Fig. 3B and 3C). Low oxygen levels (3% O2, 24 h) down-regulated BCRP mRNA and protein by 60% and 40%, respectively (Figs. 4A and B). Consequently, BCRP function was reduced by 3% O2 as demonstrated by a 2-fold increase in the cellular retention of Hoechst 33342 (Fig. 4C).
Fig. 3. Activation of HIF-1α signaling in BeWo cells by hypoxia.
Following 24 h exposure of BeWo cells to hypoxia (3% O2), (A) HIF-1α protein levels were quantified by ELISA. (B) HIF-1α target gene (VEGF and GLUT1) expression was assessed by qPCR and normalized to RPL13A. (C) GLUT1 protein expression was determined by western blot and β-ACTIN was used as a loading control. Data are presented as mean ± SE (n=3-4). Asterisks (*) represent statistically significant differences (p<0.05) compared to 20% O2 cells.
Fig. 4. Down-regulation of BCRP in BeWo cells by hypoxia.
BeWo cells were exposed to hypoxia (3% O2) for 24 h, following which the cells were processed for (A) BCRP and RPL13A mRNA expression by qPCR. (B) Protein expression of BCRP was determined by western blot and β-ACTIN was used as a loading control. (C) BCRP function was assessed by measuring the cellular retention of Hoechst 33342 (5 µM). Intracellular fluorescence was quantified by a Cellometer Vision automated cell counter. Data are presented as mean ± SE (n=3-4). Asterisks (*) represent statistically significant differences (p< 0.05) compared to 20% O2 cells.
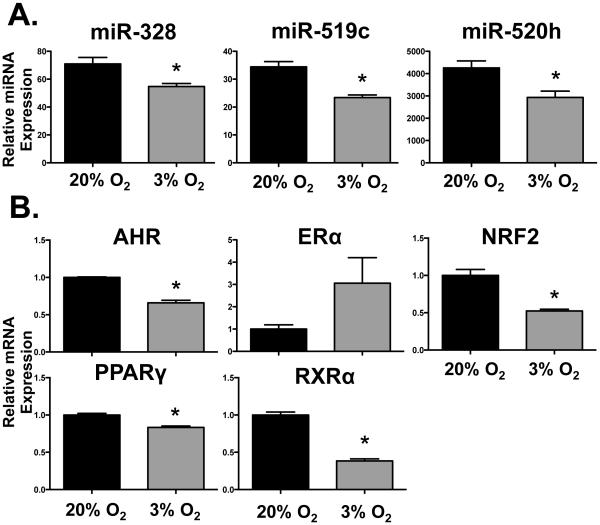
To elucidate a potential mechanism for the reduction of BCRP expression following activation of the HIF-1α pathway, we quantified the expression of critical miRNAs and transcription factors. All three BCRP regulatory miRNAs were reduced by 25 to 30% in response to hypoxia (Fig. 5A) suggesting that they were not responsible for the decrease in BCRP expression. By comparison, exposure of BeWo cells to 3% O2 for 24 h significantly lowered the mRNA expression of AHR, NRF2, PPARγ and RXRα between 20-60% (Fig. 5B). Meanwhile, there was a trend for a 3-fold induction of ERα mRNA in response to 3% O2 (Fig. 5B).
Fig. 5. Effect of hypoxia on BCRP-related miRNA and transcription factor mRNA expression in BeWo cells.
Following 24 h exposure of BeWo cells to hypoxia (3% O2), (A) BCRP-targeted miRNA (miR-328, −519c, and −520) expression and (B) mRNA expression of transcription factors (AhR, ERα, NRF2, PPARγ, RXRα) was determined by qPCR. miRNAs were normalized to U6 and mRNAs were normalized to RPL13A. Data are presented as mean ± SE (n=3-5). Asterisks (*) represent statistically significant differences (p< 0.05) compared to 20% O2 cells.
