Abstract
The hypothalamic-pituitary-adrenal (HPA) axis undergoes critical developments during adolescence. Therefore, stressors experienced during this period potentially have long-term effects on adult HPA axis function. We hypothesized that adolescent intermittent ethanol (AIE) exposure would affect adult HPA axis function, resulting in altered responses to an alcohol challenge in young adults or adults. To test these hypotheses, male rats were exposed to alcohol vapor for 6 h per day from post-natal day (PND) 28–42, then acutely challenged with alcohol intragastrically (3.2–4.5 g/kg) in young adults (PND 70) or adults (PND 90). Overall, we observed blunted HPA axis responses to an alcohol challenge due to AIE exposure. Specifically, AIE tended to inhibit the alcohol-challenge-induced increase in plasma corticosterone (CORT) concentrations in young adult and adult rats. As well, AIE significantly blunted the alcohol challenge-induced arginine vasopressin (Avp) mRNA expression in the paraventricular nucleus (PVN) of the hypothalamus of adult rats. Results of the present study are similar to what we have previously shown, that these changes in PVN responsiveness may result from AIE-induced alterations in adrenergic neurons in brain stem regions C1–C3 known to project to the PVN. AIE elevated the number of colocalized c-fos/phenylethanolamine N-methyltransferase (PNMT)-positive cell bodies in the C1 region of adult rats. Together, this data suggests that AIE exposure produces alterations in male HPA axis responsiveness to administration of an acute alcohol challenge that may be long-lasting.
Keywords: Alcohol, Corticosterone, Crf, Avp, PNMT
INTRODUCTION
Alcohol drinking is generally initiated during adolescence when the brain is still developing. In comparison to adults, adolescents are less sensitive to the aversive properties of alcohol, while they are more sensitive to the positive rewarding effects of alcohol (Spear and Varlinskaya, 2010). Genetic vulnerability, stress exposure or previous alcohol use may exacerbate these sensitivities to the aversive and rewarding aspects of alcohol (Spear and Varlinskaya, 2010). The effects of exposure to alcohol during adolescence are varied and could contribute to increased alcohol consumption in adulthood; these consequential effects may involve changes in the mesolimbic dopaminergic and glutamatergic systems (Pascual et al., 2009), impairment of neurogenesis (Morris et al., 2010), and differences in brain damage than is found in adults (Crews et al., 2000). Interestingly, adolescents also seem to be more susceptible to the effects of stress on alcohol drinking (Siegmund et al., 2005, Füllgrabe et al., 2007). Our laboratory is interested in alcohol’s effects on the central circuits known to regulate stress response – the hypothalamic-pituitary-adrenal (HPA) axis, which includes the paraventricular nucleus (PVN) of the hypothalamus and adrenergic brain stem regions that provide catecholamine inputs to the hypothalamus. It is widely known that alcohol exposure alters corticotropin releasing factor (Crf) activity in the PVN (Rivier et al., 1984, Rivier et al., 1990) with reported increases in PVN neuronal activity (Lee et al., 2000b) as well as corticosterone (CORT) release from the adrenal glands in response to alcohol consumption (Richardson et al., 2008). Our lab has also shown that alcohol causes changes in catecholaminergic cell numbers and activation following alcohol exposure (Logrip et al., 2013). However, it is important to distinguish between the response of a brain structure to alcohol itself, and the consequence of previous exposure to alcohol on the HPA axis’ ability to respond to an additional stressor such as an alcohol challenge in young adulthood and adulthood. We have examined the effects of adolescence binge drinking (self-administration) and adolescent intermittent ethanol (AIE) exposure (vapor chambers) on the brain stress response in young adulthood. In adolescent self-administering binge-drinking animals, we found a decrease in the number of Crf cells in the young adult amygdala (Allen et al., 2011a), a brain region that is important in conveying the emotional component of the stress response (Pich et al., 1995). Additionally, we have shown that AIE exerts long-term effects on the ability of the PVN to respond to an alcohol challenge in young adulthood, possibly mediated by catecholaminergic input from the brain stem to the PVN as seen by changes in activation (measured via c-fos immunoreactivity) of phenylethanolamine N-methyltransferase (PNMT) neurons (Allen et al., 2011b, Logrip et al., 2013). These results prompted us to investigate whether AIE exposure similarly or differentially affects the response to a distinct acute stressor, an alcohol challenge in young adult and adult male rats.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague Dawley rats (n = 45) were obtained from Harlan Laboratory (San Diego, CA, USA). Animals were housed three or four per cage with food and water ad libitum in a humidity- and temperature-controlled vivarium under a 12 h light/12 h dark light cycle with lights off at 1800. All experiments were carried out in the morning and met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by The Salk Institute Animal Care and Use Committee.
