Abstract
After leaving the testis, sperm have not yet acquired the ability to move progressively and are unable to fertilize oocytes. To become fertilization-competent they must go through an epididymal maturation process in the male, and capacitation in the female tract. Epididymal maturation can be defined as those changes occurring to sperm in the epididymis that render the sperm the ability to capacitate in the female tract. As part of this process, sperm cells undergo a series of biochemical and physiological changes that require incorporation of new molecules derived from the epididymal epithelium, as well as post-translational modifications of endogenous proteins synthesized during spermiogenesis in the testis. This review will focus on epididymal maturation events, with emphasis in recent advances in the understanding of the molecular basis of this process.
Keywords: sperm, epididymis, epididymosomes, maturation, signal transduction
1. INTRODUCTION
Spermatogenesis is the process by which functional sperm cells are produced in the testis. After this process is completed, sperm are morphologically highly differentiated; however, they are unable to move progressively and have not yet acquired fertilizing capacity. To become fertilizing competent, sperm need to undergo two extra-testicular maturational processes, one in the male reproductive tract, known as epididymal sperm maturation, which will be the focus of this review, and the second in the female tract, known as capacitation. Both of them are associated with sequential biochemical changes occurring in different sperm compartments. Of these two extra-testicular maturational processes, more is known about capacitation. This process was discovered independently by Chang and Austin in 1951 (Austin 1951, Chang 1951). Soon after, in vitro capacitation media were developed and the process extensively studied. The discovery of in vitro capacitation was a necessary step for the consequent development of in vitro fertilization (IVF) and facilitated the understanding of the signaling mechanisms involved in this process. For more information on capacitation, see reviews on this topic (Aitken et al. 2013, Buffone et al. 2014, Gervasi et al. 2016, Miller et al. 2015, Nishigaki et al. 2014, Santi et al. 2013).
On the other hand, the concept of epididymal maturation was developed throughout the years. Changes in motility occurring to spermatozoa during epididymal transit were first described in 1913 (Tournade 1913) and some years later, epididymal transit was linked with the acquisition of fertility (Young 1931). Little was done on this topic until the 1960’s when in a series of elegant manuscripts Bedford and other groups demonstrated the relevance of the epididymis and the epididymal milieu for the sperm acquisition of progressive motility and fertilizing capacity (Bedford 1963a, Bedford 1963b, Bedford 1967, Blandau et al. 1964, Orgebin-Crist 1967, Orgebin-Crist 1968, Orgebin-Crist 1969). Despite these earlier discoveries, contrary to capacitation, development of in vitro models of epididymal maturation had remained elusive. Consequently, the molecular changes regulating this process have been more difficult to investigate than those playing a role during capacitation.
The epididymis is a highly segmented organ that can be divided into four main anatomical regions: initial segment (proximal region, closest to the testis), caput (region between the initial segment and the corpus), corpus (middle portion), and cauda (distal region connected with the vas deferens). Although the initial segment is a hallmark of the epididymis in rodent species, its presence in other mammals has not been clearly described. Each of these segments displays differential expression of genes and maintains distinct luminal ion concentrations, which are essential to regulate the steps of sperm maturation. Most studies on sperm maturation have relied on the comparison between immature sperm taken from caput and mature sperm obtained from the cauda epididymidis. Some studies have also added corpus sperm as an intermediate maturational state. Alternatively, some approaches have used in vitro incubation of intact or demembranated caput spermatozoa in the presence of metabolites and pharmacological agents (Lindemann 1978, Mohri et al. 1980, Treetipsatit et al. 1982). Finally, more recent relevant evidence has been derived from the use of knock-out mouse models (Andersen et al. 2003, Huang et al. 2005, Joseph et al. 2010, Koch et al. 2015, Krapf et al. 2012, Krutskikh et al. 2012, Miyata et al. 2015, Xu et al. 2014). Altogether, these approaches have provided a starting point towards a working model of epididymal maturation signaling pathways. This review is written from the sperm’s perspective; however, the relevance of other aspects of the epididymis should not be overlooked, and excellent supplementary reading can be found in recent reviews (Bedford 2015, Belleannee et al. 2012, Da Silva et al. 2015, Dacheux et al. 2014, Shum et al. 2011).
2. SPERM CHANGES OCCURRING DURING EPIDIDYMAL TRANSIT
2.1. Acquisition of progressive movement
Cauda epididymal sperm suspended in appropriate saline buffers display progressive motility, but testicular and caput epididymal sperm do not. However, if immature sperm are demembranated in the presence of low concentrations of the non-ionic detergent Triton X-100, and then reactivated with ATP and cAMP, they become motile (Mohri et al. 1980, Yeung 1984). Although the motility achieved by caput sperm in these conditions is similar in intensity to that of cauda sperm, they exhibit different flagellar bending (Vadnais et al. 2013, Yeung 1984). The possibility of inducing motility in demembranated testicular and caput sperm indicates that immature sperm have functional flagellar machinery. In support of this conclusion are epididymal duct ligation experiments showing that spermatozoa retained in the caput region for an extended period become able to move progressively (Bedford 1967, Orgebin-Crist 1967). Despite these findings, it is still unclear which biochemical changes occur to the sperm during epididymal transit that render these cells capable of moving. More recent studies have started to address this problem through comparison and manipulation of signaling pathways in sperm taken from different regions of the epididymis (see Section 3 below).
2.2. Migration of the cytoplasmic droplet (CD)
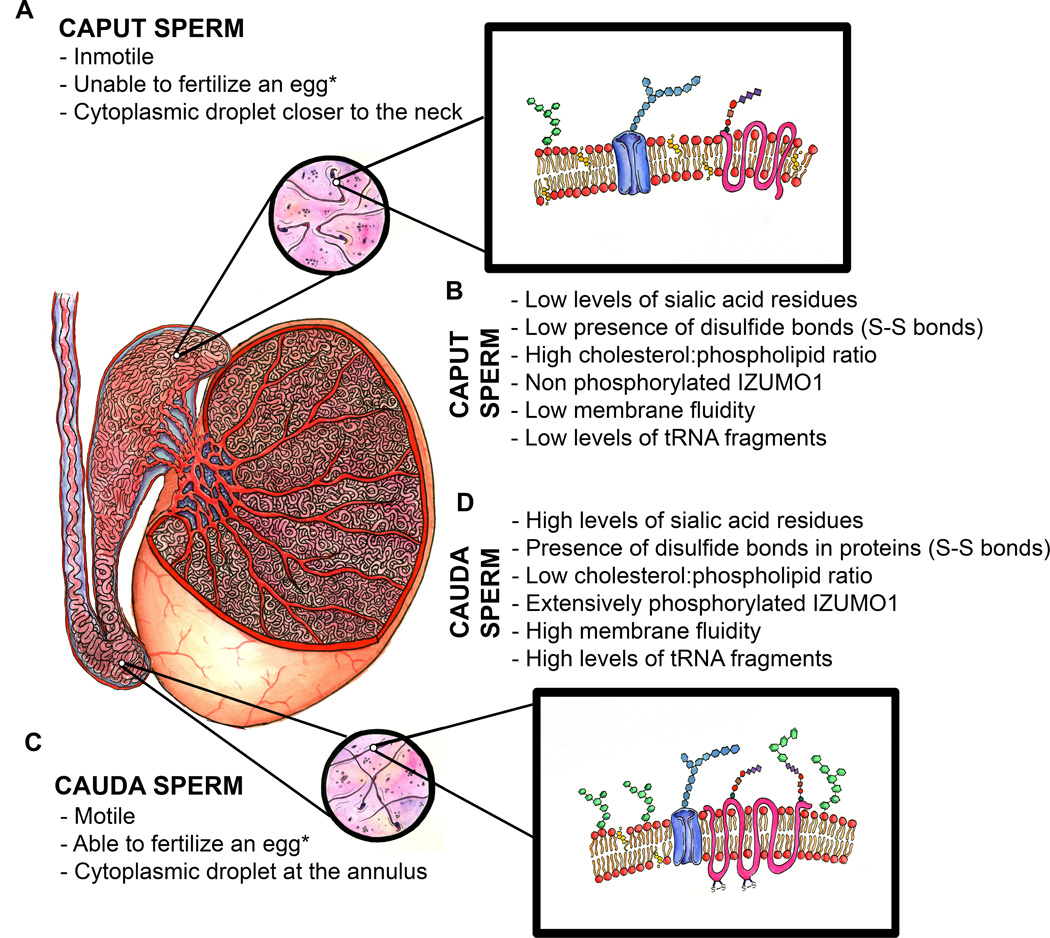
Although the ability to move progressively is one of the better characterized endpoints, maturation is also associated with other morphological, biochemical and physiological changes occurring to the sperm in their transit through the epididymis (Figure 1). Morphologically, the most obvious change is the migration of the cytoplasmic droplet (CD) from the sperm neck towards the annulus, which connects the mid-piece with the principal piece (Cooper 2011). The CD comprises germ cell cytoplasm remaining from the spermiogenesis process. In recent years, high resolution helium electron-microscopic techniques have shown that spermatozoa in the epididymis may interact with the surrounding epithelium. Images obtained with this technique clearly show the interaction of the sperm CD with many vesicle-like structures that might correspond to epididymosomes (see below) (Paunescu et al. 2014).
Figure 1.
Schematic representation of a mammalian testis, epididymis and sperm.* after incubation in conditions that support sperm capacitation. A) Principal morphological and functional characteristics of immature caput sperm. B) Molecular characteristics of immature caput sperm. C) Principal morphological and functional characteristics of mature cauda sperm. D) Molecular characteristics of mature cauda sperm.
Depending on the species, mature sperm may lose this organelle upon ejaculation. Whether retention of the CD in mature sperm has a functional role is not known yet (Cooper 2011). Sperm retention of CD has been associated with reduced fertility in boar and bull (reviewed in Sutovsky et al. 2010). Despite these results, a recent study (Yuan et al. 2013) presented evidence that mouse cauda epididymal sperm bearing CD were more motile that those without the organelle. Proteomic analyses of purified mouse CD revealed the presence of a large number of enzymes associated with energy metabolism (Au et al. 2015, Yuan et al. 2013). Another protein found in CDs is TEX101, a GPI-anchored protein in the plasma membrane of testicular sperm. This protein has been shown to be essential for processing of the metalloprotease ADAM3, an enzyme required for sperm transport through the utero-tubal junction (UTJ) (Yamaguchi et al. 2009). Consistently, knock-out male mice models lacking TEX101 are infertile, they do not have ADAM3 in their sperm plasma membrane and are deficient in sperm transport through the UTJ (Fujihara et al. 2013). TEX101 is released from most of the sperm flagellum by tACE before these cells leave the testis (Takayama et al. 2005). However, a fraction of this protein remains in detergent-resistant structures from the cytoplasmic droplet (Miranda et al. 2009, Sleight et al. 2005, Takayama et al. 2005, Yuan et al. 2013). Whether TEX101 from the CD has a role in sperm maturation is not known.
In addition to metabolism, CDs have also been proposed to regulate sperm ion homeostasis. CDs contain K+, Cl− and water channels (Cooper 2011) which have been proposed to play a role in regulation of sperm volume during transport through epididymal regions characterized by milieus of differing osmolality. Intracellular Ca2+ is also accumulated in this organelle (Ecroyd et al. 2004). Considering that caput sperm do not undergo tyrosine phosphorylation (Visconti et al. 1995), Ecroyd et al. (2004) has hypothesized that high Ca2+ found in CD is responsible for maintaining low levels of tyrosine phosphorylation in immature sperm (Ecroyd et al. 2004). This hypothesis is consistent with findings that Ca2+ has a biphasic role controlling phosphorylation pathways in sperm (Navarrete et al. 2015). Finally, although not directly related to a functional aspect of sperm epididymal maturation the CD has been used in the last decade to develop standard electrophysiological methods to measure sperm channels in the whole-cell conformation (Kirichok et al. 2006). Although electrophysiological measurements of ion fluxes have been performed in mouse sperm before by patch clamping the sperm head (Espinosa et al. 1998), this technique is time-consuming and experimentally challenging. The discovery by Kirichok et al. (2006) that the CD can be used to make high resistance seals in sperm from the caput, corpus and most recently cauda, has been essential for a more sophisticated understanding of how sperm channels behave (Figueiras-Fierro et al. 2013, Kirichok et al. 2006, Lishko et al. 2013, Lishko et al. 2010, Strunker et al. 2011).