Regulation of GLUT1 and BCRP expression in placentas from a high altitude
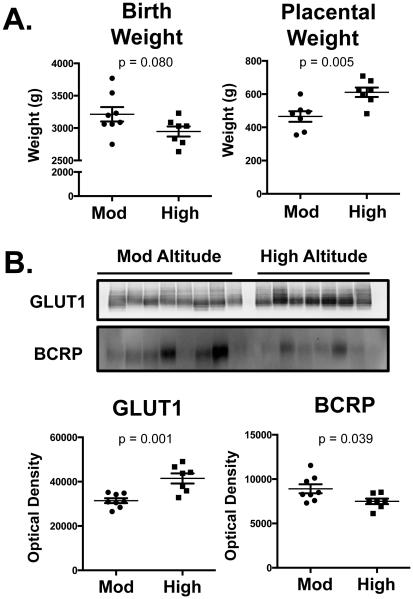
Placentas were obtained from women at moderate (1600m, n=8) and high altitudes (3100 m, n=7) as previously described [26]. Placentas from the 3100m altitude exhibited greater weights compared to the moderate altitude placentas (Fig. 6A) and infants born at the higher altitude tended to have reduced birth weights similar to published findings [26]. Western blot analysis of microvillous membranes revealed that placentas from a high altitude (3100 m) exhibited 30% higher GLUT1 protein expression than moderate altitude (1600 m) placentas (Fig. 6B). Additionally, BCRP protein expression was reduced by approximately 20% in high altitude (3100 m) placentas as compared to those from a moderate altitude (1600 m).
Fig. 6. Regulation of BCRP and GLUT1 expression in high altitude placentas.
In microvillous membranes isolated from placentas from women living at a moderate (Mod, 1600 m) or high altitude (3100 m), GLUT1 and BCRP protein expression was determined by western blot. Equal protein loading was confirmed after staining western blots with Coomassie blue dye. Data are presented as mean ± SE (n=7-9) Asterisks (*) represent statistically significant differences (p <0.05) compared to moderate altitude samples (Mod).
Discussion
The current study characterized the influence of hypoxic conditions on placental BCRP expression and function. Treatment of BeWo cells with CoCl2 or 3% O2 activated the HIF-1α transcription factor pathway, while reducing BCRP expression and function. Altered expression of known transcription factors following 3% O2 exposure, likely underlie the hypoxia-mediated down-regulation of BCRP in placental cells. Finally, microvillous membranes isolated from term placentas of high altitude pregnancies (3100 m), exhibited reduced levels of BCRP concurrently with the up-regulation of the HIF-1α target, GLUT1. Our data shed light on the regulation of the human placental BCRP transporter by hypoxia, which may be important in hypoxia-related pathologies of pregnancy such as preeclampsia and growth restriction.
Proper human placental development is dependent upon tightly-regulated oxygen concentrations [30, 31]. Through the 1st trimester, placental invasion occurs at a low oxygen tension (~20 mmHg, 2-3% O2) as the uterine spiral arterioles are plugged by extravillous trophoblasts. During this period, the HIF-1α protein is preferentially located in villous cytotrophoblasts, as syncytiotrophoblasts contain the von Hippel-Lindau tumor suppressor protein, which ubiquitinates HIF-1α and targets it for proteasomal degradation [32]. By weeks 10 to 12, maternal blood flow to the placenta is established, thereby increasing the placental oxygen tension (~55 mmHg, 8% to 10% O2). Immunohistochemical analysis suggests that BCRP staining is less intense in cytotrophoblasts and syncytiotrophoblasts from 1st trimester placentas than in term placentas [11]. Western blot analysis has also revealed lower BCRP protein expression earlier in pregnancy compared to term in whole tissue homogenates [33]. Others have reported variable BCRP expression between placentas that precluded analysis of gestational age-related changes [34]. Nonetheless, these data generally support a trend for lower BCRP protein levels early in gestation when oxygen levels are low which is consistent with our observations that activation of HIF-1α is inversely related to the expression of BCRP in placental cells.
More recent work has begun to address the ability of hypoxia to regulate BCRP expression in placental explants. Exposure of 1st trimester explants to varying levels of O2 (3, 8 and 20%) revealed that higher O2 tension was associated with greater BCRP mRNA expression [11]. These data are consistent with the findings in the current study. However, immunohistochemical analysis demonstrated BCRP protein expression to be up-regulated in 1st trimester explants at low O2 levels [11]. The disconnect between the mRNA and protein findings in this prior study were not clear and may represent an acute stress of explants to the substantial change in oxygen concentrations. Time-dependent differences in acute and chronic responses to hypoxia have been demonstrated previously, operating through distinct signaling pathways [35]. Using term placental explants, the same laboratory demonstrated that low O2 concentrations (3%) had no effect on BCRP mRNA or protein expression, suggesting that responses to hypoxia are dependent upon the stage of placental development [36].
