Abstract
To more fully characterize the clinical and pathological spectrum of a recently described tumor entity of the sinonasal tract characterized by loss of nuclear expression of SMARCB1 (INI1), we analyzed 39 SMARCB1-deficient sinonasal carcinomas collected from multiple medical centers. The tumors affected 23 males and 16 females with an age range of 19 to 89 years (median, 52). All patients presented with locally advanced disease (T3, n=5; T4, n=27) involving the sinuses (mainly ethmoid) with variable involvement of the nasal cavity. Thirty patients received surgery and/or radiochemotherapy with curative intent. At last follow-up, 56% of patients died of disease 0 to 102 months after diagnosis (median, 15), 2 were alive with disease, and 1 died of an unrelated cause. Only 9 patients (30%) were alive without disease at last follow-up (range, 11-115 months; median, 26). The original diagnosis of retrospectively identified cases was most often sinonasal undifferentiated carcinoma (n=14) and non-keratinizing/basaloid squamous cell carcinoma (n=5). Histologically, most tumors displayed either a predominantly basaloid (61%) or plasmacytoid/rhabdoid morphology (36%). The plasmacytoid/rhabdoid form consisted of sheets of tumor cells with abundant, eccentrically placed eosinophilic cytoplasm, while similar cells were typically rare and singly distributed in the basaloid variant. Glandular differentiation was seen in a few tumors. None of the cases showed squamous differentiation or surface dysplasia. By immunohistochemistry, the tumors were positive for pancytokeratin (97%), CK5 (64%), p63 (55%) and CK7 (48%); and they were negative for NUT (0%). Epstein-Barr virus and high risk human papillomavirus was not detected by in situ hybridization. Immunohistochemical loss of SMARCB1 (INI1) expression was confirmed for all 39 tumors. Investigation of other proteins in the SWI/SNF complex revealed co-loss of SMARCA2 in 4 cases, but none were SMARCA4- or ARID1A-deficient. Of 27 tumors with SMARCB1 FISH analysis, 14 showed homozygous (biallelic) deletions and 7 showed heterozygous (monoallelic) deletions. SMARCB1-deficient sinonasal carcinoma represents an emerging poorly differentiated/undifferentiated sinonasal carcinoma that 1) cannot be better classified as another specific tumor type, 2) has consistent histopathological findings (albeit with some variability) with varying proportions of plasmacytoid/rhabdoid cells, and 3) demonstrates an aggressive clinical course. This entity should be considered in any difficult-to-classify sinonasal carcinoma, as correct diagnosis will be mandatory for optimizing therapy and for further delineation of this likely underdiagnosed disease.
Keywords: SMARCB1-deficient sinonasal carcinoma, rhabdoid carcinoma, basaloid carcinoma, SMARCB1, INI1, sinonasal tract, SMARCA2, SMARCA4, ARID1A
INTRODUCTION
Sinonasal tract malignancies are uncommon, representing no more than 5% of all head and neck cancers.1,2 Several recent studies and reviews have emphasized the propensity of this relatively small anatomic area of the body to develop a plethora of histogenetically and biologically distinctive, but morphologically highly overlapping neoplasms.3,4 Since the original description of sinonasal undifferentiated carcinoma (SNUC) as a distinctive and highly aggressive sinonasal carcinoma5, advancing molecular biology techniques have permitted more precise tumor classification based on recurring biological and genetic alterations.6 Consequently, the group of SNUCs has been diminishing as new specific entities have emerged including NUT-rearranged carcinoma7,8, HPV-related adenoid cystic-like carcinoma9,10, and adamantinoma-like Ewing sarcoma.11
In 2014, Agaimy et al12 and Bishop et al13 independently described a variant of sinonasal carcinoma characterized by loss of nuclear SMARCB1 expression. Since those initial descriptions, only two additional small series and a few case reports have been published on SMARCB1-deficient sinonasal carcinomas.14-20, To more fully characterize the nature of this tumor type including its complete morphologic spectrum, its clinical behavior and its biology, we updated our previously reported experience and prospectively collected new cases from our own practices and from multiple other institutions.
MATERIALS AND METHODS
The study received Johns Hopkins institutional review board approval (IRB00096402) and the ethical vota for retrospective translational research studies of the FAU, Erlangen, Germany. The cases consisted of tumors retrieved from the routine surgical pathology files and contributed to the consultation files of the Institute of Pathology, University Hospital of Erlangen, Germany and the Pathology Department at The Johns Hopkins University. Of these, 11 cases were reported in the original descriptions of the entity but follow-up was updated, additional immunohistochemistry was performed, and missing molecular studies were completed. In total, 28 of the 39 cases had not been previously published.
Tumor specimens were fixed in buffered formalin and embedded for routine histological examination. Immunohistochemical studies were performed on 3-μm sections cut from paraffin blocks using a fully automated system (“Benchmark XT System”, Ventana Medical Systems Inc, 1910 Innovation Park Drive, Tucson, Arizona, USA) and the following antibodies: pancytokeratin (clone AE1/AE3, 1:40, Zytomed, Berlin, Germany), CK7 (OV-TL, 1:1000, Biogenex), p63 (4A4, 1:100, Zytomed), CK5 (clone XM26, 1: 50, Zytomed), chromogranin A (clone LK2H10, 1:500, Beckman-Coulter GmbH), synaptophysin (clone SY38, 1:50, Dako), CD56 (clone MRQ-42, 1:100, CELL MARQUE), CD117 (polyclonal rabbit antibody, 1:100; Dako), p16 (clone JC8, 1:100, Santa Cruz Biotechnology), anti-NUT (clone C52B1, 1:45, Cell Signaling), SMARCB1 (INI1) (MRQ-27, 1:50, Zytomed), SMARCA2 (polyclonal antibody, 1:100, Atlas Antibodies AB, Stockholm, Sweden), SMARCA4 (anti-BRG1 antibody, clone EPNCIR111A, 1:100, Abcam; Cambridge, UK) and ARID1A (rabbit polyclonal antibody, ab97995, 1:100; Abcam). Epstein Barr virus (EBV) in-situ hybridization (EBER 1/2 probes, ZytoVision, Bremerhaven, Germany) was performed according to the manufacturer instructions. Human papillomavirus (HPV) testing was performed using either PCR-based method or RNA in-situ hybridization (ISH) by the RNAscope method as detailed previously.12,13 Assessment of the staining results of the SWI/SNF components was done as recently described21, i.e. only the nuclei of viable tumor tissue (away from necrotic areas) were assessed. As a control, the presence of a homogeneous strong nuclear staining of stromal fibroblasts, inflammatory cells, vascular endothelial cells or normal epithelial cells in the background was a prerequisite for assessable staining in the tumor. Three staining grades were defined: intact (strong staining in the neoplastic cells that is similar to normal background cells), lost (indicating clean neoplastic cell nuclei as opposed to strong staining in normal cells) and reduced if very weak but still discernible as opposed to strong staining in normal cells). Tumors with an admixture of these three patterns were specifically reported. Cases with absent or very weak staining in the normal background cells were considered equivocal or not assessable (no results=NR).
