Abstract
Basement membranes are protein-rich extracellular matrices (ECM) that are essential for epithelial and endothelial tissue structure and function. Aging and disease cause changes in the physical properties and ECM composition of basement membranes, which has spurred research to develop methods to repair and/or regenerate these tissues. An area of critical clinical need is the cornea, where failure of the endothelium leads to stromal edema and vision loss. Here we developed an engineered basement membrane (EBM) that consists of a dense layer of collagen IV and/or laminin approximately 5–10nm thick, created using surface-initiated assembly, conformally attached to a collagen I film. These EBMs were used to engineer a corneal endothelium (CE) that mimicked the structure of Descemet’s membrane with a thin stromal layer, towards use as a graft for lamellar keratoplasty. Results showed that bovine and human CE cells formed confluent monolayers on the EBM, expressed ZO-1 at the cell-cell borders and achieved a density of ~1600 cells mm−2 for 28 and 14 days, respectively. These results demonstrated that our technique was capable of fabricating EBMs with structural and compositional properties that mimic native basement membranes and that our EBM may be a suitable carrier for engineering transplant quality CE grafts.
Keywords: basement membrane, Descemet’s membrane, corneal endothelium, biomimetic
Graphical abstract
Human and bovine corneal endothelial cells are cultured on engineered basement membranes (EBM) designed to mimic the structure of the native Descemet’s membrane. Culturing these cells on the EBMs increases monolayer cell density compared to standard compressed collagen I gels. Additionally the cells on the EBMs have more robust ZO-1 and more cortical F-actin stress fibers.
1. Introduction
Basement membranes are dense sheets of extracellular matrix (ECM) composed of laminin, collagen type IV and other proteins, which underlie every epi- and endothelial tissue in the body and provide important structural and functional cues to the cells.[1] Changes to the structure, thickness, or composition of the ECM in basement membranes is associated with disease and/or dysfunction in a wide range of tissues.[2] Here we are focused on the corneal endothelium (CE) at the posterior of the cornea, specifically Descemet’s membrane, the basement membrane that supports the monolayer of non-proliferative CE cells.[3] The CE is responsible for nutrient transport and maintaining corneal clarity by pumping fluid out of the corneal stroma. Disease or injury to the CE results in irreversible swelling of the stroma causing corneal blindness, [3,4] and is responsible for ~ 40% of corneal transplants in the US.[5] The most common CE disease is Fuchs’ dystrophy, typically affecting both eyes and characterized by a significant increase in Descemet’s membrane thickness and a decrease in cell density that causes swelling of the stroma.[6] Additionally, during normal aging the protein composition of the Descemet’s membrane changes [7] and the membrane gradually thickens,[8] in association with a decrease in CE cell density.[9] Importantly, once the cell density decreases to less than 500 cells mm−2, the CE loses its ability to pump enough fluid out of the stroma, leading to corneal opacification and blindness.[4]
The current treatment for corneal blindness due to Fuchs’ dystrophy and other CE dysfunction is corneal transplantation. Full thickness penetrating keratoplasty that was the standard of care 5–10 years ago has now widely been replaced by lamellar transplant techniques including Descemet’s stripping endothelial keratoplasty (DSEK) and Descemet’s membrane endothelial keratoplasty (DMEK). These newer methods transplant just the Descemet’s membrane and the CE layer for DMEK or also a thin layer of stroma for DSEK, currently account for more than 75% of transplants to repair CE dysfunction and are the most common keratoplasty procedures performed in the US.[5] These surgeries are indeed successful in restoring corneal clarity and vision [10]; however, the availability of donor corneas remains limited worldwide and donated tissue exhibits variability due to age and other factors.[10b, 11] To address this, a potential approach is to tissue engineer a transplant quality CE from cultured CE cells and a suitable carrier (scaffold) that could be implanted using the currently established DSEK/DMEK methods. Researchers have investigated scaffolds fabricated from collagen type I (COL1) [12], gelatin [13], hyaluronic acid [14], and chitosan [15] as well as tissue-based such as decellularized corneas [16], corneas denuded of the CE [17], decellularized amniotic membranes [18], or anterior lens capsule.[19] These approaches have demonstrated that CE monolayers can be formed in vitro, but recreating the structure and ECM composition of native basement membrane remains a challenge. Decellularized or denuded corneas would appear to be the most desirable scaffold, but suffer from donor-to-donor variability in the structure, composition and mechanics of the ECM. This is due in part because they are derived from corneas that were deemed unsuitable for transplant and putting healthy CE cells on a dysfunctional carrier could lead to premature graft failure. Thus there remains a significant need to reproducibly engineer a transplant quality CE with well-defined structure, composition and function that can be implanted using established DMEK/DESK surgical techniques.