In the present studies, the down-regulation of BCRP in the placenta by hypoxia was somewhat surprising since investigations in other cell types and tissues demonstrated that HIF-1α can directly transactivate the human ABCG2 gene at specific response elements (−1059 to −1055 bp, −194 bp to −190 bp, and −116 to −112 bp) [9, 37]. Because of this disconnect, we explored the expression of transcriptional and epigenetic pathways that could explain BCRP down-regulation in placental cells during hypoxia. We anticipated the up-regulation of miR-328, −519c, and −520h, which could lead to BCRP mRNA; however, the opposite was observed suggesting that altered miRNA regulation was an unlikely mechanism in the current study.
Transcription factors directly regulate BCRP expression through activation of response elements in the promoter of the ABCG2 gene. These factors include NRF2 [16, 17], AHR [12, 13], PPARγ [18, 19], ERα [14, 15], as well as RXRα, a common nuclear receptor partner for activated transcription factors. Interestingly, the mRNA expression of these transcription factors, with the exception of ERα, decreased in response to 3% O2 and provide candidate regulators that may underlie the down-regulation of BCRP during hypoxia. Prior work by Wang et al. has demonstrated that mRNA levels of Bcrp in mouse placentas correlate with a number of transcription factors including HIF-1α, AhR and ERβ [10]. While these data infer a relationship, the ability of some transcription factors such as HIF-1α to regulate expression of a transgene is dependent upon activation of the signaling pathway rather than the relative abundance of mRNAs. Notably, when trophoectoderm cells were exposed to low oxygen conditions, HIF-1α protein was increased but no change in mRNA expression was observed suggesting little dependence upon transcriptional regulation [38]. Like Wang et al., we have observed a coordinated regulation of AHR and BCRP mRNAs. Both genes declined in response to hypoxia in BeWo cells as well as explants obtained from third trimester healthy placentas (data not shown). It is important to note that while Wang et al. observed a correlation between AhR and Bcrp mRNAs in mice, prior work has shown that pharmacological activation of AhR does not alter Abcg2 transcription in mice (including placenta) whereas the human AHR receptor can transactivate the ABCG2 gene and increase expression [39]. Supporting AHR as a mediator of BCRP down-regulation, it is known that hypoxia reduces the transcriptional activity of AHR [40, 41] in part due to competition for the common heterodimer partner, aryl hydrocarbon receptor nuclear translocator [40]. Therefore, dysregulation of placental AHR signaling by HIF-1α activation is a likely mechanism for the decline in BCRP expression and function in BeWo cells.
A number of gestational disorders that are complicated by low oxygen tension result in reduced placental BCRP expression. For example, BCRP expression was reduced in placentas from preeclamptic pregnancies that were accompanied by HELLP syndrome [42]. This finding corresponded with a significant decrease in birth weight (~25%) in infants born to mothers with preeclampsia and HELLP. Likewise, levels of BCRP mRNA were reduced ~40% in placentas from pregnancies affected by fetal growth restriction in the absence of preeclampsia compared to placentas from gestational age matched controls [43]. The authors postulated that reduced BCRP expression in fetal growth restriction may sensitize the placenta to increased apoptosis due to altered responses to cytokines. The general association between reduced birth weight and lower BCRP expression was also observed in the current study investigating healthy placentas from high altitude that experience preplacental hypoxia in the absence of pathological disease. Taken together, these findings suggest that activation of human HIF-1α signaling reduces placental BCRP expression in vitro and in vivo likely by dysregulating critical transcriptional pathways, such as AHR.
Supplementary Material
Highlights.
Cobalt chloride and low oxygen activate HIF-1α signaling in BeWo placental cells.
BCRP expression and function are reduced by HIF-1α signaling in BeWo placental cells.
BCRP protein expression is reduced in placentas from high altitude pregnancies.
Down-regulation of BCRP in the placenta may increase fetal exposure to chemicals.
Acknowledgments
Funding
This work was supported by the National Institutes of Environmental Health Sciences [Grants ES020522, ES005022, ES007148], a component of the National Institutes of Health. Kristin Bircsak was supported by predoctoral fellowships from the American Foundation for Pharmaceutical Education and Pharmaceutical Research and Manufacturers of America. High altitude studies were supported by the National Science Foundation [Grant BCS 0309142 to S.Z.], and the National Institute of Child Health and Human Development, a component of the National Institutes of Health [Grants HD042737 to S.Z. and HD046982 to N.I.].
