Abstract
Analysis of the N-ethyl-N-nitrosourea (ENU)-induced repro42 mutation previously identified spermatogenesis associated 22 (Spata22) as a gene required for meiotic progression and fertility in both male and female mice, but its specific contribution to the process was unclear. Here we report on a novel, null allele of Spata22 (Spata22Gt) and confirm its requirement for germ cell development. Similar to repro42 mutant mice, histological and mating analyses indicate that gametogenesis is profoundly affected in Spata22Gt/Gt males and females, resulting in infertility. Cytological examination confirms that germ cells do not progress beyond zygonema and meiotic arrest is linked to impairment of both synapsis and DNA repair. Analysis of SPATA22 distribution reveals that it localizes to foci associated with meiotic chromosomes during prophase I and that the number of foci peaks at zygonema; there are also more SPATA22 foci in oocytes than in spermatocytes. Furthermore, SPATA22 co-localizes with a number of proteins involved in meiotic recombination, including RAD51, DMC1 and MLH1, and is present until mid-pachynema, suggesting a role in resolution of recombination intermediates. In fact, SPATA22 co-localizes with MLH1 in more than 20% of foci at pachynema. Analysis of Spata22Gt/Gt meiocytes confirms that SPATA22 is required for localization of MEIOB but not RPA, two proteins known to interact with SPATA22, and immunoblotting corroborates that production of MEIOB is indeed decreased in the absence of SPATA22. Together, these data suggest that SPATA22 is required for both meiotic recombination and synapsis during meiosis in mice.
Keywords: Spata22, recombination, synapsis, prophase I, meiosis, spermatogenesis, oogenesis, infertility, mouse
INTRODUCTION
In many species, meiosis is a defining event of gametogenesis. This specialized type of cell division ensures accurate chromosome segregation and genetic diversity; failure to execute meiosis accurately can result in infertility and/or aneuploidy. Meiotic arrest is often observed in human patients with idiopathic infertility (Matzuk and Lamb, 2008). Our understanding of male- and female- factor infertility could therefore benefit from deciphering the genetic program needed for proper completion of meiosis.
Following DNA replication, meiosis involves two successive rounds of chromosome segregation resulting in separation of homologous chromosomes and then sister chromatids during meiosis I (MI) and meiosis II (MII), respectively. Accurate chromosome segregation requires three highly coordinated events during prophase of meiosis I: homologous chromosome pairing, synapsis and recombination. In fungi, mammals and plants, these events are intrinsically linked and sterility is commonly observed when any of these processes are disrupted (Cohen et al., 2006; Handel and Schimenti, 2010; Zickler and Kleckner, 2015). The synaptonemal complex (SC) plays an integral role in synchronizing these events. The SC is a tripartite proteinaceous scaffold consisting of two lateral elements (LE) and a central element (CE) (Costa and Cooke, 2007; Fraune et al., 2012; Yang and Wang, 2009). A number of proteins required for SC assembly have been identified and gene inactivation studies conducted in mice have revealed their unique requirement for fertility. Interestingly, sexual dimorphism in timing and execution of meiotic events has been observed in many species, most notably in mammals. In the male mouse, meiotic prophase first begins around postnatal day 8 and spermatogenesis proceeds continuously throughout the lifespan of the individual (Bellve et al., 1977; Griswold, 2016). In the female mouse, cells enter meiosis in the fetal ovary, around embryonic day 13.5, but completion of prophase I is halted prior to birth; meiosis remains arrested until hormonal stimulation triggers resumption of the first meiotic division in a small cohort of oocytes (Pepling, 2006). Differences in gene-specific requirements, recombination rates, crossover placement and frequency of aneuploidy have been noted between sexes, suggesting mechanistic variations in execution and control of meiotic events (Hunt and Hassold, 2002; Morelli and Cohen, 2005).
In mammals, meiotic recombination is initiated following epigenetic marking of recombination sites by the histone methyltransferase PRDM9 (Prdm9, PR domain containing 9) (Baudat et al., 2010; Parvanov et al., 2010) and subsequent induction of DNA double strand breaks (DSBs) by the endonuclease SPO11 (Spo11, SPO11 meiotic protein covalently bound to DSB) (Keeney et al., 1997; Lam and Keeney, 2015). Processing of DSBs leads to resection of their 5’ends, yielding 3’-single-stranded DNA (ssDNA) overhangs; these are coated by RPA (replication protein A) to protect them against degradation and formation of secondary structures (Baudat et al., 2013; Ribeiro et al., 2015). The strand exchange proteins RAD51 (Rad51, RAD51 homolog) and DMC1 (Dmc1, DMC1 dosage suppressor of mck1 homolog, meiosis-specific homologous recombination) mediate invasion of the 3’ ssDNA overhang into the duplex of the homologous chromosome, resulting in displacement of one strand of the homolog, D-loop formation and elongation of the invading 3’end (Baudat et al., 2013). The fate of the recombination intermediate then depends on how it is processed. If the D-loop extending towards the second end captures the other 3’-ssDNA overhang and a double Holiday junction is formed, a crossover (CO) is established. Alternatively, if the elongated invading strand reanneals with the 3’-ssDNA overhang that was not involved in strand invasion from the same chromatid (single-end strand invasion), it results in a non-crossover (NCO) (Baudat et al., 2013). Much of our understanding of meiotic recombination in mammals comes from the study of model organisms such as yeast. However, certain critical factors in yeast are dispensable in mice and many genes required for meiotic recombination in mammals do not have orthologues in yeast, suggesting some evolutionary divergence in the execution of meiotic recombination (Hunter, 2015; Keeney et al., 2014; Lam and Keeney, 2015).
We recently characterized the ENU-induced mutation repro42 and identified spermatogenesis associated 22 (Spata22) as a vertebrate-specific gene required for meiotic progress and fertility in mice (La Salle et al., 2012). We showed that expression of Spata22 is predominantly restricted to germ cells of both sexes, and that the SPATA22 protein is absent in repro42 mutant gonads. repro42 mutant mice and Spata22-deficient rats display severe gonadal hypoplasia caused by arrest during prophase I of meiosis (Ishishita et al., 2013; La Salle et al., 2012). Progression through meiosis is halted due to aberrant SC formation, chromosome asynapsis and impaired DSB repair. In the rat, SPATA22 appears to be required for maintenance of RAD51 at recombination nodules, the sites of DSB repair (Ishishita et al., 2014). SPATA22 was also recently shown to be part of a complex that localizes to recombination intermediates and contains MEIOB (Meiob, meiosis specific with OB domains), another novel meiosis-specific protein, as well as RPA (Rpa, replication protein A) (Luo et al., 2013). Meiob-null mice exhibit a phenotype of meiotic arrest and sterility nearly identical to repro42 mutant males and females (La Salle et al., 2012; Luo et al., 2013; Souquet et al., 2013). Remarkably, localization and stability of MEIOB and SPATA22 seem to be interdependent (Luo et al., 2013). Whereas MEIOB appears to participate in second-end capture via its ability to bind ssDNA and its 3’-5’ exonuclease activity, the exact contribution of SPATA22 to meiosis remains to be elucidated.
Here we confirm the essential function of Spata22 during meiosis and its requirement for fertility using a novel mouse allele. We uncover sex-specific differences in the distribution of SPATA22 in oocytes and spermatocytes and reveal the dynamics of SPATA22 localization at recombination intermediates. Finally, our analysis of Spata22-deficient oocytes and spermatocytes indicates that SPATA22 is essential for localization of one of its interacting partners, MEIOB, in addition to being required for DNA repair and synapsis.
MATERIALS AND METHODS
Mice
All mice used in this study were maintained under standard conditions by the investigators at Midwestern University (MWU; Downers Grove, IL) in accordance with the National Institutes of Health and U.S. Department of Agriculture standards; all procedures conducted were approved by the MWU Institutional Animal Care and Use Committee (IACUC). For timed pregnancies, the day a vaginal plug was found was designated as 0.5 day postcoitum (dpc), while the day of birth was designated as 0 day postpartum (dpp). All mice used for experiments were genotyped by PCR amplification of genomic DNA extracted from tail tissue.
Two mutations for Spata22 were used in this study. The first one was the previously described repro42 mutation induced in a C57BL6/J (B6) background (La Salle et al., 2012). For this study, a congenic line on C3H (N10) was established; all Spata22repro42 offspring and control wild-type littermates used for biological analyses were from the congenic line. The second mutation was the knockout-first allele Spata22tm1a(KOMP)Wtsi obtained from the Knockout Mouse Project (KOMP) Repository (UC Davis, Davis, CA) (Skarnes et al., 2011); details on the allele can be found at http://www.mousephenotype.org/data/alleles/MGI:2685728/tm1a(KOMP)Wtsi. Chimeras were generated by microinjection of a C57BL/6N derived JM8.N4 ES cell line carrying the Spata22tm1a(KOMP)Wtsi allele, herein referred to as the Spata22Gt allele, into B6(Cg)-Tyrc-2J/J blastocysts by the Reproductive Sciences Services at The Jackson Laboratory (Bar Harbor, ME). Two chimeric males with germ line transmission were bred to B6 females to establish individual Spata22Gt lines (lines 172 and 174, respectively). Since initial phenotype analysis did not identify any differences between the two lines, line 174 was chosen to perform the analyses described in this study. Offspring homozygous for the Spata22Gt allele were compared to heterozygous and wild-type littermate controls in all biological analyses performed, unless otherwise indicated (see Supplemental Table S1 for genotyping primer sequences).