Here we report, for the first time, a technique capable of bottom up engineering substrates that mimic the native structure and composition of basement membranes. Utilizing surface initiated assembly (SIA) techniques [20], we have fabricated an engineered basement membrane (EBM) composed of a layer of basement membrane ECM proteins that is approximately 5–10 nm thick, supported by a compressed COL1 gel that is approximately 10 μm thick (Figure 1). The dense ECM top layer of the EBM is designed to recapitulate the structure and composition of Descemet’s membrane, which in vivo consists predominantly of collagen type IV (COL4), collagen type VIII and laminin (LAM) and other ECM components in lesser amounts.[7a, 21] Previously we reported that COL4 with or without LAM adsorbed on a soft, synthetic gel-like substrate enhanced the expansion of bovine CE cells while maintaining phenotype.[22] Based on this, we created an EBM consisting of a COL4+LAM layer on a COL1 film to mimic Descemet’s membrane and a thin layer of stroma with an overall thickness of ~10 μm, similar in composition to a DSEK graft but more similar to a DMEK graft in thickness. Initial studies to screen conditions showed that bovine CE cells on the EBMs formed high density monolayers with continuous ZO-1 (tight junction protein) at the cell-cell border as compared cells on controls. We then followed this by seeding primary human CE cells on both COL4 and COL4+LAM EBMs, and determined that formation of a high density monolayer is enhanced on the COL4 EBM and associated with continuous ZO-1 at the borders, similar to the CE in vivo. Together these results show that the EBMs we have developed can support formation of high density CE monolayers from bovine and human cells and that the unique structure and composition of the EBM provides significant improvements over existing approaches.
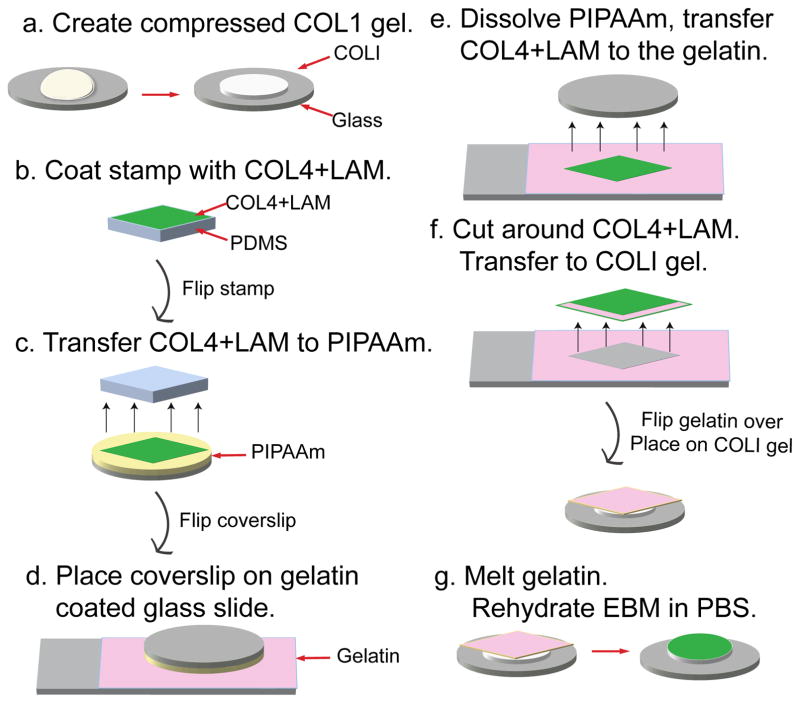
Figure 1. Schematic diagram of the EBM fabrication process.
(a) A 6 mg/mL collagen I gel (COL1) is compressed in a humid incubator at 37 °C for 3 hours. Once compressed, it is dried in a biohood. (b) Flat, featureless PDMS stamps are coated in a mixture of 50 μg/mL collagen IV and 50 μg/mL laminin in PBS (COL4+LAM), for 1 hour. (c) The square sheet of COL4+LAM is brought into conformal contact with a PIPAAm coated coverslip for 1 hour. (d) The coverslip is flipped over so the COL4+LAM sheet is placed down onto a gelatin coated glass slide. (e) The PIPAAm is dissolved by immersing the slide and coverslip in 25 °C distilled water for 5 minutes. (f) The gelatin is cut around the COL4+LAM sheet, peeled away from the glass slide and placed COL4+LAM side down onto the dried compressed COL1 film. (g) The COL4+LAM sheet is transferred to the COL1 by melting the gelatin in an incubator at 37 °C for 45 minutes, followed by rinsing twice with warm PBS, an additional incubation with warm PBS for 45 min and rinsing twice with warm PBS.