2.3. Changes in the sperm proteome
During epididymal transit, sperm undergo changes in their protein, lipid and sugar content. Considering that sperm are translationally silent, proteins appearing in them as a consequence of their maturation in the epididymis are thought to be synthesized by the epididymal epithelium and then incorporated to the sperm cells. The mechanisms involved in the transfer of epididymal proteins to the sperm are not fully understood. Three complementary hypotheses are: 1) absorption of soluble proteins secreted by the epithelium into the epididymal fluid (Cuasnicu et al. 2002); 2) transfer of exosomes released from the epididymal epithelium; these are known as epididymosomes and proposed to contain proteins, non-coding RNA and distinct set of lipids to be transferred to the sperm while they pass through different regions of the epididymis (Belleannee 2015, Sharma et al. 2016, Sullivan et al. 2013); 3) recent high resolution imaging revealed tight connections between the sperm cell and the apical epididymal epithelial surface (Paunescu et al. 2014). These results suggest that molecules can be transferred directly from the epididymis to sperm through these contact points by mechanisms not yet described. From these putative mechanisms of molecular transfer from the epididymal epithelium to the sperm, epididymosomes have gained increasing attention during the last decade.
Epididymosomes are proposed to be the major way of transferring proteins, and other molecules to sperm. However, the exact mechanisms by which this transfer of molecules between epididymosomes vesicles and sperm occur is poorly understood (Sullivan et al. 2013). In this section we give basic information about epididymosomes. For a better understanding of this topic, we encourage the reader to check excellent reviews published in recent years (Martin-DeLeon 2015, Sullivan et al. 2013).
The epididymal epithelium comprises different epithelial cell types including principal cells, narrow cells (found only in the initial segment), clear cells and basal cells (Hermo et al. 2002). Each of these cell types has a specific structure and function that varies depending on their localization along the epididymis (for review see Breton et al. 2016, Hermo et al. 2002). In addition, communication between these different cellular types generates a luminal microenvironment appropriate for sperm maturation and storage in the epididymis (Shum et al. 2011). Principal cells secrete proteins to the epididymal lumen by both merocrine and apocrine mechanisms (Hermo et al. 2002). Merocrine secretion involves membrane fusion between Golgi-derived vesicles and the cell plasma membrane allowing the release of the vesicle contents to the extracellular space (Farkas 2015). Conversely, apocrine secretions involve loss of apical protrusions into the lumen containing cytoplasm and secretory materials that can be pre-contained in vesicles or dissolved in the cytoplasm (Farkas 2015). Apocrine secretion is the mechanism by which epididymosomes are believed to be released by principal cells into the lumen of the epididymis (Sullivan et al. 2007). Epididymosomes are small membranous vesicles (25–300 nm in diameter) that contain different proteins, lipids and non-coding RNAs (Belleannee et al. 2013, Martin-DeLeon 2015, Sullivan et al. 2013). Epididymosomes collected from different segments of the epididymis are highly heterogeneous in size and content (Rejraji et al. 2006), which may explain differential protein transfer to the sperm. Proteins transferred from epididymosomes to sperm can either be incorporated into the plasma membrane of sperm (Kirchhoff et al. 1996) or to intracellular structures (Eickhoff et al. 2001, Frenette et al. 2005). Nevertheless, the exact mechanism by which epididymosomes transfer proteins to sperm remains elusive (Sullivan et al. 2013).
A variety of proteins with diverse proposed functions have been shown to be transferred to the sperm by epididymosomes during epididymal transit, and has been recently reviewed (Sullivan et al. 2013). Some of these acquired proteins are involved in the development of sperm functions such as sperm motility (Eickhoff et al. 2004, Eickhoff et al. 2001, Frenette et al. 2003, Frenette et al. 2004, Murta et al. 2016); sperm capacitation (Krapf et al. 2012); the acrosome reaction (Joshi et al. 2013); sperm-zona pellucida interaction (Frenette et al. 2001) and fertilization (Caballero et al. 2012, Gibbs et al. 2010, Oh et al. 2005). Other acquired proteins are proposed to tag defective sperm; examples of them are: ubiquitin (Sutovsky et al. 2001) and epididymal sperm binding protein 1 (ELSPBP1) (D’Amours et al. 2012), which are hypothesized to be transferred to defective or dead sperm during epididymal transit. Moreover, it has been recently proposed that epididymosomes can also be part of a mechanism for protein removal. The expression of the protein dicarbonyl L-xylulose reductase (DCXR) is higher in cauda epididymosomes than in those from caput, and is lower in cauda sperm than in caput sperm, suggesting that this protein is removed by epididymosomes from the sperm during sperm maturation (Akintayo et al. 2015).
Although the mechanisms of molecule transfer to the sperm are not clear, different methods have been used to characterize proteins secreted by different regions of the epididymis and to identify which of these proteins are found in mature sperm. Recent studies with mass spectrometry have compared proteomes from immature caput sperm with those of mature cauda sperm (Table 1). In some of these studies, proteins were initially separated by using two-dimensional polyacrylamide electrophoresis (PAGE) (Dacheux et al. 2014, Ijiri et al. 2011, Ijiri et al. 2014). In others, mass spectrometry was done on total sperm extracts (Baker et al. 2012, Labas et al. 2015). These studies have revealed differences in the relative abundance of proteins as well as post-translational modifications occurring to sperm during epididymal transit (Baker et al. 2012, Baker et al. 2005, Ijiri et al. 2011, Kameshwari et al. 2010). Characterization of protein profiles from spermatozoa from different epididymal regions provides grounds for the understanding of the sperm maturation process. However, in most cases, these investigations have been limited by the lack of in vitro models to test predictions of the role of particular proteins in sperm function. In some cases, epididymal proteins have been transferred in vitro to caput epididymal sperm (Caballero et al. 2013); however, functional implications of the in vitro transfer have not yet been thoroughly investigated.
Table 1. Comparative proteomic during sperm maturation.
Summary of works that present detailed proteomic results comparing caput and cauda sperm of different species.
| Identification of abundance of proteins | |||
|---|---|---|---|
| Samples analyzed | Species | Technique used | Reference |
| Cytoplasmic droplets |
Mouse | Isolation of cytoplasmic droplets by sucrose gradient followed by MALDI-TOF/TOF MS. |
Yuan et al. 2013 |
| Rat | Isolation of cytoplasmic droplets |
Au et al. 2015 | |
| Whole sperm | Boar | Intact cell, detergent- soluble, and detergent- insoluble protein purification. MALDI-TOF MS. |
Labas et al. 2015 |
| Hamster | MALDI-MS/MS. | Kameshwari et al. 2010 | |
| Mouse | Two-dimensional Fluorescence difference gel electrophoresis (2D–DIGE) followed by MALDI- TOF/TOF MS. |
Ijiri et al. 2011 | |
| Sperm surface proteins |
Boar | Labeling and purification of surface protein with sulfo- NHSS-SS-biotin. 1D or 2D electrophoresis followed by MS. |
Belleannee et al. 2011 |
| Epididymosomes |
Human | 1D electrophoresis followed by LC-QToF MS. |
Thimon et al. 2008 |
| Bull | 1D electrophoresis followed by ES-MS/MS |
Girouard et al. 2011 | |
| Identification of post-translational modifications of proteins | |||
| Samples analyzed | Species | Technique used | Reference |
| Phosphorylation | Rat | TiO2 enrichment of phosphopeptides followed by LC-MS. |
Baker et al. 2012 |
| Non-identified posttranslational modification |
Rat | 2D–DIGE followed by MALDI-TOF MS. |
Baker et al. 2005 |
| Thiol status of proteins |
Mouse | 2D–DIGE MALDI-TOF/TOF MS. |
Ijiri et al. 2014 |
In addition to changes in protein content, it has been shown that epididymal maturation is associated with post-translational protein changes, including phosphorylation and oxidation of thiol groups. It has been hypothesized that during sperm maturation oxidation of protein thiol groups gradually stabilizes sperm structures such as the nucleus and tail components, by formation of disulfide bonds (S–S) (Bedford et al. 1974a, Bedford et al. 1974b, Calvin et al. 1971). Most of these studies have focused on the analysis of stabilization by S-S bonds of nuclear sperm protamines (Marushige et al. 1975, Pellicciari et al. 1983, Saowaros et al. 1979) or sperm membrane proteins (Mercado et al. 1976) during epididymal transit. Later on, Shalgi et al. (1989) reported that the formation of S-S bonds (with no changes in net content of disulfide and thiols) was increased in sperm proteins from tails and heads of rat sperm during epididymal maturation (Shalgi et al. 1989). Consistently, more recent analyses have applied two-dimensional fluorescence difference gel electrophoresis (Ijiri et al. 2014) or two dimensional electrophoresis (Dias et al. 2014), coupled to proteomic approaches, to assess thiol changes in sperm proteins between caput and cauda epididymal sperm in both the mouse and stallion. Both works identified proteins localized in the sperm tail that increase in S-S during maturation (Dias et al. 2014, Ijiri et al. 2014). As most of the identified proteins are related to structural and cytoskeletal functions, the stabilization of proteins during sperm maturation by oxidation of thiol groups could have an important function related to the stabilization of the flagellum for the subsequent acquisition of sperm motility.
Besides proteins, epididymosomes deliver fragments of tRNAs to mammalian sperm during sperm maturation (Sharma et al. 2016). These tRNA fragments have been shown to affect the metabolism of the offspring and were proposed to participate in paternal epigenetic inheritance (Chen et al. 2016, Sharma et al. 2016). Interestingly, there is tRNA cleavage in the epididymis and cauda sperm present high levels of these tRNA fragments when compared with immature caput sperm (Sharma et al. 2016).
2.4. Changes in the sperm surface
In addition to the acquisition of new proteins, spermatozoa undergo molecular changes on their surface during epididymal maturation. These alterations include addition, removal and/or modification of external sugars and lipids of the sperm plasma membrane. Glycoproteins and polysaccharides form an interface between the sperm and its external environment is known as the glycocalyx (Schroter et al. 1999, Tecle et al. 2015). Similarly to the strategies used for identification of changes occurring in the sperm proteome, comparative studies of the glycocalyx of sperm from different epididymal regions have been made. Identification of sugar residues can involve taking advantage of the affinity of different lectins for specific terminal saccharide residues (Cummings 1994). Lectins are macromolecules highly specific for sugar moieties that play a role in biological recognition. This property has been exploited in several cell types to identify terminal saccharides using a variety of approaches including lectin cytochemistry, lectin flow cytometry (Magargee et al. 1988), lectin fluorescence (Magargee et al. 1988), lectin blots (Srivastava et al. 1991), lectin agglutination (Hammerstedt et al. 1982, Nicolson et al. 1972) and lectin microarrays (Xin et al. 2014).
Studies in several species have shown that epididymal maturation is associated with an increase or a decrease in the accessibility of specific lectins to the sperm glycocalyx (Magargee et al. 1988, Nicolson et al. 1977, Srivastava et al. 1991) (see Table 2 for a summary of published data). Epididymal luminal fluid contains high concentrations of soluble glycohydrolases and glycosyltransferases, which are thought to be involved in the alteration of sperm surface glycoconjugates (Tulsiani 2006). A net-negative change in the external sperm surface charge during epididymal maturation has been shown (Bedford 1963a, Yanagimachi et al. 1972). It is considered that the increase in negative charge is due to changes in sugar moieties; in particular, the incorporation of negatively-charged sialic acid (Calvo et al. 2000, Holt 1980). Consistent with this hypothesis, higher concentrations of sialic acid are present in cauda epididymal luminal fluid when compared to caput luminal fluid (Singh et al. 2009, Yanagimachi et al. 1972).
Table 2. Changes in the composition of sperm glycocalyx during epididymal maturation in different species.