Complementation analysis and fertility testing
The origin of the repro42 mutation was confirmed genetically by analyzing the reproductive phenotype of progeny obtained from a cross between heterozygous repro42 mice and heterozygous Spata22Gt/+ mice. Since the polymorphic markers normally used to identify the repro42 mutation were uninformative because both lines were maintained on the B6 background, PCR amplification of the area containing the mutation followed by comparative restriction digestion of the amplicon were utilized to identify the repro42 allele (see Supplemental Table S1 for primer sequences). The repro42 mutation changes an Ags I restriction enzyme site to an Ase I site; genomic DNA was therefore digested with both Ags I (SibEnzyme, West Roxbury, MA) and Ase I (New England Biolabs, Ipswich, MA) according to the manufacturers’ instructions. Single- and double- heterozygous males and females (minimum n=3 per genotype, per sex) were mated with control B6 mice of proven fertility for a period of 5 months. The number of litters and offspring produced were recorded. Body weights and paired testis weights were recorded upon completion of the study, and testes and ovaries were collected for histological analysis as described below. The reproductive phenotype of wild-type, heterozygous and homozygous Spata22Gt as well as repro42 congenic adult males and females was also assessed as described above (minimum n=4 per genotype, per sex).
Histological analysis
Testes and ovaries were fixed by immersion in Bouin's fixative (Sigma, St. Louis, MO) for 2 to 5 hours for prepubertal testes and adult ovaries, or overnight for adult testes; fixed tissues were dehydrated and paraffin-embedded. Sections (7-μm thick) were cut, mounted on glass slides, deparaffinized with xylene, stained with Periodic Acid Schiff (PAS) or eosin and counterstained with hematoxylin following standard procedures. A Leica DM5500 B upright microscope was used to visualize the slides, and images were acquired using a Leica DFC450 C camera and LAS version 4.5.0 software (Leica Microsystems, Buffalo Grove, IL). A minimum of an n=3 biological replicates were analyzed per developmental time point, genotype and sex.
Preparation of enriched male germ cell populations
Mixed germ cell preparations were obtained from 15 dpp and 18 dpp wild-type and homozygous mutant testes as previously described (La Salle et al., 2008). Briefly, detunicated testes were digested in 0.5mg/ml collagenase (Sigma) and then 0.5 mg/ml trypsin (Sigma) supplemented with DNaseI (USB, Cleveland, OH) in Krebs-Ringer bicarbonate (KRB) media; germ cells were subsequently released by pipetting. Single-cell suspensions were filtered through 80 μm Nitex mesh (Sefar America, Depew, NY) and washed three times in KRB. After the final wash, cells were counted and resuspended in KRB media prior to being processed in subsequent applications.
Surface-spread chromatin and indirect immunofluorescence microscopy
Enriched male germ cells obtained as described above were used in the preparation of spermatocyte spread chromatin as previously described (La Salle et al., 2008) with the following modification: a series of spreads was prepared with solutions at pH 7.4 to allow for analysis of MLH1 distribution. Oocyte spread chromatin was also prepared as previously described once timed pregnancies were established between B6 mice or Spata22Gt/+ females and males (La Salle et al., 2012); fetal ovaries were collected from 14.5 dpc, 15.5 dpc and 17.5 dpc female fetuses. All slides were stored at −20°C before being processed for immunofluorescence labeling.
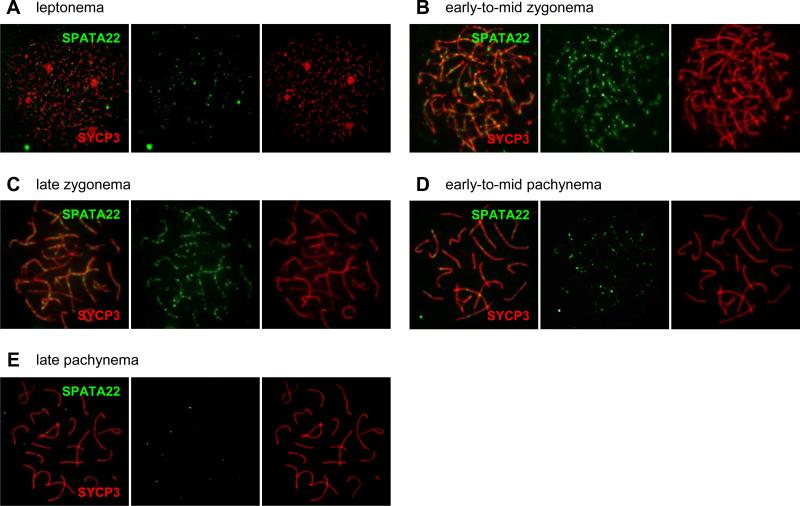
Solutions used for immunolabeling of surface-spread chromatin were prepared in phosphate buffered saline (PBS) pH 7.4 and procedures were carried out at room temperature as previously described (La Salle et al., 2008). Slides were blocked for one hour in ADB and then incubated with the appropriate primary antibodies (see Supplemental Table S2 for a list of primary antibodies) diluted in ADB. At least one well per slide received only ADB to serve as a negative control for primary antibody specificity. For double immunolabeling, primary antibodies were incubated concomitantly overnight and slides were incubated with a 1:1500 dilution of the appropriate secondary antibodies conjugated to AlexaFluor 488 or 594 (Molecular Probes/ ThermoFisher Scientific, Grand Island, NY) in a humidified dark chamber. For triple immunolabeling, individual primary and corresponding secondary antibodies were incubated successively (1:1500 dilution of secondary antibody conjugated to AlexaFluor 488 or 594 (Molecular Probes/ ThermoFisher Scientific) or Dylight 650 (ThermoFisher Scientific)). Slides were mounted with SlowFade Gold Antifade Mountant containing DAPI (Molecular Probes/ ThermoFisher Scientific) and imaged using a Leica DM5500B upright microscope equipped with a 63X/1.4 NA oil immersion objective; fluorescence images were captured using a Leica DCF365 FX camera with the LAS AF software (Leica Microsystems). All immunolabeling experiments were conducted three times for spermatocytes (n=3 per genotype), and twice for oocytes (minimal n=2 per genotype). For determination of SPATA22 distribution at each substage of prophase I in both spermatocytes and oocytes, only foci associated with developing axial/lateral elements or fully developed SCs (as identified by the localization pattern of SYCP3) were included in the analysis. The number of foci in each nucleus was counted by three individuals; triplicate counts varied by less than 12% (equivalent to ± 16 foci) and were averaged; nuclei were then staged by two individuals to ensure accuracy of staging. Total SPATA22 foci counts at each substage are presented as mean ± SD; the number of nuclei counted at each substage can be found in Table 1.
Table 1.
Number of SPATA22 foci in spermatocytes and oocytes at each substage of prophase I
| Substage | Spermatocytes | Oocytes | ||||
|---|---|---|---|---|---|---|
| N | Average # of foci | Range | N | Average # of foci | Range | |
| Leptotene | 13 | 69 ± 11 | 44-83 | 10 | 24 ± 8 | 15-44 |
| Early-to-mid zygotene | 21 | 113 ± 20 | 87-162 | 13 | 234 ± 63 | 151-389 |
| Late zygotene | 16 | 134 ± 14 | 109-160 | 15 | 148 ± 26 | 106-187 |
| Early-to-mid pachytene | 25 | 81 ± 16 | 60-108 | 24 | 90 ± 28 | 51-157 |
| Late pachytene | 13 | 25 ± 12 | 6-43 | 10 | 10 ± 3 | 5-16 |
| Diplotene | 4 | ND | ND | -- | -- | -- |
ND = Not detected
RNA extraction and RT-PCR
Total RNA was extracted from snap-frozen whole testes collected from 15 dpp wild-type, heterozygous, and homozygous mutant males using the RNeasy extraction kit with DNaseI treatment as described by the manufacturer (Qiagen, Valencia, CA). Primers were designed to span introns and were used in one-step RT-PCR reactions (OneStep RT-PCR kit, Qiagen) (see Supplemental Table S1 for a list of primers, amplicon length and annealing temperatures). Reactions were performed using 10 ng (for Actb) or 50 ng (for meiotic genes except Mlh1 which required 100 ng) of RNA according to the manufacturer's instruction with the following modifications: denaturation for 30 sec, annealing for 30 sec and extension for 60 sec for a total of 28 cycles, followed by a final extension step of 10 minutes. Individual PCR products were then mixed with Actb PCR products in a 1:1 ratio with the exception of Dmc1 (similar amplicon size). Products were separated by electrophoresis following standard procedures and images were acquired using the Bio-Rad Chemiluminescence MP imaging station (Bio-Rad, Hercules, CA). Densitometry analyses were conducted in triplicate (n=3 biological replicates) using the Image Lab 4.1 Build 16 software (Bio-Rad); expression of individual meiotic genes was normalized to that of Actb across genotypes to determine expression ratios relative to wild-type littermate controls.