Intermittent alcohol vapor exposure
Adolescent rats [post-natal day (PND) 28–42] were exposed to alcohol vapor in an airtight chamber system provided by La Jolla Alcohol Research, Inc. (La Jolla, CA, USA, http://www.ljari.com), as described in detail previously (Lee et al., 2000a, 2000b). Rats were exposed to alcohol as vapor daily for 6 h (0700–1300; AIE) or air [Control (CON)] for the duration of the 15-day adolescent period (PND 28–42). After each daily exposure, the rats were returned to clean housing cages. Blood alcohol levels (BALs) were obtained and monitored on alternate days to maintain BALs at approximately 200 mg/dL. This exposure paradigm was used to generate controlled daily cycles of alcohol intoxication and withdrawal during the adolescent period as described previously (Logrip et al., 2013).
Blood alcohol levels
Blood samples for BAL measurements were obtained from the tails of all animals, including the air-exposed rats to control for any procedural stress effects. Blood plasma (5 μL) was used to determine BALs via an Analox AM 1 analyzer (Analox Instruments Ltd., Lunenburg, MA, USA (Lee et al., 2000a, 2000b). The precision of this assay is 1–2%, the sensitivity is 0.1 mg/dL and the curve is linear up to 400 mg/dL.
Corticosterone assay
Plasma corticosterone levels were measured in unextracted plasma from iv blood according to manufacturer’s instructions using a widely applied commercial double antibody RIA kit for rat and mouse samples (MPBiomedicals, Diagnostic Division, Orangeburg, NY, USA, catalog # 07120103). Specifically, blood was collected into chilled plastic tubes containing EDTA (0.6 mg/500 mL whole blood) and centrifuged. The resulting plasma was aspirated and a 5 μL aliquot immediately diluted into 995 μL corticosterone assay buffer, 1/200 final dilution per manufacturers instructions, and stored frozen until thawing once for CORT analysis. All samples from a single experiment were analyzed in the same CORT RIA and all samples displayed B/B0 of 90–20%. The reliable range of the assay is 10 to 1000 ng/mL with an intra-assay coefficient of variance of <10%. This assay is highly specific for corticosterone; the cross-react with desoxycorticosterone is 0.34%, testosterone is 0.1% and cortisol is 0.05%.
Animal surgery and alcohol injection
AIE and control male rats were implanted with ig or iv catheters upon reaching PND 62–63 or PND 68–69, respectively, under isoflurane anesthesia (Butler Animal Health Supply, Dublin, OH, USA) [see (Ogilvie et al., 1997) for methods], and were allowed to recover from surgery in individual cages for 7–8 days (ig) or 1–2 days (iv) before experimentation. On the day of the experiment (PND 70 or 90), the animals were placed in individual buckets with wood chip bedding in a quiet room with extension cannulae connected to a syringe containing heparinized saline such that the animals could be injected without being handled, to prevent any procedural stress. They were free-moving and left undisturbed for 2–3 h in an effort to acclimatize rats, then administered 3.2–4.5 g/kg alcohol (< 20% v/v in water) via the ig cannula. The alcohol challenge dose corresponds to that previously used in our laboratory (Lee et al., 2001, Lee and Rivier, 2003, Seo and Rivier, 2003). Injections were slowly infused over a 2-min period because of the large volume needed to be administered.
Acute Stressor
Plasma CORT concentration in young adult (PND 70) rats was determined to confirm a hormonal stress response to alcohol – a previously published stressor known to elicit changes in HPA-related brain circuitry [i.e., alcohol challenge; (Logrip et al., 2013)]: n = 6–7/group]. We also determined CORT concentration in adult animals (PND 70 or 90) to identify a hormonal stress response to an alcohol challenge; blood was collected from control (air: n = 4–7) and treatment (AIE: n = 4–7) male rats at 0, 60, 120, 180 and 240 min (PND 70) and at 0, 60, 120 and 210 min (PND 90) after an alcohol challenge (3.2–3.6 g/kg, ig). Finally, the effects of AIE exposure on the brain stress response to an alcohol challenge in adult animals was examined. Following an alcohol challenge in adulthood, the expression of Crf or arginine vasopressin (Avp) mRNA in the PVN of the same animals was examined (air: n = 4–5 and AIE: n = 6). In addition, the number of PNMT cells in the brain stem (C1–C3) region (air: n = 6 and AIE: n = 6) was studied.