2. Results and Discussion
2.1 Engineered basement membranes mimic the laminar structure of native basement membranes and stroma
Using SIA, we fabricated EBMs in order to mimic a DSEK graft, which is composed of a thin portion of the COL1 rich stroma, Descemet’s membrane and adherent CE cells. The SIA process is able to create thin layers of dense ECM protein (Figure 1) by first partially unfolding ECM proteins in solution onto a PDMS stamp through hydrophobic interactions, and then transferring the ECM proteins in the partially unfolded state to a thermoresponsive poly(N-isopropylacrylamide) (PIPAAm) surface through microcontact printing, as previously described.[20, 23] The partially unfolded ECM proteins are then assembled in to a dense, free-standing insoluble matrix when the PIPAAm swells during the thermally-trigged dissolution process, by first hydrating the PIPAAm at 40 °C in PBS and then decreasing below the lower critical solution temperature of ~32 °C.[20, 23] We found that the gelatin carrier was critical to achieve reliable transfer of the dense ECM sheet of COL4 or COL4+LAM to the COL1 gels, as direct transfer from PIPAAm to COL1 was inconsistent due small thermal fluctuations causing premature PIPAAm dissolution. To confirm the laminar structure of the EBM fabricated via SIA, we used multiphoton imaging to visualize the fluorescently labeled COL4 as well as the COL1 by second harmonic generation. Individual COL1 fibers were not visible in the control COL1 films (Figure 2a). The COL1 layer of the EBM exhibited this same structure and the maximum intensity projection showed that the COL4+LAM layer completely covered the underlying COL1 (Figure 2b). The orthogonal cross-section views of COL1 (Figure 2c) and EBM (Figure 2d) confirmed that the top COL4+LAM ECM layer of the EBM was conformally adhered to the COL1 film. There was also minimal overlap of the two layers, indicating that the COL4+LAM layer did not penetrate into the COL1 film, thus forming a distinct, dense continuous mat on top of the COL1. Confocal microscopy also confirmed that COL1 and EBMs were approximately 10 μm in thickness. While the COL4+LAM ECM layer of the EBM appeared to be ~1 μm in thickness, previous studies have shown that the thickness of SIA layers of ECM is below the resolution limit of optical microscope (<1 μm), requiring other techniques for accurate thickness measurement.
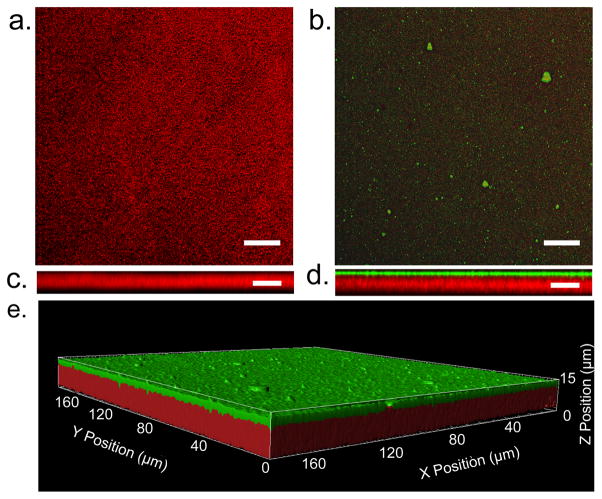
Figure 2. EBMs have a structure biomimetic to a native basement membrane, featuring a single layer of COL4+LAM following the contour of the underlying COL1.
Multiphoton images showing the structure of the EBM compared to a COL1 film. (a) A maximum intensity projection of the backward second-harmonic generation signal (red) from the COL1 film. Scale bar: 25 μm (b) Maximum intensity projection of the EBM showing the second-harmonic generation of the underlying COL1 film (red) and the single layer of ECM (COL4+LAM) shown in green. Scale bar: 25 μm (c) An orthogonal view of COL1 showing the porous nature of the gel. Scale bar: 10 μm (d) An orthogonal view of the EBM. The ECM layer sits on top and follows the contour of COL1and no overlap of the two (yellow) is seen. Scale bar: 10 μm (e) 3D rendering illustrating the layered structure of the EBM.