Protein extraction and immunoblotting
Protein lysates were prepared from freshly isolated whole testes collected from 15 dpp wild-type, heterozygous, homozygous mutant and B6 males using RIPA buffer (Pierce/ThermoFisher Scientific, Grand Island, NY) supplemented with a protease inhibitor cocktail (Pierce/ThermoFisher Scientific) according to the manufacturer's recommendations. Protein concentration was determined using the BCA protein assay following the manufacturer's instructions (Pierce/ThermoFisher Scientific). Total protein in reducing sample buffer (15 μg per lane) was fractionated by SDS-polyacrylamide gel electrophoresis and either stained with GelCode (Pierce/ThermoFisher Scientific) to assess loading or transferred to Immobilon-P PVDF membranes (Millipore, Billerica, MA). Membranes were blocked overnight at 4 °C in 5% non-fat dried milk diluted in PBS with 0.1% Tween 20 (PBST), and incubated with the appropriate primary antibody diluted in blocking buffer for 1 hour at room temperature (see Supplemental Table S2 for a list of primary antibodies). Membranes were washed with PBST, followed by incubation with a 1:25,000-1:35,000 dilution of the appropriate anti-species IgG-HRP (horseradish peroxidase) -conjugated secondary antibody (Invitrogen/ThermoFisher Scientific). Membranes were washed with PBST and then exposed to the SuperSignal West Femto Western Blotting detection solution according to the manufacturer's instructions (Pierce/ThermoFisher Scientific). Chemiluminescence was revealed using a Bio-Rad Chemiluminescence MP imaging station. To confirm loading of equal amounts of proteins on gel, gels electrophoresed under identical conditions were stained with GelCode Blue Stain Reagent (Pierce/ThermoFisher Scientific). Individual membranes were also stripped and reprobed with an antibody against GAPDH (see Supplemental Table S2) as described above and exposed to the Western Lightning ECL Pro kit (Perkin Elmer, Waltham, MA). Densitometry analyses were conducted in triplicate (n=3 biological replicates) using the Image Lab 4.1 Build 16 software (Bio-Rad); protein expression was normalized to that of GAPDH across genotypes to determine expression ratios relative to wild-type littermate controls.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6.05 (GraphPad Software, Inc. La Jolla, CA). A paired Student t-test was used to assess differences in testis- and body- weights between wild-type and mutant animals; an unpaired Student t-test was used when relative expression differences were considered across genotypes; a one-way Anova was used to evaluate differences in foci numbers at different stages of prophase I in spermatocytes or oocytes; and a two-way Anova was used to compare foci counts between oocytes and spermatocytes. Data are presented as mean ± standard deviation (SD). A p-value of less than 0.05 (p < 0.05) was considered significant.
RESULTS
Spata22 is Essential for Male and Female Fertility
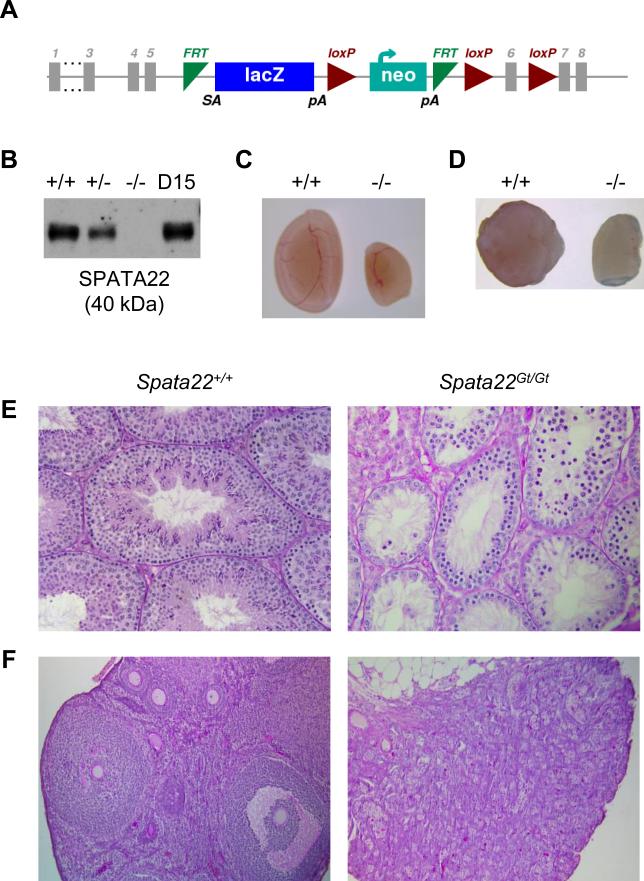
We used a novel targeted mutation allele, the Spata22Gt allele, to further assess the requirement for Spata22 during germ cell development (Fig. 1A). Immunoblotting analysis confirmed the absence of SPATA22 protein in homozygous mutant testes, indicating that the Spata22Gt mutation produces a null allele (Fig. 1B). Initial phenotypic analysis of Spata22Gt/Gt males and females revealed no perceptible gross phenotypic differences. Nonetheless, as we previously observed with repro42 mutant mice (La Salle et al., 2012), Spata22Gt/Gt males and females were sterile despite presenting normal mating behavior (Supplemental Table S3). The average paired testis weights of adult mutant males was 24.6% of wild-type littermate controls (Fig. 1C and Supplemental Table S4). Spata22Gt/Gt females also presented with smaller ovaries when compared to wild-type littermate females (Fig. 1D). Histological evaluation of Spata22Gt/Gt seminiferous tubules revealed absence of spermatids and spermatozoa, as well as some types of spermatocytes (Fig. 1E). Similarly, while all stages of follicular development were observed in adult wild-type ovaries, Spata22Gt/Gt ovaries lacked oocytes and contained degenerated follicles (Fig. 1F).
Figure 1. Gonadal failure is caused by absence of mature germ cells in adult Spata22Gt/Gt male and female mice.
(A) Schematic representation of the Spata22Gt allele (adapted from the Spata22tm1a(KOMP)Wtsi allele map found at http://www.mousephenotype.org). An embryonic stem cell line harboring a knockout-first reporter cassette inserted between exons 5 and 6 of the Spata22 gene was obtained from the International Knockout Mouse Consortium; mice carrying the Spata22Gt allele were generated as described in the Materials and Methods section. (B) Analysis of protein extracts prepared from wild type (+/+), heterozygous (+/−), and homozygous mutant (−/−) testes, as well as D15 B6 control testes. The SPATA22 protein is absent in homozygous mutant testes, confirming that the Spata22Gt allele is a null allele. The molecular weight of SPATA22 (in kDa) is indicated below the immunoblot. (C, D) Adult Spata22Gt/Gt testes (C-right) and ovaries (D-right) are significantly smaller than those of wild type littermate controls (C- and D- left, respectively). (E) Testis sections from adult wild type males contain spermatogonia, spermatocytes and spermatids (left), whereas only spermatogonia and some spermatocytes are visible in Spata22Gt/Gt sections (right). (F) All stages of follicular development are observed in adult wild type female ovary sections (left), whereas only degenerated follicles are present in Spata22Gt/Gt ovaries (right). D, postnatal day. Original magnification: ×400.
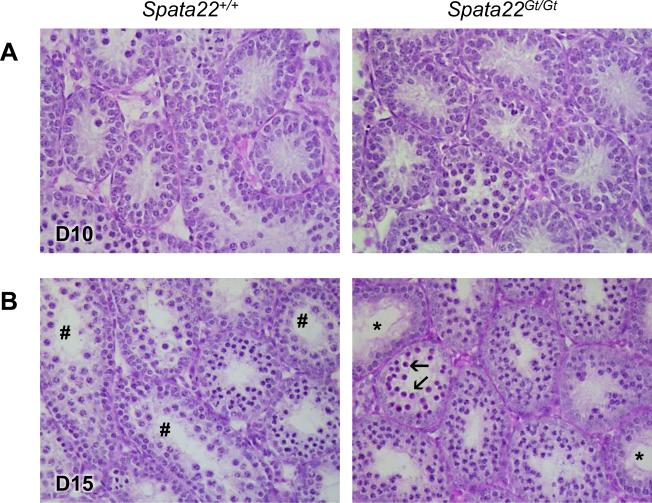
We next performed a histological examination of the first wave of spermatogenesis to define when spermatogenic defects first became apparent in Spata22Gt/Gt males. As we had previously observed in repro42 mutant males, there were no obvious differences in testis cellular composition or morphology between wild-type and mutant littermates at 10 dpp (Fig. 2A; (La Salle et al., 2012)). Differences in cellular composition became apparent by 12 dpp in mutant males, and these were even more prominent by 15 dpp (Fig. 2B and data not shown). Pachytene spermatocytes were absent in Spata22Gt/Gt seminiferous tubules and numerous cells with highly condensed nuclei (heteropycnotic nuclei) were visible. Seminiferous tubules showing cellular depletion typical of stage IV arrest were also observed in mutant testis sections. Absence of spermatocytes beyond the zygotene stage as well as post-meiotic germ cells was confirmed in 21 dpp Spata22Gt/Gt testis sections (data not shown). This phenotype of germ cell depletion observed in Spata22Gt/Gt mice was identical to the one previously observed in repro42 mutant mice, suggesting meiotic arrest in this mutant mouse line as well (La Salle et al., 2012).
Figure 2. Loss of germ cells is caused by meiotic arrest prior to pachynema in Spata22Gt/Gt testes.