Immunohistochemistry
Young adult and adult rats were deeply anesthetized by an ip injection of 35% chloral hydrate (Ogilvie et al., 1997) followed by transcardial perfusion with 0.9% NaCl for 2 min and 4% cold paraformaldehyde (PFA) for 18 min. Brains were placed in 4% PFA, then cryoprotected by overnight incubation in a 10% sucrose/4% PFA solution prior to coronal sectioning by microtome at a thickness of 30 μm. All sections were maintained in an antifreeze solution (50% 0.1 M phosphate buffered saline, 20% glycerol, 30% ethylene glycol) at −20°C until analysis. Briefly, double DAB immunohistochemistry (IHC) staining was performed on free-floating sections as described previously (Choi et al., 2008, Allen et al., 2011b) with a rabbit anti-c-fos antibody (1:10,000, Calbiochem, San Diego, CA, USA) stained black and a sheep anti-PNMT antibody (1:7500, Chemicon/Millipore, Billerica, MA, USA) stained brown. Sections were mounted on gelatin-coated sub slides, dehydrated, and coverslipped using DPX mounting medium (Electron Microscopy Sciences, Hatfield, PA). Negative controls without primary or secondary antibody were included. The black (c-fos) stain indicates activated nuclei whereas the brown PNMT-ir stain shows cytoplasmic signals. Individual and colocalized immuno-labeled cells were counted using a 20X dry objective in 3–6 sections throughout each brain region examined, and the average values per section for each rat were determined by brain region. The data are expressed as both the total number of PNMT-positive cells per section and as the number of c-fos-positive cells colocalized with PNMT-positive cell bodies.
In situ hybridization
As we observed the blunting effect of AIE on CORT responses to alcohol challenges, we further hypothesized that AIE would also decrease Crf and Avp mRNA expression in the PVN of adult male rats during the alcohol challenge. To test this, Crf and Avp mRNA levels were assessed in adult male rats at the end of the 2-h alcohol administration (4.5 g/kg, ig). In situ hybridization was performed according to a previously published protocol [see (Lee et al., 2000a, 2000b) for method].
Imaging
A Nikon optical system, Eclipse E600 microscope (Nikon Instruments Inc., Melville, NY, USA) equipped with a Micro*Color filter (Model RGB-MS-C, CRI Inc., Woburn, MA, USA) and CoolSNAP camera (Photometrics, Tucson, AZ, USA), coupled to a PC was used to capture images for IHC and in situ sections. IHC images were obtained with a 20X objective, while autoradiographic images were captured under 100X magnification. Image Pro Plus software (version 4.5.029, Media Cybernetics Inc., Bethesda, MD, USA) was used to obtain the densitometric analyses of the autoradiographic signals. Gray level measurements (optical density) were taken under dark-field illumination of hybridized sections in the PVN.
Statistical analysis
For all analyses which contained repeated measures over time or different brain reagions 2-way ANOVA for repeated measures with Sidak’s multiple comparisons test between groups at individual time points was used. All samples with n>4 were tested for normal distribution employing the Shapiro-Wilk or Kolmogorov-Smirnov test and visual evaluation using QQ plots. Equality of variances was tested using the Brown-Forsythe method. Single moderate violations were considered acceptable taking in account the relatively high robustness of ANOVA against minor violations especially when equal sample sizes are employed. However, in order to reduce probability for type-I errors post-hoc tests were amended by non-parametric tests for comparisons containing non-normally distributed samples and by Welch-corrected t-tests when variances between samples were significantly unequal. For simple comparisons between two samples unpaired t-tests were used when samples displayed normal distribution (determined by Kolmogorov-Smirnov test) and equal variances (Brown-Forsythe). Otherwise they were amended by non-parametric (Mann-Whitney) or Welch-corrected t-tests. Satistics were performed using Microsoft Excel 2013 (Microsoft Corp., Redmond, WA, USA) and Graphpad Prism 6.0 (Graphpad Software Inc., La Jolla, CA, USA). For all tests P<0.05 was considered significant. Where applicable, significant differences indicated in figures are based on corrected/non-parametric p-values.
