Abstract
Opioid dependence leads to physical dependence and addiction which finally results in profound medical, psychological and social dysfunction. One of the useful medications for opioid dependence is buprenorphine, the partial opioid agonist, which is used alone or in combination with naloxone. However, buprenorphine is the victim of its own success due to its illicit use and accidental poisoning in children. Also, buprenorphine typically requires daily self-administration and its effectiveness heavily depends on patient adherence. So, poor treatment adherence results in ineffective treatment manifesting as craving and withdrawal symptoms. Short-term use of buprenorphine in opioid dependence is also often followed by relapse. Buprenorphine when used sublingually often results in inadequate or fluctuating blood concentrations and poorer treatment retention compared with methadone. All of these led to the development of Probuphine®, a polymeric matrix composed of ethylene vinyl acetate and buprenorphine in the form of implants, that are implanted subdermally in office practice and deliver the active drug over 6 months. Buprenorphine release from such implant is fairly consistent, avoiding plasma peaks and troughs, and the implant is also reported to be safe. In this review article, we have highlighted these aspects of treatment of opioid addiction, stressing on the pharmacology of buprenorphine and Probuphine®, and relevant clinical trials addressing the efficacy and safety of Probuphine®. This sustained-release implantable formulation of buprenorphine has the potential to be a suitable alternative to daily or alternate day sublingual buprenorphine which can thereby eliminate the need for daily supervision, minimizing fluctuations in plasma concentrations, and allowing these patients to reduce clinic or pharmacy visits.
Keywords: addiction, buprenorphine, implant, opioid dependence, Probuphine®
Opioid addiction and buprenorphine
Opioid use leads to physical dependence, medical and psychological disorders and social dysfunction. Drugs are effective for the treatment of opioid dependence given the unique effects of opioids on the brain and the availability of medications that can interfere with these effects [O’Connor, 2010; Ling et al. 2010]. While counseling is critical for all substance abuse treatment, opioid dependence is uniquely susceptible to drug therapy. Patients and care givers are often inclined to short-term medication rather than following a rigorous longer-term maintenance [O’Connor, 2010]. However, for most opioid-dependent patients there are no such shortcuts. It has been conclusively demonstrated that detoxification has exceedingly high long-term failure rates and is not beneficial as opioid maintenance [Masson et al. 2004; Woody and Metzger, 2011].
One of the useful drugs for opioid dependence is the partial opioid agonist buprenorphine. When used alone, or in combination with naloxone (buprenorphine–naloxone) in a sublingual tablet formulation, buprenorphine improves drug use-related outcomes in a manner similar to methadone [Johnson et al. 2000] with a much better safety profile even at high doses [Umbricht et al. 2004]. The naloxone component is not much absorbed sublingually, but is included to block opioid effects if intravenous use is tried [O’Connor, 2010]. Buprenorphine was approved by the United States Food and Drug Administration (FDA) in 2002 and its use has increased considerably. Along with enhanced safety, buprenorphine has the advantage of being available through prescription through office practices, thus expanding the availability of maintenance treatment outside the confines of rigid programs [Arfken et al. 2010]. Subsequent research has expanded knowledge on how [Fiellin et al. 2006] and in whom [Woody et al. 2008] buprenorphine can be used most effectively.
Owing to its high affinity for the μ receptor, buprenorphine inhibits the reinforcing effect of exogenous opioids. The ceiling effect of buprenorphine’s μ-agonistic activity reduces the potential for drug overdose and confers low toxicity even at high doses. Buprenorphine pharmacotherapy has proven to be a suitable treatment approach that supports recovery from addiction while reducing or curtailing the use of opioids [Ling et al. 2012]. Although the misuse liability persists in the monotherapy, it is limited if naloxone is present. When injected, naloxone precipitates opioid withdrawal in opioid-dependent individuals and inhibits injection of the buprenorphine plus naloxone combination product [Ling et al. 2012].
Before initiating buprenorphine treatment, it is important to establish a diagnosis of opioid dependence and review the risks and benefits of treatment. Urine drug screening is a valuable tool to collect objective evidence of recent opioid use. Prior to induction, patients should abstain from short-acting opioids for at least 12 h and exhibit mild-to-moderate objective signs of opioid withdrawal (reflected by a Clinical Opiate Withdrawal Scale score of ⩾9) [Wesson and Ling, 2003] before administration of the first dose of buprenorphine, to avoid causing withdrawal symptoms during the induction period [Ling et al. 2012].
Pharmacology of buprenorphine
Pharmacokinetics
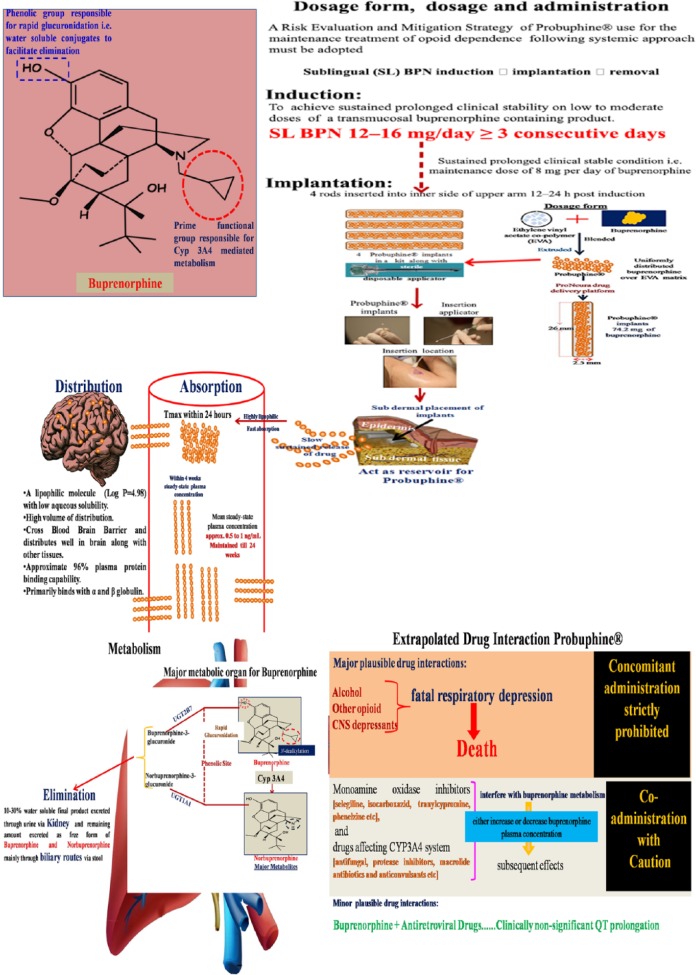
For opioid addiction, buprenorphine is most commonly prescribed as a tablet or film containing buprenorphine hydrochloride mixed in a 4:1 ratio with naloxone for sublingual administration [Ling et al. 2012]. Buprenorphine has poor oral bioavailability but high sublingual bioavailability [Compton et al. 2006]. Peak plasma concentration occurs approximately 90 min after absorption, with a mean half-life of 37 h. It is metabolized in the liver via N-dealkylation and glucuronidation, with the resulting active metabolite norbuprenorphine conjugating with glucuronic acid and finally excreted in feces and urine [Chiang and Hawks, 2003]. Acute administration results in small amounts of the metabolite in plasma, while chronic dosing results in increased plasma levels of norbuprenorphine, the only biologically active metabolite [Kuhlman et al. 1998].
In the combination product, naloxone does not affect the pharmacokinetics of buprenorphine [Harris et al. 2004]. Naloxone undergoes direct glucuronidation to naloxone 3-glucoronide, as well as N-dealkylation and reduction of the 6-oxo group. Sublingual absorption of both buprenorphine and naloxone is subjected to inter-individual variations, but this variability is found to be clinically insignificant when they are administered to opioid-dependent individuals. Both the maximum concentration and the area under curve of buprenorphine increase in a linear fashion within the 4–16 mg dose range, but the increase is not directly dose-proportional. All metabolites are virtually undetectable by 11 days after administration [Ling et al. 2012].