(A) Histological differences are not detectable in cross-sections of D10 Spata22Gt/Gt testes (right) when compared to cross-sections of wild type testes (left). (B) At D15,tubules containing spermatogonia and spermatocytes up to the pachytene stage (number sign) are visible in wild type testes (left). However, differences in germ cell content are visible in mutant testes (right), which are devoid of pachytene spermatocytes and instead contain germ cells with condensed nuclei (arrows). Tubules with stage IV arrest (stars) are also observed. D, postnatal day. Original magnification: ×400.
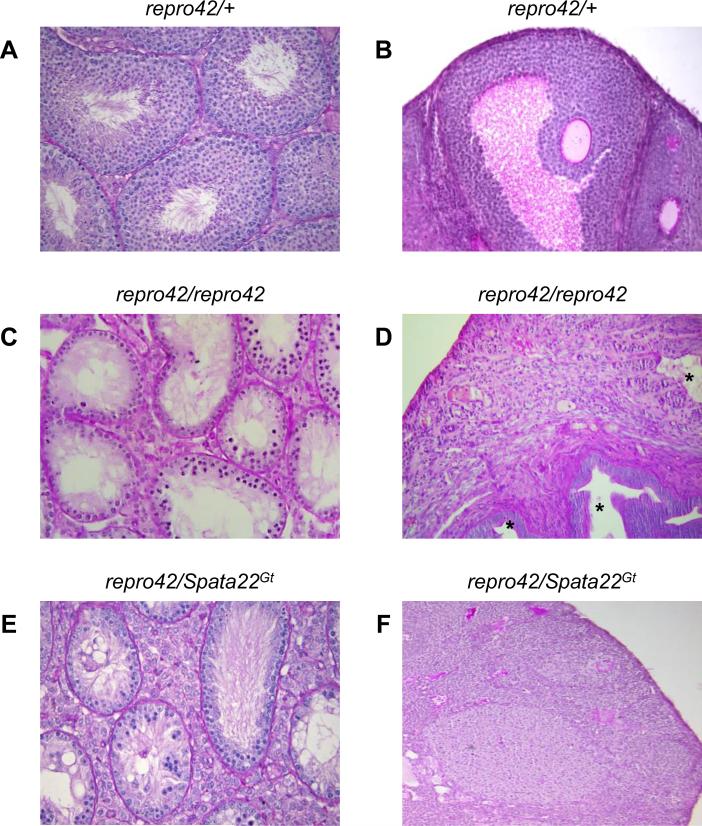
Since the Spata22Gt allele is the result of a targeted mutation in the Spata22 gene, we used it in a complementation test to genetically confirm that the repro42 phenotype is caused by the previously identified T-to-A transversion in exon 8 of Spata22 (La Salle et al., 2012). Compound heterozygous (repro42/Spata22Gt) males and females appeared overtly normal and presented no obvious phenotypic aberrations aside from sterility (Supplemental Table S3). Similarly to Spata22Gt/Gt and repro42/repro42 males (Figs. 1E and 3C), adult repro42/Spata22Gt males had smaller testes, and histological evaluation of testis sections revealed an absence of certain spermatocytes, spermatids, and spermatozoa (Fig. 3E; Supplemental Table S4). Like adult Spata22Gt/Gt and repro42/repro42 females (Figs. 1F and 3D), adult repro42/Spata22Gt females had smaller ovaries, lacked oocytes, and contained degenerated follicles as well as cysts (Fig. 3F). These analyses genetically confirmed that repro42 and Spata22Gt are alleles of the same gene, formally establishing the repro42 mutation as the Spata22repro42 allele (hereafter referred as such).
Figure 3. Mutation of Spata22 causes the repro42 phenotype.
(A, B) Representative adult testis (A) and ovary (B) cross-sections from repro42/+ displaying normal spermatogenesis and oogenesis, respectively. (C, D) Severe loss of germ cells is observed in cross-sections of adult Spata22repro42/repro42 testes (C) and ovaries (D). Cysts (stars) are visible in certain mutant ovaries. (E, F) Compound heterozygous repro42/Spata22Gt mice reveal meiotic arrest and severe germ cell depletion in repro42/Spata22Gt males (E), and a complete absence of oocytes in repro42/Spata22Gt females (F), demonstrating lack of complementation of the repro42 and Spata22Gt alleles. Original magnification: ×400.
SPATA22 is Required for Synapsis and DNA Repair in Spermatocytes and Oocytes
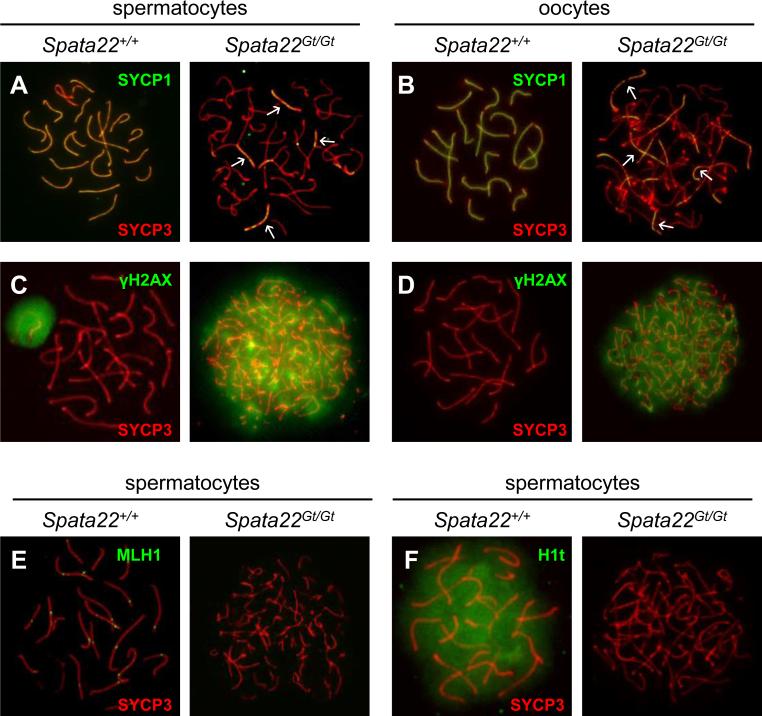
We previously determined that Spata22repro42/repro42 germ cells arrest at a late zygotene-like stage due to aberrant SC formation, synapsis and DNA repair (La Salle et al., 2012). To determine if meiotic progression was similarly compromised in Spata22Gt/Gt germ cells, expression and localization of specific meiotic markers was assessed in spread chromatin prepared from mutant spermatocytes and oocytes. Assembly of the SC and extent of synapsis were first evaluated by ascertaining the labeling patterns of the LE protein SYCP3 (Sycp3, synaptonemal complex protein 3) and the transverse filament protein SYCP1 (Sycp1, synaptonemal complex protein 1), which bridges the gap between one LE and the CE. SYCP3, a protein of the axial and lateral elements of the SC, presents a distinctive localization pattern used to define the substage of prophase I (La Salle et al., 2008). Here, the SYCP3 pattern revealed that fully formed LEs were established in Spata22Gt/Gt germ cells, indicating that mutant cells progress to the zygotene stage (Fig. 4A-B). However, completely individualized SCs were never observed in mutant spermatocytes or oocytes, suggesting that cells do not progress to pachynema. The labeling pattern of SYCP1 further supported this observation, revealing that formation of the SC and synapsis were indeed severely impaired (Fig. 4A-B). A complete array of fully synapsed homologs was never observed in Spata22Gt/Gt meiocytes, unlike wild-type pachytene spermatocytes and oocytes. Interestingly, whereas most mutant spermatocytes only presented short discontinuous stretches of CE-associated SYCP1 (Fig. 4A), longer continuous segments of SYCP1 were visible in mutant oocytes (Fig. 4B).
Figure 4. Synapsis and DNA repair are severely impaired in Spata22Gt/Gt spermatocytes and oocytes.
(A, B) Co-localization of SYCP1 (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (A) and oocytes (B). The pattern of yellow color representing co-labeling with anti-SYCP1 and anti-SYCP3 represents the extent of synapsis (white arrows). (C, D) Detection of γH2AX (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (C) and oocytes (D). The pattern of SYCP3 determines the prophase I substage. Whereas wild type germ cells reach pachynema, mutant cells appear to arrest at zygonema. The pattern of γH2AX reflects DSB formation and repair; persistence of γH2AX suggests DSBs are induced but not repaired in mutant germ cells. (E) Localization of MLH1 (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes. MLH1 is found at crossover sites in wild-type pachytene spermatocytes but is undetectable in mutant spermatocytes. (F) Detection of the testis-specific histone H1t (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes. H1t, which becomes expressed by mid-pachynema in wild type spermatocytes, is never observed in mutant cells, confirming that arrest takes place prior to the mid-pachytene stage. Original magnification: ×630.