We next used AFM to examine the nanoscale structure of the COL1 film, the COL4+LAM layer on PIPAAm prior to transfer to the COL1 film and the EBM. AFM of COL1 at a 10 μm scan size revealed an isotropic network of fibers are mainly unorganized and unaligned (Figure 3a), and at a 1 μm scan size the characteristic banding of the COL1 fibrils was visible (Figure 3d). In comparison, AFM of the COL4+LAM on PIPAAm at a 10 μm scan size revealed a dense layer of protein (Figure 3b), that in previous studies was determined to be 5–10 nm thick.[20, 23] At a 1 μm scan size, the COL4+LAM on PIPAAm (Figure 3e) had a mesh-like structure resembling that reported for native basement membrane, specifically the structure of Descemet’s membrane from SEM data published by Abrams et al.[24] The EBM (Figure 3c) surface structure was almost identical to that of COL1 at both the 10- and 1 μm scan sizes, appearing as isotropic fibers with the banding of the underlying COL1 fibrils still visible (Figure 3f). Importantly, the surface topography due to the underlying COL1 dominated the surface structure and obscured the mesh-like structure of the COL4+LAM. We confirmed the banding of the COL1 fibrils from the phase signal of the 1 μm AFM scan size (Figure 3g), and by plotting phase intensity along the length of the fibrils (Figure 3h) verified a 60–80 nm spacing, which was in agreement with the known COL1 band width of 64 nm.[25] Finally, using the AFM topographic scans we calculated the RMS roughness and showed that at both the 10 and 1 μm scan sizes the surface structure was comparable between COL1 and EBM, which both were significantly rougher than the COL4+LAM layer on PIPAAm (Figure 3i and 3j). This indicated that the dominating physical features of both the EBM and COL1 were the COL1 fibers (red arrows in Figure 3d and 3f), and confirmed that the COL4+LAM layer was extremely thin, closely following the topography of the COL1 film. The ability of the COL4+LAM layer created using SIA to conformally adhere to a surface is in agreement with our previous reported results, which also established that SIA layers of ECM protein are distinctly different than ECM absorbed from liquid, both in terms nanostructure and structural integrity.[26]
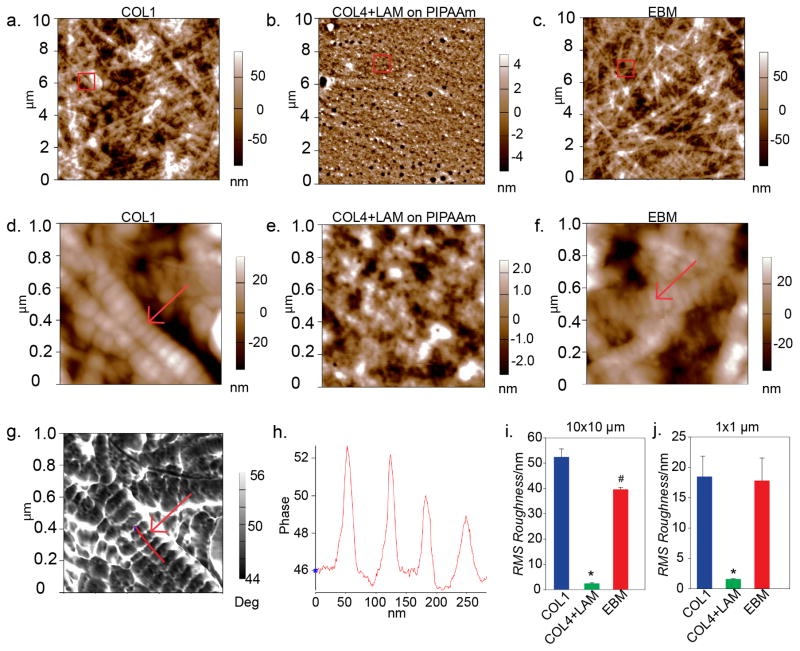
Figure 3. The EBM has a nanostructure similar to a native basement membrane.
Atomic force micrographs showing the nanostructure of COL1 (a, d), COL4+LAM assembled on PIPAAm (b, e) and EBM (c, f). The red boxes indicate the area of the 10 μm × 10 μm scan that was selected for the 1 μm × 1 μm scan. (a) The COL1 gel has short fibers creating a network with no orientation and an average RMS roughness of 52.468 ± 3.178 nm. (b) The COL4+LAM assembled on the PIPAAm is much smoother, with an average RMS roughness of 2.435 ± 0.279 nm, which resembles that of the native basement membrane mesh-like structure. (c) The finished EBM has a surface structure that resembles that of COL1 with an average RMS roughness on the same order of magnitude at 39.665 ± 0.783 nm. (d) A 1 μm × 1 μm scan shows the structure of the COL1 fibers in the gel indicated by the red arrow with an average RMS roughness of 18.472 ± 3.375 nm. (e) The mesh like network of the COL4+LAM can be seen at a 1 μm × 1 μm scan size which has an RMS roughness of 1.610 ± 0.0404 nm. (f) A closer view of the finished EBM shows the surface structure. The similarity between COL1 and EBM is highlighted by the arrows in D and F indicating the structure of the COL1 fibers dominates the topography in both the COL1 and EBM. At the 1 μm × 1 μm scan size, the EBM has an average RMS roughness similar to that of COL1, 17.718 ± 3.726 nm. (g) The corresponding phase image from panel (D), showing a trace (red line) used to measure the width of the banding of the COL1 fibers. (H) A graph that corresponds to the red trace line on panel G showing the change in phase height as the AFM cantilever went over the edges of the bands on the COL1 fiber. The distance between the peaks corresponds to the width in nanometers of each band. (I) A graph showing the average RMS roughness of the samples at the 10 μm scan size. The data was compared using a One-way ANOVA with Tukey test (p<0.05). The RMS roughness of the COL4+LAM on PIPAAm is significantly lower compared to both the EBM and COL1 (*, p < 0.001) and RMS roughness of the EBM is significantly lower, though on the same order of magnitude, as COL1 (#, p <0.001). (J) A graph showing the average RMS roughness of the samples at the 1 μm scan size. The data was compared using a One-way ANOVA with Tukey test (p<0.05). The RMS roughness of the COL4+LAM on PIPAAm is significantly lower compared to both the EBM and COL1 (*, p = 0.001).
