Abstract
Serum uric acid (SUA) has long been associated with increased cardiovascular risk, with arterial stiffness proposed as a mediator. However, evidence on the association between SUA and arterial stiffness is limited to contradicting cross-sectional studies. In this analysis, we examined the longitudinal relationship between SUA and pulse wave velocity (PWV), a measure of arterial stiffness, in a community-dwelling population. We studied 446 women and 427 men participating in the Baltimore Longitudinal Study of Aging, with 1409 and 1434 observations, respectively over an average period of 6 years. At baseline, women and men mean age was 65±13 and 68±13 year, mean SUA 4.6±1.1 and 5.7±1.3 mg/dL, mean PWV 8.1±1.7 and 8.6±1.9 m/sec, respectively (p<0.0001). In gender-stratified models accounting for age, blood pressure, renal function, metabolic measures and medications, there was a significant interaction between SUA and follow-up time in men (β=0.69, p=0.0002) but not in women. Men, but not women, in the highest gender-specific SUA tertile at baseline (SUA≥6.2 in men, SUA≥4.9 mg/dL in women) had a greater rate of PWV increase over time than those in the lowest tertiles (β=0.997, p=0.012). This gender difference was lost when the distribution of SUA in men and women was made comparable by excluding hyperuricemic men (SUA≥6.2 mg/dL). In conclusion, higher SUA was associated with greater increase in PWV in men but not women; this association was lost when men with SUA≥6.2 mg/dL were not included, suggesting a threshold for SUA association with arterial stiffness, which is more frequently reached in men.
Keywords: Serum uric acid, hyperuricemia, pulse wave velocity, arterial stiffness, longitudinal cohort study
INTRODUCTION
Serum uric acid (SUA) is a well-known marker of cardiovascular damage 1 and much experimental and clinical evidence support the possibility that an elevated SUA level may independently lead to or worsen hypertension 2. However, the mechanisms linking hyperuricemia to hypertension remain uncertain at present, and include endothelial dysfunction and arterial stiffening through increased oxidative stress, renin-angiotensin-aldosterone system activity and inflammation 3.
Carotid-femoral pulse wave velocity (PWV) is the gold standard measurement of arterial stiffness, and it is independently associated with the incidence and progression of hypertension 4-6. Nonetheless, only few studies have investigated the relationship between SUA and PWV, and these are mainly cross-sectional with contrasting findings. Some have suggested an independent positive association between SUA and PWV 7-9 while others have demonstrated that this association loses significance after adjustment for measures of renal function and body fat 10-13. More recently, two large studies found a stronger cross-sectional relationship between SUA and PWV in men than in women 14, 15. The gender difference in the association between SUA and PWV is particularly important given the higher SUA level in men, and their accelerated arterial stiffness with aging 6.
In light of previous study results, we hypothesized a direct independent longitudinal relationship between SUA and PWV that is more pronounced in men given their higher values of SUA. We tested this hypothesis performing gender-stratified linear mixed-effect analyses using data from the Baltimore Longitudinal Study of Aging (BLSA), a prospective cohort study with repeated measures of SUA, PWV, and other cardiovascular risk factors. Such information will not only elucidate one potential mechanism relating SUA to cardiovascular risk but could also help in determining whether targeting SUA might be useful to slow the progression of arterial stiffness and reduce the significant cardiovascular risk associated with it.
METHODS
Study sample
The study population was derived from the BLSA. This is an ongoing prospective study of community-dwelling elderly individuals, who regularly undergo a 2- to 3-day-long follow-up visit for the monitoring of a variety of medical conditions, including cardiovascular risk factors, organ damage and diseases. At each study visit, SUA was measured from a blood sample usually drawn in the morning of the first day, whereas PWV was measured within the next few days of study visit, according to the patient-specific schedule. For the present analysis, from the original cohort of 1351 subjects with 4831 follow-up visits, we selected only participants with at least two longitudinal visits with complete data of the variables of interest (i.e. SUA and PWV) and covariates (see below). We excluded visits before 2003 where PWV had been measured with a Doppler device 6. Between May 2003 and December 2014 repeated measurements were performed in a subset of 873 BLSA participants, with a total of 2843 repeated concurrent PWV and SUA measures. The distribution of female and male participants and follow-up visits by age groups at study entry is presented in Supplemental Table S1. The 478 participants that were excluded from the analysis were in equal part men (n=236) and women (n=242), had a lower number of prospective visits (total=655) and were generally older (mean age 73±11 years) and had greater comorbidity than those who were included.
The BLSA protocol was approved by the Institutional Review Board of record at the time of data collection (MedStar Health Research Institute, Baltimore, MD or National Institute of Environmental Health Sciences, NC). Participants were given a detailed description of the study and consented to participate.
Carotid-femoral PWV
Carotid-femoral PWV was calculated as the distance traveled by the pulse wave from the carotid to the femoral artery measured over the body surface divided by the time difference between the feet of carotid and femoral arterial waveforms gated to ECG. Details of the PWV protocol measurement are available in the Supplement Material, including the process applied to standardize PWV measures obtained from 2 different devices across the study period (Complior SP device [Artech Medical, Paris, France] from 2003 to 2010, and SphygmoCor system [AtCor Medical, Sydney, Australia] since 2011) 6.