Presence of DSBs and initiation of the cascade leading to their repair was also examined in Spata22Gt/Gt germ cells using the phosphorylated form of histone H2AFX (commonly known as γH2AX). γH2AX accumulates at DSBs upon their induction at leptonema and gradually disappears as breaks are repaired during zygonema; in spermatocytes, it becomes restricted to the XY body at pachynema (Mahadevaiah et al., 2001). While γH2AX became restricted to the XY body in wild-type pachytene spermatocytes, it persisted across the chromatin of Spata22Gt/Gt spermatocytes as well as that of mutant oocytes (Fig. 4C-D), confirming that DSBs were induced at the onset of meiosis but were not repaired before meiotic arrest. We also determined that MLH1 (Mlh1, mutL homolog 1), a protein involved in formation of crossovers between homologous chromosomes and usually expressed at pachynema (Baker et al., 1996; Edelmann et al., 1996), could not be detected in mutant spermatocytes (Fig. 4E). To confirm that meiotic arrest occurs prior to the mid-pachytene stage during spermatogenesis, we evaluated the presence of the mid-pachynema marker histone HIST1H1T (also known as H1t) in mutant spermatocytes (Cobb et al., 1999; Drabent et al., 1993; Inselman et al., 2003). H1t was never detected in Spata22Gt/Gt spermatocytes, confirming that arrest takes place prior to mid-pachynema in the male (Fig. 4F). Absence of a γH2AX-positive domain indicative of the XY body in mutant spermatocytes further confirmed that mutant cells do not reach pachynema (Fig. 4C). Taken together, these observations are consistent with a role for SPATA22 in synapsis and DNA repair in meiocytes of both sexes.
Recruitment of SPATA22 to Meiotic Chromosomes is Prominent at Zygonema
We previously showed that SPATA22 localizes to foci along meiotic chromosomes in mouse spermatocytes (Luo et al., 2013). Although the spatiotemporal distribution pattern of SPATA22 was examined in rat spermatocytes (Ishishita et al. 2014), it had not been defined in mouse germ cells. Because this is essential for inferring function, we further determined the localization dynamics of SPATA22 by immunolabeling spermatocyte and oocyte spread chromatin with an antibody against SPATA22. The labeling pattern of SYCP3 provided precise definition of the prophase I substage of each nucleus. In both spermatocytes and oocytes, SPATA22 localized to foci along axial and/or lateral elements during leptonema, zygonema and pachynema (Fig. 5A-E and Supplemental Fig. S1). In spermatocytes, SPATA22 was absent in early leptotene nuclei but became detectable by late leptonema (Fig. 5A and Table 1), and was present in foci arrays along SCs at zygonema and early pachynema (Fig. 5B-D). Most SPATA22 foci disappeared by the late pachytene stage and could not be detected at the diplotene stage (Fig. 5E and Table 1). The number of SPATA22 foci peaked at late zygonema (average of 134 ± 14 foci per cell), which was statistically different from the number of foci counted in early-to-mid zygotene spermatocytes (compared to 113 ± 20 foci, p <0.001) (Table 1). The overall distribution profile of SPATA22 in fetal oocytes was similar to that observed in spermatocytes, with SPATA22 foci most prominent at the zygotene stage (Supplemental Fig. S1 and Table 1). However, the number of SPATA22 foci differed quite remarkably between oocytes and spermatocytes from leptonema to early-to-mid zygonema. Whereas more SPATA22 foci were detected in leptotene spermatocytes than in oocytes at the same stage (69 ± 11 foci in spermatocytes compared to 24 ± 8 foci in oocytes, p<0.0001), the number of SPATA22 foci increased drastically in early-to-mid zygotene oocytes and was significantly higher than in spermatocytes at the same stage (234 ± 63 foci in oocytes compared to 113 ± 20 in spermatocytes, p <0.0001). Interestingly, the number of foci in late zygotene oocytes decreased to levels comparable to those observed in late zygotene spermatocytes (148 ± 26 foci compared to 134 ± 14 foci in spermatocytes, p= 0.06). Overall, the focal distribution pattern of SPATA22 suggests that this protein is recruited to recombination intermediates during prophase I in both male and female germ cells.
Figure 5. SPATA22 localizes to foci along meiotic chromosomes.
(A-E) Distribution of SPATA22 foci in spermatocytes during leptonema (A), early-to-mid-zygonema (B), late zygonema (C), early-to-mid pachynema (D) and late pachynema (E). SPATA22 (green) localizes to foci along axial/lateral elements identified by the SC protein SYCP3 (red). The overlay (left), SPATA22 labeling only (middle), and SYCP3 labeling only (right) are presented for each substage. Information on number of nuclei analyzed at each stage and distribution range can be found in Table 1. Original magnification: ×630.
SPATA22 Co-Localizes with Meiotic Recombination Factors
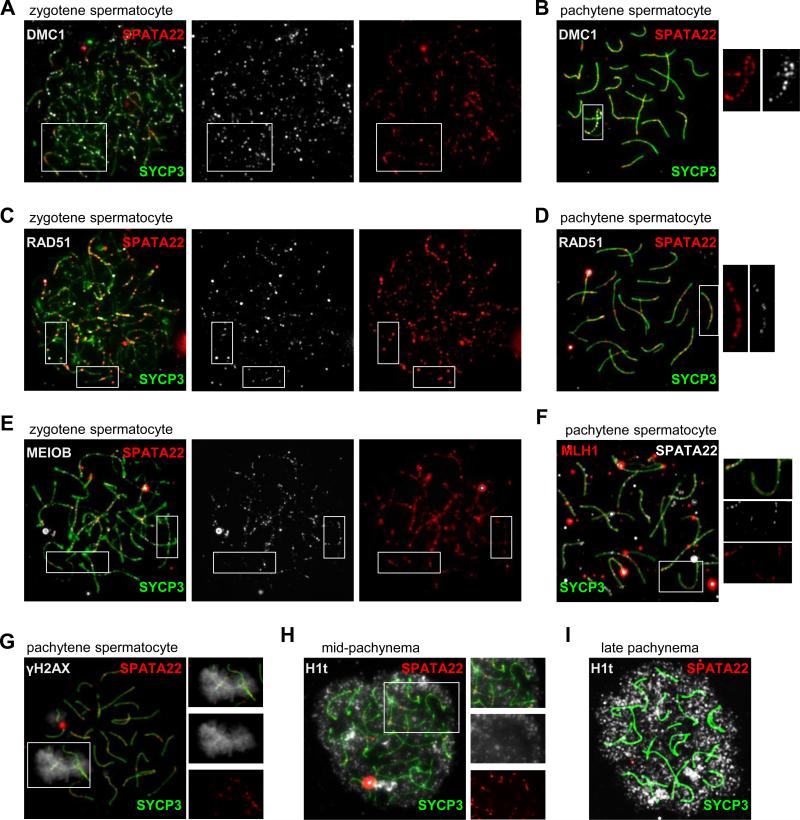
Our characterization of the MEIOB-SPATA22-RPA complex in mouse meiotic germ cells and observations made in rat spermatocytes demonstrating co-localization of SPATA22 with proteins involved in meiotic recombination (Ishishita et al., 2014) prompted us to determine the temporal relationship between SPATA22 and various meiotic recombination factors. Spread chromatin from spermatocyte was thus sequentially immunolabeled with antibodies against SYCP3 (to define the prophase I substage), SPATA22, and either DMC1, RAD51, MEIOB or MLH1 (Fig. 6A-F).
Figure 6. SPATA22 co-localizes with meiotic recombination factors.
SPATA22 co-localizes with DMC1, RAD51, MEIOB and MLH1 on meiotic chromosomes. The substage of each nucleus is identified by the pattern of SYCP3 (green). (A) DMC1 (white) and SPATA22 (red) are both present in foci along forming LEs (left panel) in zygotene spermatocytes but are not always found in the same foci (compare boxed area in middle and right panel). (B) By pachynema, DMC1 (white) and SPATA22 (red) are found in overlapping foci along the X chromosome as well as the pseudoautosomal region (boxed area, merged panel). Enlarged views of SPATA22 (left) and DMC1 (right) labeling in this area are shown. (C) RAD51 (white) and SPATA22 (red) are present in foci along forming LEs (left panel) in zygotene spermatocytes but are not always found in the same foci (compare boxed area in middle and right panel). (D) By pachynema, RAD51 (white) and SPATA22 (red) are found in overlapping foci along all SCs. Enlarged views of SPATA22 (left) and RAD51 (right) labeling of the boxed area in the merged panel are shown. (E) MEIOB (white) and SPATA22 (red) are present in foci along forming SCs (left panel) in zygotene spermatocytes but are not always found in the same foci (compare boxed area in middle and right panel). (F) For the most part, MLH1 (red) and SPATA22 (white) do not overlap in pachytene spermatocytes. However, a few foci appear to contain both proteins, as observed in the enlarged views of the merged image (top), SPATA22 labeling (middle) and MLH1 labeling (bottom) of the boxed area. (G) SPATA22 (red) localizes to foci along the XY chromosomal axes identified by γH2AX (white) labeling. Enlarged views of the merged image (top), γH2AX labeling (middle) and SPATA22 foci (bottom) of the boxed area demonstrate that SPATA22 foci are present in the XY body. (H) SPATA22 (red) is still present at midpachynema since foci are found in H1t (white)-positive cells. Enlarged views of the merged image (top), H1t labeling (middle) and SPATA22 labeling (bottom) of the boxed area are presented on the right. (I) Absence of SPATA22 is some H1t-positive late pachytene spermatocytes. Original magnification: ×630.