2.2 Bovine CE monolayers on the engineered basement membranes have continuous ZO-1 and cortical F-actin filaments
We next investigated whether cultured bovine CE cells could form a high density monolayer and express ZO-1 (tight junction protein necessary for pump function) on the EBM. Bovine CE monolayers were cultured on the EBMs and two control sample types, (i) COL1 films with no modification (COL1) and (ii) COL1 films with gelatin melted over top (COL1+gelatin) to ensure that the gelatin used during fabrication (Figure 1g) did not have an effect on the cell response to the EBMs. Cells were maintained for up to 4 weeks to allow the bovine CE cells to form tight junctions, organize their cytoskeleton and to investigate the stability of the COL4+LAM layer of the EBM. After 1 week in culture (Figure 4a), the bovine CE cells on the EBM exhibited continuous ZO-1 expression at all cell borders. In contrast, the cells on COL1 and COL1+gelatin exhibited minimal ZO-1 at the cell borders. The F-actin cytoskeleton on all 3 sample types was unorganized with stress fibers spanning across the cell bodies. After 4 weeks in culture (Figure 4b), the F-actin stress fibers on all 3 sample types were more organized and located cortically, a characteristic of CE cells in vivo.[22] The greatest difference observed at week 4 between the conditions was the continuous ZO-1 at the cell borders for CE cells on the EBM, comparable to CE cells in vivo.[22] In contrast, on COL1 and COL1+gelatin, the ZO-1 was punctate and discontinuous at the cell-cell borders. Thus, the presence of the COL4+LAM layer of the EBM improved the structure and expression of phenotypic CE markers. This was most likely due to the biochemical rather than structural properties of the COL4+LAM layer, as the AFM analysis (Figure 3) showed that the morphology and roughness of the COL1 film and EBM were similar.
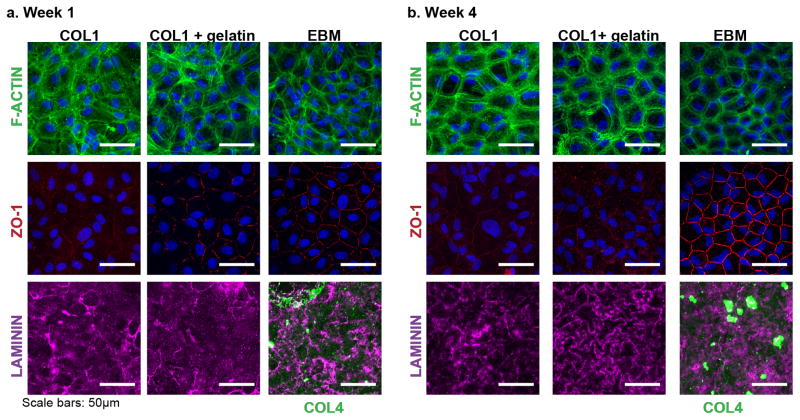
Figure 4. Bovine CE monolayers on EBMs have continuous ZO-1 expression and remodel the ECM layer of the EBM.
Representative confocal microscopy images after weeks 1 (a) and 4 (b) in culture. (a) At week 1, the F-actin cytoskeletal structure on all 3 sample types is unorganized with more F-actin stress fibers across the cell bodies. Bovine CE cells monolayers on the EBM have more continuous ZO-1 present at the borders of the cells, whereas bovine CE cells on both control samples have very little ZO-1 present. On the EBM, the original labeled COL4 is still present, showing the cells have not completely remodeled the EBM, but the LAM structure is different from the original ECM sheet, showing the cells are remodeling the ECM or laying down their own matrix. Similarly, LAM is present on COL1and COL1+gelatin. (b) By 4 weeks, the F-actin stress fibers of cells on all sample types are more organized and located cortically. Bovine CE cells on the EBM express continuous ZO-1at the borders resembling CE cells in vivo. In contrast, cells on the COL1 and COL1+gelatin have much less and non-continuous ZO-1. The LAM structure on all 3 samples is different from that at week 1, indicating the cells are remodeling the matrix they have begun to assemble. The original labeled COL4 is still present on the EBM.