Serum Uric Acid
Blood samples were collected in the morning after participants had been fasting for at least 12 hours and sitting for 15 minutes. Aliquots of serum were immediately obtained and stored at−80°C. Serum uric acid (mg/dL) was measured using enzymatic–colorimetric methods (Bayer, GmbH, Leverkusen, Germany). The lower limits of detection were 0.2 mg/dL, range 0.2 to 25.0 mg/dL, intra-assay and interassay coefficients of variation were equal to .5% and 1.7%, respectively 16. SUA was also expressed in gender-specific tertile at study entry. In women, the limit values of each tertile were the following: SUA <4 mg/dL (n=152, visits=469), SUA 4-4.8 mg/dL (n=145, visits=465), SUA ≥4.9 mg/dL (n=149, visits=475). In men, the limit values of each tertile were the following: SUA <5.1 mg/dL (n=136, visits=458), SUA 5.1-6.1 mg/dL (n=143, visits=494), SUA ≥6.2 mg/dL (n=148, visits=482).
Covariates of interest
Oscillometric brachial blood pressure was measured at the time of PWV using an appropriately sized cuff, and hypertension was defined as a blood pressure ≥140/90 mmHg or the use of antihypertensive medications. The heart rate was recorded before measuring PWV from the electrocardiogram. Body mass index was calculated as body weight divided by squared height (kg/m2). Waist circumference was measured at the minimal abdominal circumference between the lower edge of the rib cage and the iliac crests, and central obesity was defined as a waist circumference >102 cm in men and >88 cm in women. Smoking was ascertained by a questionnaire and participants who had never smoked >100 cigarettes were considered as non-smokers. Diabetes mellitus was diagnosed according to the 2011 American Diabetes Association criteria 17 or the use of diabetes medications. The estimated glomerular filtration rate (eGFR) was calculated by the simplified modification of diet in renal disease (MDRD) formula and expressed as mL/min/1.73 m2. Fasting serum samples were also used to assay plasma glucose and plasma lipoproteins, and low-density lipoprotein cholesterol concentrations were estimated by using the Friedewald formula. Hypercholesterolemia was defined as total serum cholesterol ≥200 mg/dL or the use of lipid-lowering medications. Use of medications was determined at each study visit according to the Anatomical Therapeutic Chemical (ATC) classification system recommended by the World Health Organization. Lipid-lowering medications included statins (C10AA) and their combination with other lipid-lowering agents (C10BA and C10BX), while antihypertensive medications included vasodilators (C01D, C03 and C04), diuretics (C03), beta-blockers (C07), calcium channel blockers (C08) and agents acting on the renin-angiotensin-aldosterone system (C09). Diabetes medications included insulin, biguanides, sulfonamides and other drugs used in diabetes (A10).
Statistical analysis
Baseline characteristics of women and men included in the analysis are presented as mean±standard deviation and ranges, or frequencies and percentages, and were compared by Student t test or chi-squared test, as appropriate. Linear mixed-effect models were implemented to assess the longitudinal association between SUA and PWV, after accounting for confounders. A linear mixed-effects regression model easily accommodates unbalanced, unequally spaced observations and, consequently, is an ideal tool for analyzing longitudinal changes in data from this observational study 18. Age was introduced in the model as entry age and follow-up time (time) to allow the detection of nonlinear changes in PWV with aging (see Supplemental Material for further details). Due to the well-known differences in PWV and SUA between women and men, all models were stratified by gender. To test for the longitudinal association between SUA and PWV, SUA and its interactions with entry age and time were introduced into a multivariate model accounting for established determinants of SUA and PWV. These covariates were chosen based on both their clinical and statistical associations with the predictor (i.e. SUA) and the outcome (i.e. PWV) demonstrated in previous literature 2, 6, 19, 20, and their availability at each study visit. Thus, the final full models, beyond SUA and its interactions with entry age and time, also included, nonwhite race, systolic blood pressure and heart rate, eGFR, waist circumference, glucose, triglycerides, LDL and HDL cholesterol, smoking, antihypertensive medications and lipid-lowering medications 6. Main determinants of PWV (i.e. systolic blood pressure, heart rate and waist circumference) and SUA (i.e. systolic blood pressure, eGFR and waist circumference) were forced into each model, then backward elimination of the remaining terms with p value >0.10 was performed. To further assess the differences in longitudinal changes in PWV with age at different SUA level, linear mixed-effect models with gender-specific baseline tertiles of SUA were also built, using the lowest gender-specific SUA tertile as the reference group. All analyses were performed using SAS for Windows (version 9.2; SAS Institute Inc, Cary, NC).