Although SPATA22 and DMC1 were both localized to foci on meiotic chromosomes at early zygonema, there was little overlap between the localization patterns of these two proteins at this stage (Fig. 6A). By the pachytene stage, DMC1 was no longer present in foci along meiotic chromosomes except along the X chromosome, including the partially synapsed region between sex chromosomes, where it co-localized with SPATA22 (Fig. 6B). Similarly, overlap between the patterns of RAD51 and SPATA22 foci was only partial at zygonema (Fig. 6C). Nonetheless, the two proteins appeared to co-localize by the pachytene stage, where most SPATA22-positive foci were also RAD51-positive (Fig. 6D). As we previously observed, focal co-localization between MEIOB and SPATA22 was extensive in spermatocytes (Fig. 6E; (Luo et al., 2013)). Interestingly, while most foci appeared to be both MEIOB- and SPATA22- positive, there were foci that contained only one protein or the other. Finally, although SPATA22 and MLH1 were usually present in different foci along meiotic chromosomes at pachynema, they co-localized in ~23% of foci (Fig. 6F and Table 2).
Table 2.
Distribution of SPATA22 and MLH1 foci at pachynema
| N | Average # of foci/nuclei (distribution range of foci) | Proportion of MLH1-only foci (distribution range of foci) | Proportion of SPATA22-only foci (distribution range of foci) | Proportion of MLH1 + SPATA22 foci (distribution range of foci) |
|---|---|---|---|---|
| 8 | 79 (58-110) | 39% (35-70) | 38% (35-65) | 23% (12-25) |
We next verified that SPATA22 indeed localized to all chromosomes and was not excluded from sex chromosomes. To this end, we assessed the presence of SPATA22 in relation to γH2AX, which becomes restricted to the XY body in wild-type pachytene spermatocytes. As we observed with SPATA22 and DMC1 co-localization in pachytene spermatocytes (Fig. 6B), we found SPATA22 foci in the γH2AX-positive XY body, confirming that SPATA22 is indeed recruited to sex chromosomes (Fig. 6G). To corroborate that SPATA22 is present until at least the mid-pachytene stage, we evaluated the presence of SPATA22 in conjunction with that of H1t, which becomes expressed by mid-pachynema in spermatocytes (Cobb et al., 1999; Drabent et al., 1993; Inselman et al., 2003). H1t-positive pachytene spermatocytes could be classified into two groups with regards to SPATA22 expression. SPATA22 foci were visible in a subset of H1t-positive cells (Fig. 6H), whereas other cells were devoid of SPATA22 despite being positive for H1t (Fig. 6I), suggesting that SPATA22 persists until at least mid-pachynema but is removed from recombination intermediates shortly thereafter.
SPATA22 Deficiency Impairs the Production and Localization of MEIOB
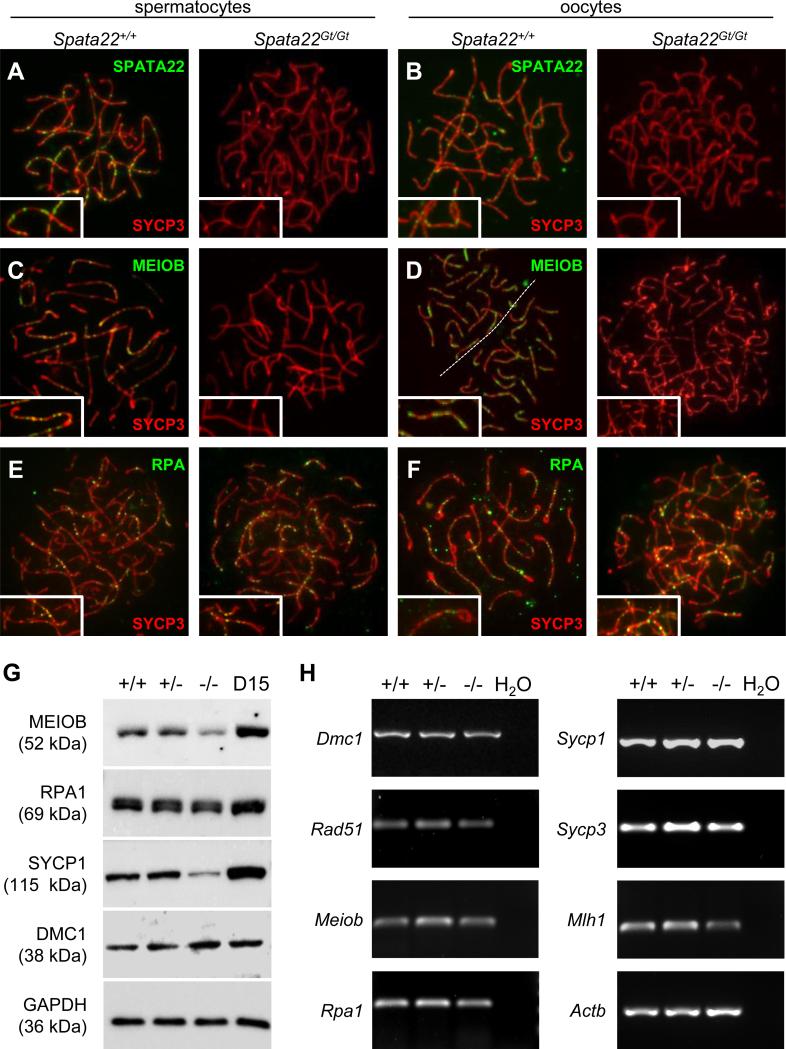
Characterization of Meiob-null spermatocytes previously demonstrated that SPATA22 localization and stability were dependent on the presence of MEIOB in germ cells, whereas localization of RPA was seemingly unaffected (Luo et al., 2013). Similarly, MEIOB foci were absent in Spata22repro42/repro42 spermatocytes. Analysis of spermatocytes isolated from the tremor rat model, which lacks a large (>200 kb) segment of rat chromosome 10 including the Spata22 gene, suggested that RPA localization was unaffected in these cells (Ishishita et al., 2014), but this had not been verified in the mouse. We thus explored the distribution dynamics of the members of the MEIOB-SPATA22-RPA complex in spread chromatin prepared from wild-type and Spata22Gt/Gt meiocytes. Both SPATA22 and MEIOB foci were visually undetectable in mutant spermatocytes and oocytes (Fig. 7A-D), whereas RPA foci were present along meiotic chromosomes in mutant cells of both sexes (Fig. 7E-F). We next investigated if the production of MEIOB and RPA proteins was compromised in Spata22Gt/Gt whole testis extracts (Fig. 7G and Supplemental Fig. S2). The level of MEIOB protein was reduced by almost half in Spata22Gt/Gt testis, but it was not completely absent, consistent with previous observations made in Spata22repro42/repro42 testis extracts (Luo et al., 2013). Both the native and phosphorylated forms of RPA1, one of the three subunit of the RPA heterotrimeric complex, were detected in mutant testis extracts at levels similar to the ones observed in wild-type and heterozygous testis extracts, suggesting that production and/or stability of RPA is not compromised in the absence of SPATA22.
Figure 7. SPATA22 deficiency impairs localization and/or stability of proteins required for meiotic recombination and synapsis.
(A, B) Localization of SPATA22 (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (A) and oocytes (B).(C, D) Localization of MEIOB (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (C) and oocytes (D). (E, F) Localization of RPA (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (E) and oocytes (F). Whereas SPATA22 and MEIOB are absent in mutant meiocytes, RPA localization remains unaffected. (G) Immunoblotting analysis of protein extracts prepared from D15 wild type (+/+), heterozygous (+/−), and homozygous mutant (−/−) testes, as well as D15 B6 control testes, with antibodies against MEIOB, RPA1, SYCP1 and DMC1. Expression of GAPDH (bottom panel) served as a loading control to normalize protein expression across genotypes. The molecular weight of each protein (in kDa) is indicated to the left of the corresponding immunoblot. (H) Analysis of RNA extracts prepared from D15 wild type (+/+), heterozygous (+/−), and homozygous mutant (−/−) testes. Transcripts for all meiotic genes assessed were amplified by RT-PCR. The name of the gene evaluated is indicated to the left of each corresponding panel. Expression of Actb was used to normalize the expression of each gene across genotypes. H2O, water control. D, postnatal day.
Finally, to gain further insight into the impact of Spata22 mutation on the meiotic process, we evaluated the expression of Dmc1, Rad51, Meiob, Rpa, Mlh1, Sycp1, and Sycp3 in RNA extracted from Spata22Gt/Gt testes. These genes were chosen because their protein product co-localizes with SPATA22 (Fig. 6) and/or a defect in their distribution was observed in SPATA22-deficient cells (Fig. 4 and 7B-F). Transcripts for all genes assessed were detected in mutant testis RNA at levels comparable to those observed in wild-type and heterozygous testes, suggesting no transcriptional inhibition in the absence of SPATA22 (Fig. 7H and Supplemental Table S5). Since localization of the CE-associated protein SYCP1 was greatly compromised in Spata22Gt/Gt germ cells of both sexes, we also assessed the presence of SYCP1 in Spata22Gt/Gt testis protein extracts. Consistent with our immunolocalization findings (Fig. 4A), the levels of SYCP1 in mutant testis extracts were considerably reduced; they reached only ~20% of those observed in wild type and heterozygous testes. In contrast, DMC1 production was unaffected (Fig. 7G and Supplemental Fig. S2).