RESULTS
Demographic and clinical features
The clinicopathological features are summarized in Table 1. The patients with SMARCB1-deficient sinonasal carcinoma included 23 males and 16 females ranging in age from 19 to 89 years (median, 52). The age range was similar for females (21-87; median, 50) and males (19-89; median, 53). Imaging revealed extensive involvement of the paranasal sinuses with or without involvement of the nasal cavity and frequent involvement of the skull base (Fig. 1). The ethmoidal cells were involved in 18 of 39 cases (46%), either isolated or (more frequently) with concurrent involvement of the frontal/sphenoidal sinuses or the nasal cavity. The nasal cavity was affected alone in 8 and with concurrent sinus involvement in 11 patients. Of 33 patients with detailed tumor staging information, 27 (82%) presented with stage T4 disease with extensive involvement of the bony confinements of the sinonasal cavities and variable infiltration into periorbital or skull base tissues. Synchronous regional lymph node involvement and distant metastases were detected in three and two patients respectively.
Table 1.
Clinicopathological features of SMARCB1-deficient sinonasal carcinomas (n=39).
| No | Age/ Sex |
Site | Original diagnosis | Predominant histology |
Initial stage | Treatment | Clinical course (months) | Outcome/ Follow -up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 F | Right anterior ethmoid, both frontal sinuses | basaloid SCC | Basaloid | T4N0M0 | Radical surgery+ CT + XRT | Lung, pericardial & pleural metastases (63) | DOD (93) |
| 2 | 52 M | Left frontal, ethmoid | CA ex Schneiderian papilloma | Plasamacytoid/rhabdoid | T4N2M0 | Radical surgery+ + CT + XRT | Regional node metastases (synchronous + metachronous) | DOD (102) |
| 3 | 28 F | Right frontal, anterior ethmoid | Undifferentiated CA | Basaloid + | T4N0M0 | Radical surgery+ CT + XRT | None | NED (115) |
| 4 | 40 F | Left nasal cavity; middle turbinate + sinuses | Oncocytic & squamoid large cell, nested | Pasamacytoid/rhabdoid | T4N0M0 | Biopsy, CT + XRT | Local recurrence (2) | AWD (24) |
| 5 | 76 F | Sinuses, NS | Anaplastic CA/NKSCC | Pasamacytoid/rhabdoid | T4N0M1 | Surgery, CT + XRT | Synchronous skin metastases bilateral thighs | DOD (4) |
| 6 | 67 F | Right ethmoidal, sphenoidal, frontal sinuses | Anaplastic CA/NKSCC | Basaloid + | T3N0M0 | Surgery + CT + XRT | Distant metastases (site not specified) | DOD (100) |
| 7 | 38 F | Sinuses+ skull base | SNUC | Pasamacytoid/rhabdoid + squamoid | T4N0M0 | Biopsy + CT | Synchronous bone metastases | AWD (4) |
| 8 | 67 M | Sinuses left | NKSCC | Basaloid | T4N+M0 | surgery+ RT, palliative CT | Regional node metastases (0), lung metastasis (9), local recurrence (13) | DOD (22) |
| 9 | 85 M | Left nasal cavity turbinate | SNUC | Basaloid | NA | Biopsy, palliative | Unknown | Unknown |
| 10 | 21 F | Left ethmoid, sphenoid | SNUC | Basaloid | T4N0M0 | Surgery + RT | Unknown | Unknown |
| 11 | 45 M | Left frontal sinus + nasal cavity | poorly differentiated adenocarcinoma | Pasamacytoid/rhabdoid with adenoid features | T4N0M0 | Surgery+ CT + XRT | None | NED (42) |
| 12 | 44 F | Nasal septum invading cribriform plate | SMARCB1-deficient carcinoma | Sarcomatoid, | T4NxM1 | Biopsy, supportive care | Lung, synchronous | DOD post-biopsy |
| 13 | 71 F | Maxillary sinus | Adenocarcinoma NOS | Basaloid, spindled, adenoid | T3 N0M0 | Surgery + XRT | Local recurrence (11) | DOD (15) |
| 14 | 46 F | Frontal and ethmoid sinuses | NKSCC | Basaloid | T4N0M0 | Surgery + XRT | None | NED (57) |
| 15 | 33 M | Nasal cavity, ethmoid sinus, frontal sinus | SNUC | Basaloid | T4N0M0 | Surgery + XRT | Local recurrence (9), distant metastases (19) | DOD (30) |
| 16 | 60 M | Ethmoid sinus | NKSCC | Basaloid | T4N0M0 | Surgery + XRT | None | Died of unclear reasons (10) |
| 17 | 78 F | Nasal cavity, ethmoid sinus | Myoepithelial carcinoma | Pasamacytoid/rhabdoid | T4N0M0 | Surgery | None | NED (26) |
| 18 | 54 F | Nasal cavity, ethmoid & maxillary sinuses | SNUC | Basaloid | T4N0M0 | Surgery+ CT + XRT | Local recurrence (12) | DOD (15) |
| 19 | 77 M | Ethmoid sinus | Myoepithelial carcinoma | Pasamacytoid/rhabdoid | T4N0M0 | Surgery + CT + XRT | Distant metastasis (10) | DOD (17) |
| 20 | 44 M | Ethmoid sinus | NKSCC | Basaloid | T4N0M0 | Surgery + CI + XRT | None | NED (23) |
| 21 | 87 F | Nasal cavity | SNUC | Basaloid | T4NXMX | Surgery + XRI | None | NED (11) |
| 22 | 52 M | Ethmoid sinus, nasal cavity, | SNUC | Basaloid | T4NXMX | Surgery | Local recurrence (10) | DOD (12) |
| 23 | 68 F | Nasal cavity | SMARCB1-deficient carcinoma | Pasamacytoid/rhabdoid | Unknown | Unknown | Unknown | Unknown |
| 24 | 24 M | Frontal sinus | SMARCB1-deficient carcinoma | Basaloid+ | T4NXMX | Surgery + CT + XRT | Local recurrence and distant metastasis (10) | DOD (14) |
| 25 | 21 M | Nasal cavity | SNUC | Basaloid | T4NXMX | Surgery | Persistent disease | DOD (6) |
| 26 | 70 M | Ethmoid sinus, sphenoid sinus | SMARCB1-deficient carcinoma | Basaloid | T4N0M0 | CT | Local progression (1) | Stopped chemo at 1 month – on hospice AWD (9) |
| 27 | 50 M | Nasal cavity, ethmoid sinus | SMARCB1-deficient carcinoma | Pasamacytoid/rhabdoid with focal glandular differentiation | T4NXMX | Surgery | Unknown | Unknown |
| 28 | 24 F | Ethmoid sinus, sphenoid sinus, nasal cavity | SMARCB1-deficient malignant neoplasm | Pasamacytoid/rhabdoid | T4N0M0 | CT | No response to treatment | DOD (1) |
| 29 | 79 M | Nasal cavity growing into anterior cranial fossa | SMARCB1-deficient carcinoma | Pasamacytoid/rhabdoid | T4N0M0 | Unknown | Unknown | Unknown |
| 30 | 53 M | Right maxillary sinus | SMARCB1-deficient carcinoma | Basaloid | T3N0M0 | Unknown | Unknown | Unknown |
| 31 | 26 M | Ethmoid | SMARCB1-deficient carcinoma | Basaloid with focal clear cell features | Unknown | Surgery + CT + XRT | None | NED (16) |
| 32 | 64 M | Nasal cavity | SMARCB1-deficient carcinoma | Basaloid | Unknown | CT + XRT | None | NED (18) |
| 33 | 19 M | Nasal cavity | SMARCB1-deficient carcinoma | Basaloid | Unknown | Unknown | Unknown | Unknown |
| 34 | 68 F | Nasal cavity | HGADCA | Pasamacytoid/rhabdoid with prominent clear cells | T4N1M0 | Unknown | Neck Lymph nodes | Unknown |
| 35 | 61 M | Nasal Cavity, Ethmoid | SNUC | Basaloid | T3N0M0 | CT+XRT, Surgery | Orbital recurrence with nodal and vertebral mets (18) | DOD (43) |
| 36 | 89 M | Nasal Caviy, Sphenoid and Ethmoid Sinuses | SMARCB1- deficient carcinoma | Pasamacytoid/rhabdoid | T3N0M0 | Surgery + XRT | Local Recurrence | DOD (6) |
| 37 | 46 M | Frontal Sinus | PD SCC | Basaloid | T1N0M0 | CT+XRT, Surgery, adjuvant CT | None | NED (43) |
| 38 | 63 M | Nasal Mass | SMARCB1-deficient carcinoma | Basaloid | unknown | Unknown | Unknown | Unknown |
| 39 | 86 M | Nasal, maxillary and ethmoid sinuses, cranial extension | SNUC | Plasamacytoid/rhabdoid | T4N0M0 | XRT and Surgery | Local Recurrence with nodal and skeletal metastases (10) | DOD (9) |
F=female; M=male; CT=chemotherapy; XRT=external beam radiotherapy; NA=not available; DOD=died of disease; NED=no evidence of disease; AWD= alive with disease; SNUC, sinonasal undifferentiated carcinoma; NKSCC, non-keratinizing squamous cell carcinoma; SCC, squamous cell carcinoma; CA, carcinoma.