2.3 Bovine CE cells remodel the engineered basement membranes and deposit their own ECM
To determine if the bovine CE cells were remodeling the COL4+LAM layer of the EBM, we examined changes in the organization of these ECM components over time using confocal microscopy. After 1 and 4 weeks in culture, the fluorescently-labeled COL4 in the EBM was still present and had not been remodeled by the cells (Figure 4a and 4b). Antibody specific to LAM 111 was used to determine if the cells remodeled the LAM in the EBM or deposited new LAM on the COL1 and COL1+gelatin. Unlike COL4, the structure of the LAM in the EBM changed drastically in 1 week and resembled the LAM produced by the bovine CE cell monolayers on the two other substrates (Figure 4a). By week 4, the structure of the LAM present on all 3 sample types had changed from having the appearance of an unorganized mat at week 1 to a more organized basement membrane like structure, indicating that the cells were producing and actively remodeling their own LAM (Figure 4b). The presence and remodeling of the LAM on all three substrates was expected, as LAM is found early in basement membrane assembly.[1b, 1c] The imaging of COL4 and LAM is not a complete characterization of the ECM assembled by the adhered CE cells, but is does establish that the difference in the monolayer structure was not due simply to the presence or absence of LAM, but rather it was the initial presence of the COL4+LAM layer of the EBM that improved morphology and phenotypic ZO-1 expression.
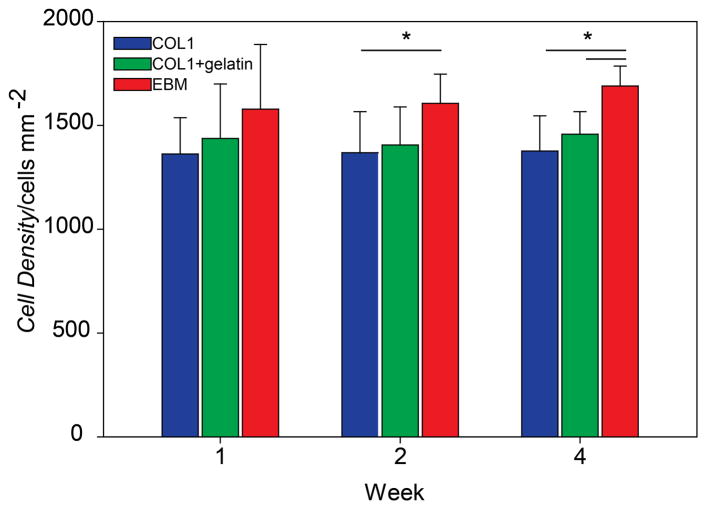
2.4 Bovine CE monolayers formed on engineered basement membranes have a significantly higher cell density compared to controls
We analyzed bovine CE cell density on all 3 substrate types over time (Figure 5) because it is an important metric to determine if a CE monolayer is suitable for implant, as it needs to be above the minimum functioning density of 500 cells mm−2.[4] At week 1, there was no statistical difference between the cell densities of the monolayers formed by CE on the 3 different substrates. After 2 weeks in culture, the monolayers formed on the EBMs had a significantly higher cell density compared to COL1 and after 4 weeks in culture, a significantly higher cell density compared to COL1 and COL1+Gelatin. Importantly, the CE cells remained in a monolayer and did not stratify, thus maintaining the phenotypic polygonal morphology of the endothelium. Further, the density of the CE monolayers on the EBMs could be further increased by increasing the initial cell seeding density. These cell density results provide further evidence that the CE cells were specifically responding to the cues from the top COL4+LAM layer of the EBM and not that of the underlying COL1.
Figure 5. Bovine CE cell monolayers cultured on EBMs increase in density overtime and have a significantly higher density compared to controls at weeks 2 and 4.
At week 1, there is no statistical difference between the cell densities of the three sample types. By week 2, the average cell density on the EBM (1606 ± 141) is significantly higher than that of the COL1 (1368 ± 198). At week 4, the average cell density on the EBM (1689 ± 96) is significantly higher than that of COL1 (1376 ± 170) and COL1+gelatin (1457 ± 108).