Pharmacodynamics
As mentioned, buprenorphine is a partial opioid agonist with a strong affinity for the μ opioid receptor and is an antagonist at the κ receptor. The high affinity for and limited intrinsic activity at the μ receptor inhibits the reinforcing effect of exogenous opioids [Walsh et al. 1995]. Although buprenorphine is a partial opioid agonist, its tight binding characteristic and slow rate of dissociation result in a prolonged clinical effect and limited physical dependence [Ling et al. 2012]. An advantage of buprenorphine as pharmacotherapy for opioid addiction is that its reinforcing effect is counterbalanced as it does not produce the ‘rush’ sought by addicted individuals. Reliably inhibiting opioid self-administration, abrupt cessation of buprenorphine dosing gradually results in an abstinence syndrome milder than that observed after cessation of methadone [Kosten et al. 1993; Ling et al. 1996], thereby facilitating discontinuation of the medication as necessary or desired.
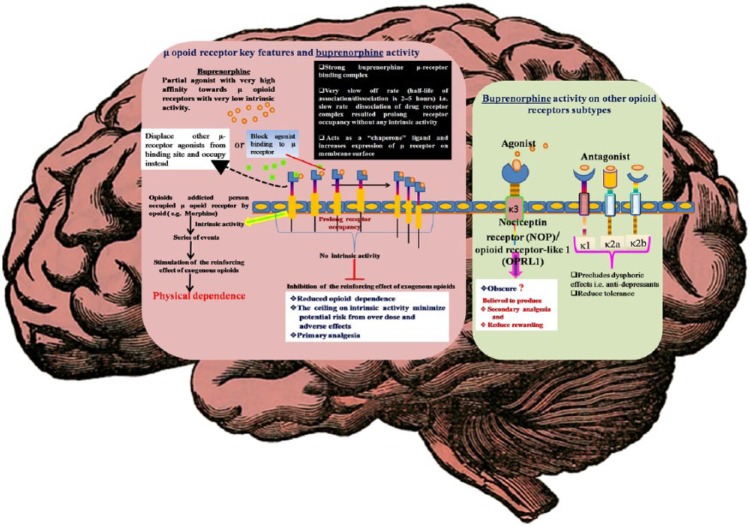
In receptor binding assays, buprenorphine has been characterized as an agonist-antagonist opioid with a partial agonist activity on μ receptors, an agonist activity on κ3 receptors and an antagonist activity on κ1, κ2a, and κ2b receptors [Pick et al. 1997; Davids and Gastpar, 2004]. It exhibits a unique profile that offers several advantages over other opioids for the management of physical dependence on opioids [Bickel and Amass, 1995; Pick et al. 1997; Barnett et al. 2001]. Its activity on the μ and κ3 receptors enables it to attenuate the physical dependence of other opioids but with a lower abuse liability [Lewis, 1985; Walsh et al. 1995; Pick et al. 1997]. This also contributes to a superior safety profile of a ceiling effect on its adverse effects (e.g. respiratory depression) [Davids and Gastpar, 2004; Law et al. 2004]. Its antagonist activity on the κ1, κ2a, and κ2b receptors precludes dysphoric effects, which also results in a low abuse liability [Pick et al. 1997; Barnett et al. 2001; Davids and Gastpar, 2004; Law et al. 2004] (Figure 1).
Figure 1.
Key pharmacodynamic aspects of buprenorphine.
NOP, nociceptin receptor; OPRL1, opioid-related nociceptin receptor 1.
Solomon and Corbit proposed opponent-process theory of motivation, where they postulated two automatically generated processes (A-process and B-process) by the central nervous system in case of any hedonic, affective or emotional state. The A-process, which could be a positive or negative hedonic response, occurs shortly after presentation of a stimulus correlates closely with the stimulus intensity; quality and duration of reinforce and eventually shows tolerance. Where, the B-process appears after the termination of A-process, which has a sluggish onset, takes time to build up and extremely slow to decay, but gets larger with repeated exposure. Based on Solomon’s hypothesis [Solomon, 1980] of the existence of proponent and opponent motivational processes, similar processes also work in the brain substrate of rewards, and hence the concept of antireward pathway was proposed [Koob and Le Moal, 2008]. This hypothesis can be applied in an addiction model, where after few exposure, positive hedonic responses in the reward pathway comes into a tolerance where the withdrawal symptoms in the antireward pathway becomes stronger, which needs the generation of new motives and new opportunities for reinforcing and energizing the specific (drug seeking) behavior [Solomon and Corbit, 1973, 1974; Koob and Le Moal, 2008]. This antireward pathway is mostly mediated through the κ opioid receptor–dynorphin system which causes significant dysphoria, and other psychotomimetic effects, etc. [Pfeiffer et al. 1986; Roth et al. 2002; Wang et al. 2010]. Buprenorphine being a κ receptor antagonist relieves these effects and also decreases the chance/urge of the individual to activate reward pathways that could lead to further consumption of the particular substance.
Also, buprenorphine has very high lipid solubility, which is equivalent to that of fentanyl [Roy et al. 1994] which allows the unimpeded transfer of buprenorphine through the blood–brain barrier [Roy et al. 1994]. Its activity on opioid receptors is quite different from the other opioids, such as morphine and methadone, leading to less cross-tolerance [Bulka et al. 2004]. Also it has few drug interactions compared with methadone. Both buprenorphine and its active metabolite, norbuprenorphine, are rapidly glucuronidated and inactivated [Davis, 2005]. It is for all of these reasons that buprenorphine is considered as the first line drug for opioid dependence.
Adverse reactions and drug interactions
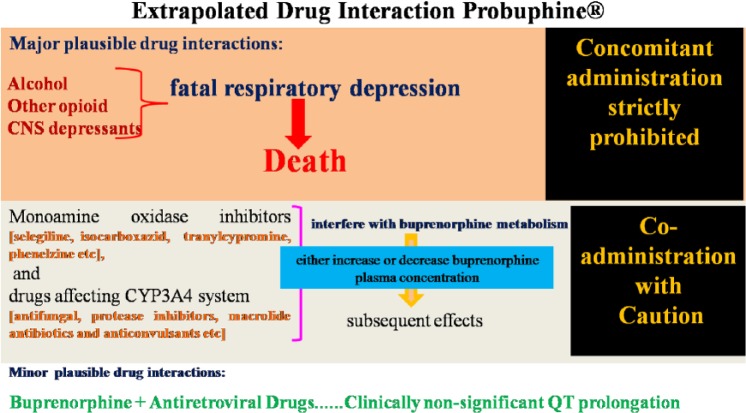
Notable adverse reactions to buprenorphine include headache, drowsiness, nausea, constipation, sleep problems, depression, anxiety, dizziness, numbness or tingling, and trouble in concentration [Doran et al. 2003]. Other adverse effects are weight gain, sweating, skin rash, itching, abdominal pain, lassitude, menstrual irregularities and decreased libido [Ling et al. 2012]. Its combination buprenorphine with alcohol, opioids or other central nervous system (CNS) depressants can result in severe respiratory depression. For example, the combination of buprenorphine with intravenous benzodiazepines may result in death due to profound respiratory depression [McCance-Katz et al. 2010]. Monoamine oxidase inhibitors and drugs affecting cytochrome P450 enzymes, specifically CYP3A4 drugs, can either increase or inhibit buprenorphine metabolism. Common drugs in this group include antifungals, protease inhibitors, macrolide antibiotics and anticonvulsants [McCance-Katz et al. 2006]. Cardiac arrhythmias can be of concern in some populations, when taken with antiretroviral drugs, buprenorphine prolongs QT interval [Anchersen et al. 2009; Baker et al. 2006] (Figure 2).