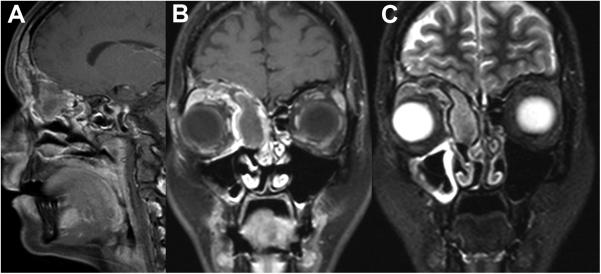
Figure 1.
MRT (case 3) revealed a mass in the right frontal sinus and anterior ethmoid cells abutting the anterior skull base. The lesion demonstrates inhomogeneous contrast enhancement with areas of necrosis on post-contrast T1w images (A and B). T2w images (C) help in differentiating tumor and surrounding sinonasal mucosa. The right eye globe is displaced latero-inferiorly.
Treatment consisted of radical surgical resection combined by chemotherapy and/or radiation in 22 patients. Four patients underwent surgery alone, and 5 patients received chemo/radiotherapy alone. Two patients received only supportive (palliative) care after biopsy diagnosis. For the remaining six patients, detailed information regarding therapy was not available. Follow-up data was available for 30 patients, and the follow-up period ranged from <1 (for those who died of disease shortly after diagnosis) to 115 months (median, 17). Distant metastases were recorded in 11 of 30 cases. The sites of distant metastases included the lungs (n = 2), pericardium (n = 1), pleura (n = 1), bone (n = 3) and soft tissues of the thighs (n = 1). They occurred at 0 (at presentation) to 63 months after diagnosis (median, 10 months). Regional failure was seen in 33% of patients, with 10 local recurrences and 3 regional recurrences to cervical lymph nodes. At last follow-up, 17 of 30 (56%) patients had died of their disease a few days to 102 months after diagnosis (median, 15 months), three were alive with disease and one died of unrelated cause 10 months later. Taken together, 20 of 30 (66%) patients with ascertained disease status or cause of death either died of their disease or were alive with disease at last follow-up. Only 9 patients (30%) were alive without evidence of disease at last follow-up (range, 11-115 months; median, 26). Of the 9 survivors, 7 received radical surgery + radiochemotherapy. The plasmacytoid/rhabdoid cell morphology (see pathologic findings below) occurred with similar frequency among the groups who had died of disease and those who survived. Notably, the basaloid and eosinophilic histology comprised 60% and 66% of patients who died of their disease, respectively.
PATHOLOGICAL FINDINGS
For the archival cases identified retrospectively, the original diagnosis was anaplastic/undifferentiated carcinoma or SNUC (n=14), non-keratinizing or basaloid squamous cell carcinoma (SCC) (n=5), myoepithelial carcinoma (n=2), adenocarcinoma, not otherwise specified (n=1), oncocytic carcinoma (n=1), poorly differentiated adenocarcinoma (n=2), and non-keratinizing SCC ex Schneiderian papilloma (n=1). The remaining prospective cases were diagnosed using the current terminology.12,13
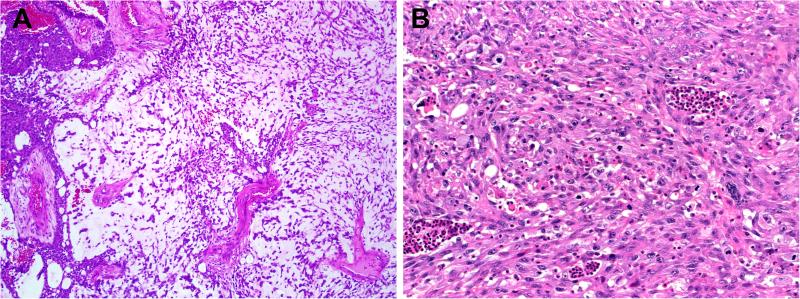
Grossly, the tumors were described to have infiltrative margins with variable exophytic papillary surface component in some cases. Histologically, the tumors had in common cellular monotony with relatively monomorphic small-to-medium sized rounded nuclei with dispersed chromatin, variably prominent nucleoli and indistinctive cytoplasmic borders. Mitotic rates were uniformly high, and necrosis was common. On occasion, the sinonasal respiratory-type epithelium was colonized by tumor, often in a pagetoid fashion (Fig. 2). Conventional squamous dysplasia/carcinoma in situ was not seen. Another common feature seen in many of the cases was the presence of non-specific, clear, “empty” cytoplasmic vacuoles (Fig. 3A)
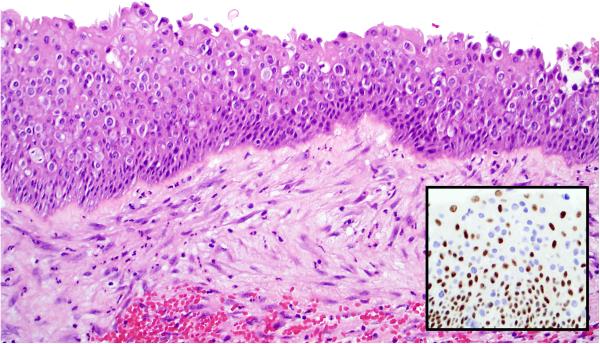
Figure 2.
While the surface epithelium overlying SMARCB1-deficient sinonasal carcinoma lacks conventional squamous dysplasia or carcinoma-in-situ, the tumors often exhibit spread into the epithelium in a pagetoid manner. This can be demonstrated by SMARCB1 immunohistochemistry which highlights absent expression in the tumor cells (inset).