RESULTS
Effect of AIE on plasma corticosterone (CORT) levels of young adult and adult male rats in response to an alcohol challenge
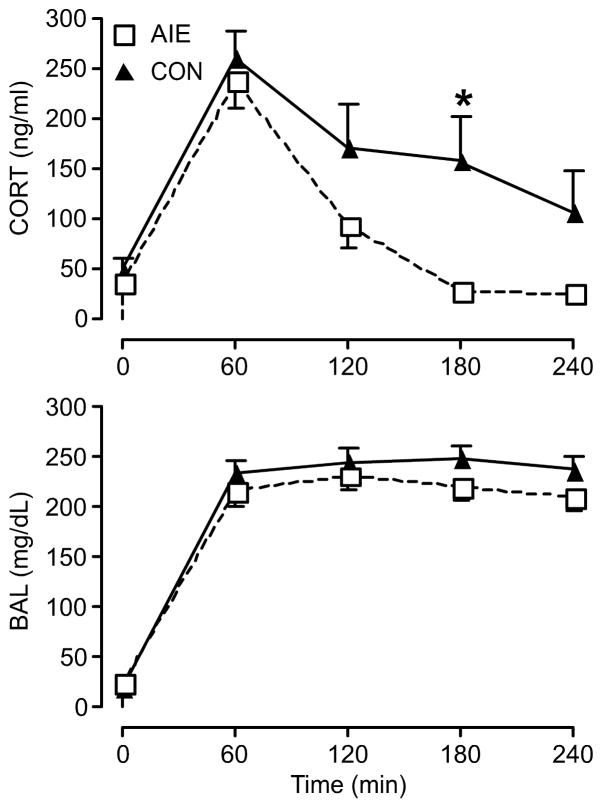
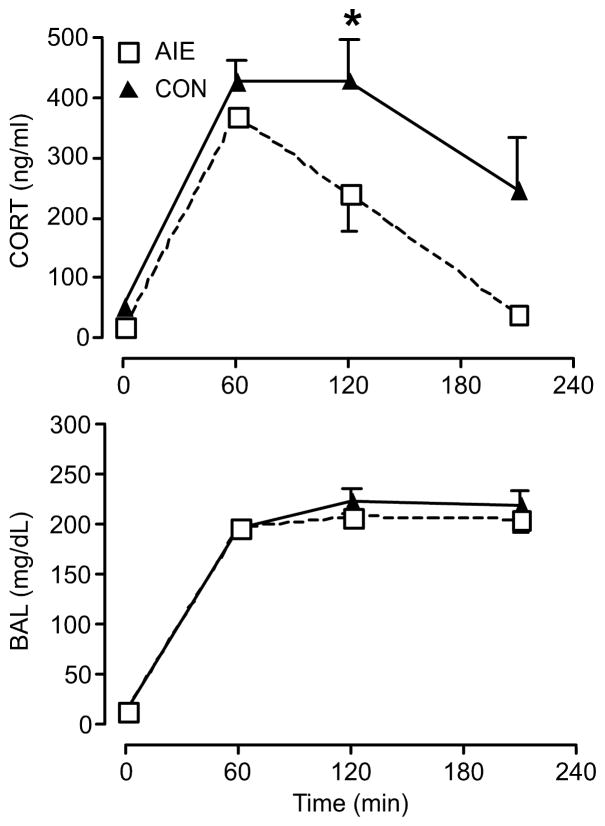
We hypothesized that exposure to alcohol during adolescence would have long-lasting effects on the adult HPA axis in response to an alcohol challenge. As predicted based on previous changes in the brain stress response (Logrip et al., 2013), the AIE group showed decreased plasma CORT concentrations in comparison to that of the control group at multiple time-points after ig alcohol injection in young adulthood (significant time × group interaction, F(4,48)=2.679 p <0.05, n=7 per group; significant difference between groups at 180 min: t=3.275, DF=60, P<0.01 (Sidak); t=2.917, df=6.236, p<0.05 (Welch-corrected t-test); Fig. 1). Furthermore, we tested to see if the blunting effects of AIE that we observed in young adult male rats were consistent with adult male rats as well (Fig. 2). As plasma CORT levels were measured at 60, 120, and 210 minutes after the injection of alcohol ig (3.2 g/kg), the AIE group again showed a significantly decreased CORT level compared to the control group (significant time × group interaction, F(3,18)=3.276, P<0.05, n=4 per group; significant difference between groups at 120 min: t=2.829, DF=24, P<0.05 (Sidak); Fig. 2, top). BALs, on the other hand, did not show any difference between AIE and control group, suggesting that differences in plasma CORT levels in adult male rats were independent of BAL discrepancies (Fig. 1 and 2, bottom). These results support the previous findings, from PVN Crf and Avp gene expression data that we published [see (Logrip et al., 2013)], suggesting that AIE blunts HPA axis activity. Together, these results show that AIE has a unique effect of inhibiting the HPA axis in response to an alcohol challenge.