Figure 2.
Clinically relevant drug interactions with buprenorphine.
CYP, cytochrome P450 enzymes.
Problems with buprenorphine formulations in opioid addiction
Several studies support the efficacy of buprenorphine in opioid withdrawal [Gowing et al. 2009]. But, in some ways, buprenorphine is a victim of its own success, as its possession in the hands of patients has unavoidably resulted in some illicit usage and diversion to individuals who are not under medical care, showing that the drug can be abused and is being used illicitly. Increasing numbers of people misusing prescription opioid pain medications have provided a new market for diverted buprenorphine. Thus, buprenorphine-prescribing doctors and regulatory agencies are increasingly concerned about the compliance and diversion of buprenorphine, which has worsened with its extensive use. Another issue attendant with the widespread use of buprenorphine is the potential for accidental poisoning in children and others who come into contact with buprenorphine which is not properly stored. The problems and concerns about buprenorphine have raised much attention despite its clear benefits and utility. Diversion, nonadherence and noncompliance with medication remain troublesome issues that require innovative solutions and diligence among clinicians [Ling et al. 2012].
Buprenorphine has a high cost relative to methadone. In addition, buprenorphine typically requires daily supervised or self-administration. Thus, particularly as office-based treatment where medication administration is generally unsupervised, efficacy relies on patient adherence. Efforts to improve adherence have been investigated, including less than daily dosing and electronic compliance monitoring; however, these approaches are seldom used [O’Connor, 2010]. Inadequate or fluctuating blood levels may explain why sublingual buprenorphine, particularly when relatively lower doses are used early in treatment, is associated with poorer treatment compliance compared with methadone [Kosten et al. 1993; Ling et al. 1996; Fischer et al. 1999; Petitjean et al. 2001].
Short-term use of buprenorphine in opioid dependence is often followed by relapse [Weiss et al. 2011]. In general, the duration of buprenorphine pharmacotherapy should be tailored individually. Patients with unstable or untreated medical or psychiatric conditions may require prolonged pharmacotherapy. Whether discontinuation is appropriate is a matter of timing and clinical judgment based on understanding the individual patient and the circumstances [Ling et al. 2012]. In the US, buprenorphine can be prescribed in office-based physician practice [Fudala et al. 2003]. However, there are concerns about diversion and nonmedical use of sublingual buprenorphine, even when a buprenorphine–naloxone combination is used [Winstock et al. 2008; Alho et al. 2007; Bruce et al. 2009]. Poor treatment adherence, resulting in craving and withdrawal symptoms that increase the likelihood of relapse, is also a concern with sublingual buprenorphine [Bell et al. 2009a,b]. To address these problems with adherence, diversion, and nonmedical use, the implantable formulations of buprenorphine were developed [Ling et al. 2012].
Long-term delivery formulations of buprenorphine
In the treatment of opioid addiction, buprenorphine is gaining favor due to its efficacy and superior safety profile compared with methadone and other drugs (e.g. L-alpha-acetylmethadol) [Strain et al. 1994; Johnson et al. 2000]. Due to its mixed agonist–antagonist effects on the opioid receptor, high doses of buprenorphine usually do not cause significant complications [Lewis, 1985; Walsh et al. 1994].
Where supervision of dosing is required, frequent visits to the clinic or pharmacy reduce patients’ independence and incur significant staff time and cost [Kakko et al. 2007; Byrne and Wodak, 2007]. These limitations of standard buprenorphine treatment have raised interest in the development of alternative formulations [Sobel et al. 2004; Lanier et al. 2007]. An implantable, long-term delivery formulation of buprenorphine could improve treatment by ensuring compliance, maintaining stable plasma blood concentrations of the drug and reducing the likelihood of abuse and diversion [Lopatko et al. 2003].
An animal study showed that buprenorphine implants maintain release in vitro that can be manipulated by washing with 95% ethanol (USP) at room temperature for 30 min. The washed implants released 2 mg/day on the first day of release, and stabilized to approximately 1 mg/day over the subsequent 13 days. They also maintained dose-proportional steady-state concentration release in vivo for 1 year with little variability and produce minimal adverse effects. The release of buprenorphine from the implants is dependent on the rate of dissolution and passive diffusion through the polymer matrix [Kleppner et al. 2006]. Studies have also shown that buprenorphine implants provide long-term stable blood concentrations, eliminating the frequent fluctuations that are observed with sublingual administration. Peak buprenorphine concentrations are generally reached within 24 h after implantation. Steady-state plasma levels are attained between 3 and 8 weeks and are maintained subsequently and no implant-related serious adverse effects are reported [Kleppner et al. 2006]. In a study by Liu and colleagues, it was found that the depot of buprenorphine base in sesame oil or oleaginous vehicles (e.g. castor oil, cottonseed oil, peanut oil and soybean oil) produced a dose-related long-lasting effect [Liu et al. 2006].
Different types of sustained-release biodegradable microcapsules for the parenteral delivery of buprenorphine have been developed using different pharmaceutical techniques [Mandal, 1999]. A subcutaneously implantable buprenorphine delivery system utilizing cholesterol–glyceryltristearate matrix for prolonged release of the drug has been described. Neither deterioration of implant nor any gross anatomic changes at implant site were apparent 12 weeks after such pellet implantation [Pontani and Misra, 1983]. A small study of depot injection buprenorphine demonstrated low plasma levels and pharmacologic activity over 6 weeks after a single injection [Sigmon et al. 2006]. Intramuscular buprenorphine has been available for many years for pain management and a sublingual liquid formulation was initially used in investigational studies prior to FDA approval for treating opioid dependence. In addition, sublingual film buprenorphine has been developed, a version of which was approved by the FDA in 2010 [O’Connor, 2010].
This long-term delivery formulation of buprenorphine is safe and well suited for treating disorders that require strict compliance, such as opioid dependence, and may also prove useful for maintaining stable plasma concentrations of drugs for treating a variety of long-term disabilities. It also reduces adverse effects associated with peak/trough blood levels, providing constant therapeutic drug levels and improving long-term outcome [Kleppner et al. 2006].
Probuphine®
This was developed by Titan Pharmaceuticals, Inc. and their partner Braeburn Pharmaceuticals. US FDA advisory committee recommended approval of Probuphine® to treat opioid addiction [Braeburn Pharmaceuticals, 2015]. Probuphine® is a polymeric matrix composed of ethylene vinyl acetate and buprenorphine in the form of match-stick sized implants (26 mm × 2.5 mm) that are implanted subdermally and deliver buprenorphine over 6 months. The dose is equivalent to about 80 mg of buprenorphine, reliably released at a measured rate according to the matrix diffusion.
The rod is placed subdermally in the upper arm in a 15 min procedure in the office, and is removed in a similar manner at the end of the treatment period. The standard implant dose, following the manufacturer’s brochure, is four rods, with a fifth and sixth rod added if additional medication is needed according to the physician’s assessment. Patients are first inducted onto sublingual buprenorphine for 3–7 days in preparation for the implant of Probuphine® rods [Ling et al. 2011a]. After insertion of the specified number of implants followed by an initial pulse release, buprenorphine release from Probuphine® remains essentially constant, until the implant is removed [White et al. 2009]. Research has documented the safety and acceptability of the transition from sublingual buprenorphine to the Probuphine® implants [Ling et al. 2011b].