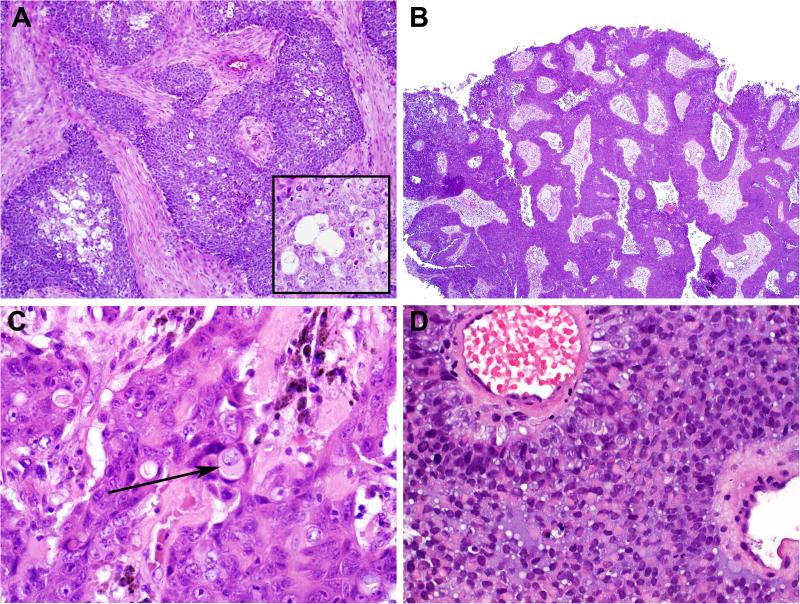
Figure 3.
The predominant histologic pattern of SMARCB1-deficient sinonasal carcinoma was basaloid, with nests of basophilic cells with high nuclear: cytoplasmic ratios growing in a desmoplastic stroma. Note also the presence of non-specific vacuoles within the tumor, a common finding (inset) (A). In some cases of basaloid SMARCB1-deficient sinonasal carcinoma, the tumor grows downward in an inverted growth pattern reminiscent of inverted Schneiderian papilloma (B). On close inspection, basaloid SMARCB1-deficient sinonasal carcinomas may demonstrate rare, singly-dispersed plasmacytoid or rhabdoid cells (arrow) (C). In one case, a basaloid SMARCB1-deficient sinonasal carcinoma became predominantly plasmacytoid/rhabdoid upon metastasizing to the lung (D).
The most common microscopic appearance (23 of 39, 59%) was that of an undifferentiated basaloid or “blue cell” tumor reminiscent of non-keratinizing SCC or SNUC growing as solid well demarcated nests and sheets of basaloid cells set within a desmoplastic stroma (Fig. 3A). In these basaloid forms of SMARCB1-deficient sinonasal carcinoma, the tumor cells had high nuclear: cytoplasmic ratios and occasional palisading of nuclei at the periphery of tumor nests. The carcinomas occasionally demonstrated inverted growth down superficial mucosal glands in a pattern reminiscent of inverted Schneiderian papilloma or carcinoma arising within an inverted Schneiderian papilloma (Fig. 3B). Despite their resemblance to basaloid or non-keratinizing SCC and a “squamoid” appearance at times, none of the basaloid cases showed overt squamous differentiation in the form of keratin pearls. In the tumors with a more basaloid morphology, a plasmacytoid/rhabdoid cell (i.e., with abundant, eccentrically placed eosinophilic cytoplasm) population was not immediately evident upon initial assessment, however, in most cases, singly dispersed rhabdoid/plasmacytoid cells could be identified with a thorough search (Fig. 3C). In one case, the rhabdoid cell component was remarkably increased in the lung metastasis (Fig. 3D).
The second most common morphologic appearance seen in 14 of 39 (36%) was that of a “pink cell tumor” at low power (Fig. 4A). In this variant, the tumor consisted of nests and sheets of predominantly plasmacytoid/rhabdoid cells (Fig. 4B). Three of these tumors displayed large oncocytic squamoid cells with frequent acantholytic-like arrangement mimicking oncocytic adenocarcinoma of salivary glands (Fig. 4). Two cases of these eosinophilic tumors were reminiscent of proximal-type epithelioid sarcoma, one of them also showed multinodular growth further mimicking epithelioid sarcoma (Fig. 4C). Two of the plasmacytoid/rhabdoid variants demonstrated variable glandular differentiation (with intraluminal mucin production in one case) (Fig. 4D).
Figure 4.
The second most common appearance of SMARCB1-deficient sinonasal carcinoma was that of an eosinophilic tumor, often growing in a nested or solid pattern (A). This form of SMARCB1-deficient sinonasal carcinoma consisted of numerous cells with abundant, pink, eccentrically placed cytoplasm that were variably plasmacytoid or rhabdoid (B). Two cases grew in a multinodular, “pseudogranulomatous” manner at low power, reminiscent of epithelioid sarcoma (C), and two of the eosinophilic SMARCB1-deficient sinonasal carcinomas exhibited areas of glandular differentiation (D).
Finally, 2 of 39 (5%) cases were difficult to place into the basaloid or plasmacytoid/rhabdoid categories. One of the tumors had a major basaloid component but also demonstrated minor components of overt glandular differentiation with mucin production and spindled cells (Fig. 5A). The other case was a pure sarcomatoid carcinoma comprised of malignant spindled cells (Fig. 5B). Both of these cases demonstrated rhabdoid cytomorphology similar to the other carcinomas.
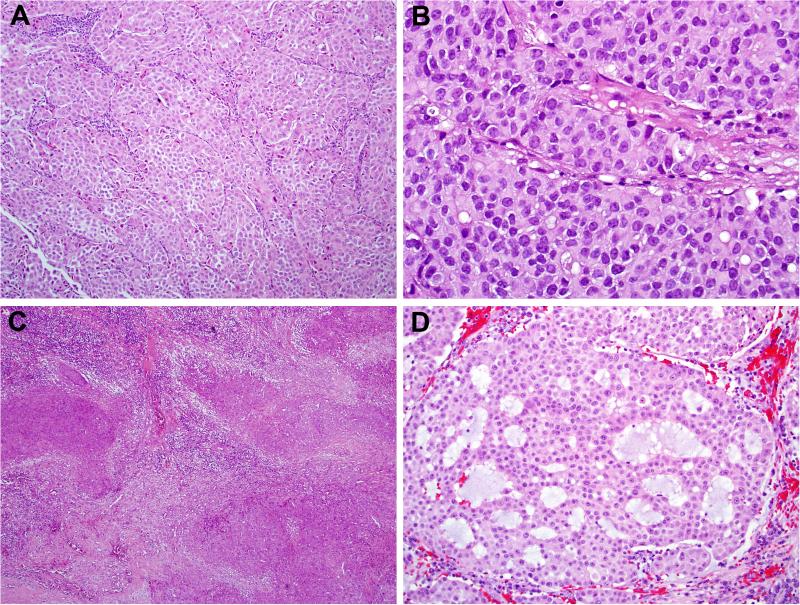
Figure 5.
Two SMARCB1-deficient sinonasal carcinomas exhibited overt spindle cell (sarcomatoid) differentiation. In one case, the sarcomatoid areas (right) were seen in addition to the more common basaloid pattern (left) (A), while the other case was as pure sarcomatoid carcinoma (B).