Fig. 1.
Following an alcohol challenge in young adulthood (3.6g/kg via ig on PND 70) adolescent intermittent ethanol (AIE) exposure significantly decreased plasma CORT concentrations in male rats compared to the control group (CON) in young adulthood. There was no significant blood alcohol level (BAL) difference observed between AIE and control groups at any given time point in young adulthood. All male rats were exposed to alcohol vapor for 6 hours per day from PND 28 to PND 42, and blood samples were collected for analysis at each time point of interest. (Top) Data are expressed as mean ± SEM CORT concentration of 7 male rats per group. *P < 0.05. (Bottom) Data are expressed as mean ± SEM BAL of 7 male rats per group.
Fig. 2.
Following an alcohol challenge in adulthood (3.2g/kg via ig on PND 90), adolescent intermittent ethanol (AIE) exposure significantly decreased plasma CORT concentrations in male rats compared to the control group (CON) in adulthood. There was no significant blood alcohol level (BAL) difference observed between AIE and control groups at any given time point in adulthood. All male rats were exposed to alcohol vapor for 6 hours per day from PND 28 to PND 42, and blood samples were collected for analysis at each time point of interest. (Top) Data are expressed as mean ± SEM CORT level of 4 male rats per group. *P < 0.05. (Bottom) Data are expressed as mean ± SEM BAL of 4 male rats per group.
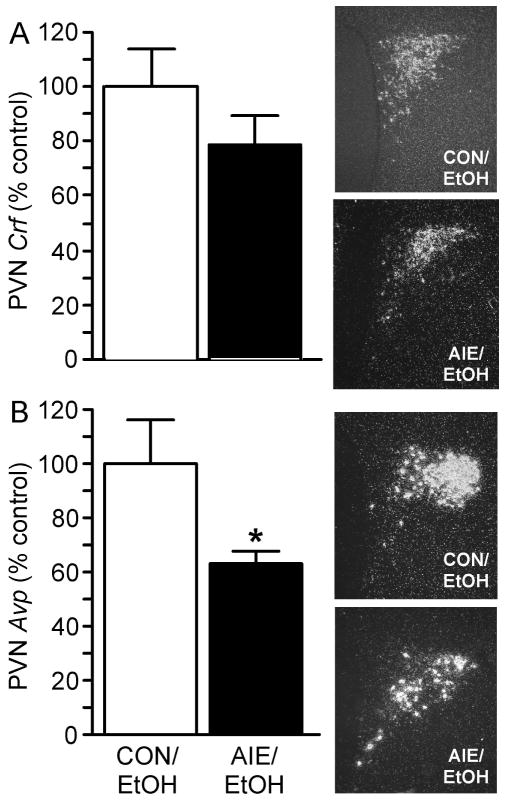
Effect of AIE on PVN Crf and Avp mRNA levels of adult male rats in response to an alcohol challenge
As illustrated in Fig. 3, PVN Avp mRNA levels were significantly decreased in the AIE group (t=2.416, df=9, P <0.05, n=5 CON, n=6 AIE). However, PVN Crf mRNA levels showed no difference between AIE and control groups (Mann-Whitney U=6, P > 0.05, n=4 CON, n=6 AIE). Together, these data support that AIE blunted PVN Avp gene expression at the end of a 2-h alcohol challenge in adult males.
Fig. 3.