RESULTS
Characteristics of the study population at baseline are presented in Table 1. The final analyses included 446 women (51%) and 427 men (49%), with 1409 and 1434 prospective observations, respectively (median number of observations per participant 3, range 2-10) over a median follow-up period of 6 years (range 1-11). At first study visit, compared to women, men were older, more likely to be white, to smoke, and had higher PWV, SUA and blood pressure values but a lower heart rate. Men also had higher plasma glucose but lower total, LDL and HDL cholesterol levels than women, and also more frequently took antihypertensive and lipid-lowering medications than women. Men were more frequently diagnosed with hypertension and diabetes mellitus than women. Fewer than 1% of study participants had a eGFR of less than 60 ml/min/1.73 m2 with no significant differences between genders. There was also no significant difference between genders in diuretic medications that could potentially modify SUA levels, including thiazide or potassium-sparing agents (ATC C03A and C03D, 15% in women vs.14% in men, p=0.75). Three men (0.7%) but no women were taking anti-gout medication at baseline (ATC M04A, p=0.09).
Table 1.
Characteristics of the study population at first study visit by gender.
| Characteristics | Women (n=446) | Men (n=427) | P value |
|---|---|---|---|
| Age at first visit, years | 65±13 (26-93) | 68±13 (27-93) | 0.0024 |
| Age ≥65 at first visit, % | 50 | 63 | <0.0001 |
| Follow-up time, years | 5.8±2.6 (1-11) | 5.8±2.6 (1-11) | 0.79 |
| Race (white), % | 61 | 72 | 0.001 |
| Serum uric acid, mg/dL | 4.6±1.1 | 5.7±1.3 | <0.0001 |
| Pulse wave velocity, m/s | 8.1±1.7 | 8.6±1.9 | <0.0001 |
| Ever smoker, % | 38 | 54 | <0.0001 |
| Systolic blood pressure, mmHg | 124±18 | 129±17 | <0.0001 |
| Diastolic blood pressure, mmHg | 64±9 | 69±10 | <0.0001 |
| Heart rate, bpm | 67±9 | 62±10 | <0.0001 |
| Waist circumference, cm | 84±10 | 96±9 | <0.0001 |
| Central obesity, % | 32 | 26 | 0.037 |
| Glucose, mg/dL | 90±12 | 95±17 | <0.0001 |
| eGFR, ml/min/1.73m2 | 93.8±12.7 | 92.9±12.7 | 0.35 |
| eGFR <60 ml/min/1.73m2 | 0.2 | 0.2 | 0.97 |
| Total cholesterol, mg/dL | 204±35 | 184±36 | <0.0001 |
| HDL cholesterol, mg/dL | 66±17 | 52±14 | <0.0001 |
| Triglyceride, mg/dL | 99±52 | 105±62 | 0.09 |
| LDL cholesterol, mg/dL | 138±37 | 132±36 | 0.019 |
| Diabetes mellitus, % | 9 | 20 | <0.0001 |
| On meds for diabetes mellitus, % | 6 | 10 | 0.0001 |
| Hypertension, % | 41 | 56 | <0.0001 |
| On antihypertensive meds, % | 38 | 52 | <0.0001 |
| Hypercholesterolemia, % | 73 | 72 | 0.75 |
| On lipid-lowering meds, % | 36 | 44 | 0.014 |
As expected, the distribution of SUA significantly differed between genders at study entry, with men showing higher SUA than women (Supplemental Figure S1). When analyzing the correlation between SUA and age at study entry, women showed increasing levels of SUA with age, particularly before the seventh decade, whereas men did not. Nonetheless, at each five-year entry age group, men displayed higher SUA than women (Supplemental Figure S2).
We tested the longitudinal association between SUA and PWV in fully adjusted gender-specific models 6 including the interaction of SUA with entry age and time (Table 2, Model 1). The coefficients of the time main term, along with the interaction terms that included it, quantify the longitudinal change in PWV and how SUA modified the magnitude of these changes. In women, we found no longitudinal association between PWV and SUA, however, there was a significant SUA*entry age interaction, indicating that higher SUA was associated with higher PWV at older entry age. In contrast, in men we found a significant SUA*time interaction, suggesting that higher SUA was associated with an accelerated increase in PWV with aging. After performing backward elimination, these associations were found to be independent of other covariates, particularly systolic blood pressure, heart rate, eGFR and waist circumference (Table 2, Model 2). A table with raw coefficients is provided in the Supplemental Material (Supplemental Table S2).
Table 2.
Longitudinal predictors of PWV determined from linear mixed-effect models in women and men.