Randomized clinical trials with Probuphine®
Saunders et al. [2005]
A phase I/II clinical study of buprenorphine implants in opioid-dependent patients showed that two buprenorphine implants were sufficient to control withdrawal and cravings in six subjects previously maintained on sublingual buprenorphine at 8 mg daily, and that four implants was sufficient to control withdrawal and cravings in six subjects previously maintained on sublingual buprenorphine at 16 mg daily. No significant adverse effects were reported in either dose group [Saunders et al. 2005].
White et al. [2009]
The goal of this study was to conduct an initial, open-label, evaluation of the safety, pharmacokinetics, and efficacy of Probuphine® in subjects with opioid dependence maintained on sublingual buprenorphine. Two doses of Probuphine® were evaluated in 12 heroin-dependent volunteers in Australia who switched from daily sublingual buprenorphine dosing to either two or four Probuphine® implants based upon their buprenorphine daily maintenance dose of 8 or 16 mg, respectively, and were monitored for 6 months. Some supplemental sublingual buprenorphine for withdrawal symptoms was administered to five subjects on an average of 5 days each over the 6 months of treatment. Probuphine® implants provided continuous steady-state delivery of buprenorphine until their removal. Withdrawal symptoms and craving remained low throughout the 6 months. For the 12 subjects, an average of 59% of urine samples were opioid-negative across the 6 months treatment period. Injection site reactions were present in half of patients, but none were serious. The observed steady-state levels of plasma concentrations of buprenorphine over the 6-month treatment period indicated that Probuphine® implants functioned as designed. After peaking within 24 h, plasma concentrations of buprenorphine decreased to the steady-state levels by 21 days. The main findings of this study were that all of the patients successfully switched from sublingual buprenorphine to Probuphine® and remained on treatment for the full 6 months period. Insertion and removal of implants appeared to be a simple procedure, which could be accomplished by a trained practitioner in an office setting [White et al. 2009].
Ling et al. [2010]
A randomized, placebo-controlled, 6-month trial [ClinicalTrials.gov identifier: NCT00447564] was conducted at 18 sites in the US between April 2007 and June 2008. A total of 163 adults diagnosed with opioid dependence were recruited, and 108 were randomized to receive buprenorphine implants and 55 to receive placebo implants. After induction with sublingual buprenorphine–naloxone tablets, patients received either four buprenorphine implants (80 mg per implant) or four placebo implants. A fifth implant was available if a threshold for rescue use of sublingual buprenorphine–naloxone treatment was exceeded. Standardized individual drug counseling was provided to all patients. The outcomes were: the percentage of urine samples negative for illicit opioids from week 1 to 16 (primary outcome) and from week 17 to 24 (secondary outcome).
Significantly more participants in the buprenorphine group completed the treatment (p < 0.001). The buprenorphine implant group had significantly more urine samples negative for illicit opioids during weeks 1 through 16 (p = 0.04) as compared with placebo. Those who received buprenorphine implants also had fewer clinician-rated (p < 0.001) and patient-rated (p = 0.004) withdrawal symptoms, had lower patient ratings of craving (p < 0.001), and experienced a greater change on clinician global ratings of severity of opioid dependence (p < 0.001) and on the clinician global ratings of improvement (p < 0.001) than those who received placebo. Minor implant site reactions were the most common adverse events: 56.5% in the buprenorphine group and 52.7% in the placebo group.
Given the known pharmacokinetics of buprenorphine, the steady-state plasma concentration levels were consistent with a constant buprenorphine release of 1–1.3 mg/day from four to five buprenorphine implants. Four to five implants were sufficient to control most craving and withdrawal symptoms with minor implant site reactions. However, only a single patient in the placebo group experienced a major implant site reaction (cellulitis). There was no evidence of unscheduled implant removal or attempted removal. It was concluded that among the subjects with opioid dependence, the use of buprenorphine implants compared with placebo resulted in less opioid use over 16 weeks as assessed by urine samples.
Several limitations of this study were reported: all patients received additional psychosocial counseling in addition to implants, the use of rescue buprenorphine–naloxone treatment complicated the interpretation of study results, the trial was not statistically powered to examine efficacy within subgroups of patients and attrition rate was high [Ling et al. 2010].
This study also received other criticisms. Both the groups received buprenorphine induction with sublingual buprenorphine–naloxone tablets, implant insertion, and up to twice-weekly counseling, which were potential confounders of the outcome. In addition, supplemental sublingual buprenorphine–naloxone was provided based on patient-reported withdrawal and craving, and otherwise when requested, thus inviting complexity (e.g. the implantation/removal procedures) or resource intensity (e.g. specialized counseling). The need to use supplemental medication indicated that risk of diversion was not completely eliminated, especially if its use was unsupervised as is common in practice.
Again because the primary analysis was conducted using an intention-to-treat approach that included all randomized patients, this could have led to erroneously assigning a greater proportion of positive and lesser proportion of negative urine samples preferentially in the placebo group [Basu and Kumar, 2011]. Ling and colleagues however replied that this measure was mandated by the FDA for the trial [Ling et al. 2011a]. Also, the primary outcome measure in this study was the proportion of illicit opioid-negative urine samples in the first 16 weeks, which appeared arbitrary [Basu and Kumar, 2011]. However, the 16-week cutoff was decided a priori by a consensus of clinical experts associated with the study and was also a registration requirement of the FDA [Ling et al. 2011b]. Regarding future research, the authors also agreed that it would be important to compare the efficacy of buprenorphine implants to sublingual buprenorphine [Ling et al. 2011b].
Moreover, because this study was performed in treatment centers with specialized counseling and close medication supervision, it provided relatively little information about how implants might be used in real office practice [Rastegar, 2011]. In the open-label phase of the landmark study by Fudala and colleagues, 55% of the participants received at least 6 months of office-based treatment with sublingual-tablet formulation of buprenorphine and naloxone in opiate addiction [Fudala et al. 2003]. In another analysis of office-based buprenorphine treatment at the Johns Hopkins Bayview Medical Center, 57% of patients remained office-compliant in treatment at 12 months [Soeffing et al. 2009].
In reply to Rastegar’s comments [Rastegar, 2011] on the interpretation of the results of this study, O’Connor mentioned that in the case of buprenorphine implants, it would be critical to ensure that they could be safely and reliably used in clinical practice and that they provide effective plasma drug levels. And regarding the concern about treatment setting, this study was performed in a treatment center environment that was quite distinct from that of office-based buprenorphine in order to evaluate this new technology in a manner that maximizes patient safety through intensive monitoring and treatment adjustments [O’Connor, 2011].
Rosenthal et al. [2013]
Another randomized clinical trial was conducted at 20 addiction treatment centers in the US with adult patients diagnosed with opiate dependence to confirm the efficacy of buprenorphine implants relative to placebo implants over 24 weeks of treatment for opioid dependence [ClinicalTrials.gov identifier: NCT01114308]. Diagnosis of opioid dependence was determined by the Mini International Neuropsychiatric Interview [Sheehan et al. 1998]. Subjects received either four buprenorphine implants (80 mg/implant) (n = 114), four placebo implants (n = 54), or open-label buprenorphine–naloxone tablets (12–16 mg/day) (n = 119). Implanting physicians were from various medical specialties with prior surgical training who received standardized training in implant insertion and removal. All implants were removed at 6 months or upon early discontinuation. Those who, at the end of the induction phase, reported significant opioid withdrawal symptoms, defined as >12 on the Clinical Opiate Withdrawal Scale [Wesson and Ling, 2003], or significant opioid craving, defined as >20 mm on a 100 mm opioid craving visual analog scale, were excluded.