Data presented has been reviewed from studies that compared sugar residues present in sperm recovered from caput and cauda epididymis. Arrows are indicative of an increase (↑) or decrease (↓) in the composition of sugars during sperm maturation. ND denotes that published data is not available. When changes in specific proteins were reported, the protein(s) molecular weight (in KDa) is specified.
| SPECIES | Bull | Goat | Monkey | Mouse | Rabbit | Ram | Rat | Stallion | |
|---|---|---|---|---|---|---|---|---|---|
| Lectin | Sugar residue | Comparison of caput vs cauda sperm. | |||||||
| Con A | D-mannose D-glucose |
↓ | = | ↑ | ↓a | =a | = | ↓b | ND |
| SBA | D-N-acetyl- galactosamine |
= | = | ND | ND | ND | ↓ | ND | ND |
| RCA | D-galactose and L- arabinose |
= | ND | ND | ND | ↓a | ↓ | ND | ↓ |
| PNA | D-galactose | ↑ (acrosome) |
= | ND | ND | ND | ↓ | ↓ (58 KDa)a | ↓ |
| WGA | Sialic acid and N-acetyl-D- glucosamine |
↓ | ↑(acrosome) | ↓ | ↑b | ↓ a ↑(acrosome)b |
↑ | ↑ (86, 47 and 37 KDa)a ↑ (acrosome)c |
↑ |
| DBA | D-N-acetyl- galactosamine |
↓ | ↑ | ND | ND | ND | ND | = | ND |
| UEA | α-L-fucose | = | ↑ | ND | ND | ND | ND | = | ↑ |
| PSA | D-mannose | ND | ND | ND | ND | ND | ND | ↓ (118 KDa)a ↓ (58 KDa)a |
ND |
| References | Arya et al. 1985 | Bains et al. 1993 | Fain-Maurel et al. 1984 | a: Schlegel et al. 1986 b: Kumar et al. 1990 |
a: Nicolson et al. 1977 b: Kumar et al. 1990 |
Magargee et al. 1988 | a: Srivastava et al. 1991 b: Olson et al. 1981 c: Kumar et al. 1990 |
Retamal et al. 2000 | |
References in the literature are indicated by letters (a), (b) or (c). Lectin abbreviations: Con A, concanavalin A; SBA, soybean agglutinin; RCA, Ricinus communis agglutinin; PNA: peanut agglutinin; WGA, wheat germ agglutinin; DBA, Dolichos biflorus agglutinin; UEA, Ulex europaeus agglutinin; PSA, Pisum sativum agglutinin.
On top of the changes observed in surface glycans, epididymal sperm maturation is associated with modifications of lipid homeostasis (see Table 3). In most species, there is an average decrease in the cholesterol:phospholipid ratio between caput and cauda sperm plasma membranes (for review on this topic see: Jones 2002, Saez et al. 2011). Consistent with the decrease in cholesterol:phospholipid ratio, fluorescence recovery after photobleaching (FRAP) assays have indicated an increase in sperm plasma membrane fluidity during epididymal maturation (Christova et al. 2004, Christova et al. 2002, Jones 2002). One of these studies compared caput and cauda sperm from several mammalian species for how changes in pH, osmolality and temperature affected membrane fluidity in different sperm compartments. The FRAP data showed an increase in membrane fluidity occurring as part of epididymal maturation and allowed the authors to propose a model of sperm lipid dynamic movement during this process Christova et al. (2004). Changes in membrane fluidity occurring during sperm maturation may be essential for later membrane events required for fertilization such as the acrosome reaction and ability to fuse with the egg oolema.
Table 3. Changes in sperm lipid content during epididymal maturation in different species.
Data reviewed from studies that compared lipids of whole cells or membrane extracts of sperm recovered from caput and cauda epididymis. Arrows are indicative of an increase (↑) or decrease (↓) in the percentage of lipid during sperm maturation; ND is indicative of no published data.
| SPECIES | Boar | Goat | Hamster | Human | Mouse | Ram | Rat |
|---|---|---|---|---|---|---|---|
|
Phospholipids out of Total phospholipids |
|||||||
| Phosphatidylcholine (PC) | ↑ | ↓ | = | ↑ | ↑a ↓b |
↑ | ↓ |
| Posphatidylethanolamine (PE) | ↓ | ↓ | = | ↓ | ↓b | ↓ | ↓ |
| Phosphatidylinositol (PI) | ↓ | ND | = | ND | ↑b | ↓ | = |
| Phosphatidylserine (PS) | ↓ | ND | = | ↓ | =b | ↓ | ↓ |
| Sphyngomyelin (SM) | ↑ | ↓ | ND | ↓ | ↑a,b | ND | ↑ |
| Sterols | |||||||
| Cholesterol | ↓ | ↑ | ↓ | ND | ↓b | ↓ | ↓ |
| Desmosterol | ↑ | ↓ | ↑ | ND | ND | ↓ | ↑b |
| Membrane Fluidity | ND | ND | ↑ | ↑ | ↑b | ↑b | ↑ |
| References | Nikolopoulou et al. 1985 | Rana et al. 1991 | Awano et al. 1993, Awano et al. 1989 | Haidl et al. 1997 | a: Pyttel et al. 2014 b: Rejraji et al. 2006 |
a: Parks et al. 1985 b: Christova et al. 2004 |
a: Aveldano et al. 1992 b: Lindenthal et al. 2001 |
Letters (a) and (b) indicate the references.
Other studies on sperm lipid content indicate an epididymal maturation-associated increase in the ratio of polyunsaturated to saturated fatty acids (Awano et al. 1993, Haidl et al. 1997, Hall et al. 1991, Nikolopoulou et al. 1985, Parks et al. 1985, Pyttel et al. 2014, Rejraji et al. 2006). Together with cholesterol and phospholipids, these polyunsaturated fatty acids affect sperm membrane fluidity and render these cells more susceptible to oxidative stress (Wathes et al. 2007). The relevance of the maturational increase in polyunsaturated fatty acids is highlighted by a recent report of Dicer1-conditional knock-out mice (Bjorkgren et al. 2012). DICER1 is an RNAase type III involved in processing non-coding RNAs, including micro RNAs (miRNA) and small interference RNAs (siRNAs) (Li et al. 2011a). When this enzyme is eliminated from the epididymal initial segment and caput regions, the mice present a male sterile phenotype (Bjorkgren et al. 2012). Sperm from these mice depicted a decrease in long-chain polyunsaturated fatty acids, and presented increased breakage of the neck and acrosomal regions, as well as a reduced ability of cauda sperm to bind to and fertilize oocytes (Bjorkgren et al. 2015). Deficiencies in polyunsaturated fatty acids in Dicer1 epididymis-specific conditional KOs suggest that changes in sperm lipid content occurring during epididymal maturation are due in part to the interaction between the sperm and epididymal epithelial secretions (Bjorkgren et al. 2015).
3. SIGNALING PATHWAYS ACTIVATED IN SPERM DURING THEIR EPIDIDYMAL TRANSIT
As a result of their high level of specialization, sperm are transcriptionally and translationally silent (Diez-Sanchez et al. 2003). Lack of sperm protein synthesis after leaving the testis suggests that regulation of epididymal maturation is controlled almost exclusively by post-translational modification of their intrinsic protein complement (Vijayaraghavan et al. 1996) or by acquisition of exogenous proteins (Caballero et al. 2012, Koch et al. 2015, Krapf et al. 2012, Martin-DeLeon 2015, Sullivan et al. 2007, Sullivan et al. 2013). One of the better studied post-translational modifications is the addition to and removal of protein phosphate groups. Reversible protein phosphorylation plays a key role as molecular switch in many cellular processes, including transduction of extracellular signals, intracellular transport, and cell cycle progression. In sperm, pharmacological or genetic loss-of-function approaches have conclusively demonstrated the role of phosphorylation cascades in the regulation of capacitation (for reviews see: Buffone et al. 2014, Visconti 2009). Less is known about the role and regulation of phosphorylation cascades in sperm during epididymal transit. Recent works have compared caput and cauda spermatozoa (Baker et al. 2012) and have shown changes in the phosphorylation status of different proteins. In most cases, further examination will be needed to understand the relevance of the phosphorylation and dephosphorylation events. However, because of the relevance of certain proteins, some of the findings might have significant implications. One of these relevant proteins found to change its phosphorylation status during epididymal transit is IZUMO1, a protein essential for sperm-egg fusion (Inoue et al. 2005). One relevant property of this protein is that it changes localization from the anterior head to the equatorial/postacrosomal region during the acrosome reaction (Miranda et al. 2009). Acrosome-reacted sperm fuses with the oocyte oolema by their equatorial segment; therefore, IZUMO1 movement is considered essential for its role in sperm-egg fusion. Using mass spectrometry to determine exact phosphorylation sites, Baker et al. recently demonstrated that IZUMO1 is extensively phosphorylated in its cytoplasmic region in cauda but not in caput epididymal sperm (Baker et al. 2012). Considering that caput sperm cells are unable to fuse with metaphase II-arrested oocytes, an attractive hypothesis is that IZUMO1 phosphorylation plays a role in IZUMO1 function, either in fusion or in its movement during the acrosome reaction. In this regard, sperm lacking the testis-specific serine/threonine kinase TSSK6 do not depict changes in IZUMO1 localization in acrosome-reacted sperm, they are unable to fuse with zona-pellucida-free oocytes, and the males are sterile in vivo and in vitro (Sosnik et al. 2009). Although more research is needed, these data are consistent with a model in which IZUMO1 phosphorylation during epididymal maturation plays a role in the acquisition of the ability of sperm to fuse and fertilize.
Besides comparison of sperm from different epididymal regions, several studies have used more direct approaches to study signaling events involved in sperm maturation. In particular, many researchers have used the acquisition of progressive motility, which is one of the most evident changes occurring to sperm during epididymal maturation. Some of these studies used mild detergent treatment to eliminate the sperm plasma membrane while leaving intact the motility machinery. These studies demonstrated that demembranated sperm become motile when ATP and cAMP are added to the incubation media. Therefore, it can be hypothesized that a protein kinase A (PRKA aka PKA) phosphorylation cascade is involved in motility regulation. However, these experiments are silent regarding the reason why intact immature sperm remain immotile in media that allow forward motility of cauda epididymal sperm even when cAMP analogues are added to the media. In 1996, Dr. Vijayaraghavan’s group showed that addition of ser/thr phosphatases inhibitors to bovine caput epididymal sperm incubated in vitro induced activation of progressive motility (Vijayaraghavan et al. 1996). Since flagellar motion patterns of caput sperm treated with the phosphatase inhibitors okadaic acid or calyculin A were similar to those from mature cauda sperm, these experiments are consistent with the hypothesis that inhibition of ser/thr phosphatases play a role in activation of sperm motility.
In eukaryotes, ser/thr phosphatases belong to three main families: PPP (Phospho Protein Phosphatases), PPM (Metallo-dependent Protein Phosphatases) and FCP (transcription initiation factor IIF-stimulated C-terminal domain Phosphatases) (Pereira et al. 2011). In sperm, only members of the PPP family have been detected. This family is constituted by seven enzymes (PPP1-PPP7), with a total of 14 catalytic subunits (see table 4). A recent profile of protein phosphatases from human sperm using western blot analyses indicated the presence of five of the catalytic isoforms in these cells: PPP1CB (aka PP1β), PPP1CC2 (PP1γ2), PPP2CA (PP2A), PPP4C (aka PPX), PPP6C (aka PP6) (Fardilha et al. 2013). PPP1CC2 has also been reported in mouse, hamster and bull sperm (Chakrabarti et al. 2007); and PPP3C (aka PP2B, aka calcineurin) has been found in mouse (Miyata et al. 2015, Navarrete et al. 2015), bovine (Wasco et al. 1984) and human sperm (Castillo Bennett et al. 2010). Each PPP subfamily presents a given sensitivity profile to a battery of toxin inhibitors. Therefore, it is possible to predict which type of PPP is involved, by analyzing the effective concentration of a given toxin inhibitor that blocks or enhances an enzyme function. For example, okadaic acid is two orders of magnitude more potent as inhibitor of PPP2A, PPP4 and PPP6 than it is for PPP1 and PPP5 subfamilies (Swingle et al. 2007). Therefore, effects observed at low nM ranges suggest a role for the former phosphatases, while those effects only observed at high nM or µM concentrations suggest involvement of PPP1C or PPP5 subfamilies. Regarding the effect of okadaic acid on motility activation of bovine caput sperm is maximal at concentrations of 5 µM suggesting the involvement of PPP1C (Vijayaraghavan et al. 1996). This ser/thr phosphatase subfamily has a testis-specific isoform, PPP1CC2, generated by alternative splicing (Sasaki et al. 1990).
Table 4. Ser/Thr-specific protein phosphatase families.