References
- [1].Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58(23):5337–9. [PubMed] [Google Scholar]
- [2].Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–64. [PubMed] [Google Scholar]
- [3].Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, Schinkel AH. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99(24):15649–54. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gedeon C, Behravan J, Koren G, Piquette-Miller M. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta. 2006;27(11-12):1096–102. doi: 10.1016/j.placenta.2005.11.012. [DOI] [PubMed] [Google Scholar]
- [5].Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Molecular pharmacology. 2005;67(5):1758–64. doi: 10.1124/mol.104.010439. [DOI] [PubMed] [Google Scholar]
- [6].Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH. Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther. 2005;312(1):144–52. doi: 10.1124/jpet.104.073916. [DOI] [PubMed] [Google Scholar]
- [7].Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Molecular pharmacology. 2007;72(4):967–75. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- [8].Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstetrics and gynecology. 1992;80(2):283–5. [PubMed] [Google Scholar]
- [9].Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279(23):24218–25. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- [10].Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, Unadkat JD, Mao Q. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors, American journal of physiology. Endocrinology and metabolism. 2006;291(6):E1295–304. doi: 10.1152/ajpendo.00193.2006. [DOI] [PubMed] [Google Scholar]
- [11].Lye P, Bloise E, Dunk C, Javam M, Gibb W, Lye SJ, Matthews SG. Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta. 2013;34(9):817–23. doi: 10.1016/j.placenta.2013.05.010. [DOI] [PubMed] [Google Scholar]
- [12].Tompkins LM, Li H, Li L, Lynch C, Xie Y, Nakanishi T, Ross DD, Wang H. A novel xenobiotic responsive element regulated by aryl hydrocarbon receptor is involved in the induction of BCRP/ABCG2 in LS174T cells. Biochem Pharmacol. 2010;80(11):1754–61. doi: 10.1016/j.bcp.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26(10):1754–63. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- [14].Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, Mao Q. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am J Physiol Endocrinol Metab. 2006;290(5):E798–807. doi: 10.1152/ajpendo.00397.2005. [DOI] [PubMed] [Google Scholar]
- [15].Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer research. 2004;64(4):1247–51. doi: 10.1158/0008-5472.can-03-3583. [DOI] [PubMed] [Google Scholar]
- [16].Singh A, Wu H, Zhang P, Happel C, Ma J, Biswal S. Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Molecular cancer therapeutics. 2010;9(8):2365–76. doi: 10.1158/1535-7163.MCT-10-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hagiya Y, Adachi T, Ogura S, An R, Tamura A, Nakagawa H, Okura I, Mochizuki T, Ishikawa T. Nrf2-dependent induction of human ABC transporter ABCG2 and heme oxygenase-1 in HepG2 cells by photoactivation of porphyrins: biochemical implications for cancer cell response to photodynamic therapy. J Exp Ther Oncol. 2008;7(2):153–67. [PubMed] [Google Scholar]
- [18].Hoque MT, Shah A, More V, Miller DS, Bendayan R. In vivo and ex vivo regulation of breast cancer resistant protein (Bcrp) by peroxisome proliferator-activated receptor alpha (Pparalpha) at the blood-brain barrier. J Neurochem. 2015;135(6):1113–22. doi: 10.1111/jnc.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, Jeney V, Ozvegy-Laczka C, Szanto A, Barta E, Balla J, Sarkadi B, Nagy L. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem. 2006;281(33):23812–23. doi: 10.1074/jbc.M604890200. [DOI] [PubMed] [Google Scholar]
- [20].Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328, −519c and −520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol. 2011;81(6):783–92. doi: 10.1016/j.bcp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang F, Xue X, Wei J, An Y, Yao J, Cai H, Wu J, Dai C, Qian Z, Xu Z, Miao Y. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103(4):567–74. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [22].Saito J, Hirota T, Furuta S, Kobayashi D, Takane H, Ieiri I. Association between DNA methylation in the miR-328 5'-flanking region and inter-individual differences in miR-328 and BCRP expression in human placenta. PLoS One. 2013;8(8):e72906. doi: 10.1371/journal.pone.0072906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. The Journal of endocrinology. 