Immunohistochemical findings
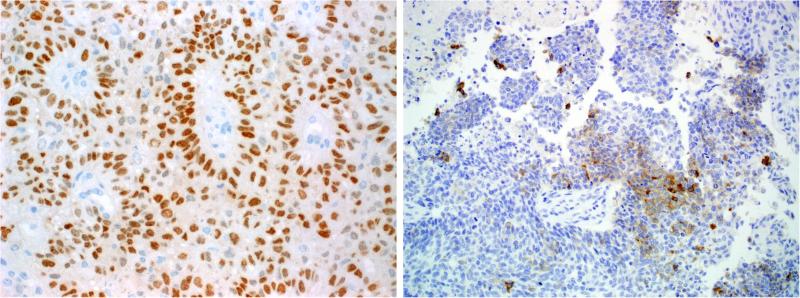
The immunohistochemical findings are summarized in Table 2. Immunohistochemistry showed consistent expression of pancytokeratin (38 of 39, 97%). The single case that was cytokeratin-negative was a tumor that was essentially identical to the other plasmacytoid/rhabdoid cases in every other respect, including at the genetic level, and could not be classified as any other tumor type. Twenty of 31 cases (64%) showed variable expression of CK5, mainly moderate to diffuse in extent. P63 was positive in 20 of 36 cases tested (55%); the immunoexpression was diffuse in 13 while it was focal in 7 cases (Fig. 6). Diffuse p63 immunoexpression was more common in the basaloid carcinomas (seen in 12 of 22) than it was in the eosinophilic forms (1 of 14). CK7 was variably positive in 15 of 31 cases (48%), but was usually focal. P16 was strongly and diffusely expressed in 4 of 29 cases tested while one additional case showed only limited focal expression. None of the cases tested for NUT immunohistochemistry (0 of 30), Epstein-Barr virus in situ hybridization (0 of 15) or oncogenic HPV by either PCR-based or in situ hybridization methods (0 of 26) was positive. A few cases were positive for neuroendocrine markers, with variable but typically focal expression of CD56 (7 of 25), synaptophysin (6 of 33) and chromogranin A (3 of 30) (Fig. 6). Five tumors co-expressed two neuroendocrine markers (4 co-expressed synaptophysin and CD56 and one case co-expressed synaptophysin + chromogranin A). Finally, CD117 was expressed in 3 of 27 cases.
Table 2.
Immunohistochemical findings in sinonasal SMARCB1-deficient carcinoma (n=39).
| No | Pan-CK | CK7 | p63 | CK5 | CHgA | SYN | CD56 | CD117 | p16 | NUT | EBV ISH | HPV PCR or ISH | SMARCB1 | SMARCA2 | SMARCA4 | ARID1A | SMARCB1 FISH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | − | − | + | − | − | − | − | + | − | − | − | Lost | Reduced | Reduced | Intact | Biallelic deletion, monosomy 22q |
| 2 | + | − | − | + | − | + | + | − | +F | − | − | − | Lost | Reduced | Intact | Intact | Biallelic deletion |
| 3 | + | − | + | + | − | − | − | − | + | − | − | − | Lost | Reduced | Intact | Intact | Monoallelic deletion + monosomy 22q |
| 4 | + | − | − | + | − | − | − | − | − | − | − | − | Lost | Reduced | intact | Intact | Intact |
| 5 | + | − | − | − | − | − | − | − | − | − | − | − | Lost | Reduced | Intact | Intact | Intact |
| 6 | + | − | + | F+ | − | − | − | − | − | − | − | − | Lost | Reduced | Intact | Intact | Biallelic deletion |
| 7 | + | − | + | F+ | − | − | ++ | − | − | − | − | − | Lost | Reduced | Intact | Intact | Monoallelic deletion |
| 8 | + | − | − | + | ND | − | ND | − | ND | − | ND | − | Lost | Reduced | NR | Intact | NR |
| 9 | + | + | − | − | ND | ND | ND | − | ND | − | ND | ND | Lost | NR | NR | Intact | NR |
| 10 | + | ND | F+ | F+ | ND | − | ND | − | ND | − | ND | ND | Lost | Reduced | NR | Intact | NR |
| 11 | + | + | − | F+ | − | − | ND | − | − | − | ND | ND | Lost | Reduced | Intact | Intact | NR |
| 12 | + | − | − | − | − | − | +F | − | − | − | − | ND | Lost | Reduced | Intact | Intact | Intact |
| 13 | + | F+ | F+ | + | − | F+ | + | − | − | − | ND | − | Lost | Reduced | Intact | Intact | Biallelic deletion |
| 14 | + | − | + | + | − | F+ | − | − | + | − | ND | − | Lost | Reduced | Intact | Intact | Biallelic deletion |
| 15 | + | + | − | − | − | − | − | − | + | − | ND | − | Lost | ND | Intact | Reduced | NR |
| 16 | + | F+ | + | + | − | − | ND | − | − | − | ND | − | Lost | Lost | Mosaic | Intact | Biallelic deletion |
| 17 | + | − | − | − | − | − | − | − | − | − | ND | − | Lost | Reduced | Intact | Intact | Monosomy 22q |
| 18 | + | − | − | − | − | − | ND | − | − | − | ND | − | Lost | Reduced | NR | Intact | NR |
| 19 | + | + | − | − | − | − | − | − | − | − | ND | − | Lost | Intact | Intact | Intact | Intact |
| 20 | + | − | + | F+ | − | − | − | − | − | − | ND | − | Lost | Lost | Intact | Intact | Monoallelic deletion + partial monosomy 22q |
| 21 | + | − | + | F+ | − | − | − | − | ND | − | Lost | Reduced | Intact | Intact | Biallelic deletion | ||
| 22 | + | F+ | + | + | − | − | − | − | − | ND | − | Lost | Reduced | Intact | Intact | Biallelic deletion | |
| 23 | + | + | ND | ND | − | + | + | − | − | ND | Lost | Intact | Intact | Intact | Monosomy 22q | ||
| 24 | + | F+ | + | F+ | F+ | − | F+ | + | − | − | − | − | Lost | Lost | Intact | Intact | Biallelic deletion + monosomy 22q |
| 25 | + | − | F+ | ND | − | − | ND | − | − | − | ND | − | Lost | Lost | Intact | Intact | NR |
| 26 | + | + | + | F+ | F+ | F+ | − | − | − | − | − | Lost | Intact | Intact | Intact | Monosomy 22q | |
| 27 | + | F+ | − | − | − | − | − | + | − | − | ND | − | Lost | Intact | Intact | NR | Equivocal, partial Monosomy 22q |
| 28 | − | − | − | − | − | − | − | ND | − | − | − | − | Lost | Reduced | Intact | ND | Biallelic deletion |
| 29 | + | F+ | F+ | ND | − | − | ND | ND | ND | ND | ND | ND | Lost | Reduced | Intact | ND | ND |
| 30 | + | ND | + | F+ | F+ | ND | ND | + | ND | ND | ND | ND | Lost | ND | ND | ND | Biallelic deletion |
| 31 | + | F+ | F+ | − | − | ND | ND | − | − | − | − | − | Lots | ND | ND | ND | Biallelic deletion |
| 32 | + | ND | ND | − | − | ND | ND | ND | − | ND | ND | ND | Lots | ND | ND | ND | Biallelic deletion |
| 33 | + | F+ | F+ | F+ | − | F+ | F+ | − | − | − | − | − | Lost | Intact | Intact | ND | Failed |
| 34 | + | ND | − | ND | − | − | ND | ND | − | ND | ND | ND | Lost | ND | ND | ND | NR |
| 35 | + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Lost | ND | ND | ND | Intact |
| 36 | + | ND | F+ | ND | ND | − | − | ND | − | ND | − | ND | Lost | ND | ND | ND | Monosomy |
| 37 | + | ND | + | ND | − | − | − | ND | − | ND | − | − | Lost | ND | ND | ND | Biallelic deletion |
| 38 | + | ND | + | + | − | − | − | ND | − | ND | ND | ND | Lost | ND | ND | ND | ND |
| 39 | + | ND | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | Lost | ND | ND | ND | Intact |
CK=cytokeratin; CHgA=chromogranin A; SYN=synaptophysin; EBV= Epstein-Barr virus; ISH=in situ hybridization; HFV=human papillomavirus; F= focal; ND= not done; NR=not reported
Figure 6.
SMARCB1-deficient sinonasal carcinomas were variably p63-positive (A; note perivascular rosette-like nuclei). A few cases expressed neuroendocrine markers like synaptophysin, typically focally (B).