This table summarizes the catalytic subunit isoforms of human PPPs. PPPs form multi-subunit proteins; nevertheless, the regulatory and scaffolding subunits are omitted. For a more comprehensive analysis of PPPs and their respective subunits, several manuscripts and reviews have been published in the last years (Pereira et al. 2011, Zhang et al. 2013, Silva et al. 2014).
| Serine/Threonine-specific Protein Phosphatases | ||||
|---|---|---|---|---|
| Family | Characteristics | Phosphatase | Catalytic Isoforms |
Alternative name |
|
PhosphoProtein Phosphatases (PPPs) |
Multi-subunit proteins. | PPP1 | PPP1CA PPP1CB PPP1CC1 PPP1CC2 |
PP1α PP1β PP1γ1 PP1γ2 |
| PPP2 | PPP2CA PPP2CB |
PP2A |
||
| PPP3 | PPP3CA PPP3CB PPP3CC |
PP2B; calcineurin |
||
| PPP4 | PPP4C | PPX | ||
| PPP5 | PPP5C | PP5 | ||
| PPP6 | PPP6C | PP6 | ||
| PPP7 | PPP7CA PPP7CB |
PPEF1 PPEF2 |
||
|
Metallo-dependent Protein Phosphatases (PPMs) |
Mg2+- or Mn2+ dependent enzymes. Single-subunit proteins. |
PPM1 | PPM1A PPM1B PPM1D |
PP2C |
| Transcription initiation factor IIF-stimulated C-terminal domain Phosphatases (FCPs) |
Require Mg2+ in the active site for catalysis. Single-subunit proteins. |
Small CTD phosphatases (SCPs) |
SCP1 SCP2 SCP3 |
NLI-interacting factor 3 NLI-interacting factor 2 NLI-interacting factor 1 |
| CTD phosphatases |
FCP1 | TFIIF- associating CTD phosphatase |
||
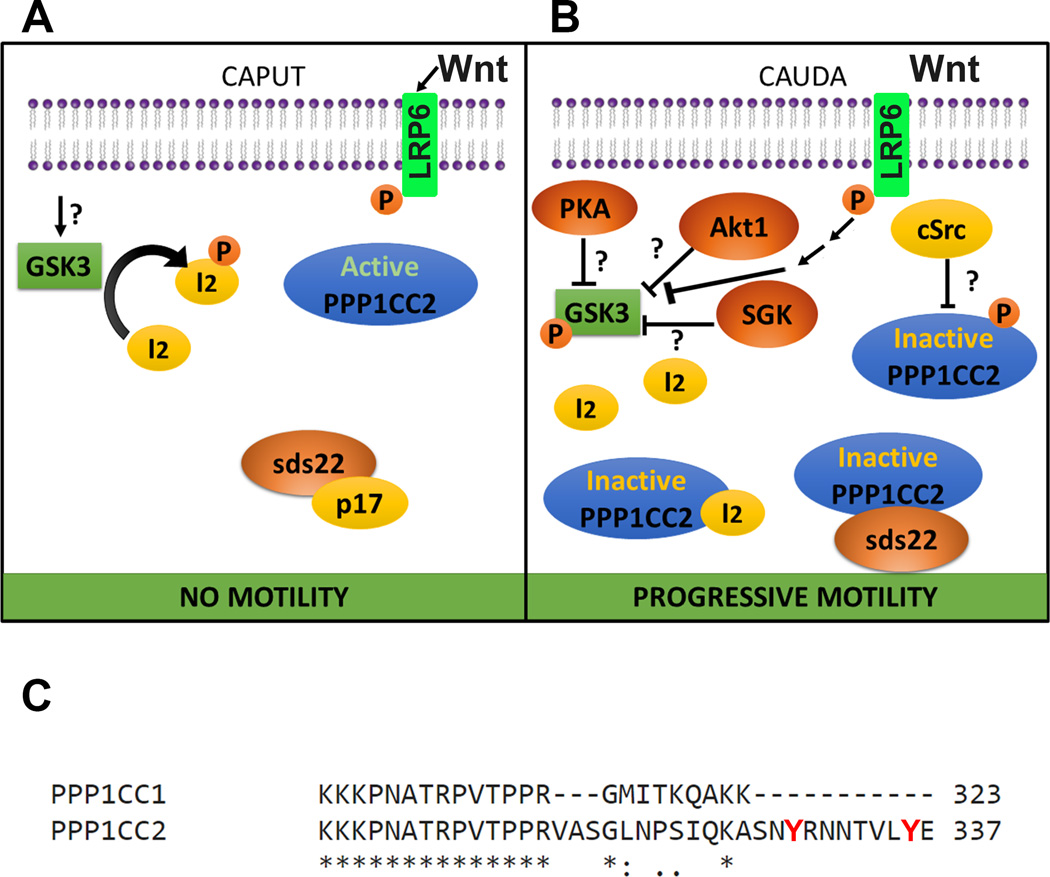
It has been shown that sperm PPP1CC2 decreases activity during epididymal maturation (Vijayaraghavan et al. 1996). Although the molecular basis of this inactivation is not well understood, it has been hypothesized that PPP1CC2 is maintained active by indirect action of glycogen synthase kinase 3 (GSK3) (Vijayaraghavan et al. 1996). This hypothesis is consistent with the finding that both GSK3α and GSK3β are present in sperm and with observations of lower GSK3 activity in cauda when compared to caput sperm (Vijayaraghavan et al. 1996). Both GSK3 kinases regulate members of the PPP1C family by phosphorylation of the protein phosphatase inhibitor 2 (I2); once phosphorylated, I2 is no longer capable of binding to and inhibiting members of the PPP1C subfamily (Hemmings et al. 1982). I2 has been reported in bovine and mouse sperm (Chakrabarti et al. 2007).
It is not clear how GSK3 becomes inactivated during epididymal maturation, but it has been proposed that active cauda epididymal sperm PKA phosphorylates GSK3, resulting in the latter’s inactivation. This possibility is consistent with experiments with anti-phospho-ser GSK3 in western blots showing that caudal sperm GSK3 has greater phosphorylation of Ser21 (for GSK3α) or Ser9 (for GSK3β) than caput GSK3 (Somanath et al. 2004). Phosphorylation of these sites is known to inactivate the respective GSK3, and both sites are known PKA substrates (Fang et al. 2000). PKA activation depends on activation of cAMP synthesis by the atypical soluble adenylyl cyclase Adcy10 (aka sAC). In this regard, cauda sperm from mice knock-out genetic models lacking sAC have reduced forward motility (Esposito et al. 2004, Hess et al. 2005).
On the other hand, it should be taken into consideration that wild type cauda epididymal sperm suspended in media that do not support sAC and PKA activation display progressive motility (Buffone et al. 2014). Therefore, the inactivating modification of Ser residues in GSK3 could be the result of phosphorylation by other serine/threonine protein kinases, including Akt (protein kinase B) (Cross et al. 1995) and/or serum glucocorticoid kinase (SGK) (Sakoda et al. 2003, Wyatt et al. 2006). Both Akt and SGK were shown to be present in mouse sperm (Vadnais et al. 2013). Their presence suggest that these kinases might play a role in GSK3 inactivation (Vadnais et al. 2013) with the consequent decrease in phosphorylated I2 resulting in PPP1CC2 inactivation (see Figure 2A and 2B for a schematic model of putative molecular pathways involved in the regulation of PPP1CC2 in caput and cauda sperm). Remarkably, knock-out mice models lacking either Akt1 or Akt2 are subfertile (Kim et al. 2012). It is also known in other biological systems, that Akt1 and SGK can be phosphorylated and activated by the 3-phosphoinositide-dependent protein kinase 1 (PDK1) (Alessi et al. 1997, Kobayashi et al. 1999). Based on the presence of PDK1 protein in caput and cauda sperm, it has been hypothesized that PDK1 induces activation of Akt1 or SGK, in a molecular pathway alternative to PKA’s, for the acquisition of sperm motility during epididymal maturation (Vadnais et al. 2013). Even though this is an attractive explanation for inactivation of GSK3 and PPP1CC2 during epididymal maturation, further investigation is required to corroborate this hypothesis.
Figure 2.
Model of putative molecular pathways involved in the regulation of PPP1CC2 and progressive sperm motility during epididymal maturation. A) CAPUT: glycogen synthase kinase 3 (GSK3) is active and phosphorylates protein I2. Once phosphorylated, I2 is not able to bind to and inactivate the ser/thr phosphatase PPP1CC2. The PPP1CC2 inhibitory protein sds22 has been shown to be complexed with p17 and cannot bind and inhibit the phosphatase. cSrc kinase is absent from caput sperm, PPP1CC2 is active and caput sperm lack motility. Wnt from epididymosomes activates the primed receptors LRP6. B) CAUDA: GSK3 is phosphorylated on ser residues, and rendered inactive by an unidentified serine/threonine kinase. Among the proposed kinases involved in GSK3 phosphorylation and inactivation are protein kinase A (PKA), RACalpha protein kinase 1 (Akt1) and serum and glucocorticoid induced kinase (SGK). All these kinases have been described in sperm. Active Wnt signaling inhibits GSK3. Due to GSK3 inactivation, protein I2 can no longer undergo phosphorylation and consequently binds to PPP1CC2, inactivating its catalytic activity. On the other hand, sds22 has been found complexed to PPP1CC2 in cauda sperm and can explain PPP1CC2 inhibition. cSrc tyrosine kinase, not present in caput sperm, is incorporated into sperm during epididymal maturation. Because PPP1CC2 is a testis-specific splicing variant containing two unique tyrosine residues in its C-terminal domain, phosphorylation of these residues by cSrc or another tyrosine kinase would also explain PPP1CC2 inactivation. Together, inactivation of PPP1CC2 leads to the ability of the sperm cell to move progressively when exposed to an appropriate medium. C) ClustalW2 sequence alignment of the human protein phosphatases PPP1CC1 and PPP1CC2. These two proteins are products of an alternative splicing and differ only in a small region of the C-terminal, as shown in the Figure. The two unique tyrosine residues (Y) in PPP1CC2 are indicated in red.
The increased phosphorylation of GSK3 in cauda sperm can be also explained by inactivation of specific ser/thr phosphatases that have this kinase as a substrate. Phosphorylated GSK3 can be substrate of the protein phosphatase PPP2CA (Hernandez et al. 2010). In this regard it has been recently shown that the catalytic activity of the protein phosphatase PPP2CA is lower in cauda than caput bovine sperm (Dudiki et al. 2015). However, solely PPP2CA inactivation is not sufficient to induce sperm motility in immature sperm (Dudiki et al. 2015).
An alternative hypothesis about GSK3 inactivation during epididymal maturation has recently arisen. Koch and collaborators proposed that Wnt signaling controls GSK3 inactivation during epididymal maturation independently of β-catenin (Koch et al. 2015). In other cell types, Wnt activates LRP6 coreceptors primed by the cyclin-dependent kinase 14 and cyclin Y (Ccny) (Davidson et al. 2009). While Ccny is ubiquitously expressed, cyclin Y-like 1 (Ccnyl1) expression is restricted to germ cells in the testis (Koch et al. 2015). Genetically modified male mice lacking Ccnyl1 are infertile due to structural and motility sperm defects (Koch et al. 2015). This brings the attention to the Wnt pathway in sperm as modulator of sperm maturation and motility. In their recent work, the authors show that Wnt is released from the epididymal epithelium in epididymosomes and sperm are responsive to Wnt via activation of LRP6 (Koch et al. 2015). Consistently with these results, incubation with Wnt3a increased the velocity of submotile mouse caput sperm and induced flagellar beating in immotile testicular human sperm (Koch et al. 2015). The model proposed is that the activation of the Wnt signaling pathway in the sperm inhibits GSK3 and promotes sperm motility by inactivation of PPP1CC2.