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Variables | β | p | β | p | β | p | β | p |
| Entry age (10 yrs) | 0.382 | <.0001 | 0.268 | <.0001 | 0.503 | <.0001 | 0.460 | <.0001 |
| Time (10 yrs) | −3.181 | 0.0002 | −3.132 | 0.0002 | −2.007 | 0.054 | −1.970 | 0.058 |
| Entry age*time | 0.647 | <.0001 | 0.638 | <.0001 | 0.565 | 0.0003 | 0.557 | 0.0001 |
| SUA (1 SD) | −0.774 | 0.023 | −0.573 | 0.079 | −0.308 | 0.429 | −0.197 | 0.015 |
| Entry age *SUA | 0.108 | 0.026 | 0.081 | 0.084 | 0.008 | 0.884 | - | - |
| Time*SUA | 0.204 | 0.207 | - | - | 0.680 | 0.0002 | 0.615 | 0.001 |
| Race (nonwhite) | 0.339 | 0.121 | - | - | 0.172 | 0.495 | - | - |
| SBP (1 SD) | 0.316 | <.0001 | 0.319 | <.0001 | 0.398 | <.0001 | 0.423 | <.0001 |
| HR (1 SD) | 0.125 | 0.025 | 0.143 | 0.009 | 0.110 | 0.090 | 0.114 | 0.073 |
| eGFR (1 SD) | −0.021 | 0.874 | −0.205 | 0.015 | −0.036 | 0.776 | −0.091 | 0.336 |
| WC (1 SD) | 0.217 | 0.002 | 0.253 | <.0001 | −0.022 | 0.766 | 0.001 | 0.990 |
| Glucose (1 SD) | −0.011 | 0.856 | - | - | −0.085 | 0.215 | - | - |
| HDL (1 SD) | −0.060 | 0.358 | - | - | −0.039 | 0.600 | - | - |
| Triglycerides(1 SD) | −0.037 | 0.572 | - | - | 0.070 | 0.413 | - | - |
| LDL (1 SD) | 0.136 | 0.022 | 0.111 | 0.044 | 0.087 | 0.244 | - | - |
| Ever smoking | −0.105 | 0.409 | - | - | 0.149 | 0.322 | - | - |
| Antihypertensive drugs | 0.068 | 0.575 | - | - | 0.150 | 0.278 | - | - |
| Lipid-lowering drugs | 0.117 | 0.291 | - | - | 0.061 | 0.645 | - | - |
β=standardized beta estimate; SD= standard deviation; SBP= systolic blood pressure; HR= heart rate; eGFR= estimated glomerular filtration rate; WC= waist circumference. * Model 1 represents the fully adjusted model, whereas Model 2 reports only the terms with p value <0.10 after backward selection; SBP, HR, eGFR and WC were forced into the reduced models, see text for details. Values of 1 SD of each continuous variable included in the model are the following: in women SUA=1.16 mg/dL, SBP 18.54 mmHg, HR= 9 bpm, WC=10.96 cm, glucose 13.18 mg/dL, HDL=16.30 mg/dL, triglyceride=48.51 mg/dL, LDL=36.42 mg/dL; in men SUA=1.29 mg/dL, SBP 17.50 mmHg, HR= 10.09 bpm, WC=12.53 cm, glucose 18.57 mg/dL, HDL=14.30 mg/dL, triglyceride=58.82 mg/dL, LDL=35.32 mg/dL.
To further dissect this differential contribution of SUA to the increase in PWV with aging in the two genders, we stratified the study population in gender-specific tertiles of SUA at study entry. When investigating the interactions of the middle and the highest SUA tertile with entry age and time (using the lowest tertile as reference) in gender-stratified linear mixed-effects models, we found a significant interaction of the highest but not of the middle SUA tertile with entry age in women, and with time in men, suggesting a threshold effect (Table 3, Models 1). Accordingly, participants in the lowest and in the middle tertile were combined in a unique reference group (Table 3, Models 2). Women in the highest SUA tertile had higher PWV at older entry age than the reference group, as evidenced by a significant entry age* highest SUA tertile interaction, but no significant differences in the rate of PWV change over time. On the contrary, men in the highest SUA tertile had an accelerated rate of PWV increase over time than the reference group, as evidenced by a significant time*highest SUA tertile interaction, but no significant differences in PWV at entry age (Table 3, Models 2).
Table 3.
Linear mixed-effects models examining the impact of SUA categories on the longitudinal changes in PWV in women and men.