SWI/SNF protein expression status
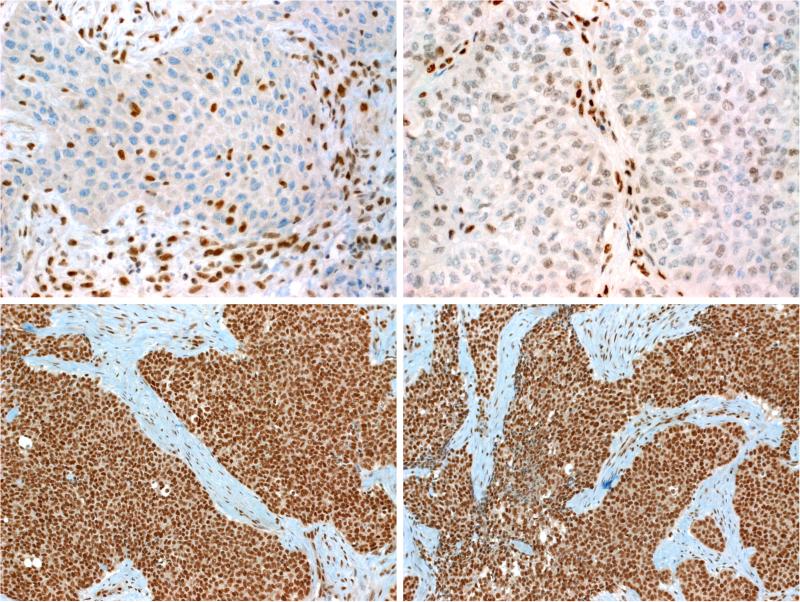
As per definition, all tumors showed complete loss of nuclear SMARCB1 (INI1) expression with retained strong reactivity in the background inflammatory, stromal and/or epithelial cells (Fig. 7A). SMARCB1 is one of the proteins in the SWI/SNF nucleosome remodeling complex, and other SMARCB1-deficient tumor types are known to have specific expression patterns for other SWI/SNF proteins. As a result, a subset of cases was also tested with additional members of the SWI/SNF complex by immunohistochemistry. Only 5 of 28 cases tested showed strong intact expression of SMARCA2, the reminder were either deficient (n=4) or showed reduced expression (n=19) (Fig. 7B). On the other hand, 24 of 26 cases with evaluable results for SMARCA4 showed intact expression (Fig. 7C) while one case was weakly positive (reduced expression) and another one contained intermingled small subpopulation of SMARCA4-negative cells imparting a mosaic-like pattern. ARID1A was intact in all (26/26; Fig. 7D) but one tumor which showed reduced expression. There was no discernable difference in the SWI/SNF expression patterns between the tumors with basaloid or eosinophilic/plasmacytoid appearances.
Figure 7.
As per definition, all tumors showed complete loss of SMARCB1 while normal stromal cells in the background stained strongly (A). SMARCA2 was frequently reduced (B) and occasionally lost. In contrast, SMARCA4 (C) and ARID1A (D) were entirely intact in all tested cases.
Molecular findings
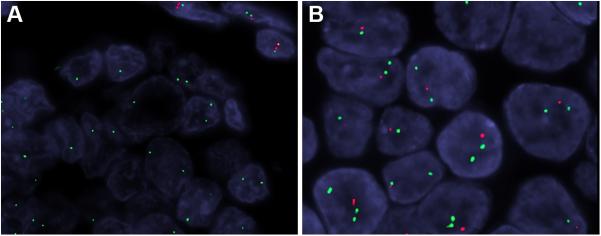
FISH testing was successful in 27 cases (the remainder were not assessable due to suboptimal and weak signal intensity). Of the 27 cases, 21 tumors (78%) showed abnormal findings with homozygous (biallelic) deletion of the SMARCB1 gene locus seen in 14 cases (Fig. 8A) and heterozygous (monoallelic) deletion in 7 cases (Fig. 8B). Six tumors showed normal signals; interestingly, all but one case of them were of the eosinophilic/plasmacytoid type. In several cases loss of one or both SMARCB1 gene locus signal was associated with loss of the corresponding centromere indicating chromosome 22q monosomy. No other-type aberrations (e.g., amplifications) were noted. Other genes involved in the SWI/SNF complex were not evaluated by FISH.
Figure 8.
By fluorescence in situ hybridization, 13 SMARCB1-deficient sinonasal carcinomas demonstrated homozygous deletion of SMARCB1, with both SMARCB1 alleles deleted (red), while 2 copies of EWSR1 (green) are present. See in contrast normal cells having 2 copies of both green and red signals (top right) (A). Six SMARCB1-deficient sinonasal carcinomas exhibited heterozygous SMARCB1 deletion, with only one copy of SMARCB1 present (red) while 2 copies of EWSR1 (green) are seen.
DISCUSSION
SMARCB1 (INI1) is a member of a large protein complex involved in chromatin remodeling and thus regulation of gene expression.22 Loss of SMARCB1 expression as a result of deletions/mutations has emerged as a defining diagnostic feature in a variety of neoplasms in children and adults, in particular malignant atypical teratoid/rhabdoid tumors of childhood23,24, epithelioid sarcoma25 and recently several epithelial tumor entities in adults and the elderly.26 SMARCB1 (INI1) immunohistochemistry has emerged as a powerful diagnostic tool to identify SMARCB1-altered neoplasms in routine surgical pathology practice.27,28 Several recent studies showed that SMARCB1 loss may occur either as the primary and sole driver genetic event (as in atypical teratoid/rhabdoid tumor, epithelioid sarcoma, etc.) or be superimposed on a preexisting genetic background (as in MSI-instable colorectal cancer and several other dedifferentiated carcinomas).21,26
The histological spectrum we described herein in conjunction with uniform SMARCB1 deficiency strongly suggests a distinctive neoplasm defined by SMARCB1 loss among other poorly differentiated sinonasal tract malignancies rather than a heterologous group of sinonasal tumors that happen to carry a shared genetic alteration. First, SMARCB1 loss has been identified as the primary and sole driver genetic event in certain tumors outside of the sinonasal tract such as atypical teratoid/rhabdoid tumor and epithelioid sarcoma. Although comprehensive genetic studies are still lacking, the one SMARCB1-deficient sinonasal carcinoma analyzed by next-generation sequencing failed to reveal any additional genetic aberrations other than homozygous SMARCB1 deletion.14,29 Second, the SMARCB1 deficient sinonasal carcinomas defied classification as some other recognized tumor type and showed no evidence of high grade transformation from a preexisting well differentiated carcinoma. They consistently lack squamous differentiation, are negative for NUT, and do not harbor the oncogenic viruses HPV or EBV. While occasional SMARCB1-deficient sinonasal carcinomas showed evidence of glandular or neuroendocrine differentiation, they do not conform to the histologic descriptions of sinonasal adenocarcinoma or neuroendocrine carcinoma. Third, SMARCB1 loss is not encountered in other well defined types of sinonasal carcinomas.12,13,15 In our previous study of sinonasal carcinomas, SMARCB1 loss was not identified in any of 133 carcinomas of surface (e.g. squamous cell carcinoma, sinonasal adenocarcinoma) or minor salivary gland origin (e.g. adenoid cystic carcinoma).12,13,15 In effect, SMARCB1 loss is an uncommon genetic alteration in sinonasal carcinomas, and it specifically localizes to a highly undifferentiated basaloid morphology with varying degrees of plasmacytoid/rhabdoid cells . It is noteworthy that complete loss of SMARCB1 immunoexpression does not completely correlate to the FISH status of the SMARCB1 gene locus. Similar to other SMARCB1-deficient neoplasms in other organs, gene mutations not detectable by FISH are likely events causing inactivation of the second allele in cases with monoallelic (heterozygous) deletions. Likewise, mutations involving both alleles are likely the cause of SMARCB1 loss in cases with normal FISH findings, but epigenetic mechanisms might play a role as well.