Inactivation of PPP1CC2 can also be explained by alternative mechanisms including binding of proteins to the catalytic subunit of phosphatases and other regulatory post-translational modifications. For example, PPP1CC2 has been shown to bind a protein homologue of the yeast protein phosphatase binding protein sds22 (Huang et al. 2002). Formation of the sds22-PPP1CC2 complexes renders the phosphatase inactive (Huang et al. 2002). Although sds22 is present in both caput and cauda sperm, sds22 is only able to bind to PPP1CC2 in cauda sperm (Mishra et al. 2003). The same group has reported that the protein 14-3-3ξ is expressed in sperm and binds to phosphorylated PPP1CC2 in cauda sperm. However, this pool of phosphorylated PPP1CC2 is active, and additional studies are required to identify the biological relevance of this protein binding (Huang et al. 2004). Furthermore, it has been shown that PPP1CC2 forms complexes with the testis-specific serine/threonine kinase 1 (TSSK1) in cauda sperm (MacLeod et al. 2014). Members of the TSSK family, including TSSK1, have been found in mouse sperm (Hao et al. 2004, Li et al. 2011b, Visconti et al. 2001). The interaction between PPP1CC2 and TSSK1 is indirect and mediated by a specific kinase substrate known as TSKS (MacLeod et al. 2014). Further investigations comparing caput and cauda sperm are required to evaluate if the PPP1CC2/TSSK1 interaction affects the phosphatase activity or participates in the acquisition of motility during sperm maturation
Recently, another ser/thr phosphatase has been proposed to play a role in epididymal maturation. Genetically modified mice lacking the Ca2+- calmodulin-dependent ser/thr phosphatase catalytic subunit PPP3CC (aka calcineurin) are infertile due to failure in penetration of the zona pellucida (Miyata et al. 2015). Computer-assisted sperm analysis experiments showed that although the motility of cauda sperm of these knock-out animals is comparable to wild type, null mice present impaired hyperactivated motility due to midpiece rigidness (Miyata et al. 2015). Consistently, administration of the calcineurin inhibitors cyclosporine A or FK506 to wild type mice for at least 5 days induced infertility due to lack of sperm midpiece flexibility and impaired hyperactivation (Miyata et al. 2015). Taking in account that the maturational process in the mouse epididymis takes 10 days, these results suggest that sperm calcineurin activity is required during sperm maturation for acquisition of proper midpiece flexibility and development of hyperactivation motility in conditions that support sperm capacitation.
Finally, the role of newly acquired enzymes synthesized by the epididymal epithelium in the regulation of sperm signaling pathways during epididymal maturation should not be ruled out. Recent work from our laboratory presented evidence that cSrc is enriched in epididymosomes and is incorporated into mouse sperm during transit through the epididymis (Krapf et al. 2012). This study also showed that cSrc KO mice display deficiencies in cauda epididymal development. Moreover, knock-out male mice of cSrc are sterile (Schwartzberg et al. 1997) and cauda sperm of cSrc-null mice have impaired motility (Krapf et al. 2010). Altogether these data suggest a role of cSrc in epididymal sperm maturation. Whether cSrc incorporation plays a role in PPP1CC2 down-regulation is not known. However, it is well-established that cSrc can phosphorylate PPP2CA, another ser/thr phosphatase, on tyrosine residues present in this phosphatase C-terminal domain. As mentioned above, PPP1CC2 is a sperm-specific splicing variant of the Ppp1cc gene that differs from PPP1CC1 only in the last 14 amino acids of the C-terminus (Fig. 2C). The sperm-specific C-terminal domain contains two tyrosine residues, indicative of a potential differential site of phosphorylation by cSrc or other protein tyrosine kinases. It can be hypothesized that, as for PPP2CA, phosphorylation of these residues changes PPP1CC2 activity. More research will be required to test this possibility.
4. CONCLUDING REMARKS
As stated in the introduction, two extra-testicular maturational processes are required for sperm to gain fertilizing capacity: epididymal maturation and capacitation. Since these are sequential processes, deficient epididymal maturation would prevent sperm to capacitate later on, either in the female tract or in vitro. For example, among the functional changes occurring during epididymal maturation, one of the better studied is the acquisition of progressive sperm motility (Yeung et al. 2002) which is a necessary step for the ability to undergo hyperactivation when exposed to capacitating conditions. Also related to the capacitation process, as part of their transit through the epididymis, sperm acquire the potential: 1) to undergo an increase in protein tyrosine phosphorylation (Visconti et al. 1995); 2) to bind to the zona pellucida (Busso et al. 2007) ; 3) to undergo the acrosome reaction (Burkin et al. 2000, Lakoski et al. 1988, Sirivaidyapong et al. 2001, Williams et al. 1991, Yeung et al. 1996); 4) to become able to fuse with the oolema (Da Ros et al. 2015, Harayama et al. 1993a, Harayama et al. 1993b, Moore et al. 1983); and 5) to fertilize the oocyte. All these events are hallmarks of capacitation which physiologically occur in the female tract. Therefore, epididymal maturation can be defined as all biochemical and physiological changes occurring during epididymal transit that render the sperm competent to undergo capacitation.
An understanding of epididymal sperm maturation at the molecular level has encountered numerous difficulties. First, the absence of methods to fully mature caput sperm in vitro has prevented researchers the use of gain- and loss-of-function approaches to analyze the necessity and sufficiency of a given signaling pathway in sperm maturation. Second, because sperm are transcriptionally and translationally inactive, genetic experiments are difficult. Although knock-out mouse models have been useful in the study of sperm signaling pathways, because sperm derive from testicular germ cells which include spermatogonia, spermatocytes and spermatids, it is rather difficult to assign a role of a particular sperm protein to different processes required for fully functional spermatozoa (e.g. spermiogenesis, epididymal maturation, and capacitation). Despite the problems inherent in experimental conditions, it is expected that modern gene editing approaches (Wijshake et al. 2014) combined with new state-of-the-art imaging techniques, such as superresolution (Chung et al. 2014), or helium ion microscopy (Paunescu et al. 2014), will facilitate the study of sperm maturational events at the molecular level.
Acknowledgments
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants (NIH RO1 HD38082 and HD44044 to P.E.V), and by the Lalor Foundation (to MGG). We would like to thank Dr. Ana Maria Salicioni for critical reading of this manuscript and to Holly Sullivan for the illustrations.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors disclose no financial conflicts of interests.
REFERENCES
- Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod. 2013;19:785–793. doi: 10.1093/molehr/gat067. [DOI] [PubMed] [Google Scholar]
- Akintayo A, Legare C, Sullivan R. Dicarbonyl L-xylulose reductase (DCXR), a “moonlighting protein” in the bovine epididymis. PLoS One. 2015;10:e0120869. doi: 10.1371/journal.pone.0120869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, Erdmann B, Mueller EC, Herz J, Otto A, Cooper TG, Willnow TE. Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem. 2003;278:23989–23995. doi: 10.1074/jbc.M302157200. [DOI] [PubMed] [Google Scholar]
- Arya M, Vanha-Perttula T. Lectin-binding pattern of bull testis and epididymis. J Androl. 1985;6:230–242. doi: 10.1002/j.1939-4640.1985.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Au CE, Hermo L, Byrne E, Smirle J, Fazel A, Kearney RE, Smith CE, Vali H, Fernandez-Rodriguez J, Simon PH, Mandato C, Nilsson T, Bergeron JJ. Compartmentalization of membrane trafficking, glucose transport, glycolysis, actin, tubulin and the proteasome in the cytoplasmic droplet/Hermes body of epididymal sperm. Open Biol. 2015:5. doi: 10.1098/rsob.150080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- Aveldano MI, Rotstein NP, Vermouth NT. Lipid remodelling during epididymal maturation of rat spermatozoa. Enrichment in plasmenylcholines containing long-chain polyenoic fatty acids of the n-9 series. Biochem J. 1992;283(Pt 1):235–241. doi: 10.1042/bj2830235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awano M, Kawaguchi A, Mohri H. Lipid composition of hamster epididymal spermatozoa. J Reprod Fertil. 1993;99:375–383. doi: 10.1530/jrf.0.0990375. [DOI] [PubMed] [Google Scholar]
- Awano M, Kawaguchi A, Morisaki M, Mohri H. Identification of cholesta-7,24-dien-3 beta-ol and desmosterol in hamster cauda epididymal spermatozoa. Lipids. 1989;24:662–664. doi: 10.1007/BF02535086. [DOI] [PubMed] [Google Scholar]
- Bains HK, Bawa SR, Pabst MA, Sehgal S. Plasma membrane alterations of maturing goat (Capra indicus) spermatozoa: lectin-binding and freeze-fracture study. Cell Tissue Res. 1993;271:159–168. doi: 10.1007/BF00297554. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Weinberg A, Naumovski N, Velkov T, Pelzing M, Dolman S, Condina MR, Aitken RJ. Analysis of phosphopeptide changes as spermatozoa acquire functional competence in the epididymis demonstrates changes in the post-translational modification of Izumo1. J Proteome Res. 2012;11:5252–5264. doi: 10.1021/pr300468m. [DOI] [PubMed] [Google Scholar]
- Baker MA, Witherdin R, Hetherington L, Cunningham-Smith K, Aitken RJ. Identification of post-translational modifications that occur during sperm maturation using difference in two-dimensional gel electrophoresis. Proteomics. 2005;5:1003–1012. doi: 10.1002/pmic.200401100. [DOI] [PubMed] [Google Scholar]
- Bedford JM. Changes in the Electrophoretic Properties of Rabbit Spermatozoa during Passage through the Epididymis. Nature. 1963a;200:1178–1180. doi: 10.1038/2001178a0. [DOI] [PubMed] [Google Scholar]
- Bedford JM. Morphological changes in rabbit spermatozoa during passage through the epididymis. J Reprod Fertil. 1963b;5:169–177. doi: 10.1530/jrf.0.0050169. [DOI] [PubMed] [Google Scholar]
- Bedford JM. Effects of duct ligation on the fertilizing ability of spermatozoa from different regions of the rabbit epididymis. J Exp Zool. 1967;166:271–281. doi: 10.1002/jez.1401660210. [DOI] [PubMed] [Google Scholar]
- Bedford JM. The epididymis re-visited: a personal view. Asian J Androl. 2015;17:693–698. doi: 10.4103/1008-682X.153297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford JM, Calvin HI. Changes in -S-S- linked structures of the sperm tail during epididymal maturation, with comparative observations in sub-mammalian species. J Exp Zool. 1974a;187:181–204. doi: 10.1002/jez.1401870202. [DOI] [PubMed] [Google Scholar]
- Bedford JM, Calvin HI. The occurrence and possible functional significance of -S-S- crosslinks in sperm heads, with particular reference to eutherian mammals. J Exp Zool. 1974b;188:137–155. doi: 10.1002/jez.1401880203. [DOI] [PubMed] [Google Scholar]
- Belleannee C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J Androl. 2015;17:730–736. doi: 10.4103/1008-682X.155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleannee C, Belghazi M, Labas V, Teixeira-Gomes AP, Gatti JL, Dacheux JL, Dacheux F. Purification and identification of sperm surface proteins and changes during epididymal maturation. Proteomics. 2011;11:1952–1964. doi: 10.1002/pmic.201000662. [DOI] [PubMed] [Google Scholar]
- Belleannee C, Calvo E, Caballero J, Sullivan R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol Reprod. 2013;89:30. doi: 10.1095/biolreprod.113.110486. [DOI] [PubMed] [Google Scholar]
- Belleannee C, Thimon V, Sullivan R. Region-specific gene expression in the epididymis. Cell Tissue Res. 2012;349:717–731. doi: 10.1007/s00441-012-1381-0. [DOI] [PubMed] [Google Scholar]