The primary efficacy endpoint was the percent of urine samples negative for opioids collected from weeks 1 to 24, examined as a cumulative distribution function. The buprenorphine implant samples cumulative distribution function was significantly different from placebo (p < 0.0001). Mean proportions of urine negative for opioids were significantly higher with buprenorphine than with implant. Buprenorphine implant subjects had a higher study completion rate relative to placebo (p < 0.0001), lower clinician-rated (p < 0.0001) and patient-rated (p < 0.0001) withdrawal, lower patient-ratings of craving (p < 0.0001), and better subjects’ (p = 0.031) and clinicians’ (p = 0.022) global ratings of improvement. Buprenorphine implant also resulted in significantly lower cocaine use (p = 0.0016). Minor implant-site reactions were comparable in the buprenorphine (27.2%) and placebo groups (25.9%). Buprenorphine implant was noninferior to buprenorphine–naloxone on percentage of urine samples negative for opioids. The lesser incidence of implant site reaction in this study can probably be explained by different exclusion criteria (low platelet count was not considered as an exclusion criteria by Ling and colleagues) and the procedure followed in the method of implantation (the experience and surgical skill of implanters, individual ethnogenetic variables, etc.).
A mentioned limitation of the noninferiority component of the study was that the comparison with buprenorphine–naloxone was unblinded. Another limitation was the use of rescue sublingual buprenorphine–naloxone across all of the groups, making it difficult to compare outcome and retention results to previous studies which did not use rescue medication. In addition, the generalization of these findings was uncertain for individuals who were also dependent on other substances, or recently received methadone or buprenorphine or opioid analgesics. It was finally concluded that, buprenorphine implants compared with placebo implants resulted in significantly less opioid use over 24 weeks, and buprenorphine implants were also found to be non-inferior to sublingual buprenorphine with regards to the proportion of urine samples negative for opioids over 24 weeks of treatment for opioid dependence [Rosenthal et al. 2013].
Rosenthal et al. [2016]
This outpatient, randomized, active-controlled, 24-week, double-blind, double-dummy trial was conducted in the US to determine whether 6-month buprenorphine implants were non-inferior to daily sublingual buprenorphine as maintenance treatment for opioid-dependent patients with stable abstinence [ClnicalTrials.gov identifier: NCT02180659]. The clinically stable patients attending the outpatient department were prescribed daily sublingual buprenorphine (8 mg/day or less) for 6 months or more. They were abstinent from opioids for 90 days or longer during those 6 months and those who showed no evidence of opioid withdrawal or illicit opioid-positive urine samples for at least 90 days prior to study entry were included. The participants were then randomized to receive sublingual buprenorphine (8 mg/day or less) plus four placebo implants or daily sublingual placebo plus four buprenorphine hydrochloride implants (80 mg). The primary endpoint was between-group difference in proportion of responders (⩾4 of 6 months without an opioid-positive urine test result and self-report). The noninferiority established for the lower bound of the 95% confidence interval was greater than −0.20 (p < 0 .025). The secondary endpoints included cumulative percentage of negative opioid urine results, abstinence, and time to first illicit opioid use. Safety parameters were also assessed. A total of 81 of 84 (96.4%) receiving buprenorphine implants and 78 of 89 (87.6%) receiving sublingual buprenorphine were responders, an 8.8% difference [one-sided 97.5% confidence interval (CI) 0.009 to ∞; p < 0 .001 for noninferiority]. Over 6 months, 72 of 84 (85.7%) receiving buprenorphine implants and 64 of 89 (71.9%) receiving sublingual buprenorphine maintained opioid abstinence (hazard ratio, 13.8; 95% CI, 0.018–0.258; p = 0.03). Non-implant-related and implant-related adverse events occurred in 48.3% and 23% of the buprenorphine implant group and in 52.8% and 13.5% of participants in the sublingual buprenorphine group, respectively. Thus, among the adult patients with opioid dependence maintaining abstinence with a stable dose of sublingual buprenorphine, the use of buprenorphine implants compared with continued sublingual buprenorphine did not result in an inferior likelihood of remaining a responder. However, the study population had an exceptionally high response rate in the control group.
Pharmacokinetics of buprenorphine implants from these clinical studies
With all of these mentioned studies, it can be inferred that buprenorphine implant has a relatively fast onset of action (peak reached within 24 h), maintains a steady concentration for weeks (steady-state plasma concentration within a month), effective blockade of opioid receptors, which persists even at very low plasma concentration of the drug, followed by gradual decrease, approaching undetectable levels (<0.10 ng/ml) by weeks and minimal residual concentration after removal of the implant. Minor implant site reactions, which were the most predominant adverse effect, can be probably avoided with better skilled professionals and improved surgical technique (Figure 3).
Figure 3.
Key pharmacokinetic aspects of Probuphine®.
CYP, cytochrome P450 enzymes; EVA, ethylene vinyl acetate; SL, sublingual.
Pharmacokinetic studies with injectable sustained release buprenorphine formulations
A pharmacokinetic study with buprenorphine implant (steady-state release in vitro: 0.5 mg/implant/day) was performed over 52 weeks in beagle dogs receiving 8, 16 or 24 subcutaneous implants. Plasma buprenorphine concentrations correlated with the number of implants administered. Peak buprenorphine concentrations were generally reached within 24 h after implantation. Steady-state plasma levels were attained between 3 and 8 weeks, and were maintained for the study duration, with a calculated mean release rate of 0.14 ± 0.04 mg/implant/day. There were no adverse effects due to buprenorphine implants [Kleppner et al. 2006]. The estimated half-life for buprenorphine after removal of the implants ranged from 9.7 to 32 h, which is within the range found for other buprenorphine administration routes [Elkader and Sproule, 2005; Kuhlman et al. 1998]. The reduction in buprenorphine concentration after removal of the implant showed that no residual drug from the implant remained [Elkader and Sproule, 2005].
In another report, data from two studies were used, in which 11 opioid-dependent volunteers each received a single subcutaneous depot injection containing 58 mg of buprenorphine, and included previously unreported detailed plasma concentration data over a 6-week time course following depot administration and examination of their relationship to pharmacodynamic indices. The buprenorphine microcapsules consisted of buprenorphine base and biodegradable PLA–PGA polymer. The rate and duration of release of the drug in the depot formulation such as the one used in these studies are controlled by many factors including particle composition, particle size and polymer properties. The mechanism of release is by diffusion of the drug from the microcapsules and is controlled initially by the thickness of the polymer wall, which then degrades after most of the drug is released. It was shown that the mean plasma buprenorphine increased gradually following depot administration, peaked at 2–3 days with a mean concentration 1.25 ng/ml, and then decreased gradually, approaching undetectable levels (<0.10 ng/ml) by 6 weeks. There was substantial between-subject consistency in several aspects of buprenorphine biodelivery, including time to first detectable blood level (4 h), peak blood level (2 days) and undetectable blood level (6–6.5 weeks). In contrast, there was marked between-subject variability in the magnitude of peak buprenorphine concentrations, ranging from 0.17 to 3.47 ng/ml. The extent of opioid blockade was tested by weekly opioid challenges with 3 mg subcutaneous hydromorphone. Subjective response and pupillary constriction were related inversely to both buprenorphine and norbuprenorphine plasma concentrations (r = 0.84–0.95). It was concluded that the depot formulation provided effective buprenorphine delivery for several weeks and that effects persisted even at fairly low buprenorphine plasma concentrations [Sigmon et al. 2006].
Another pharmacokinetic study evaluating the disposition of [15, 16(n)-3H] buprenorphine in the rat after a single 0.2 mg/kg intravenous bolus dose and continuous administration via a subcutaneously implantable long-acting delivery system, emphasized the importance of high-affinity binding of buprenorphine in brain and subsequent slow dissociation as a prime factor in its prolonged agonist/antagonist effects and higher potency than other opioid agonists [Pontani et al. 1985].