DISCUSSION
Here, we characterized a novel null allele of the Spata22 gene, Spata22Gt, which fails to complement our previously published Spata22repro42 allele (La Salle et al., 2012). In addition to demonstrating allelism, our data confirm that Spata22 is essential for germ cell development. As we previously reported in Spata22repro42/repro42 mice, Spata22Gt/Gt mice are infertile due to arrest of germ cell development prior to pachynema during prophase I of meiosis. Impaired synapsis and DSB repair in Spata22Gt/Gt spermatocytes and oocytes demonstrate that SPATA22 is essential for meiotic progression in both sexes. Consistent with a role during prophase I of meiosis, SPATA22 localizes to foci along meiotic chromosomes predominantly at zygonema in meiocytes of both sexes, implying that SPATA22 acts during processing of DSBs during meiotic recombination. Assessment of protein complexes at recombination intermediates shows that SPATA22 co-localizes with DMC1, RAD51, MEIOB and MLH1 to varying degrees, which further supports such a role. Our analysis of Spata22Gt/Gt spermatocytes also corroborates that MEIOB localization requires SPATA22, whereas RPA localization does not, confirming that MEIOB and SPATA22 localization is interdependent. The Spata22Gt allele thus provides a novel model to study SPATA22 function and requirement during gametogenesis.
Concordance of the Spata22 expression profiles and mutant phenotypes of meiotic arrest in male and female gonads implies that SPATA22 belongs to a group of proteins contributing to a sexually conserved feature of meiosis in mammals (this study and (Ishishita et al., 2013; La Salle et al., 2012)). Spata22Gt/Gt meiocytes arrest during prophase I of meiosis at a stage resembling late zygonema. Although DSBs are induced normally in the absence of SPATA22, their persistence, as indicated by prolonged detection of γH2AX, suggests impeded DNA repair. The initial steps of meiotic recombination appear to take place normally, as RAD51 and RPA are both loaded at recombination nodules; however, crossovers never form in mutant spermatocytes (this study and (Ishishita et al., 2014; La Salle et al., 2012)). Furthermore, despite the apparent normal elaboration of lateral elements, only short stretches of SYCP1 are observed, indicating that formation of the SC is also incomplete. Therefore, both synapsis and progression of meiotic recombination are impaired in Spata22Gt/Gt meiocytes. Elimination of spermatocytes at stage IV and of oocytes shortly after birth supports the activation of a DNA damage-dependent response leading to elimination of germ cells upon abrogation of SPATA22 function (Barchi et al., 2005; Di Giacomo et al., 2005). Mutations in other DNA repair genes, such as Atm (ataxia telangiectasia mutated), Dmc1, Msh4 (mutS homolog 4), and Msh5 (mutS homolog 5), hinder synapsis and progression through meiosis in both germ lines (Di Giacomo et al., 2005; Edelmann et al., 1999; Kneitz et al., 2000; Pittman et al., 1998; Yoshida et al., 1998). For example, mice deficient in DMC1, a meiosis-specific RecA homolog which mediates homologous chromatid invasion following 3’ to 5’ resection of DSBs, are infertile and present a phenotype of meiotic arrest nearly identical to the one observed in both Spata22repro42/repro42 and Spata22Gt/Gt mice (Pittman et al., 1998; Yoshida et al., 1998). Therefore, aberrant synapsis in Spata22Gt/Gt meiocytes is likely the consequence of defective DNA repair.
Still, the possible relationship between components of the SC and SPATA22 remains to be explored. Although limited synapsis is commonly observed when meiotic recombination is disrupted, the possibility that certain constituents of the SC and the recombination machinery directly interacts remains largely untested in mammals. Limited studies have reported interactions between RAD51 and SYCP1, as well as between RAD51 and the CE protein SYCE2 (Syce2, synaptonemal complex central element protein 2) (Bolcun-Filas et al., 2009; Tarsounas et al., 1999). Exploring further the interdependency between the recombination machinery and the SC should delineate the network of interactions required to support both of these essential meiotic processes.
Examination of SPATA22 distribution during prophase I of meiosis lends further support to a role for this protein in meiotic recombination. We, as well as others, previously showed that SPATA22 localizes to foci along meiotic chromosomes in mouse and rat spermatocytes (Ishishita et al., 2014; Luo et al., 2013). Here we found that SPATA22 foci are predominant at zygonema in both oocytes and spermatocytes, although SPATA22 first appears at late leptonema and persists until mid-pachynema. Overall, our findings are consistent with the observed distribution profile of SPATA22 in rat spermatocytes (Ishishita et al., 2014). However, the number of foci recorded in rat spermatocytes, especially during zygonema, is consistently higher than we have found in mouse spermatocytes. Species-specific differences may well account for these discrepancies, as variations in the number of DSBs induced at the onset of meiosis have been reported among different inbred strains of male mice (Baier et al., 2014). The time course and focal localization pattern noted for SPATA22 are consistent with those observed for recombination-related proteins involved in resolving DNA-DNA interactions prior to reciprocal recombination, such as RPA, MSH4 and BLM (Blm, Bloom syndrome, RecQ helicase-like) (Moens et al., 2002; Moens et al., 2007). SPATA22 is likely recruited to recombination intermediates following DMC1 and RAD51, which are components of early nodules, since SPATA22 rarely co-localizes to the same sites as these proteins at zygonema. In accordance with progressive repair of DSBs as cells proceed through prophase I, the number of SPATA22 foci gradually decreases as cells reach pachynema. Interestingly, DMC1 and RAD51 both co-localize with SPATA22 along meiotic chromosomes at pachynema, suggesting that SPATA22 may interact with these proteins at this stage and/or participate in nucleofilament formation leading to strand invasion and resolution of recombination intermediates as previously proposed (Ishishita et al., 2014). SPATA22 remains associated with meiotic chromosomes until at least the midpachytene stage, where it can be found with MLH1 in more than 20% of foci. Taken together, our results indicate that SPATA22 most likely functions at transitional nodules during meiotic recombination, prior to crossing-over (Moens et al., 2007).
Unexpectedly, we found sexual dimorphism in the distribution of SPATA22 along chromosomal axes between male and female meiocytes. The average number of SPATA22 foci noted was significantly higher in oocytes than in spermatocytes by approximately two-fold at early-to-mid zygonema. These findings concord with observations made in human meiocytes, whereby sex-specific differences in the number of RAD51 foci, used to estimate the number of SPO11-induced DSBs, are seen between spermatocytes and oocytes (Gruhn et al., 2013). At leptonema, the mean number of RAD51 foci is significantly lower in male germ cells, with most spermatocytes exhibiting fewer than 200 RAD51 foci and almost all oocytes presenting more than 200 foci, providing evidence that there are more DSBs induced in oocytes than spermatocytes at the onset of meiosis in humans. Our results support a similar trend in the mouse, although SPATA22 presumably functions downstream of RAD51. The number of SPATA22 foci remains higher in oocytes until mid-pachynema; difficulties in precisely staging mid-to-late pachytene meiocytes most likely account for the trend reversal observed at this stage. Alternatively, temporal and quantitative differences in recruitment of SPATA22 to meiotic chromosomes may reflect variations in the mode of action of SPATA22 between sexes. However, this is unlikely since obvious sex-specific phenotypic differences have yet to be noted in either Spata22repro42/repro42 or Spata22Gt/Gt meiocytes (La Salle et al., 2012).
Recently, we identified MEIOB as a meiosis-specific protein that associates with SPATA22 and RPA during prophase I in the mouse (Luo et al., 2013). MEIOB binds single-stranded DNA, possesses 3’-5’ exonuclease activity, and is thought to be involved in second homologue end processing during meiotic recombination in mammals. Meiob- and Spata22- null mice present with analogous phenotypes (La Salle et al., 2012; Luo et al., 2013; Souquet et al., 2013). Meiob−/− mice are infertile due to meiotic arrest caused by aberrant DSB repair as well as asynapsis. Furthermore, both RPA and RAD51 are loaded normally on meiotic chromosomes but their removal is impaired, whereas MLH1 is never detected in mutant cells. Here we confirmed that localization and stability of MEIOB and SPATA22 are indeed reciprocally interdependent, whereas RPA, the other member of the complex, persists along SCs and is thus stabilized in the absence of SPATA22, similarly to what we previously observed in Meiob-null spermatocytes (Luo et al., 2013). The persistence of DSBs and severe synaptic defects triggered by inactivation of Spata22 and Meiob suggest that both of these proteins are normally required for proper progression through meiosis, most likely through their co-expression and interaction. Surprisingly, not all sites containing MEIOB include SPATA22 (and vice versa), raising the possibility that these proteins have other interacting partners and/or accomplish additional functions independent of their interaction. Our results certainly indicate that SPATA22 could interact with both RAD51 and DMC1 during prophase I. Observations made in the tremor rat model also suggest that SPATA22 could function in RAD54L- (Rad54l, RAD54 like) and RAD54B- (Rad54b, RAD54 homolog B) mediated heteroduplex formation and D-loop disruption by interacting with RPA, although this remains untested (Ishishita et al., 2014).