- Bjorkgren I, Gylling H, Turunen H, Huhtaniemi I, Strauss L, Poutanen M, Sipila P. Imbalanced lipid homeostasis in the conditional Dicer1 knockout mouse epididymis causes instability of the sperm membrane. FASEB J. 2015;29:433–442. doi: 10.1096/fj.14-259382. [DOI] [PubMed] [Google Scholar]
- Bjorkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M, Sipila P. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS One. 2012;7:e38457. doi: 10.1371/journal.pone.0038457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandau RJ, Rumery RE. The Relationship of Swimming Movements of Epididymal Spermatozoa to Their Fertilizing Capacity. Fertil Steril. 1964;15:571–579. doi: 10.1016/s0015-0282(16)35401-2. [DOI] [PubMed] [Google Scholar]
- Breton S, Ruan YC, Park YJ, Kim B. Regulation of epithelial function, differentiation, and remodeling in the epididymis. Asian J Androl. 2016;18:3–9. doi: 10.4103/1008-682X.165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Wertheimer EV, Visconti PE, Krapf D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim Biophys Acta. 2014;1842:2610–2620. doi: 10.1016/j.bbadis.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin HR, Miller DJ. Zona pellucida protein binding ability of porcine sperm during epididymal maturation and the acrosome reaction. Dev Biol. 2000;222:99–109. doi: 10.1006/dbio.2000.9707. [DOI] [PubMed] [Google Scholar]
- Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS. A novel function for CRISP1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biol Reprod. 2007;77:848–854. doi: 10.1095/biolreprod.107.061788. [DOI] [PubMed] [Google Scholar]
- Caballero J, Frenette G, D’Amours O, Belleannee C, Lacroix-Pepin N, Robert C, Sullivan R. Bovine sperm raft membrane associated Glioma Pathogenesis-Related 1-like protein 1 (GliPr1L1) is modified during the epididymal transit and is potentially involved in sperm binding to the zona pellucida. J Cell Physiol. 2012;227:3876–3886. doi: 10.1002/jcp.24099. [DOI] [PubMed] [Google Scholar]
- Caballero JN, Frenette G, Belleannee C, Sullivan R. CD9-positive microvesicles mediate the transfer of molecules to Bovine Spermatozoa during epididymal maturation. PLoS One. 2013;8:e65364. doi: 10.1371/journal.pone.0065364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin HI, Bedford JM. Formation of disulphide bonds in the nucleus and accessory structures of mammalian spermatozoa during maturation in the epididymis. J Reprod Fertil Suppl. 1971;13:65–75. [PubMed] [Google Scholar]
- Calvo A, Pastor LM, Bonet S, Pinart E, Ventura M. Characterization of the glycoconjugates of boar testis and epididymis. J Reprod Fertil. 2000;120:325–335. [PubMed] [Google Scholar]
- Castillo Bennett J, Roggero CM, Mancifesta FE, Mayorga LS. Calcineurin-mediated dephosphorylation of synaptotagmin VI is necessary for acrosomal exocytosis. J Biol Chem. 2010;285:26269–26278. doi: 10.1074/jbc.M109.095752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Cheng L, Puri P, Soler D, Vijayaraghavan S. Protein phosphatase PP1 gamma 2 in sperm morphogenesis and epididymal initiation of sperm motility. Asian J Androl. 2007;9:445–452. doi: 10.1111/j.1745-7262.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng GH, Peng H, Zhang X, Zhang Y, Qian J, Duan E, Zhai Q, Zhou Q. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- Christova Y, James P, Mackie A, Cooper TG, Jones R. Molecular diffusion in sperm plasma membranes during epididymal maturation. Mol Cell Endocrinol. 2004;216:41–46. doi: 10.1016/j.mce.2003.10.075. [DOI] [PubMed] [Google Scholar]
- Christova Y, James PS, Cooper TG, Jones R. Lipid diffusion in the plasma membrane of mouse spermatozoa: changes during epididymal maturation, effects of pH, osmotic pressure, and knockout of the c-ros gene. J Androl. 2002;23:384–392. [PubMed] [Google Scholar]
- Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell. 2014;157:808–822. doi: 10.1016/j.cell.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG. The epididymis, cytoplasmic droplets and male fertility. Asian J Androl. 2011;13:130–138. doi: 10.1038/aja.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cuasnicu PS, Cohen DJ, Ellerman DA, Busso D, Da Ros VG, Morgenfel MM. Changes in specific sperm proteins during epididymal maturation. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York, Boston, Dordrecht, London, Moscow: Kluwer Academic/Plenum Publishers; 2002. pp. 389–403. [Google Scholar]
- Cummings RD. Use of lectins in analysis of glycoconjugates. Methods Enzymol. 1994;230:66–86. doi: 10.1016/0076-6879(94)30008-9. [DOI] [PubMed] [Google Scholar]
- D’Amours O, Frenette G, Bordeleau LJ, Allard N, Leclerc P, Blondin P, Sullivan R. Epididymosomes transfer epididymal sperm binding protein 1 (ELSPBP1) to dead spermatozoa during epididymal transit in bovine. Biol Reprod. 2012;87:94. doi: 10.1095/biolreprod.112.100990. [DOI] [PubMed] [Google Scholar]
- Da Ros VG, Munoz MW, Battistone MA, Brukman NG, Carvajal G, Curci L, Gomez-ElIas MD, Cohen DB, Cuasnicu PS. From the epididymis to the egg: participation of CRISP proteins in mammalian fertilization. Asian J Androl. 2015;17:711–715. doi: 10.4103/1008-682X.155769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Smith TB. Exploring the role of mononuclear phagocytes in the epididymis. Asian J Androl. 2015;17:591–596. doi: 10.4103/1008-682X.153540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux JL, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction. 2014;147:27–42. doi: 10.1530/REP-13-0420. [DOI] [PubMed] [Google Scholar]
- Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of wnt receptor activation. Dev Cell. 2009;17:788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Dias GM, Lopez ML, Ferreira AT, Chapeaurouge DA, Rodrigues A, Perales J, Retamal CA. Thiol-disulfide proteins of stallion epididymal spermatozoa. Anim Reprod Sci. 2014;145:29–39. doi: 10.1016/j.anireprosci.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Diez-Sanchez C, Ruiz-Pesini E, Montoya J, Perez-Martos A, Enriquez JA, Lopez-Perez MJ. Mitochondria from ejaculated human spermatozoa do not synthesize proteins. FEBS Lett. 2003;553:205–208. doi: 10.1016/s0014-5793(03)01013-5. [DOI] [PubMed] [Google Scholar]
- Dudiki T, Kadunganattil S, Ferrara JK, Kline DW, Vijayaraghavan S. Changes in Carboxy Methylation and Tyrosine Phosphorylation of Protein Phosphatase PP2A Are Associated with Epididymal Sperm Maturation and Motility. PLoS One. 2015;10:e0141961. doi: 10.1371/journal.pone.0141961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecroyd H, Asquith KL, Jones RC, Aitken RJ. The development of signal transduction pathways during epididymal maturation is calcium dependent. Dev Biol. 2004;268:53–63. doi: 10.1016/j.ydbio.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Eickhoff R, Baldauf C, Koyro HW, Wennemuth G, Suga Y, Seitz J, Henkel R, Meinhardt A. Influence of macrophage migration inhibitory factor (MIF) on the zinc content and redox state of protein-bound sulphydryl groups in rat sperm: indications for a new role of MIF in sperm maturation. Mol Hum Reprod. 2004;10:605–611. doi: 10.1093/molehr/gah075. [DOI] [PubMed] [Google Scholar]
- Eickhoff R, Wilhelm B, Renneberg H, Wennemuth G, Bacher M, Linder D, Bucala R, Seitz J, Meinhardt A. Purification and characterization of macrophage migration inhibitory factor as a secretory protein from rat epididymis: evidences for alternative release and transfer to spermatozoa. Mol Med. 2001;7:27–35. [PMC free article] [PubMed] [Google Scholar]
- Espinosa F, de la Vega-Beltran JL, Lopez-Gonzalez I, Delgado R, Labarca P, Darszon A. Mouse sperm patch-clamp recordings reveal single Cl- channels sensitive to niflumic acid, a blocker of the sperm acrosome reaction. FEBS Lett. 1998;426:47–51. doi: 10.1016/s0014-5793(98)00305-6. [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain-Maurel MA, Dadoune JP, Reger JF. A cytochemical study on surface charges and lectin-binding sites in epididymal and ejaculated spermatozoa of Macaca fascicularis. Anat Rec. 1984;208:375–382. doi: 10.1002/ar.1092080308. [DOI] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardilha M, Ferreira M, Pelech S, Vieira S, Rebelo S, Korrodi-Gregorio L, Sousa M, Barros A, Silva V, da Cruz e Silva OA, da Cruz e Silva EF. “Omics” of human sperm: profiling protein phosphatases. OMICS. 2013;17:460–472. doi: 10.1089/omi.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas R. Apocrine secretion: New insights into an old phenomenon. Biochim Biophys Acta. 2015:1850. doi: 10.1016/j.bbagen.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Figueiras-Fierro D, Acevedo JJ, Martinez-Lopez P, Escoffier J, Sepulveda FV, Balderas E, Orta G, Visconti PE, Darszon A. Electrophysiological evidence for the presence of cystic fibrosis transmembrane conductance regulator (CFTR) in mouse sperm. J Cell Physiol. 2013;228:590–601. doi: 10.1002/jcp.24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette G, Legare C, Saez F, Sullivan R. Macrophage migration inhibitory factor in the human epididymis and semen. Mol Hum Reprod. 2005;11:575–582. doi: 10.1093/molehr/gah197. [DOI] [PubMed] [Google Scholar]
- Frenette G, Lessard C, Madore E, Fortier MA, Sullivan R. Aldose reductase and macrophage migration inhibitory factor are associated with epididymosomes and spermatozoa in the bovine epididymis. Biol Reprod. 2003;69:1586–1592. doi: 10.1095/biolreprod.103.019216. [DOI] [PubMed] [Google Scholar]
- Frenette G, Lessard C, Sullivan R. Polyol pathway along the bovine epididymis. Mol Reprod Dev. 2004;69:448–456. doi: 10.1002/mrd.20170. [DOI] [PubMed] [Google Scholar]
- Frenette G, Sullivan R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol Reprod Dev. 2001;59:115–121. doi: 10.1002/mrd.1013. [DOI] [PubMed] [Google Scholar]
- Fujihara Y, Tokuhiro K, Muro Y, Kondoh G, Araki Y, Ikawa M, Okabe M. Expression of TEX101, regulated by ACE, is essential for the production of fertile mouse spermatozoa. Proc Natl Acad Sci U S A. 2013;110:8111–8116. doi: 10.1073/pnas.1222166110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi MG, Visconti PE. Chang’s meaning of capacitation: A molecular perspective. Mol Reprod Dev. 2016 doi: 10.1002/mrd.22663. [DOI] [PubMed] [Google Scholar]
- Gibbs GM, Lo JC, Nixon B, Jamsai D, O’Connor AE, Rijal S, Sanchez-Partida LG, Hearn MT, Bianco DM, O’Bryan MK. Glioma pathogenesis-related 1-like 1 is testis enriched, dynamically modified, and redistributed during male germ cell maturation and has a potential role in sperm-oocyte binding. Endocrinology. 2010;151:2331–2342. doi: 10.1210/en.2009-1255. [DOI] [PubMed] [Google Scholar]
- Girouard J, Frenette G, Sullivan R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int J Androl. 2011;34:e475–e486. doi: 10.1111/j.1365-2605.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- Haidl G, Opper C. Changes in lipids and membrane anisotropy in human spermatozoa during epididymal maturation. Hum Reprod. 1997;12:2720–2723. doi: 10.1093/humrep/12.12.2720. [DOI] [PubMed] [Google Scholar]
- Hall JC, Hadley J, Doman T. Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. J Androl. 1991;12:76–87. [PubMed] [Google Scholar]
- Hammerstedt RH, Hay SR, Amann RP. Modification of ram sperm membranes during epididymal transit. Biol Reprod. 1982;27:745–754. doi: 10.1095/biolreprod27.3.745. [DOI] [PubMed] [Google Scholar]
- Hao Z, Jha KN, Kim YH, Vemuganti S, Westbrook VA, Chertihin O, Markgraf K, Flickinger CJ, Coppola M, Herr JC, Visconti PE. Expression analysis of the human testis-specific serine/threonine kinase (TSSK) homologues. A TSSK member is present in the equatorial segment of human sperm. Mol Hum Reprod. 2004;10:433–444. doi: 10.1093/molehr/gah052. [DOI] [PubMed] [Google Scholar]
- Harayama H, Kusunoki H, Kato S. Capacity of goat epididymal spermatozoa to undergo the acrosome reaction and subsequent fusion with the egg plasma membrane. Reprod Fertil Dev. 1993a;5:239–246. doi: 10.1071/rd9930239. [DOI] [PubMed] [Google Scholar]
- Harayama H, Kusunoki H, Kato S. Capacity of rete testicular and cauda epididymal boar spermatozoa to undergo the acrosome reaction and subsequent fusion with egg plasma membrane. Mol Reprod Dev. 1993b;35:62–68. doi: 10.1002/mrd.1080350111. [DOI] [PubMed] [Google Scholar]