This comprehensive study incorporated all cases of SMARCB1-deficient sinonasal carcinoma diagnosed in a large number of collaborative institutions. We found that this tumor affects the sexes equally over a wide age range (19 to 87, mean 52) and may have a predilection for the ethmoid sinuses. In addition, in this series, SMARCB1-deficient sinonasal carcinoma behaved in an aggressive manner, with 54% of patients succumbing to their disease 0 to 102 months following diagnosis (median, 16). These findings are in agreement with the other published cases of this disease (Table 3).14-20 This large series also confirms the view that the majority of SMARCB1-deficient sinonasal carcinomas display prominent basaloid features mimicking basaloid SCC, SNUC, or other “small blue round cell” tumors. A tumor dominated by plasmacytoid/rhabdoid features is a common morphologic variant as well. However, in this extended study, we uncovered the uncommon occurrence of unusual morphological variants including tumors with variable adenoid features and mucin production that warranted the original diagnosis of adenocarcinoma in some cases. Finally, two cases demonstrated a variable component with frankly sarcomatoid features (focal in one and dominant in the other case). The morphologic profile of SMARCB1-deficient sinonasal carcinomas appears to be broader than previously anticipated. Indeed, these observations highlight the wider histomorphological spectrum of this entity and the need to include SMARCB1 in the immunohistochemical marker panel used in the workup of poorly differentiated or difficult-to-classify sinonasal tract malignancies.
Table 3.
Clinicopathological features of SMARCB1-deficient sinonasal carcinomas published by other authors (n=15).
| No | Authors/ref | Age/ Gender |
Site | Original diagnosis | Predominant reported histology |
Initial stage | Treatment | Disease course (months) |
Outcome/ Follow- up (months) |
SMARCB1 FISH |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jamshidi et al14 | 32 M | Sphenoid, posterior ethmoid cells | poorly diff SCC | Basaloid | T4N0 | surgery + CT + XRT | mediastinal + retroperitoneal node metastases (13) | Local recurrence + metastases (13) ; DOD (19) | Homologous deletion (NGS) |
| 2 | Bell et al15 | 64 F | frontal sinus | SNUC | Undifferentiated/basaloid | T4N0 | surgery + CRT | None | NED (3) | ND |
| 3 | Bell et al15 | 75 M | nasal cavity | Basaloid SCC | basaloid | T4N0 | Induction CT + XRT, surgery+ XRT | Local recurrence | AWD (12) | ND |
| 4 | Bell et al15 | 51 F | left nasal passage, temporal fossa, sphenoid | SNUC | Undifferentiated/basaloid | T4N0 | Surgery + CT + XRT | Local recurrence; distant metastases (NR) | DOD (24) | ND |
| 5 | Bell et al15 | 33 F | anterior skull base | high-grade malignant germ cell tumor | Undifferentiated | T4N0 | surgery + CRT | Local recurrence | AWD (33) | ND |
| 6 | Zeng et al16 | 86 M | left maxillary sinus + nasal cavity | SNUC | Basaloid, eosinophilic cells | PT4pN0MX | Radical surgery + neck | None | Died of colorectal cancer (21) | ND |
| 7 | Baressi et al17 | 16 F | nasal cavities, left frontal & ethmoid sinuses, skull base | Atypical teratoid/rhabdoid tumor | Rhabdoid | T4 | surgery | Metastasis to lung+ bones | DOD (9) | SMARCB1 mutation |
| 8 | Shatzkes et al20 | 35 F | NA | Carcinoma with squamoid features | NA | T4bN0M0 | NA | NA | NED (10) | NA |
| 9 | Shatzkes et al20 | 45 M | NA | Adenocarcinoma | NA | T4b | NA | NA | NED (48) | NA |
| 10 | Shatzkes et al20 | 50 F | NA | PD SCC + papillary features | NA | T4aN0M0 | NA | NA | NED (9) | NA |
| 11 | Shatzkes et al20 | 72 M | NA | SMARCB1-deficient CA | NA | T4aN0M0 | NA | NA | NED (12) | NA |
| 12 | Shatzkes et al20 | 43 M | NA | PD SCC | NA | T1NXMX | NA | NA | NED (9) | NA |
| 13 | Shatzkes et al20 | 62 M | NA | SNUC | NA | T4bN0M1 | NA | NA (M1 unspecified) | NED (3) | NA |
| 14 | Wasserman et al20 | 34 M | Maxillary sinus + maxillary alveolus + orbital floor | SNUC | Rhabdoid | T4 N0 M0 | Surgery + CRT | Metastasis to lung, pleura, bone, liver | DOD (26) | ND |
| 15 | Wasserman et al20 | 56 F | Nasal cavity + cranial cavity + cavernous sinus | Rhabdoid | T4b NX MX | CRT | None | AWD (12) | ND |
M=male; F=female; SCC=squamous cell carcinoma; SNUC= sinonasal undifferentiated carcinoma; CT=chemotherapy; XRT=external beam radiotherapy; DOD=died of disease; NED=no evidence of disease; AWD=alive with disease; ND=not done; NA= not available.
An extended immunohistochemical panel performed in this study revealed some unexpected findings. A subset of SMARCB1-deficient sinonasal carcinomas, particularly the basaloid form, demonstrate diffuse p63 immunoreactivity that may result in a misdiagnosis of non-keratinizing/basaloid SCC or NUT midline carcinoma.7,8 However, SMARCB1-deficient sinonasal carcinoma lacks overt squamous differentiation and does not exhibit squamous surface dysplasia. Uncommon but a potential pitfall is the partial expression of neuroendocrine markers (seen in a small subset of cases). Thus, the mere presence of neuroendocrine differentiation by immunohistochemistry, particularly if the expression is focal, does not exclude the diagnosis of SMARCB1-deficient sinonasal carcinoma. Further, a small subset of cases diffusely express p16 which may cause confusion with an HPV-related SCC. However, all cases of SMARCB1-deficient sinonasal carcinoma tested for oncogenic HPV infection have been negative. The phenotypic features and the growth patterns of these tumors strongly point to an epithelial origin and argue for classifying these neoplasms as carcinomas and not as “proximal-type epithelioid sarcoma” or “atypical teratoid/rhabdoid tumors”. That being said, a single case was completely negative for all cytokeratins. While the absence of cytokeratin expression is unexpected and counter-intuitive for a carcinoma, this case conformed in every other way to the histologic, immunophenotypic, and molecular findings of the other SMARCB1-deficient sinonasal carcinomas. As a result, in the setting of a sinonasal tumor that morphologically resembles SMARCB1-deficient sinonasal carcinoma, SMARCB1 immunohistochemistry should be considered even in the absence of cytokeratin expression. Although a SMARCB1-deficient neoplasm from another site could theoretically metastasize to the sinonasal area, the rarity of these entities in other organs in general and the consistent predominantly basaloid pattern of SMARCB1-deficient sinonasal carcinomas allow for this distinction.