Safety concerns with buprenorphine implants
Minor implant site reactions were the most common adverse events in the trial conducted by Ling and colleagues (56.5% in the buprenorphine group and 52.7% in the placebo group) [Ling et al. 2010]. Similar minor implant-site reactions were also observed in trials by Rosenthal and colleagues (27.2% in the buprenorphine group and 25.9% in the placebo group) [Rosenthal et al. 2013] and White and colleagues (half of the patients with buprenorphine implants) [White et al. 2009]. An animal study was conducted to determine the safety of a compounded sustained-release formulation of buprenorphine, compared with effects of regular buprenorphine, for postoperative analgesia in rabbits. No major adverse effects were detected with the sustained release formulation [DiVincenti et al. 2016].
However, another study comparing the bioavailability of buprenorphine with the biodegradation of lipid-encapsulated subcutaneous drug pellets showed that the drug implants can retain significant and unintended reservoirs of drugs without causing any signs of edema or inflammation at the implant site. Regardless of the composition of the delivery system itself, long-term histopathological studies of the subcutaneous space are warranted to look for toxicities resulting from such implants (may be due to drug-bound delivery vehicles) [Guarnieri et al. 2014].
Where do we finally stand with Probuphine®
Apart from Probuphine®, lofexidine (primarily for managing opioid withdrawal symptoms), a combination of sublingual film form of buprenorphine and naloxone (Suboxone®) and injectable depot form of naltrexone (Vivitrol®) (for consistent opioid receptor blockade for 1 month between administrations) have been tried in the US for opioid dependence [Ling et al. 2011a]. Novel formulations of buprenorphine using a polymer microcapsule depot sustained-release technology [Sobel et al. 2004] and biodegradable polymer microcapsule technology Sigmon et al. [2006] have been developed. In contrast to Probuphine®, concentrations of buprenorphine using this depot formulation are undetectable by 6 weeks. The depot formulation may be more appropriate for use in detoxification or withdrawal management [Sigmon et al. 2006], whereas Probuphine® is intended for use in long-term treatment following sublingual buprenorphine.
One great advantage of the implantable formulation is that the effect of buprenorphine can be terminated rapidly by removal of Probuphine®. The implants are likely to reduce the amount of illicit diversion, as, once placed under skin, the implants will be difficult to remove. In addition, the fact that Probuphine® is a matrix system rather than a reservoir means that it is very difficult to extract buprenorphine for illicit intravenous use [White et al. 2009; Ling et al. 1998].
The optimal way to administer Probuphine®, whether always following induction with sublingual buprenorphine or not, also should be examined in future studies. Another important issue that awaits investigation is the nature and amount of regular clinical contact that is needed once Probuphine® has been implanted. The role of psychosocial therapy in conjunction with Probuphine® should also be investigated. Additional studies are also needed to explore the range of patients for whom implants may be appropriate [White et al. 2009; Ling et al. 1998].
Apart from opioid dependence, regarding cocaine use, earlier post hoc findings [Montoya et al. 2004] were replicated suggesting a beneficial effect of buprenorphine implant in reducing cocaine use in opioid-dependent subjects. It is unknown whether this effect is an indirect result of buprenorphine treatment of opioid dependence or is a direct pharmacological effect. The potential utility of implantable buprenorphine in cocaine-use disorders, at least among opioid-dependent individuals, deserves further attention [Montoya et al. 2004]. However, in a double-blind, placebo-controlled trial it was demonstrated that buprenorphine implants result in significantly lower cocaine use over 24 weeks as compared to placebo, suggesting a beneficial effect of buprenorphine implants in reducing cocaine use in opioid-dependent subjects [Rosenthal et al. 2013].
Although the safety and efficacy of buprenorphine implant has been confirmed in several studies, how many patients and physicians would prefer buprenorphine implants instead of the available forms of the medicine is a big question and has never been measured in these studies. Physicians may be reluctant to prescribe implants when other easily administrable formulations are available. In the different trials, physicians across a variety of specialties safely performed the procedure with training. It is possible that clinicians would identify certain patients more suitable for either a sublingual form of the medicine or the implant. For example, clinicians could decide to recommend implants for patients with young children in the home, patients with a pattern of inconsistent adherence to prescriptions or patients who may repeatedly misplace sublingual formats. Balancing individual patient and physician concerns with the burgeoning need to minimize harm resulting from opioid abuse and diversion [Centers for Disease Control and Prevention, 2010, 2012; Johanson et al. 2012] is a question for clinicians, future clinical research and for public health policy [Rosenthal et al. 2013]. The cost of Probuphine® might also hinder its overspread use.
Even then, it can be said that Probuphine® holds particular promise for increasing access to efficacious pharmacotherapy for opioid dependence particularly in rural geographic areas. Because they require only semiannual administration and provide opioid maintenance while simultaneously reducing needs for take-home doses and minimizing risk for potential nonadherence, abuse, and diversion. Reductions in the number of visits reduce the burden of time and travel for patients, thereby making it easier for the patients to participate in various usual pro-social activities (e.g. employment, educational opportunities, and family responsibilities). Reductions in clinician burden and attenuated risks of nonadherence and diversion could also increase physicians’ comfort level with Probuphine® treatment, making them more willing to treat opioid-dependent patients [Sigmon, 2014].
Acknowledgments
The authors acknowledge Dr Jacob Peedicayil, Professor, Department of Pharmacology and Clinical Pharmacology, Christian Medical College, Vellore, India for giving valuable suggestions during manuscript preparation.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Preeti Barnwal, Jamia Hamdard (Hamdard University) - Department of Medical Elementology and Toxicology, Faculty of Science, New Delhi, India.
Saibal Das, Department of Pharmacology and Clinical Pharmacology, Christian Medical College, Vellore 632002, India.
Somnath Mondal, Department of Clinical and Experimental Pharmacology - Calcutta School of Tropical Medicine, Kolkata, India.
Anand Ramasamy, Swamy Vivekanandha College of Pharmacy - Department of Pharmacology, Namakkal, India.
Tanay Maiti, Christian Medical College - Department of Psychiatry, Vellore, India.
Arunava Saha, Christian Medical College - Student (MBBS), Vellore, India.
References
- Alho H., Sinclair D., Vuori E., Holopainen A. (2007) Abuse liability of buprenorphine–naloxone tablets in untreated IV drug users. Drug Alcohol Depend 88: 75–78. [DOI] [PubMed] [Google Scholar]
- Anchersen K., Clausen T., Gossop M., Hansteen V., Waal H. (2009) Prevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: a mortality assessment study. Addiction 104: 993–999. [DOI] [PubMed] [Google Scholar]
- Arfken C., Johanson C., di Menza S., Schuster C. (2010) Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: national surveys of physicians. J Subst Abuse Treat 39: 96–104. [DOI] [PubMed] [Google Scholar]
- Baker J., Best A., Pade P., McCance-Katz E. (2006) Effect of buprenorphine and antiretroviral agents on the QT interval in opioid-dependent patients. Ann Pharmacother 40: 392–396. [DOI] [PubMed] [Google Scholar]
- Barnett P., Rodgers J., Bloch D. (2001) A meta-analysis comparing buprenorphine to methadone for treatment of opiate dependence. Addiction 96: 683–690. [DOI] [PubMed] [Google Scholar]
- Basu D., Kumar V. (2011) Buprenorphine implants and opioid dependence. JAMA 305: 253–255. [DOI] [PubMed] [Google Scholar]
- Bell J., Butler B., Lawrance A., Batey R., Salmelainen P. (2009b) Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend 104: 73–77. [DOI] [PubMed] [Google Scholar]
- Bell J., Trinh L., Butler B., Randall D., Rubin G. (2009a) Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction 104: 1193–1200. [DOI] [PubMed] [Google Scholar]
- Bickel W., Amass L. (1995) Buprenorphine treatment of opioid dependence: a review. Exp Clin Psychopharmacol 3: 477–489. [Google Scholar]
- Braeburn Pharmaceuticals. (2015) Titan Pharmaceuticals and Braeburn Pharmaceuticals Announce FDA Advisory Committee Recommends Approval of Probuphine, First 6-Month Implant to Treat Opioid Addiction [online]. Available at: https://braeburnpharmaceuticals.com/titan-pharmaceuticals-and-braeburn-pharmaceuticals-announce-fda-advisory-committee-recommends-approval-of-probuphine-first-6-month-implant-to-treat-opioid-addiction/ (accessed 20 May 2016).