Exposure to intermittent alcohol vapor during adolescence significantly decreased Avp mRNA levels in the PVN of adult male rats (PND 90) compared to controls following an alcohol challenge. Male rats exposed to adolescent intermittent ethanol (AIE) vapor or air (CON) were perfused 2 h after administration of alcohol challenge (4.5 g/kg, ig), and in situ hybridization was performed to quantify Crf and Avp mRNA levels in the PVN. (A) Quantification of PVN Crf mRNA levels by densitometric analysis of autoradiographic signals (arbitrary units, left) and representative dark-field photomicrographs of PVN Crf mRNA levels in adult male rats on PND 90 (100x magnification, right). (B) Quantification of PVN Avp mRNA levels by densitometric analysis of autoradiographic signals (arbitrary units, left) and representative dark-field photomicrographs of PVN Avp mRNA levels in male rats on PND 90 (100x magnification, right). Data in histograms are expressed as mean ± SEM optical density, expressed as percent control relative to CON/EtOH (ethanol) average for the given experiment from 4–6 rats per treatment. *P < 0.05.
Effect of AIE on brain stem PNMT levels of adult male rats in response to an alcohol challenge
Based on the observed effect of AIE on PVN Crf or Avp mRNA expression of adult male rats in response to an alcohol challenge, we hypothesized that AIE would affect brain stem PNMT neuron activation of adult male rats in response to an alcohol challenge as well. To test this, we performed c-fos/PNMT IHC on adult male rats’ brain stem, following alcohol administration (4.5 g/kg, ig). As shown in Fig. 4, the AIE group showed significantly elevated colocalization of c-fos-ir and PNMT-ir cells/section in the C1 medullary region (significant brain region × group interaction, F(2,20)=9.343, P < 0.01, n=6 per group; post-hoc comparison at C1: t=3.566, DF=30, P<0.01), in comparison to the control group. For both C2 and C3 regions of the brain stem, there was no statistically significant difference observed between AIE and control group in activation of PNMT-expressing neurons in the brain stem. Together, these data indicate that AIE differentially altered medullary brain stem regions in adult male rats following an alcohol challenge, suggesting a central role for catecholamines in modulating the long-term consequences of AIE on the adult HPA axis.
Fig. 4.
Exposure to intermittent alcohol vapor during adolescence significantly increased the number of colocalized c-fos and PNMT immunoreactive (ir) cells/section in the C1 region of the brain stem of male rats following alcohol challenge in adulthood (PND 90). Male rats exposed to adolescent intermittent ethanol (AIE) or air (CON) were perfused 2 h after administration of the alcohol challenge (4.5 g/kg, ig). (A) Double immunohistochemical staining labeled c-fos-containing nuclei black and PNMT-containing cell bodies brown in C1–C3 regions of the brain stem. Representative images were captured using a 20x dry objective (scale bar = 100 μm). (B) Quantification of PNMT-positive cell numbers (top) and PNMT-positive cells containing c-fos staining (bottom) are expressed as mean ± SEM cells/section for 6 male rats per treatment. **P < 0.01.
DISCUSSION
The overall goal of this study was to determine if AIE exposure exerts long-term effects on stress response and brain stress circuits in adult animals subjected to a distinct acute stressor – alcohol challenge. In line with previous findings in young adult male rats exposed to alcohol during adolescence, we found that alcohol illicits a stress response (CORT) and that AIE exposure causes changes in the brain stress circuitry to an alcohol challenge in adult male rats. Abnormal stress responses could have repercussions, such as changes in immune function (Segerstrom and Miller, 2004), which could lead to a decrease in overall health or survivorship.
Intermittent alcohol exposure during adolescence blunts the CORT response to an alcohol challenge. Interestingly, Przybycien-Szymanska et al. (2011) found the opposite result; animals exposed to ethanol (single dose or binge-like) during puberty (PND 37–44) had greater CORT concentrations than control animals and this finding suggested that when subjected to a stressor (ethanol), the HPA axis of young adult (PND 75) animals (Male Wistar rats) exposed to ethanol during puberty was more responsive. It is likely these differences are due to the more invasive method of ethanol administration (ip injection vs. ig cannulation administration) of the Przybycien-Szymanska et al. (2011) study as the intensity and type of stressor differs between the studies, with the less intense stress occurring with present methodology. Despite these discrepancies, it is evident that adolescent drinking can alter the adult HPA axis response to stress.