Finally, immunohistochemical investigation of additional SWI/SNF complex proteins revealed occasional loss of SMARCA2, another catalytic subunit of the SWI/SNF complex in 4 cases, but co-loss of SMARCA4 was never observed. These findings are consistent with recent studies highlighting concurrent co-inactivation of two or more members of the SWI/SNF complex as a consequence of genetic mutation affecting SMARCB1 (epithelioid sarcoma and rhabdoid gastrointestinal carcinomas with variable loss of other SWI/SNF subunits other than SMARCA4)21,30,31 or involving SMARCA4 mutations (small cell carcinoma of the ovary, hypercalcemic type with dual loss of SMARCA4 and SMARCA2).32 The mechanisms responsible for the observed loss of additional SWI/SNF components (such as SMARCA2) are currently unknown.
The current series combined with additional published cases (Table 3) underlines the aggressive behavior of SMARCB1-deficient sinonasal carcinoma with almost two-thirds of patients with detailed information either succumbing to their disease, usually within 2 years after diagnosis, or alive with disease under palliative therapy. However, the biology of the disease seems to be somewhat heterogeneous as several cases with similarly advanced disease stage at initial diagnosis survived for several years following aggressive multimodal therapy. With restriction, there is some evidence that cases without metastatic disease at the time of diagnosis who received aggressive post-surgical radiochemotherapy tend to have a better outcome. In line with this notion, a recent study pointed to dramatic response of SMARCA4-deficient non-small cell lung carcinoma to platinum-based chemotherapy.33 These observations (also made in a few cases in our series) suggest the possibility of enhanced chemosensitivity of some of SWI/SNF-deficient epithelial neoplasms and merit future verification.
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
REFERENCES
- 1.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877–85. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 2.Haerle SK, Gullane PJ, Witterick IJ, et al. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am. 2013;24:39–49. doi: 10.1016/j.nec.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Mills SE, Fechner RE. “Undifferentiated” neoplasms of the sinonasal region: differential diagnosis based on clinical, light microscopic, immunohistochemical, and ultrastructural features. Semin Diagn Pathol. 1989;6:316–28. [PubMed] [Google Scholar]
- 4.Stelow EB, Jo VY, Mills SE, et al. A histologic and immunohistochemical study describing the diversity of tumors classified as sinonasal high-grade nonintestinal adenocarcinomas. Am J Surg Pathol. 2011;35:971–80. doi: 10.1097/PAS.0b013e31821cbd72. [DOI] [PubMed] [Google Scholar]
- 5.Frierson HF, Jr, Mills SE, Fechner RE, et al. Sinonasal undifferentiated carcinoma. An aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10:771–9. [PubMed] [Google Scholar]
- 6.Bishop JA. Recently described neoplasms of the sinonasal tract. Semin Diagn Pathol. 2016;33:62–70. doi: 10.1053/j.semdp.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–34. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 8.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–21. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–92. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop JA, Ogawa T, Stelow EB, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–44. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop JA, Alaggio R, Zhang L, et al. Adamantinoma-like Ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol. 2015;39:1267–74. doi: 10.1097/PAS.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agaimy A, Koch M, Lell M, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014;38:1274–81. doi: 10.1097/PAS.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38:1282–9. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamshidi F, Pleasance E, Li Y, Shen Y, et al. Diagnostic value of next-generation sequencing in an unusual sphenoid tumor. Oncologist. 2014;19:623–30. doi: 10.1634/theoncologist.2013-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell D, Hanna EY, Agaimy A, et al. Reappraisal of sinonasal undifferentiated carcinoma: SMARCB1 (INI1)-deficient sinonasal carcinoma: a single-institution experience. Virchows Arch. 2015;467:649–656. doi: 10.1007/s00428-015-1853-1. [DOI] [PubMed] [Google Scholar]
- 16.Zeng M, Chen C, Yang S, et al. SMARCB1 (INI1)-deficient sinonasal carcinoma: a newly described entity. Int J Clin Exp Pathol. 2016;9:3454–3458. [Google Scholar]
- 17.Barresi V, Branca G, Raso A, et al. Atypical teratoid rhabdoid tumor involving the nasal cavities and anterior skull base. Neuropathology. 2016;36:283–289. doi: 10.1111/neup.12271. [DOI] [PubMed] [Google Scholar]
- 18.Agaimy A, Geddert H, Märkl B, et al. SMARCB1(INI1)-Deficient Sinonasal Carcinomas: Expanding the Morphological Spectrum of a Recently Described Entity. Lab Invest. 95:318A–318A. [Google Scholar]
- 19.Shatzkes DR, Ginsberg LE, Wong M, et al. Imaging Appearance of SMARCB1 (INI1)- Deficient Sinonasal Carcinoma: A Newly Described Sinonasal Malignancy. AJNR Am J Neuroradiol. 2016 Jul 7; doi: 10.3174/ajnr.A4841. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserman JK, Dickson BC, Perez-Ordonez B, de Almeida JR, Irish JC, Weinreb I. INI1 (SMARCB1)-Deficient Sinonasal Carcinoma: A Clinicopathologic Report of 2 Cases. Head Neck Pathol. 2016 Sep 19; doi: 10.1007/s12105-016-0752-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agaimy A, Daum O, Märkl B, et al. SWI/SNF Complex-deficient Undifferentiated/ Rhabdoid Carcinomas of the Gastrointestinal Tract. A Series of 13 Cases highlighting Mutually Exclusive Loss of SMARCA4 and SMARCA2 and Frequent Co-inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol. 2016;40:544–53. doi: 10.1097/PAS.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 22.Peterson CL, Dingwall A, Scott MP. Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc Natl Acad Sci U S A. 1994;91:2905–8. doi: 10.1073/pnas.91.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biegel JA, Zhou JY, Rorke LB, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–9. [PubMed] [Google Scholar]
- 24.Biegel JA, Tan L, Zhang F, et al. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8:3461–7. [PubMed] [Google Scholar]
- 25.Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542–50. doi: 10.1097/PAS.0b013e3181882c54. [DOI] [PubMed] [Google Scholar]
- 26.Agaimy A. The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity. Adv Anat Pathol. 2014;21:394–410. doi: 10.1097/PAP.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 27.Judkins AR. Immunohistochemistry of INI1 expression: a new tool for old challenges in CNS and soft tissue pathology. Adv Anat Pathol. 2007;14:335–9. doi: 10.1097/PAP.0b013e3180ca8b08. [DOI] [PubMed] [Google Scholar]
- 28.Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol. 2011;35:e47–63. doi: 10.1097/PAS.0b013e31822b325b. [DOI] [PubMed] [Google Scholar]
- 29.Hasselblatt M, Isken S, Linge A, et al. High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer. 2013;52:185–90. doi: 10.1002/gcc.22018. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Fan XS, Xia QY, et al. Concurrent loss of INI1, PBRM1, and BRM expression in epithelioid sarcoma: implications for the contributions of multiple SWI/SNF complex members to pathogenesis. Hum Pathol. 2014;45:2247–54. doi: 10.1016/j.humpath.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Rao Q, Xia QY, Wang ZY, et al. Frequent co-inactivation of the SWI/SNF subunits SMARCB1, SMARCA2 and PBRM1 in malignant rhabdoid tumours. Histopathology. 2015;67:121–9. doi: 10.1111/his.12632. [DOI] [PubMed] [Google Scholar]
- 32.Karnezis AN, Wang Y, Ramos P, et al. Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. 2016;238:389–400. doi: 10.1002/path.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell EH, Chakraborty AR, Mo X, et al. SMARCA4/BRG1 is a novel prognostic biomarker predictive of cisplatin-based chemotherapy outcomes in resected non-small cell lung cancer. Clin Cancer Res. 2016;22:2396–404. doi: 10.1158/1078-0432.CCR-15-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]