Despite conservation of the most prominent features of meiosis between yeast and mammals, many key steps of meiotic recombination remain poorly explained in mammals. The severe and profound meiotic defects observed in Spata22-deficient mice demonstrate that SPATA22 plays a crucial role during prophase I of meiosis. Our results indicate that SPATA22 is required for meiotic recombination following induction and initial processing of DSBs. Studies aimed at delineating the functional domains of SPATA22 and identifying additional interacting partners should provide insight into the mechanism of action of this meiotic recombination factor.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jessica Landaiche, Rabiya Ghani and Chelsea Schonert for making some of the initial observations related to this work, as well as Patrick Davis, Angelica Lorenzo, Erica Yothment, Evan Hale, and Michael Lewis for their technical assistance. We are grateful to Drs. P. Jeremy Wang (University of Pennsylvania Perelman School of Medicine) and Mary Ann Handel (The Jackson Laboratory) for kindly providing the MEIOB antibody, and the SYCP3 and H1t antibodies, respectively. We also thank Dr. Elizabeth Snyder (The Jackson Laboratory) for help with histological procedures. We acknowledge the Scientific Services of the Jackson Laboratory as well as the Office of Research and Sponsored Programs and the Animal Resources of Midwestern University for their outstanding support. Finally, we are greatly indebted to Dr. Joshua Gasiorowski (Midwestern University) and Drs. Mary Ann Handel and Laura Reinholdt (The Jackson Laboratory) for their critical comments on this manuscript. This study was supported by funds provided by Midwestern University (to SL). Mutant mouse alleles were produced by the Reproductive Genomics Program at The Jackson Laboratory with support from the NIH (HD42137).
Footnotes
The authors have nothing to declare.
EH, NM, VD, ZF, SB and KH performed the experiments; EH, VD and SL designed the research study and analyzed the data; EH and SL wrote the manuscript; all authors reviewed the manuscript.
REFERENCES
- Baier B, Hunt P, Broman KW, Hassold T. Variation in genome-wide levels of meiotic recombination is established at the onset of prophase in mammalian males. PLoS Genet. 2014;10:e1004125. doi: 10.1371/journal.pgen.1004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, Ashley T, Liskay RM. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- Barchi M, Mahadevaiah S, Di Giacomo M, Baudat F, de Rooij DG, Burgoyne PS, Jasin M, Keeney S. Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol. 2005;25:7203–7215. doi: 10.1128/MCB.25.16.7203-7215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F, Buard J, Grey C, Fledel-Alon A, Ober C, Przeworski M, Coop G, de Massy B. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science. 2010;327:836–840. doi: 10.1126/science.1183439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation. Nat Rev Genet. 2013;14:794–806. doi: 10.1038/nrg3573. [DOI] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. Journal of Cell Biology. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E, Hall E, Speed R, Taggart M, Grey C, de Massy B, Benavente R, Cooke HJ. Mutation of the mouse Syce1 gene disrupts synapsis and suggests a link between synaptonemal complex structural components and DNA repair. PLoS Genet. 2009;5:e1000393. doi: 10.1371/journal.pgen.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb J, Cargile B, Handel MA. Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev Biol. 1999;205:49–64. doi: 10.1006/dbio.1998.9101. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Pollack SE, Pollard JW. Genetic analysis of chromosome pairing, recombination, and cell cycle control during first meiotic prophase in mammals. Endocr Rev. 2006;27:398–426. doi: 10.1210/er.2005-0017. [DOI] [PubMed] [Google Scholar]
- Costa Y, Cooke HJ. Dissecting the mammalian synaptonemal complex using targeted mutations. Chromosome Res. 2007;15:579–589. doi: 10.1007/s10577-007-1142-1. [DOI] [PubMed] [Google Scholar]
- Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci U S A. 2005;102:737–742. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabent B, Bode C, Doenecke D. Structure and expression of the mouse testicular H1 histone gene (H1t). Biochim Biophys Acta. 1993;1216:311–313. doi: 10.1016/0167-4781(93)90162-7. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, Pollard JW, Kolodner RD, Kucherlapati R. Meiotic pachytene arrest in MLH1-deficient mice. Cell. 1996;85:1125–1134. doi: 10.1016/s0092-8674(00)81312-4. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard JW, Kucherlapati R. Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet. 1999;21:123–127. doi: 10.1038/5075. [DOI] [PubMed] [Google Scholar]
- Fraune J, Schramm S, Alsheimer M, Benavente R. The mammalian synaptonemal complex: Protein components, assembly and role in meiotic recombination. Experimental Cell Research. 2012;318:1340–1346. doi: 10.1016/j.yexcr.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Griswold MD. Spermatogenesis: The Commitment to Meiosis. Physiol Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhn JR, Rubio C, Broman KW, Hunt PA, Hassold T. Cytological Studies of Human Meiosis: Sex-Specific Differences in Recombination Originate at, or Prior to, Establishment of Double-Strand Breaks. PLoS ONE. 2013;8:e85075. doi: 10.1371/journal.pone.0085075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–136. doi: 10.1038/nrg2723. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- Hunter N. Meiotic Recombination: The Essence of Heredity. Cold Spring Harb Perspect Biol. 2015;7:a016618. doi: 10.1101/cshperspect.a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselman A, Eaker S, Handel MA. Temporal expression of cell cycle-related proteins during spermatogenesis: establishing a timeline for onset of the meiotic divisions. Cytogenetic and Genome Research. 2003;103:277–284. doi: 10.1159/000076813. [DOI] [PubMed] [Google Scholar]
- Ishishita S, Inui T, Matsuda Y, Serikawa T, Kitada K. Infertility associated with meiotic failure in the tremor rat (tm/tm) is caused by the deletion of spermatogenesis associated 22. Exp Anim. 2013;62:219–227. doi: 10.1538/expanim.62.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishishita S, Matsuda Y, Kitada K. Genetic evidence suggests that Spata22 is required for the maintenance of Rad51 foci in mammalian meiosis. Sci Rep. 2014;4:6148. doi: 10.1038/srep06148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- Keeney S, Lange J, Mohibullah N. Self-organization of meiotic recombination initiation: general principles and molecular pathways. Annu Rev Genet. 2014;48:187–214. doi: 10.1146/annurev-genet-120213-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneitz B, Cohen PE, Avdievich E, Zhu L, Kane MF, Hou H, Jr., Kolodner RD, Kucherlapati R, Pollard JW, Edelmann W. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev. 2000;14:1085–1097. [PMC free article] [PubMed] [Google Scholar]
- La Salle S, Palmer K, O'Brien M, Schimenti JC, Eppig J, Handel MA. Spata22, a novel vertebrate-specific gene, is required for meiotic progress in mouse germ cells. Biol Reprod. 2012;86:45. doi: 10.1095/biolreprod.111.095752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Salle S, Sun F, Zhang XD, Matunis MJ, Handel MA. Developmental control of sumoylation pathway proteins in mouse male germ cells. Dev Biol. 2008;321:227–237. doi: 10.1016/j.ydbio.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam I, Keeney S. Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb Perspect Biol. 2015;7:a016634. doi: 10.1101/cshperspect.a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Yang F, Leu NA, Landaiche J, Handel MA, Benavente R, La Salle S, Wang PJ. MEIOB exhibits single-stranded DNA-binding and exonuclease activities and is essential for meiotic recombination. Nature Communications. 2013;4:2788. doi: 10.1038/ncomms3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Kolas NK, Tarsounas M, Marcon E, Cohen PE, Spyropoulos B. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. Journal of Cell Science. 2002;115:1611–1622. doi: 10.1242/jcs.115.8.1611. [DOI] [PubMed] [Google Scholar]
- Moens PB, Marcon E, Shore JS, Kochakpour N, Spyropoulos B. Initiation and resolution of interhomolog connections: crossover and non-crossover sites along mouse synaptonemal complexes. Journal of Cell Science. 2007;120:1017–1027. doi: 10.1242/jcs.03394. [DOI] [PubMed] [Google Scholar]
- Morelli MA, Cohen PE. Not all germ cells are created equal: aspects of sexual dimorphism in mammalian meiosis. Reproduction. 2005;130:761–781. doi: 10.1530/rep.1.00865. [DOI] [PubMed] [Google Scholar]
- Parvanov ED, Petkov PM, Paigen K. Prdm9 controls activation of mammalian recombination hotspots. Science. 2010;327:835. doi: 10.1126/science.1181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, Handel MA, Schimenti JC. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1:697–705. doi: 10.1016/s1097-2765(00)80069-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro J, Abby E, Livera G, Martini E. RPA homologs and ssDNA processing during meiotic recombination. Chromosoma. 2016;125:265–276. doi: 10.1007/s00412-015-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquet B, Abby E, Herve R, Finsterbusch F, Tourpin S, Le Bouffant R, Duquenne C, Messiaen S, Martini E, Bernardino-Sgherri J, Toth A, Habert R, Livera G. MEIOB targets single-strand DNA and is necessary for meiotic recombination. PLoS Genet. 2013;9:e1003784. doi: 10.1371/journal.pgen.1003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarsounas M, Morita T, Pearlman RE, Moens PB. RAD51 and DMC1 form mixed complexes associated with mouse meiotic chromosome cores and synaptonemal complexes. J Cell Biol. 1999;147:207–220. doi: 10.1083/jcb.147.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Wang PJ. The Mammalian synaptonemal complex: a scaffold and beyond. Genome Dyn. 2009;5:69–80. doi: 10.1159/000166620. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T. The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell. 1998;1:707–718. doi: 10.1016/s1097-2765(00)80070-2. [DOI] [PubMed] [Google Scholar]
- Zickler D, Kleckner N. Recombination, Pairing, and Synapsis of Homologs during Meiosis. Cold Spring Harb Perspect Biol. 2015;7:a016626. doi: 10.1101/cshperspect.a016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.