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Variables | β | p | β | p | β | p | β | p |
| Entry age (10 yrs) | 0.132 | 0.146 | 0.191 | 0.011 | 0.456 | <.0001 | 0.459 | <.0001 |
| Time (10 yrs) | −3.129 | 0.0003 | −3.129 | 0.0002 | −2.611 | 0.014 | −2.692 | 0.010 |
| Entry age*time | 0.639 | <.0001 | 0.638 | <.0001 | 0.609 | <.0001 | 0.604 | <.0001 |
| Low SUA tertile Women <4 Men <5.1 |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mid SUA tertile Women 4-4.8 Men 5.1-6.1 |
−0.921 | 0.246 | 0 | 0 | −0.184 | 0.354 | 0 | 0 |
| High SUA tertile Women ≥4.9 Men ≥6.2 |
−1.962 | 0.020 | −1.557 | 0.042 | −0.226 | 0.271 | −0.126 | 0.474 |
| Entry age *Mid SUA tertile | 0.140 | 0.243 | - | - | - | - | - | - |
| Entry age *High SUA tertile | 0.300 | 0.015 | 0.238 | 0.033 | - | - | - | - |
| Time *Mid SUA tertile | - | - | - | - | −0.233 | 0.610 | - | - |
| Time *High SUA tertile | - | - | - | - | 0.877 | 0.057 | 0.997 | 0.012 |
| SBP (1 SD) | 0.321 | <.0001 | 0.323 | <.0001 | 0.413 | <.0001 | 0.416 | <.0001 |
| HR (1 SD) | 0.149 | 0.007 | 0.143 | 0.009 | 0.112 | 0.077 | 0.113 | 0.074 |
| eGFR (1 SD) | −0.188 | 0.023 | −0.191 | 0.020 | −0.090 | 0.329 | −0.083 | 0.366 |
| WC (1 SD) | 0.241 | 0.0002 | 0.238 | 0.0001 | −0.006 | 0.935 | −0.011 | 0.866 |
| LDL (1 SD) | 0.110 | 0.045 | 0.105 | 0.055 | - | - | - | - |
For abbreviations see Table 2. The value “0” indicate the reference group. Models were adjusted for variables shown, plus race, glucose, HDL cholesterol, triglycerides, HDL cholesterol, smoking, and antihypertensive and lipid-lowering drugs; results are reported for terms with p value <0.10 after backward selection; SBP, HR, eGFR and WC were forced into the reduced models, see text for details. SUA values are expressed in mg/dL. Full model results before backward selection are presented in Supplemental Table S3.
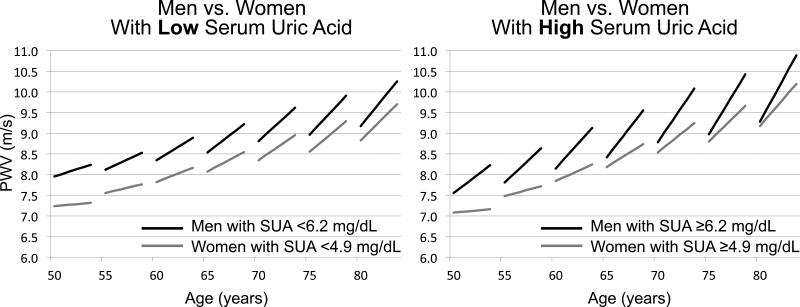
Figure 1 shows predicted longitudinal trajectories of PWV increase over time in women vs. men belonging to the lowest and the middle gender-specific SUA tertiles (Figure 1, panel on the left) and to the highest gender-specific SUA tertile (Figure 1, panel on the right). Whereas there was no differential contribution of SUA to the longitudinal increase in PWV between women and men with low SUA, men with high SUA had an accelerated rate of PWV increase over time compared to women with higher SUA (Figure 1), suggesting a gender-specific independent causative role of SUA in the longitudinal increase in PWV only in men.
Figure 1. Predicted longitudinal changes in PWV by gender and SUA categories.
Predicted longitudinal changes in pulse wave velocity (PWV) in women and men in the lowest and mid gender-specific serum uric acid (SUA) tertiles (left panel), and in the highest gender-specific SUA tertile (right panel) at study entry, obtained from Model 2 in Table 3. Note in the right panel the faster increase in PWV (i.e. steeper slope) with aging in men compared to women in the highest gender-specific SUA tertile. In contrast, note in the left panel that the rate of change did not significantly differ (i.e. lines are parallel) in men and women in the lowest and mid gender-specific SUA tertile.
We therefore performed sensitivity analyses to evaluate whether the higher SUA thresholds generally reached by men as compared to women could explain this gender difference. We selected a sample including only men in the lowest-middle SUA tertiles at baseline (SUA <6.2 mg/dL) and women in the middle-highest SUA tertiles at baseline (SUA ≥4 mg/dL). This sample included 294 women with 940 prospective observations, and 279 men with 952 prospective observations, with women having significantly higher mean baseline SUA values than men (5.1±0.9 vs. 4.9±0.8, p=0.03). The longitudinal association between SUA and PWV became not statistically significant in men (SUA*time β=0.46, p=0.11) and remained not statistically significant in women (SUA*time β=0.23, p=0.34). In addition, to further substantiate the potential cause-effect relationship between hyperuricemia and arterial stiffness, we repeated the analysis using lagged independent variables (i.e. correlating prior-visit SUA and covariates with next-visit PWV), and the results remained substantially unchanged (see Supplemental Table S4).
DISCUSSION
This is the first analysis investigating the longitudinal association between SUA and PWV in a community-dwelling population of elderly and mostly Caucasian individuals. We disclosed a significant independent relationship between increased SUA and increased PWV over time. This association was restricted to men, and possibly explained by the higher SUA levels in men than in women throughout their lifespan, rather than gender-specific susceptibility to the effects of SUA.