- Bruce R., Govindasamy S., Sylla L., Kamarulzaman A., Altice F. (2009) Lack of reduction in buprenorphine injection after introduction of co-formulated buprenorphine/naloxone to the Malaysian market. Am J Drug Alcohol Abuse 35: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulka A., Kouya P., Bottiger Y., Svensson J., Xu X., Wiesenfeld-Hallin Z. (2004) Comparison of the antinociceptive effect of morphine, methadone, buprenorphine and codeine in two substrains of Sprague-Dawley rats. Eur J Pharmacol 492: 27–34. [DOI] [PubMed] [Google Scholar]
- Byrne A., Wodak A. (2007) Data do not support buprenorphine as a first-line treatment for addiction. Am J Psychiatry 164: 1757–1758. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2010) Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004–2008. MMWR Morb Mortal Wkly Rep 59: 705–709. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2012) Buprenorphine prescribing practices and exposures reported to a poison center–Utah, 2002–2011. MMWR Morb Mortal Wkly Rep 61: 997–1001. [PubMed] [Google Scholar]
- Chiang C., Hawks R. (2003) Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend 70(Suppl. 2): S39–S47. [DOI] [PubMed] [Google Scholar]
- Compton P., Ling W., Moody D., Chiang N. (2006) Pharmacokinetics, bioavailability and opioid effects of liquid versus tablet buprenorphine. Drug Alcohol Depend 82: 25–31. [DOI] [PubMed] [Google Scholar]
- Davids E., Gastpar M. (2004) Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol 14: 209–216. [DOI] [PubMed] [Google Scholar]
- Davis M. (2005) Buprenorphine in cancer pain. Support Care Cancer 13: 878–887. [DOI] [PubMed] [Google Scholar]
- DiVincenti L., Jr., Meirelles L., Westcott R. (2016) Safety and clinical effectiveness of a compounded sustained-release formulation of buprenorphine for postoperative analgesia in New Zealand white rabbits. J Am Vet Med Assoc 248: 795–801. [DOI] [PubMed] [Google Scholar]
- Doran C., Shanahan M., Mattick R., Ali R., White J., Bell J. (2003) Buprenorphine versus methadone maintenance: a cost-effectiveness analysis. Drug Alcohol Depend 71: 295–302. [DOI] [PubMed] [Google Scholar]
- Elkader A., Sproule B. (2005) Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet 44: 661–680. [DOI] [PubMed] [Google Scholar]
- Fiellin D., Pantalon M., Chawarski M., Moore B., Sullivan L., O’Connor P., et al. (2006) Counseling plus buprenorphine–naloxone maintenance therapy for opioid dependence. N Engl J Med 355: 365–374. [DOI] [PubMed] [Google Scholar]
- Fischer G., Gombas W., Eder H., Jagsch R., Peternell A., Stuhlinger G., et al. (1999) Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction 94: 1337–1347. [DOI] [PubMed] [Google Scholar]
- Fudala P., Bridge T., Herbert S., Williford W., Chiang C., Jones K., et al. (2003) Office-based treatment of opiate addiction with a sublingual tablet formulation of buprenorphine and naloxone. N Engl J Med 349: 949–958. [DOI] [PubMed] [Google Scholar]
- Gowing L., Ali R., White J. (2009) Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev 3: CD002025. [DOI] [PubMed] [Google Scholar]
- Guarnieri M., Tyler B., DeTolla L., Zhao M., Kobrin B. (2014) Subcutaneous implants for long-acting drug therapy in laboratory animals may generate unintended drug reservoirs. J Pharm Bioallied Sci 6: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D., Mendelson J., Lin E., Upton R., Jones R. (2004) Pharmacokinetics and subjective effects of sublingual buprenorphine, alone or in combination with naloxone: lack of dose proportionality. Clin Pharmacokinet 43: 329–340. [DOI] [PubMed] [Google Scholar]
- Johanson C., Arfken C., di Menza S., Schuster C. (2012) Diversion and abuse of buprenorphine: findings from national surveys of treatment patients and physicians. Drug Alcohol Depend 120: 190–195. [DOI] [PubMed] [Google Scholar]
- Johnson R., Chutuape M., Strain E., Walsh S., Stitzer M., Bigelow G. (2000) A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med 343: 1290–1297. [DOI] [PubMed] [Google Scholar]
- Kakko J., Grönbladh L., Svanborg K., Von Wachenfeldt J., Rück C., Rawlings B., et al. (2007) A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry 164: 797–803. [DOI] [PubMed] [Google Scholar]
- Kleppner S., Patel R., McDonough J., Lauren C. (2006) In vitro and in vivo characterization of a buprenorphine delivery system. JPP 58: 295–302. [DOI] [PubMed] [Google Scholar]
- Koob G., Le Moal M. (2008) Addiction and the brain antireward system. Annu Rev Psychol 59: 29–53. [DOI] [PubMed] [Google Scholar]
- Kosten T., Schottenfeld R., Ziedonis D., Falcioni J. (1993) Buprenorphine versus methadone maintenance for opioid dependence. J Nerv Ment Dis 181: 358–364. [DOI] [PubMed] [Google Scholar]
- Kuhlman J., Jr., Levine B., Johnson R., Fudala P., Cone E. (1998) Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction 93: 549–559. [DOI] [PubMed] [Google Scholar]
- Lanier R., Umbricht A., Harrison J., Nuwayser E., Bigelow G. (2007) Evaluation of a transdermal buprenorphine formulation in opioid detoxification. Addiction 102: 1648–1656. [DOI] [PubMed] [Google Scholar]
- Law F., Myles J., Daglish M., Nutt D. (2004) The clinical use of buprenorphine in opiate addiction: evidence and practice. Acta Neuropsychiatr 16: 246–274. [DOI] [PubMed] [Google Scholar]
- Lewis J. (1985) Buprenorphine. Drug Alcohol Depend 14: 363–372. [DOI] [PubMed] [Google Scholar]
- Ling W., Casadone P., Bigelow G., Kampman K., Patkar A., Bailey G., et al. (2010) Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA 304: 1576–1583. [DOI] [PubMed] [Google Scholar]
- Ling W., Charuvastra C., Collins J., Batki S., Brown L., Kintaudi P., et al. (1998) Buprenorphine maintenance treatment of opiate dependence: a multicenter randomized clinical trial. Addiction 93: 475–486. [DOI] [PubMed] [Google Scholar]
- Ling W., Mooney L., Torrington M. (2012) Buprenorphine for opioid addiction. Pain Manag 2: 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W., Mooney L., Zhao M., Nielsen S., Torrington M., Miotto K. (2011a) Selective review and commentary on emerging pharmacotherapies for opioid addiction. Substance Abuse Rehabil 2: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W., Rosenthal R., Beebe K. (2011b) Buprenorphine implants and opioid dependence—reply. JAMA 305: 253–255. [DOI] [PubMed] [Google Scholar]
- Ling W., Wesson D., Charuvastra C., Klett J. (1996) A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry 53: 401–407. [DOI] [PubMed] [Google Scholar]
- Liu K., Kao C., Liu S., Sung K., Kuei C., Wang J., et al. (2006) Novel depots of buprenorphine have a long-acting effect for the management of physical dependence to morphine. JPP 58: 337–344. [DOI] [PubMed] [Google Scholar]
- Lopatko O., White J., Huber A., Ling W. (2003) Opioid effects and opioid withdrawal during a 24 h dosing interval in patients maintained on buprenorphine. Drug Alcohol Depend 69: 317–22. [DOI] [PubMed] [Google Scholar]
- Luty J., O’Gara C., Sessay M. (2005) Is methadone too dangerous for opiate addiction? BMJ 331: 1352–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal T. (1999) Development of biodegradable drug delivery system to treat addiction. Drug Dev Ind Pharm 25: 773–779. [DOI] [PubMed] [Google Scholar]