2.5 Human CE cells form a high density monolayer, express ZO-1 and have cortical F-actin filaments on engineered basement membranes
As human cells are more clinically relevant, we next investigated whether primary human CE cells would form high-density monolayers on the EBMs. First, human CE cells were isolated from 2 pairs of donor corneas, seeded onto 2 different COL4+LAM EBMs and cultured for 25 or 36 days (Table 1). However, on both samples the human CE cells did not reach confluence. After these samples were fixed and stained, it was evident that the cells did not express ZO-1, even in small areas where there were confluent patches of cells and had F-actin stress fibers across the cell bodies (Figure 6a). Next, we tested the human CE cells on COL4 EBMs, based on our previous results for CE expansion that demonstrated COL4 coated PDMS supported high-density monolayer formation.[22] To determine if there were any physical differences between the two different EBMs, we used multiphoton fluorescent imaging and atomic force microscopy to investigate the micro- and nanoscopic structure of the COL4 EBMs (Figure S1). The results showed that both EBMs had similar structure and strongly suggest that a top layer of EBM containing COL4 or COL4+LAM provides important biochemical cues that support formation of a high-density endothelial monolayer with polygonal cell morphology and phenotypic ZO-1 expressions at the cell-cell borders. Human CE cells were isolated from one cornea of two separate donors, and cultured on two separate COL4 EBMs for 14 days (Table 1). On both samples the human CE cells formed confluent monolayers with cell densities of approximately 1700 cells mm−2 and 760 cells mm−2, which importantly was greater than the minimum functional density for the CE of 500 cells mm−2. Furthermore, the human CE cells expressed continuous ZO-1 at the cell borders, and had primarily cortical F-actin filaments (Figure 6b), similar to in vivo human CE. Surprisingly, the fluorescently-labeled COL4 layer of the EBM was no longer visible at 14 days, indicating that the cells had remodeled it. This is distinctly different than the behavior of bovine CE cells, in which COL4 still remained at 28 days.
Table 1. Human donor CE information and summary of EBMs results.
This table details the donor age, sex and race, as well as the number of donor CEs and cell density of the CEs used for each of the 4 EBM samples. It also summarizes the qualitative and quantitative results of the human CE cells on the two different EBM types.
| Donor | Age | Sex | Race | # of donor corneas | CE Densities [cells mm−2] | EBM Type Seeded | Days in culture | Cell Morphology on EBM | Confluent [Yes or No] | Confluent Cell Density [cells mm−2] | ZO-1 Present [Yes or No] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | Male | African American | 2 | 2857/2747 | COL4+LAM | 25 | Elongated | No | N/A | No |
| 2 | 29 | Female | Caucasian | 2 | 3086/3003 | COL4+LAM | 36 | Elongated | No | N/A | No |
| 3 | 34 | Male | Caucasian | 1 | 2915 | COL4 | 14 | Polygonal | Yes | 760 | Yes |
| 4 | 28 | Female | Caucasian | 1 | 2882 | COL4 | 14 | Polygonal | Yes | 1700 | Yes |
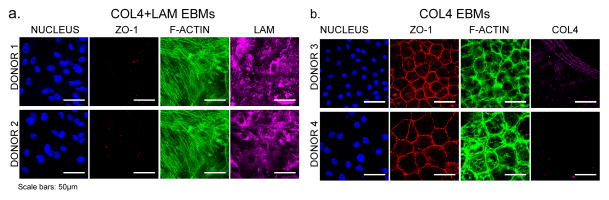
Figure 6. Human CE cells form a high density monolayer and express ZO-1 when cultured on a COL4 EBM.
(a) In contrast, human CE cells isolated from two corneas from two separate donors were cultured for >25 days on two COL4+LAM EBMs (1 donor pair of corneas per EBM) and in each case failed to become for a full confluent monolayer, expressed no ZO-1 at the cell borders, and the F-actin cytoskeleton of the cells has large stress fibers spanning across the cell bodies. The LAM was antibody labeled and shows the structure of the LAM does not match that which was initially present in the EBM or what was produced by the bovine CE cells. (b) Human CE cells isolated from single corneas from two separate donors were cultured for 14 days on two COL4 EBMs (1 donor per EBM) and formed monolayers with a densities of 1700 cells mm−2 (top row) and 760 cells mm−2. The cells express continuous ZO-1 at the cell borders. The F-actin cytoskeleton shows very organized stress fibers located cortically. In most regions of the EBM the COL4 (labeled during fabrication) was gone indicating the cells had completely remodeled the COL4, however it did remain in some areas. Scale bars: 50μm.