These data further expand the growing body of literature investigating a potential independent contribution of SUA to the progressive stiffening of the arterial tree observed with aging. Some previous cross-sectional analyses showed no association between SUA and PWV after adjusting for measures of renal function and body fat 10-13, whereas others showed a positive independent association 7-9. To date it remains unclear whether hyperuricemia has a causal effect or is simply a marker for other cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes 2. Yet, our present findings demonstrate an accelerated increase in PWV with aging in male individuals with higher SUA values, which was independent of major confounders including age, blood pressure, renal function and metabolic measures. Interestingly, two recent large cross-sectional studies, despite intrinsic limitations, also found a significant independent relationship between SUA and PWV only in men 14, 15. Men have SUA levels higher than women at all ages 21, a finding that we confirmed in our study population (Supplemental Figure S2). While this gender difference has generally been attributed to the uricosuric effect of estrogens in premenopausal women 22 and possibly to impaired renal clearance and excess production of uric acid in male patients, especially in the presence of visceral fat obesity or metabolic syndrome 23, the pathophysiology and clinical meaning of this finding has never been clearly elucidated 24. The gender-specific and time-dependent effect of SUA on PWV we report here might be due to the higher levels of SUA in men. In fact, when we selected a sample of men with baseline SUA levels comparable to those reported in women (i.e. <6.2 mg/dL), the significance of the longitudinal association with PWV was lost.
Consistent with the results of the continuous analysis, higher SUA levels at baseline were associated with an increased longitudinal rate of change in PWV in men. Interestingly, this result did not apply to male participants with baseline SUA values <6 mg/dL, which corresponds to the highest limit of the mid SUA tertile in men (<6.2 mg/dL) and is commonly considered as a cutoff criterion for definition of asymptomatic hyperuricemia 25. Thus, it appears as if there is a threshold effect for SUA on arterial stiffness, and men cross that threshold more frequently, hence the high PWV acceleration. In addition, this finding suggests that it is likely the long-term exposure to high levels of SUA that matters, even in the absence of symptoms. Accordingly, the 2-step process proposed by Feig 26 seems to fit well with recently reported findings in children 27, which suggest that time exposure to elevated SUA may lead to vascular remodeling and persistent hypertension as a long time consequence of functional and transient vasoconstriction.
Arterial stiffness is the consequence of a complex interaction between structural and geometric properties of the arterial wall and dynamic effects of the distending pressure. Its main determinants are aging and blood pressure 6, 20. The healthy properties of the vascular wall rely on the dynamic interplay of production and degradation of collagen and elastin. Deregulation of this balance may be stimulated by an inflammatory milieu leading to increased arterial stiffness 28 and contribute to the increase of blood pressure 4, 5. Several possible pathophysiological mechanisms linking uric acid and arterial stiffness have been proposed, including that uric acid may stimulate the inflammatory pathways 29 and promote vascular smooth muscle cells (VSMC) proliferation by entering cells 30. In particular, SUA has been shown to induce VSMC proliferation and oxidative stress by stimulating the vascular renin-angiotensin system 31. Uric acid also promotes endothelial dysfunction through inactivation of NO and impairment of endothelial cells proliferation 32. In addition to these molecular and animal models that support a direct metabolic effect of soluble urate, many recent studies have shown that hyperuricemia predict the development of hypertension 19. Furthermore, some preliminary data in children suggest that urate lowering treatment might reduce and normalize blood pressure 33, 34. Thus, the vascular damage our findings suggest to be induced by hyperuricemia may be a novel mechanism contributing to the development of essential hypertension. In resume, known biological mechanisms linking SUA to increased arterial stiffness might justify our findings of an increased baseline PWV in older female with higher SUA levels and of a steeper increase in PWV over time in men in the highest SUA tertile. Our data provide the first human model suggesting a robust pathogenetic mechanism linking SUA to the increase of blood pressure (or to the onset of hypertension).
Our findings supply some insight into the mechanism relating SUA to cardiovascular disease and the growing evidence of possible benefit from reducing asymptomatic hyperuricemia. Several large observational studies have demonstrated an independent association between SUA and cardiovascular outcomes 2, 21, 35. While reducing SUA in humans using hypouricemic drugs has not been found to independently improve cardiovascular outcomes 36, recently published large-scale studies show a favorable effect of reducing SUA production by pharmacologic inhibition of xanthine-oxidase on all cause mortality and cerebrovascular events 37, 38. In addition, preliminary data suggest that xanthine-oxidase inhibitors might induce relative reversibility of uric-acid related target organ damage 39.
Our study has some limitations as well as several strengths that should be mentioned. First, as a population-based study, the results of this analysis should be interpreted with caution in terms of implications on the direction of causality and applicability to individual patients. The purpose of this study is to reveal whether there is a longitudinal association between SUA and PWV and guide future studies to further examine this association. However, the effect size, using standardized coefficients, suggest that the effect of SUA in men is quite noticeable, with the effect of one standard deviation of SUA on the acceleration in the longitudinal increase in PWV being even greater than the effect of additional 10 years of age at study entry (β=0.615 vs. β=0.557, respectively, see Table 2, Model 2). The relatively large effect size might suggest a potential role for interventions targeting SUA in the future. Second, the relatively small sample size (especially when categorizing SUA subgroups) and the relatively healthy population of our cohort prevented stratifying the analysis by health conditions, which limits the generalizability of this cohort to other non-healthy populations. Another limitation is that, following the recommendation available at the beginning of the study, PWV was measured after at least 30-minute abstention from caffeine intake; to minimize biases to the longitudinal trends, we did not alter the protocol to accommodate the more recent recommended abstention period of 3 hours. However, PWV examinations are performed on BLSA participants during their 2-3 day stay in the hospital unit, within a controlled and comfortable environment, and far from habitual coffee time (breakfast or lunch).