2004;183(1):145–54. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- [24].Baumann MU, Zamudio S, Illsley NP. Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1, American journal of physiology. Cell physiology. 2007;293(1):C477–85. doi: 10.1152/ajpcell.00075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, Post M, Illsley NP, Caniggia I. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. The American journal of pathology. 2007;170(6):2171–9. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta. 2006;27(1):49–55. doi: 10.1016/j.placenta.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bircsak KM, Gupta V, Yuen PY, Gorczyca L, Weinberger BI, Vetrano AM, Aleksunes LM. Genetic and Dietary Regulation of Glyburide Efflux by the Human Placental Breast Cancer Resistance Protein Transporter. J Pharmacol Exp Ther. 2016;357(1):103–13. doi: 10.1124/jpet.115.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [29].Bircsak KM, Gibson CJ, Robey RW, Aleksunes LM. Assessment of drug transporter function using fluorescent cell imaging, Current protocols in toxicology / editorial board. Mahin D. Maines. 2013;57:6. doi: 10.1002/0471140856.tx2306s57. Unit 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–72. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- [31].Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- [32].Ietta F, Wu Y, Winter J, Xu J, Wang J, Post M, Caniggia I. Dynamic HIF1A regulation during human placental development. Biology of reproduction. 2006;75(1):112–21. doi: 10.1095/biolreprod.106.051557. [DOI] [PubMed] [Google Scholar]
- [33].Yeboah D, Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Canadian journal of physiology and pharmacology. 2006;84(12):1251–8. doi: 10.1139/y06-078. [DOI] [PubMed] [Google Scholar]
- [34].Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages, American journal of physiology. Regulatory, integrative and comparative physiology. 2005;289(4):R963–9. doi: 10.1152/ajpregu.00173.2005. [DOI] [PubMed] [Google Scholar]
- [35].Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW, Lambin P, Koumenis C, Sonenberg N, Wouters BG. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25(5):1114–25. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Javam M, Audette MC, Iqbal M, Bloise E, Gibb W, Matthews SG. Effect of oxygen on multidrug resistance in term human placenta. Placenta. 2014;35(5):324–30. doi: 10.1016/j.placenta.2014.02.010. [DOI] [PubMed] [Google Scholar]
- [37].Cheng GM, To KK. Adverse Cell Culture Conditions Mimicking the Tumor Microenvironment Upregulate ABCG2 to Mediate Multidrug Resistance and a More Malignant Phenotype. ISRN Oncol. 2012;2012:746025. doi: 10.5402/2012/746025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jeong W, Bazer FW, Song G, Kim J. Expression of hypoxia-inducible factor-1 by trophectoderm cells in response to hypoxia and epidermal growth factor. Biochem Biophys Res Commun. 2016;469(2):176–82. doi: 10.1016/j.bbrc.2015.11.091. [DOI] [PubMed] [Google Scholar]
- [39].Tan KP, Wang B, Yang M, Boutros PC, Macaulay J, Xu H, Chuang AI, Kosuge K, Yamamoto M, Takahashi S, Wu AM, Ross DD, Harper PA, Ito S. Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2) Mol Pharmacol. 2010;78(2):175–85. doi: 10.1124/mol.110.065078. [DOI] [PubMed] [Google Scholar]
- [40].Vorrink SU, Severson PL, Kulak MV, Futscher BW, Domann FE. Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines. Toxicol Appl Pharmacol. 2014;274(3):408–16. doi: 10.1016/j.taap.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Khan S, Liu S, Stoner M, Safe S. Cobaltous chloride and hypoxia inhibit aryl hydrocarbon receptor-mediated responses in breast cancer cells. Toxicol Appl Pharmacol. 2007;223(1):28–38. doi: 10.1016/j.taap.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jebbink J, Veenboer G, Boussata S, Keijser R, Kremer AE, Elferink RO, van der Post J, Afink G, Ris-Stalpers C. Total bile acids in the maternal and fetal compartment in relation to placental ABCG2 expression in preeclamptic pregnancies complicated by HELLP syndrome. Biochim Biophys Acta. 2015;1852(1):131–6. doi: 10.1016/j.bbadis.2014.11.008. [DOI] [PubMed] [Google Scholar]
- [43].Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, Keelan JA. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB J. 2007;21(13):3592–605. doi: 10.1096/fj.07-8688com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.