Exposure to intermittent alcohol vapor during adolescence significantly decreased Avp mRNA expression in the PVN of adult male rats following alcohol challenge in adulthood. In contrast, while Przybycien-Szymanska et al. (2011) found no significant difference in PVN Avp mRNA between control rats and young adult (PND 75) male rats with a history of alcohol injections during adolescence, the authors did find that adolescent alcohol-experienced rats had elevated PVN Crf mRNA after an acute alcohol challenge administered in young adulthood. Previous studies in our laboratory on AIE where young adult animals were challenged with alcohol identified the opposite result; Allen et al. (2011b) found a decrease in PVN Crf in young adult male (Sprague Dawley) rats (PND 61–62) rather than a decrease of Avp expression. Again, it is possible that the differences between our findings and those of the Przybycien-Szymanska et al. (2011) study are due to different methods of alcohol challenge. Taken together, these findings support our central hypothesis that AIE exerts long-term neuroendocrine effects.
Considering that we found a decrease in CORT concentration following an acute stressor in adult rats exposed to AIE, it is not surprising that we found a decrease in Avp mRNA levels in the PVN. Recently, Jasnic et al. (2013) found direct evidence of the role of Avp in regulating pituitary adrenocorticotropic hormone secretion which correlated with CORT concentration in male Wistar rats. This result supports our finding that exposure to alcohol during adolescence decreases the ability of the brain to mount a stress response to an acute stressor in adulthood and this is likely due to the hypothalamic blunting of Avp which consequently decreases the adrenal release of CORT into circulation.
An alcohol challenge in adulthood following AIE exposure increased the stress response of adrenergic cells in the brain stem area which innervates the hypothalamus (see summary of studies in Table 1). Specifically, AIE increased PNMT neuron activation in the C1 region of the brain stem of adult male rats following adult alcohol challenge. As well, exposure to intermittent alcohol vapor during adolescence increased PNMT neuron activation and the number of PNMT-ir cells/section in the C2 region of the brain stem of male (Sprague Dawley) rats following young adult alcohol challenge (Logrip et al., 2013). Similarly, Allen et al. (2011a) found a decrease in PNMT neuron activation in the C3 region of the brain stem of young adult male (Sprague Dawley) rats (PND 61–62) that voluntarily consumed alcohol in a binge-like manner during adolescence. Other researchers who examine the effects of stress on the heart, found an increase in PNMT expression in the ventricles of the heart of rats and suggested that this finding could provide a molecular mechanism that contributes to a modified physiological response, which is necessary for stress responsivity (Spasojevic et al., 2011). These multiple lines of evidence of changes in the catecholaminergic system suggest that central catecholamines play an important role in modulating the long-term consequence of AIE exposure on the HPA axis.
Table 1.
Exposure to alcohol during adolescence significantly changes the number and activation of adrenergic cells [phenylethanolamine N-methyltransferase (PNMT)] in the brain stem area (which innervates the hypothalamus – the site of stress response initiation) following an alcohol challenge in young adult and adult male rats. Note: post-natal day (PND).
| Treatment Group (Citation) | PNMT-ir cells per section | c-fos-ir/PNMT-ir cells per section |
|---|---|---|
| Vapor | C1: - | C1: - |
| PND 61–62 | C2: ↑ | C2: - |
| (Allen et al., 2011b) | C3: - | C3: - |
| Vapor | C1: - | C1: ↑ |
| PND 70–71 | C2: ↑ | C2: - |
| (Logrip et al., 2013) | C3: - | C3: - |
| Vapor | C1: - | C1: ↑ |
| PND 90–91 | C2: - | C2: - |
| (Present Study) | C3: - | C3: - |
| Self-Administration | C1: - | C1: - |
| PND 61–62 | C2: - | C2: - |
| (Allen et al., 2011a) | C3: - | C3: ↑ |
Overall, exposure to alcohol during adolescence alters (1) corticosterone release following an alcohol challenge, (2) Avp mRNA levels in the PVN after alcohol challenge, and (3) adrenergic cell activation in the brainstem following an alcohol challenge. All of these findings suggest that the HPA axis is vulnerable to the effects of alcohol during adolescence. Because the adolescent brain is still developing it is possible that alcohol permanently alters the brain during development and prevents it from being able to mount a stress response in the future. Based on our results, it may be that the adrenergic neurons that project to the PVN are activated in a greater number during alcohol consumption in adulthood, which then decreases the ability of the hypothalamus to respond to that stressor. Therefore, adults that were exposed to alcohol during adolescence may have irrevocably altered the ability of their brain to respond to stressful events (e.g., alcohol drinking in adulthood). However, future studies are required to further investigate the role of the HPA axis system and the catecholaminergic brain stem regions in modulating the future consequences of AIE exposure on adult’s HPA axis system.