Among the strengths, the wide age distribution and the similar number of observations and follow-up time between the two genders allowed us to perform the necessary gender-stratified analysis. In addition, the relatively low cardiovascular risk profile of our sample (i.e. only few participants with chronic kidney disease and low prevalence of diabetes mellitus and central obesity) likely permitted the isolation of the potential independent causal effect of a single molecule such as SUA on PWV. More importantly, it's very unlikely that this effect may be due or mediated by treatments, with less than 15% of the sample taking medications that could potentially affect SUA.
PERSPECTIVES
Our study, showing that subclinical SUA levels might affect the worsening of PWV over time, provides the first demonstration of a relationship between increased SUA levels and vascular damage, namely arterial stiffness, in a longitudinal setting. To better elucidate the role of uric acid lowering treatment in the context of cardiovascular risk factors, our findings should be further tested for the effect of lowering SUA on PWV in randomized controlled trials.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1) What Is New
This is the first analysis investigating the longitudinal association between SUA and PWV in a community-dwelling population of elderly individuals. A significant independent relationship exist mainly in men between SUA and an increase in PWV over time.
2) What Is Relevant?
Our present findings demonstrate an accelerated increase in PWV with aging in male individuals with higher SUA values, which was independent of major confounders including age, blood pressure, renal function and metabolic measures. This relationship may suggest causality, as the observed increase in PWV follows long-term exposure to asymptomatic hyperuricemia.
Summary
We found a steeper increase in PWV over time in men in the highest serum uric acid tertile. This association was restricted to men, and possibly explained by the higher SUA levels in men than in women throughout their lifespan. These data further expands the growing body of literature investigating a potential independent contribution of SUA to the progressive stiffening of the arterial tree observed with aging. Moreover, our study provides, for the first time, clinical evidence supporting pathogenetic mechanisms linking SUA to the onset of hypertension.
Acknowledgments
None
Sources of funding: This research was supported by the Intramural Research Program of the NIH, National Institute on Aging (USA).
Footnotes
Disclosures
None
REFERENCES
- 1.Viazzi F, Parodi D, Leoncini G, Parodi A, Falqui V, Ratto E, Vettoretti S, Bezante GP, Del Sette M, Deferrari G, Pontremoli R. Serum uric acid and target organ damage in primary hypertension. Hypertension. 2005;45:991–996. doi: 10.1161/01.HYP.0000161184.10873.ea. [DOI] [PubMed] [Google Scholar]
- 2.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavishi C, Messerli FH, Rimoldi SF. Serum uric acid in primary hypertension: From innocent bystander to primum movens? Hypertension. 2016;67:845–847. doi: 10.1161/HYPERTENSIONAHA.116.07056. [DOI] [PubMed] [Google Scholar]
- 4.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: The Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Li Y, Sheng CS, Huang QF, Zheng Y, Wang JG. Association of serum uric acid with aortic stiffness and pressure in a chinese workplace setting. Am J Hypertens. 2010;23:387–392. doi: 10.1038/ajh.2009.277. [DOI] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Xaplanteris P, Vyssoulis G, Bratsas A, Baou K, Tzamou V, Aznaouridis K, Dima I, Lazaros G, Stefanadis C. Association of serum uric acid level with aortic stiffness and arterial wave reflections in newly diagnosed, never-treated hypertension. Am J Hypertens. 2011;24:33–39. doi: 10.1038/ajh.2010.111. [DOI] [PubMed] [Google Scholar]
- 9.Tsai WC, Huang YY, Lin CC, Li WT, Lee CH, Chen JY, Chen JH. Uric acid is an independent predictor of arterial stiffness in hypertensive patients. Heart Vessels. 2009;24:371–375. doi: 10.1007/s00380-008-1127-9. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Marcos MA, Recio-Rodriguez JI, Patino-Alonso MC, Agudo-Conde C, Rodriguez-Sanchez E, Gomez-Sanchez L, Gomez-Sanchez M, Garcia-Ortiz L, Vasorisk g. Relationship between uric acid and vascular structure and function in hypertensive patients and sex-related differences. Am J Hypertens. 2013;26:599–607. doi: 10.1093/ajh/hps097. [DOI] [PubMed] [Google Scholar]
- 11.Mule G, Riccobene R, Castiglia A, D'Ignoto F, Ajello E, Geraci G, Guarino L, Nardi E, Vaccaro F, Cerasola G, Cottone S. Relationships between mild hyperuricaemia and aortic stiffness in untreated hypertensive patients. Nutr Metab Cardiovasc Dis. 2014;24:744–750. doi: 10.1016/j.numecd.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Cicero AF, Salvi P, D'Addato S, Rosticci M, Borghi C. Brisighella Heart Study g. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: Data from the Brisighella Heart Study. J Hypertens. 2014;32:57–64. doi: 10.1097/HJH.0b013e328365b916. [DOI] [PubMed] [Google Scholar]