- Marsch L. (1998) The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction 93: 515–532. [DOI] [PubMed] [Google Scholar]
- Masson C., Barnett P., Sees K., Delucchi K., Rosen A., Wong W., et al. (2004) Cost and cost-effectiveness of standard methadone maintenance treatment compared to enriched 180-day methadone detoxification. Addiction 99: 718–726. [DOI] [PubMed] [Google Scholar]
- McCance-Katz E., Moody D., Morse G., Friedland G., Pade P., Baker J., et al. (2006) Interactions between buprenorphine and antiretrovirals. The nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis 43: S224–S234. [DOI] [PubMed] [Google Scholar]
- McCance-Katz E., Sullivan L., Nallani S. (2010) Drug Interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict 19: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya I., Gorelick D., Preston K., Schroeder J., Umbricht A., Cheskin L., et al. (2004) Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther 75: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor P. (2010) Advances in the treatment of opioid dependence: continued progress and ongoing challenges. JAMA 304: 1612–1614. [DOI] [PubMed] [Google Scholar]
- O’Connor P. (2011) Buprenorphine implants and opioid dependence—reply JAMA 305: 253–255. [DOI] [PubMed] [Google Scholar]
- Petitjean S., Stohler R., Deglon J., Livoti S., Waldvogel D., Uehlinger C., et al. (2001) Double-blind randomized trial of buprenorphine and methadone in opiate dependence. Drug Alcohol Depend 62: 97–104. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Brantl V., Herz A., Emrich H. (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233: 774–776. [DOI] [PubMed] [Google Scholar]
- Pick C., Peter Y., Schreiber S., Weizman R. (1997) Pharmacological characterization of buprenorphine, a mixed agonist-antagonist with κ 3 analgesia. Brain Res 744: 41–46. [DOI] [PubMed] [Google Scholar]
- Pontani R., Misra A. (1983) A long-acting buprenorphine delivery system. Pharmacol Biochem Behav 18: 471–474. [DOI] [PubMed] [Google Scholar]
- Pontani R., Vadlamani N., Misra A. (1985) Disposition in the rat of buprenorphine administered parenterally and as a subcutaneous implant. Xenobiotica 15: 287–297. [DOI] [PubMed] [Google Scholar]
- Rastegar D. (2011) Buprenorphine implants and opioid dependence. JAMA 305: 253–255. [DOI] [PubMed] [Google Scholar]
- Rosenthal R., Ling W., Casadonte P., Vocci F., Bailey G., Kampman K., et al. (2013) Buprenorphine implants for treatment of opioid dependence: randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction 108: 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R., Lofwall M., Kim S., Chen M., Beebe K., Vocci F., et al. (2016) Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: a randomized clinical trial. JAMA 316: 282–290. [DOI] [PubMed] [Google Scholar]
- Roth B., Baner K., Westkaemper R., Siebert D., Rice K., Steinberg S., et al. (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A 99: 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Roos E., Sharma K. (1994) Transdermal delivery of buprenorphine through cadaver skin. J Pharm Sci 83: 126–130. [DOI] [PubMed] [Google Scholar]
- Saunders J., White J., Bell J., Williamson P., Makowska M., Lissin D., et al. (2005) Treatment of Opiate Dependence with Probuphine [buprenorphine implant]. International Society for Addiction Medicine [ISAM] VII Annual Conference, April 21–24, Mar del Plata, Argentina. [Google Scholar]
- Sheehan D., Lecrubier Y., Sheehan K., Amorim P., Janavs J., Weiller E., et al. (1998) The Mini-International Neuropsychiatric Interview [M.I.N.I.]: the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59: 22–33. [PubMed] [Google Scholar]
- Sigmon S. (2014) Access to treatment for opioid dependence in rural America Challenges and future directions. JAMA Psychiatry 71: 359–360. [DOI] [PubMed] [Google Scholar]
- Sigmon S., Moody D., Nuwayser E., Bigelow G. (2006) An injection depot formulation of buprenorphine: extended biodelivery and effects. Addiction 101: 420–422. [DOI] [PubMed] [Google Scholar]
- Sobel B., Sigmon S., Walsh S., Johnson R., Liebson I., Nuwayser E., et al. (2004) Open-label trial of an injection depot formulation of buprenorphine in opioid detoxification. Drug Alcohol Depend 73: 11–22. [DOI] [PubMed] [Google Scholar]
- Soeffing J., Martin L., Fingerhood M., Jasinski D., Rastegar D. (2009) Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat 37: 426–430. [DOI] [PubMed] [Google Scholar]
- Solomon R. (1980) The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychol 35: 691–712. [DOI] [PubMed] [Google Scholar]
- Solomon R., Corbit J. (1973) An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol 81: 158–171. [DOI] [PubMed] [Google Scholar]
- Solomon R., Corbit J. (1974) An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev 81: 119–145. [DOI] [PubMed] [Google Scholar]
- Strain E., Stitzer M., Liebson I., Bigelow G. (1994) Comparison of buprenorphine and methadone in the treatment of opioid dependence. Am J Psychiatry 151: 1025–1030. [DOI] [PubMed] [Google Scholar]
- Umbricht A., Huestis M., Cone E., Preston K. (2004) Effects of high-dose intravenous buprenorphine in experienced opioid abusers. J Clin Psychopharmacol 24: 479–487. [DOI] [PubMed] [Google Scholar]
- Walsh S., Preston K., Bigelow G., Stitzer M. (1995) Acute administration of buprenorphine in humans: partial agonist and blockade effect. J Pharmacol Exp Ther 274: 361–372. [PubMed] [Google Scholar]
- Walsh S., Preston K., Stitzer M., Cone E., Bigelow G. (1994) Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther 55: 569–580. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sun J., Tao Y., Chi Z., Liu J. (2010) The role of kappa-opioid receptor activation in mediating antinociception and addiction. Acta Pharmacol Sin 31: 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R., Potter J., Fiellin D., Byrne M., Connery H., Dickinson W., et al. (2011) Adjunctive counseling during brief and extended buprenorphine–naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry 68: 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson D., Ling W. (2003) The clinical opiate withdrawal scale [COWS]. J Psychoactive Drugs 35: 253–259. [DOI] [PubMed] [Google Scholar]
- White J., Bell J., Saunders J., Williamson P., Makowska M., Farquharson A., et al. (2009) Open-label dose-finding trial of buprenorphine implants (Probuphine) for treatment of heroin dependence. Drug and Alcohol Depend 103: 37–43. [DOI] [PubMed] [Google Scholar]
- Winstock A., Lea T., Sheridan J. (2008) Prevalence of diversion and injection of methadone and buprenorphine among clients receiving opioid treatment at community pharmacies in New South Wales, Australia. Int J Drug Policy 19: 450–458. [DOI] [PubMed] [Google Scholar]
- Woody G.E., Poole S., Subramaniam G., Dugosh K., Bogenschutz M., Abbott P., et al. (2008) Extended vs short-term buprenorphine–naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA 300: 2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody G.E., Metzger D.S. (2011) Injectable extended-release naltrexone for opioid dependence. Lancet 378: 664–665. [DOI] [PubMed] [Google Scholar]