- Hemmings BA, Resink TJ, Cohen P. Reconstitution of a Mg-ATP-dependent protein phosphatase and its activation through a phosphorylation mechanism. FEBS Lett. 1982;150:319–324. doi: 10.1016/0014-5793(82)80760-6. [DOI] [PubMed] [Google Scholar]
- Hermo L, Robaire B. Epidydimal cell types and their functions. In: Robaire B, Hinton BT, editors. The Epidydims: from molecules to clinical practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 81–102. [Google Scholar]
- Hernandez F, Langa E, Cuadros R, Avila J, Villanueva N. Regulation of GSK3 isoforms by phosphatases PP1 and PP2A. Mol Cell Biochem. 2010;344:211–215. doi: 10.1007/s11010-010-0544-0. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt WV. Surface-bound sialic acid on ram and bull spermatozoa: deposition during epididymal transit and stability during washing. Biol Reprod. 1980;23:847–857. doi: 10.1095/biolreprod23.4.847. [DOI] [PubMed] [Google Scholar]
- Huang Z, Khatra B, Bollen M, Carr DW, Vijayaraghavan S. Sperm PP1gamma2 is regulated by a homologue of the yeast protein phosphatase binding protein sds22. Biol Reprod. 2002;67:1936–1942. doi: 10.1095/biolreprod.102.004093. [DOI] [PubMed] [Google Scholar]
- Huang Z, Myers K, Khatra B, Vijayaraghavan S. Protein 14-3-3zeta binds to protein phosphatase PP1gamma2 in bovine epididymal spermatozoa. Biol Reprod. 2004;71:177–184. doi: 10.1095/biolreprod.104.027284. [DOI] [PubMed] [Google Scholar]
- Huang Z, Somanath PR, Chakrabarti R, Eddy EM, Vijayaraghavan S. Changes in intracellular distribution and activity of protein phosphatase PP1gamma2 and its regulating proteins in spermatozoa lacking AKAP4. Biol Reprod. 2005;72:384–392. doi: 10.1095/biolreprod.104.034140. [DOI] [PubMed] [Google Scholar]
- Ijiri TW, Merdiushev T, Cao W, Gerton GL. Identification and validation of mouse sperm proteins correlated with epididymal maturation. Proteomics. 2011;11:4047–4062. doi: 10.1002/pmic.201100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri TW, Vadnais ML, Huang AP, Lin AM, Levin LR, Buck J, Gerton GL. Thiol changes during epididymal maturation: a link to flagellar angulation in mouse spermatozoa? Andrology. 2014;2:65–75. doi: 10.1111/j.2047-2927.2013.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- Jones R. Plasma membrane composition and organisation during maturation of spermatozoa in the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 405–416. [Google Scholar]
- Joseph A, Shur BD, Ko C, Chambon P, Hess RA. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor alpha knockout mouse. Biol Reprod. 2010;82:958–967. doi: 10.1095/biolreprod.109.080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CS, Suryawanshi AR, Khan SA, Balasinor NH, Khole VV. Liprin alpha3: a putative estrogen regulated acrosomal protein. Histochem Cell Biol. 2013;139:535–548. doi: 10.1007/s00418-012-1044-y. [DOI] [PubMed] [Google Scholar]
- Kameshwari DB, Bhande S, Sundaram CS, Kota V, Siva AB, Shivaji S. Glucose-regulated protein precursor (GRP78) and tumor rejection antigen (GP96) are unique to hamster caput epididymal spermatozoa. Asian J Androl. 2010;12:344–355. doi: 10.1038/aja.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Omurtag K, Moley KH. Decreased spermatogenesis, fertility, and altered Slc2A expression in Akt1−/− and Akt2−/− testes and sperm. Reprod Sci. 2012;19:31–42. doi: 10.1177/1933719111424449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff C, Hale G. Cell-to-cell transfer of glycosylphosphatidylinositol-anchored membrane proteins during sperm maturation. Mol Hum Reprod. 1996;2:177–184. doi: 10.1093/molehr/2.3.177. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339(Pt 2):319–328. [PMC free article] [PubMed] [Google Scholar]
- Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt Signaling Governs Epididymal Sperm Maturation. Cell. 2015;163:1225–1236. doi: 10.1016/j.cell.2015.10.029. [DOI] [PubMed] [Google Scholar]
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 2010;285:7977–7985. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf D, Ruan YC, Wertheimer EV, Battistone MA, Pawlak JB, Sanjay A, Pilder SH, Cuasnicu P, Breton S, Visconti PE. cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev Biol. 2012;369:43–53. doi: 10.1016/j.ydbio.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutskikh A, Poliandri A, Cabrera-Sharp V, Dacheux JL, Poutanen M, Huhtaniemi I. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. 2012;26:4198–4209. doi: 10.1096/fj.12-205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GP, Laloraya M, Agrawal P, Laloraya MM. The involvement of surface sugars of mammalian spermatozoa in epididymal maturation and in vitro sperm-zona recognition. Andrologia. 1990;22:184–194. doi: 10.1111/j.1439-0272.1990.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Labas V, Spina L, Belleannee C, Teixeira-Gomes AP, Gargaros A, Dacheux F, Dacheux JL. Analysis of epididymal sperm maturation by MALDI profiling and top-down mass spectrometry. J Proteomics. 2015;113:226–243. doi: 10.1016/j.jprot.2014.09.031. [DOI] [PubMed] [Google Scholar]
- Lakoski KA, Carron CP, Cabot CL, Saling PM. Epididymal maturation and the acrosome reaction in mouse sperm: response to zona pellucida develops coincident with modification of M42 antigen. Biol Reprod. 1988;38:221–233. doi: 10.1095/biolreprod38.1.221. [DOI] [PubMed] [Google Scholar]
- Li L, Liu Y. Diverse small non-coding RNAs in RNA interference pathways. Methods Mol Biol. 2011a;764:169–182. doi: 10.1007/978-1-61779-188-8_11. [DOI] [PubMed] [Google Scholar]
- Li Y, Sosnik J, Brassard L, Reese M, Spiridonov NA, Bates TC, Johnson GR, Anguita J, Visconti PE, Salicioni AM. Expression and localization of five members of the testis-specific serine kinase (Tssk) family in mouse and human sperm and testis. Mol Hum Reprod. 2011b;17:42–56. doi: 10.1093/molehr/gaq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB. A cAMP-induced increase in the motility of demembranated bull sperm models. Cell. 1978;13:9–18. doi: 10.1016/0092-8674(78)90133-2. [DOI] [PubMed] [Google Scholar]
- Lindenthal B, Aldaghlas TA, Kelleher JK, Henkel SM, Tolba R, Haidl G, von Bergmann K. Neutral sterols of rat epididymis. High concentrations of dehydrocholesterols in rat caput epididymidis. J Lipid Res. 2001;42:1089–1095. [PubMed] [Google Scholar]
- Lishko P, Clapham DE, Navarro B, Kirichok Y. Sperm patch-clamp. Methods Enzymol. 2013;525:59–83. doi: 10.1016/B978-0-12-397944-5.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- MacLeod G, Shang P, Booth GT, Mastropaolo LA, Manafpoursakha N, Vogl AW, Varmuza S. PPP1CC2 can form a kinase/phosphatase complex with the testis-specific proteins TSSK1 and TSKS in the mouse testis. Reproduction. 2014;147:1–12. doi: 10.1530/REP-13-0224. [DOI] [PubMed] [Google Scholar]
- Magargee SF, Kunze E, Hammerstedt RH. Changes in lectin-binding features of ram sperm surfaces associated with epididymal maturation and ejaculation. Biol Reprod. 1988;38:667–685. doi: 10.1095/biolreprod38.3.667. [DOI] [PubMed] [Google Scholar]
- Martin-DeLeon PA. Epididymosomes: transfer of fertility-modulating proteins to the sperm surface. Asian J Androl. 2015;17:720–725. doi: 10.4103/1008-682X.155538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marushige Y, Marushige K. Transformation of sperm histone during formation and maturation of rat spermatozoa. J Biol Chem. 1975;250:39–45. [PubMed] [Google Scholar]
- Mercado E, Carvajal G, Reyes A, Rosado A. Sulfhydryl groups on the spermatozoa membrane. A study with a new fluorescent probe for SH groups. Biol Reprod. 1976;14:632–640. doi: 10.1095/biolreprod14.5.632. [DOI] [PubMed] [Google Scholar]
- Miller MR, Mansell SA, Meyers SA, Lishko PV. Flagellar ion channels of sperm: similarities and differences between species. Cell Calcium. 2015;58:105–113. doi: 10.1016/j.ceca.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Miranda PV, Allaire A, Sosnik J, Visconti PE. Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm. Biol Reprod. 2009;80:897–904. doi: 10.1095/biolreprod.108.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Somanath PR, Huang Z, Vijayaraghavan S. Binding and inactivation of the germ cell-specific protein phosphatase PP1gamma2 by sds22 during epididymal sperm maturation. Biol Reprod. 2003;69:1572–1579. doi: 10.1095/biolreprod.103.018739. [DOI] [PubMed] [Google Scholar]
- Miyata H, Satouh Y, Mashiko D, Muto M, Nozawa K, Shiba K, Fujihara Y, Isotani A, Inaba K, Ikawa M. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science. 2015;350:442–445. doi: 10.1126/science.aad0836. [DOI] [PubMed] [Google Scholar]
- Mohri H, Yanagimachi R. Characteristics of motor apparatus in testicular, epididymal and ejaculated spermatozoa. A study using demembranated sperm models. Exp Cell Res. 1980;127:191–196. doi: 10.1016/0014-4827(80)90426-7. [DOI] [PubMed] [Google Scholar]
- Moore HD, Hartman TD, Pryor JP. Development of the oocyte-penetrating capacity of spermatozoa in the human epididymis. Int J Androl. 1983;6:310–318. doi: 10.1111/j.1365-2605.1983.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Murta D, Batista M, Silva E, Trindade A, Henrique D, Duarte A, Lopes-da-Costa L. Notch signaling in the epididymal epithelium regulates sperm motility and is transferred at a distance within epididymosomes. Andrology. 2016;4:314–327. doi: 10.1111/andr.12144. [DOI] [PubMed] [Google Scholar]
- Navarrete FA, Garcia-Vazquez FA, Alvau A, Escoffier J, Krapf D, Sanchez-Cardenas C, Salicioni AM, Darszon A, Visconti PE. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J Cell Physiol. 2015;230:1758–1769. doi: 10.1002/jcp.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson GL, Usui N, Yanagimachi R, Yanagimachi H, Smith JR. Lectin-binding sites on the plasma membranes of rabbit spermatozoa. Changes in surface receptors during epididymal Maturation and after ejaculation. J Cell Biol. 1977;74:950–962. doi: 10.1083/jcb.74.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson GL, Yanagimachi R. Terminal saccharides on sperm plasma membranes: identification by specific agglutinins. Science. 1972;177:276–279. doi: 10.1126/science.177.4045.276. [DOI] [PubMed] [Google Scholar]
- Nikolopoulou M, Soucek DA, Vary JC. Changes in the lipid content of boar sperm plasma membranes during epididymal maturation. Biochim Biophys Acta. 1985;815:486–498. doi: 10.1016/0005-2736(85)90377-3. [DOI] [PubMed] [Google Scholar]
- Nishigaki T, Jose O, Gonzalez-Cota AL, Romero F, Trevino CL, Darszon A. Intracellular pH in sperm physiology. Biochem Biophys Res Commun. 2014;450:1149–1158. doi: 10.1016/j.bbrc.2014.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Woo JM, Choi E, Kim T, Cho BN, Park ZY, Kim YC, Kim DH, Cho C. Molecular, biochemical, and cellular characterization of epididymal ADAMs, ADAM7 and ADAM28. Biochem Biophys Res Commun. 2005;331:1374–1383. doi: 10.1016/j.bbrc.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Olson GE, Danzo BJ. Surface changes in rat spermatozoa during epididymal transit. Biol Reprod. 1981;24:431–443. doi: 10.1095/biolreprod24.2.431. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist MC. Sperm maturation in rabbit epididymis. Nature. 1967;216:816–818. doi: 10.1038/216816a0. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist MC. Maturation of spermatozoa in the rabbit epididymis: delayed fertilization in does inseminated with epididymal spermatozoa. J Reprod Fertil. 1968;16:29–33. doi: 10.1530/jrf.0.0160029. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist MC. Studies on the function of the epididymis. Biol Reprod. 1969;1(Suppl 1):155–175. doi: 10.1095/biolreprod1.supplement_1.155. [DOI] [PubMed] [Google Scholar]
- Parks JE, Hammerstedt RH. Development changes occurring in the lipids of ram epididymal spermatozoa plasma membrane. Biol Reprod. 1985;32:653–668. doi: 10.1095/biolreprod32.3.653. [DOI] [PubMed] [Google Scholar]
- Paunescu TG, Shum WW, Huynh C, Lechner L, Goetze B, Brown D, Breton S. High-resolution helium ion microscopy of epididymal epithelial cells and their interaction with spermatozoa. Mol Hum Reprod. 2014;20:929–937. doi: 10.1093/molehr/gau052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari C, Hosokawa Y, Fukuda M, Manfredi Romanini MG. Cytofluorometric study of nuclear sulphydryl and disulphide groups during sperm maturation in the mouse. J Reprod Fertil. 1983;68:371–376. doi: 10.1530/jrf.0.0680371. [DOI] [PubMed] [Google Scholar]