Highlights.
AIE blunts the young adult and adult corticosterone response to alcohol.
AIE blunts paraventricular nucleus Avp expression response to alcohol challenge.
AIE increases adult brain stem PNMT activation response to alcohol challenge.
Acknowledgments
The authors would like to thank Sarah Im, Thomas Cho, Edward Vuong, Dylan Pham, and Debbie Doan for technical assistance. The project described was supported by U01-AA019973-NADIA Project from the National Institute on Alcohol Abuse and Alcoholism.
Abbreviations
- AIE
adolescent intermittent ethanol
- Avp
arginine vasopressin
- BAL
blood alcohol level
- CON
control group
- CORT
corticosterone
- Crf
corticotropin releasing factor
- HPA
hypothalamic-pituitary-adrenal
- ir
immunoreactive
- ig
intragastric
- iv
intravenous
- PVN
paraventricular nucleus
- PNMT
phenylethanolamine N-methyltransferase
- PND
post-natal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain Behav Immun. 2011a;25(Suppl 1):S50–60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Rivier CL, Lee SY. Adolescent alcohol exposure alters the central brain circuits known to regulate the stress response. Neuroscience. 2011b;182:162–168. doi: 10.1016/j.neuroscience.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lee S, Rivier C. Novel role of adrenergic neurons in the brain stem in mediating the hypothalamic-pituitary axis hyperactivity caused by prenatal alcohol exposure. Neuroscience. 2008;155:888–901. doi: 10.1016/j.neuroscience.2008.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Braun C, Hoplight B, Switzer R, 3rd, Knapp D. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Füllgrabe M, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Jasnic N, Djordjevic J, Vujovic P, Lakic I, Djurasevic S, Cvijic G. The effect of vasopressin 1b receptor (V1bR) blockade on HPA axis activity in rats exposed to acute heat stress. J Exp Biol. 2013;216:2302–2307. doi: 10.1242/jeb.082842. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Long-term influence of an initial exposure to alcohol on the rat hypothalamic-pituitary axis. Alcoholism: Clin Exper Res. 2003;27:1463–1470. doi: 10.1097/01.ALC.0000086065.06203.DD. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcoholism: Clin Exper Res. 2000a;24:110–122. [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis of rats exposed to alcohol in utero: role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000b;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt ED, Tilders FJH, Rivier C. Effect of repeated exposure to alcohol on the response of the hypothalamic-pituitary adrenal axis of the rat: I. Role of changes in hypothalamic neuronal activity. Alcoholism: Clin Exper Res. 2001;25:98–105. [PubMed] [Google Scholar]
- Logrip M, Rivier C, Lau C, Im S, Vaughan J, Lee S. Adolescent alcohol exposure alters the rat adult hypothalamic-pituitary-adrenal axis responsiveness in a sex-specific manner. Neuroscience. 2013;235:174–186. doi: 10.1016/j.neuroscience.2012.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S, Eaves D, Smith A, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob G, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska M, Mott N, Paul C, Gillespie R, Pak T. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS One. 2011;6:e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Rivier C, Imaki T, Vale W. Prolonged exposure to alcohol: effect on CRF mRNA levels, and CRF- and stress-induced ACTH secretion in the rat. Brain Res. 1990;520:1–5. doi: 10.1016/0006-8993(90)91685-a. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DO, Rivier C. Interaction between alcohol and nitric oxide on ACTH release in the rat. Alcoholism: Clin Exper Res. 2003;27:989–996. doi: 10.1097/01.ALC.0000071737.84882.C4. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer M, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Spasojevic N, Gavrilovic L, Dronjak S. Regulation of catecholamine-synthesising enzymes and beta-adrenoceptors gene expression in ventricles of stressed rats. Physiol Res. 2011;60:S171–176. doi: 10.33549/physiolres.932173. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]