- 13.Wijnands JM, Boonen A, van Sloten TT, Schram MT, Sep SJ, Koster A, van der Kallen CJ, Henry RM, Dagnelie PC, Stehouwer CD, van der Linden S, Arts IC. Association between serum uric acid, aortic, carotid and femoral stiffness among adults aged 40-75 years without and with type 2 diabetes mellitus: The Maastricht study. J Hypertens. 2015;33:1642–1650. doi: 10.1097/HJH.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 14.Mehta T, Nuccio E, McFann K, Madero M, Sarnak MJ, Jalal D. Association of uric acid with vascular stiffness in the Framingham Heart Study. Am J Hypertens. 2015;28:877–883. doi: 10.1093/ajh/hpu253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baena CP, Lotufo PA, Mill JG, Cunha Rde S, Bensenor IJ. Serum uric acid and pulse wave velocity among healthy adults: Baseline data from the brazilian longitudinal study of adult health (elsa-brasil). Am J Hypertens. 2015;28:966–970. doi: 10.1093/ajh/hpu298. [DOI] [PubMed] [Google Scholar]
- 16.Sutin AR, Cutler RG, Camandola S, Uda M, Feldman NH, Cucca F, Zonderman AB, Mattson MP, Ferrucci L, Schlessinger D, Terracciano A. Impulsivity is associated with uric acid: Evidence from humans and mice. Biol Psychiatry. 2014;75:31–37. doi: 10.1016/j.biopsych.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. J Gerontol A Biol Sci Med Sci. 2009;64:215–222. doi: 10.1093/gerona/gln024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33:1729–1741. doi: 10.1097/HJH.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network N-i. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. European Heart Journal. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 21.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES i epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 22.Anton FM, Garcia Puig J, Ramos T, Gonzalez P, Ordas J. Sex differences in uric acid metabolism in adults: Evidence for a lack of influence of estradiol-17 beta (e2) on the renal handling of urate. Metabolism. 1986;35:343–348. doi: 10.1016/0026-0495(86)90152-6. [DOI] [PubMed] [Google Scholar]
- 23.Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, Matsuzawa Y. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism. 1998;47:929–933. doi: 10.1016/s0026-0495(98)90346-8. [DOI] [PubMed] [Google Scholar]
- 24.Barbieri L, Verdoia M, Schaffer A, Marino P, Suryapranata H, De Luca G, Novara Atherosclerosis Study G. Impact of sex on uric acid levels and its relationship with the extent of coronary artery disease: A single-centre study. Atherosclerosis. 2015;241:241–248. doi: 10.1016/j.atherosclerosis.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol. 2014;26:186–191. doi: 10.1097/BOR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 26.Feig DI. Hyperuricemia and hypertension. Adv Chronic Kidney Dis. 2012;19:377–385. doi: 10.1053/j.ackd.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Viazzi F, Rebora P, Giussani M, Orlando A, Stella A, Antolini L, Valsecchi MG, Pontremoli R, Genovesi S. Increased serum uric acid levels blunt the antihypertensive efficacy of lifestyle modifications in children at cardiovascular risk. Hypertension. 2016;67:934–940. doi: 10.1161/HYPERTENSIONAHA.115.06852. [DOI] [PubMed] [Google Scholar]
- 28.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 29.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, Endou H, Lipkowitz M, Abramson R, Mu W, Johnson RJ. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol. 2005;25:425–433. doi: 10.1159/000087713. [DOI] [PubMed] [Google Scholar]
- 31.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular reninangiotensin system. J Hypertens. 2008;26:269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 32.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced c-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 33.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60:1148–1156. doi: 10.1161/HYPERTENSIONAHA.112.196980. [DOI] [PubMed] [Google Scholar]
- 35.Niskanen LK, Laaksonen DE, Nyyssonen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: A prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 36.Vinik O, Wechalekar MD, Falzon L, Buchbinder R, van der Heijde DM, Bombardier C. Treatment of asymptomatic hyperuricemia for the prevention of gouty arthritis, renal disease, and cardiovascular events: A systematic literature review. J Rheumatol Suppl. 2014;92:70–74. doi: 10.3899/jrheum.140465. [DOI] [PubMed] [Google Scholar]
- 37.Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford) 2009;48:804–806. doi: 10.1093/rheumatology/kep069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, Touyz RM, Dawson J. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension. 2016;67:535–540. doi: 10.1161/HYPERTENSIONAHA.115.06344. [DOI] [PubMed] [Google Scholar]
- 39.Sezai A, Soma M, Nakata K, Hata M, Yoshitake I, Wakui S, Hata H, Shiono M. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH trial). Circ J. 2013;77:2043–2049. doi: 10.1253/circj.cj-13-0082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.