- Pereira SR, Vasconcelos VM, Antunes A. The phosphoprotein phosphatase family of Ser/Thr phosphatases as principal targets of naturally occurring toxins. Crit Rev Toxicol. 2011;41:83–110. doi: 10.3109/10408444.2010.515564. [DOI] [PubMed] [Google Scholar]
- Pyttel S, Nimptsch A, Bottger J, Zschornig K, Jakop U, Wegener J, Muller K, Paasch U, Schiller J. Changes of murine sperm phospholipid composition during epididymal maturation determined by MALDI-TOF mass spectrometry. Theriogenology. 2014;82:396–402. doi: 10.1016/j.theriogenology.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Rana AP, Majumder GC, Misra S, Ghosh A. Lipid changes of goat sperm plasma membrane during epididymal maturation. Biochim Biophys Acta. 1991;1061:185–196. doi: 10.1016/0005-2736(91)90284-f. [DOI] [PubMed] [Google Scholar]
- Rejraji H, Sion B, Prensier G, Carreras M, Motta C, Frenoux JM, Vericel E, Grizard G, Vernet P, Drevet JR. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol Reprod. 2006;74:1104–1113. doi: 10.1095/biolreprod.105.049304. [DOI] [PubMed] [Google Scholar]
- Retamal C, Urzua J, Lorca C, Lopez ML, Alves EW. Changes in the plasma membrane proteins of stallion spermatozoa during maturation in the epididymis. J Submicrosc Cytol Pathol. 2000;32:229–239. [PubMed] [Google Scholar]
- Saez F, Ouvrier A, Drevet JR. Epididymis cholesterol homeostasis and sperm fertilizing ability. Asian J Androl. 2011;13:11–17. doi: 10.1038/aja.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda H, Gotoh Y, Katagiri H, Kurokawa M, Ono H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Fukushima Y, Abe M, Shojima N, Kikuchi M, Oka Y, Hirai H, Asano T. Differing roles of Akt and serum- and glucocorticoid-regulated kinase in glucose metabolism, DNA synthesis, and oncogenic activity. J Biol Chem. 2003;278:25802–25807. doi: 10.1074/jbc.M301127200. [DOI] [PubMed] [Google Scholar]
- Santi CM, Orta G, Salkoff L, Visconti PE, Darszon A, Trevino CL. K+ and Cl- channels and transporters in sperm function. Curr Top Dev Biol. 2013;102:385–421. doi: 10.1016/B978-0-12-416024-8.00014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saowaros W, Panyim S. The formation of disulfide bonds in human protamines during sperm maturation. Experientia. 1979;35:191–192. doi: 10.1007/BF01920608. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Shima H, Kitagawa Y, Irino S, Sugimura T, Nagao M. Identification of members of the protein phosphatase 1 gene family in the rat and enhanced expression of protein phosphatase 1 alpha gene in rat hepatocellular carcinomas. Jpn J Cancer Res. 1990;81:1272–1280. doi: 10.1111/j.1349-7006.1990.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel RA, Hammerstedt R, Cofer GP, Kozarsky K, Freidus D, Williamson P. Changes in the organization of the lipid bilayer of the plasma membrane during spermatogenesis and epididymal maturation. Biol Reprod. 1986;34:379–391. doi: 10.1095/biolreprod34.2.379. [DOI] [PubMed] [Google Scholar]
- Schroter S, Osterhoff C, McArdle W, Ivell R. The glycocalyx of the sperm surface. Hum Reprod Update. 1999;5:302–313. doi: 10.1093/humupd/5.4.302. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL, Xing L, Hoffmann O, Lowell CA, Garrett L, Boyce BF, Varmus HE. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 1997;11:2835–2844. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalgi R, Seligman J, Kosower NS. Dynamics of the thiol status of rat spermatozoa during maturation: analysis with the fluorescent labeling agent monobromobimane. Biol Reprod. 1989;40:1037–1045. doi: 10.1095/biolreprod40.5.1037. [DOI] [PubMed] [Google Scholar]
- Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F, Song L, Carone BR, Ricci EP, Li XZ, Fauquier L, Moore MJ, Sullivan R, Mello CC, Garber M, Rando OJ. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell cross talk in the epididymis: control of luminal acidification. J Androl. 2011;32:576–586. doi: 10.2164/jandrol.111.012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JV, Freitas MJ, Fardilha M. Phosphoprotein phosphatase 1 complexes in spermatogenesis. Curr Mol Pharmacol. 2014;7:163–146. doi: 10.2174/1874467208666150126154222. [DOI] [PubMed] [Google Scholar]
- Singh S, Malini T, Rengarajan S, Balasubramanian K. Impact of experimental diabetes and insulin replacement on epididymal secretory products and sperm maturation in albino rats. J Cell Biochem. 2009;108:1094–1101. doi: 10.1002/jcb.22337. [DOI] [PubMed] [Google Scholar]
- Sirivaidyapong S, Bevers MM, Gadella BM, Colenbrander B. Induction of the acrosome reaction in dog sperm cells is dependent on epididymal maturation: the generation of a functional progesterone receptor is involved. Mol Reprod Dev. 2001;58:451–459. doi: 10.1002/1098-2795(20010401)58:4<451::AID-MRD14>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sleight SB, Miranda PV, Plaskett NW, Maier B, Lysiak J, Scrable H, Herr JC, Visconti PE. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation. Biol Reprod. 2005;73:721–729. doi: 10.1095/biolreprod.105.041533. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Jack SL, Vijayaraghavan S. Changes in sperm glycogen synthase kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation. J Androl. 2004;25:605–617. doi: 10.1002/j.1939-4640.2004.tb02831.x. [DOI] [PubMed] [Google Scholar]
- Sosnik J, Miranda PV, Spiridonov NA, Yoon SY, Fissore RA, Johnson GR, Visconti PE. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci. 2009;122:2741–2749. doi: 10.1242/jcs.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Olson GE. Glycoprotein changes in the rat sperm plasma membrane during maturation in the epididymis. Mol Reprod Dev. 1991;29:357–364. doi: 10.1002/mrd.1080290407. [DOI] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–491. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146:21–35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Lovercamp K. Molecular markers of sperm quality. Soc Reprod Fertil Suppl. 2010;67:247–256. doi: 10.7313/upo9781907284991.021. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Thompson WE, Schatten G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J Cell Sci. 2001;114:1665–1675. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- Swingle M, Ni L, Honkanen RE. Small-Molecule inhibitors of Ser/Thr Protein Phosphatases. In: Moorhead G, editor. Protein Phosphatase Protocols. Totowa, NJ: Humana Press Inc; 2007. pp. 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama T, Mishima T, Mori M, Ishikawa T, Takizawa T, Goto T, Suzuki M, Araki Y, Matsubara S, Takizawa T. TEX101 is shed from the surface of sperm located in the caput epididymidis of the mouse. Zygote. 2005;13:325–333. doi: 10.1017/S0967199405003394. [DOI] [PubMed] [Google Scholar]
- Tecle E, Gagneux P. Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol Reprod Dev. 2015;82:635–650. doi: 10.1002/mrd.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod. 2008;23:1698–1707. doi: 10.1093/humrep/den181. [DOI] [PubMed] [Google Scholar]
- Tournade A. Différence de motilité des spermatozoides prélevés dans les divers segments de l’épididyme. C R Soc Biol. 1913;74:738–739. [Google Scholar]
- Treetipsatit N, Chulavatnatol M. Effects of ATP, cAMP and pH on the initiation of flagellar movement in demembranated models of rat epididymal spermatozoa. Exp Cell Res. 1982;142:495–499. doi: 10.1016/0014-4827(82)90397-4. [DOI] [PubMed] [Google Scholar]
- Tulsiani DR. Glycan-modifying enzymes in luminal fluid of the mammalian epididymis: an overview of their potential role in sperm maturation. Mol Cell Endocrinol. 2006;250:58–65. doi: 10.1016/j.mce.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Vadnais ML, Aghajanian HK, Lin A, Gerton GL. Signaling in sperm: toward a molecular understanding of the acquisition of sperm motility in the mouse epididymis. Biol Reprod. 2013;89:127. doi: 10.1095/biolreprod.113.110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, Greengard P. Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol Reprod. 1996;54:709–718. doi: 10.1095/biolreprod54.3.709. [DOI] [PubMed] [Google Scholar]
- Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A. 2009;106:667–668. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Hao Z, Purdon MA, Stein P, Balsara BR, Testa JR, Herr JC, Moss SB, Kopf GS. Cloning and chromosomal localization of a gene encoding a novel serine/threonine kinase belonging to the subfamily of testis-specific kinases. Genomics. 2001;77:163–170. doi: 10.1006/geno.2001.6628. [DOI] [PubMed] [Google Scholar]
- Wasco WM, Orr GA. Function of calmodulin in mammalian sperm: presence of a calmodulin-dependent cyclic nucleotide phosphodiesterase associated with demembranated rat caudal epididymal sperm. Biochem Biophys Res Commun. 1984;118:636–642. doi: 10.1016/0006-291x(84)91350-0. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]
- Wijshake T, Baker DJ, van de Sluis B. Endonucleases: new tools to edit the mouse genome. Biochim Biophys Acta. 2014;1842:1942–1950. doi: 10.1016/j.bbadis.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Williams RM, Graham JK, Hammerstedt RH. Determination of the capacity of ram epididymal and ejaculated sperm to undergo the acrosome reaction and penetrate ova. Biol Reprod. 1991;44:1080–1091. doi: 10.1095/biolreprod44.6.1080. [DOI] [PubMed] [Google Scholar]
- Wyatt AW, Hussain A, Amann K, Klingel K, Kandolf R, Artunc F, Grahammer F, Huang DY, Vallon V, Kuhl D, Lang F. DOCA-induced phosphorylation of glycogen synthase kinase 3beta. Cell Physiol Biochem. 2006;17:137–144. doi: 10.1159/000092075. [DOI] [PubMed] [Google Scholar]
- Xin AJ, Cheng L, Diao H, Wang P, Gu YH, Wu B, Wu YC, Chen GW, Zhou SM, Guo SJ, Shi HJ, Tao SC. Comprehensive profiling of accessible surface glycans of mammalian sperm using a lectin microarray. Clin Proteomics. 2014;11:10. doi: 10.1186/1559-0275-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Washington AM, Hinton BT. PTEN signaling through RAF1 proto-oncogene serine/threonine kinase (RAF1)/ERK in the epididymis is essential for male fertility. Proc Natl Acad Sci U S A. 2014;111:18643–18648. doi: 10.1073/pnas.1413186112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Muro Y, Isotani A, Tokuhiro K, Takumi K, Adham I, Ikawa M, Okabe M. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol Reprod. 2009;81:142–146. doi: 10.1095/biolreprod.108.074021. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Noda YD, Fujimoto M, Nicolson GL. The distribution of negative surface charges on mammalian spermatozoa. Am J Anat. 1972;135:497–519. doi: 10.1002/aja.1001350405. [DOI] [PubMed] [Google Scholar]
- Yeung CH. Effects of cyclic AMP on the motility of mature and immature hamster epididymal spermatozoa studied by reactivation of the demembranated cells. Gamete Research. 1984;9:99–114. [Google Scholar]
- Yeung CH, Cooper TG. Acquisition and development of sperm motility upon maturation in the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 417–434. [Google Scholar]
- Yeung CH, Cooper TG, Weinbauer GF. Maturation of monkey spermatozoa in the epididymis with respect to their ability to undergo the acrosome reaction. J Androl. 1996;17:427–432. [PubMed] [Google Scholar]
- Young WC. A study of the function of the epididymis. III. Funtional changes undergone by spermatozoa during their passage through the epididymis and vas deferens in the guinea-pig. The Journal of Experimental Biology. 1931;8:151–162. [Google Scholar]
- Yuan S, Zheng H, Zheng Z, Yan W. Proteomic analyses reveal a role of cytoplasmic droplets as an energy source during epididymal sperm maturation. PLoS One. 2013;8:e77466. doi: 10.1371/journal.pone.0077466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Yogesha SD, Mayfield JE, Gill GN, Zhang Y. Viewing serine/threonine protein phosphatases through the eyes of drug designers. FEBS J. 2013;280:4739–4760. doi: 10.1111/febs.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]