The results for the human CE cells (Table 1 and Figure 6b) demonstrate that the COL4-only EBM can be used to tissue engineer human CE monolayers with densities adequate for potential use in a DSEK or DMEK procedure. In terms of cell density, our results are comparable to those achieved by other researchers that have engineered human corneal endothelium. For example, Lai et al cultured human CE cells on PIPAAm-coated Petri dishes and released them once monolayers formed in order to produce scaffold-free, free-standing CE sheets.[27] These CE sheets maintained structural integrity through cell-cell adhesions and some secreted matrix, with reported densities of ~2500 cells mm−2. In another approach, Levis et al cultured human CE cells on plastic compressed COL1 gels termed RAFTs, and achieved densities of ~2000 cells mm−2.[12b] From both of these examples, as well as our results, it is clear that human CE cells can be engineered into a corneal endothelium with clinically relevant cell density and express ZO-1 at the cell-cell borders. However, our EBMs have the unique advantage of being able to tailor the composition of the basement membrane-like ECM layer onto which the CE cells are adhered, as well as the thickness of the underlying stroma-like COL1 film. This provides independent control of the adhesion layer and mechanical support layer. This is potentially important, because clinical data comparing DSEK and DMEK grafts has shown that having a thinner or absent stromal layer improves visual acuity[28] and lowers graft rejection rates[.29] Thus, EBMs could be optimized to minimize the COL1 film thickness to reduce the potential for rejection and inflammation while still maximizing its thickness for ease of handling and support of the CE monolayer. Determining this optimal thickness, as well as the functional performance versus other approaches, ultimately requires in vivo functional testing in an animal model, and will be the focus of future studies.
2.6 Understanding the complex interaction between engineered basement membrane composition and cell type
The source of the CE cells dictated not only which ECM protein composition supported the best monolayer formation, but also which of the initial ECM sheet proteins was remodeled and how. Specifically, bovine CE cells formed high density monolayers on COL4+LAM EBMs, whereas human CE cells did not form monolayers on the COL4+LAM EBMs, but did form high density monolayers with continuous ZO-1 on the COL4 EBM. Additionally, bovine CE cells did not appear to remodel the COL4 from the engineered sheet, but did remodel the LAM. In contrast, for the human cells the fluorescently-labeled COL4 layer of the EBM was no longer visible at day 14, suggesting it was degraded by the cells. In addition to the species of the cell source, there are other potential factors that could be responsible for the differences in behavior exhibited between bovine and human cells in response to the EBMs. For example the age of and consistency between donor corneas and the source species can affect the way the cells interact with the substrate. The bovine corneas were a more consistent cell source in terms of donor age (all were 1–3 years old) and environmental factors (all were raised on the same farm under the same conditions in terms of food, weather, activity level, exposure to the elements etc.) compared to the human donor corneas which came from donors with a wide range in age and unknown environmental factors. A more consistent human cell source will be needed for future studies focused on investigating the species specific differences in response to the EBMs. Additionally, the cell behavior in response to the EBMs could be an effect of source species of the ECM proteins or the ECM isoforms used to engineer the ECM. In the current study, we used COL4 from human placenta and LAM from mouse tumors, future studies will be focused investigating ECM proteins produced by different species. Further, we will focus on using ECM isoforms that are more specific to the native Descement’s membrane, such as collagen VIII, which is a major component of Descemet’s membrane and LAM isoforms 511 and 521 as recent studies have shown that expanding human CE cells on laminin isoforms 511 and 521 increased their cell density by up to 100%.[30]
3. Conclusions
In this study, we fabricated an EBM composed of a dense COL4 or COL4+LAM layer ~10 nm thick integrated on top of a COL1 film ~10 μm thick in order to closely mimic the physical structure of the acellular portion of a DSEK graft. Bovine CE cells cultured on the EBM formed higher density monolayers that exhibited more continuous ZO-1 staining and cortical F-actin filaments at the borders compared to controls. Additionally, human CE cells cultured on the COL4 EBM formed a monolayer with tight junctions and cortical F-actin filaments. These results indicate the EBMs have the potential to be used clinically as the acellular portion of grafts in DSEK and DMEK surgeries. Additionally, we have previously shown the ability to use a range of ECM proteins in SIA based techniques, including fibronectin and fibrinogen, suggesting that we can fabricate EBMs designed to mimic the protein composition of the native basement membrane of multiple tissues. Thus, we believe EBMs are a highly adaptable platform with the potential for broad application in the engineering human endothelial and epithelial tissues.
4. Experimental Section
Fabrication of engineered basement membranes
To fabricate the EBMs, SIA was used to transfer a thin layer of ECM (COL4 or COL4+LAM) onto a compressed COL1 film (Figure 1). To create the compressed COL1 film, we first prepared 6 mg mL−1 COL1 solution from a higher concentration stock solution (>9 mg mL−1, BD Biosciences). The COL1 solution was pipetted onto a glass coverslip with a silicone mold on top to define the shape of the gel as a 9 mm diameter circle. The COL1 solution was gelled for three hours in a humidified cell culture incubator at 37 °C, resulting in auto-compression of the gel.[31] Following gelation, the silicone molds were removed and the gels were dried in a cell culture hood (Figure 1A) to form the COL1 films. Subsequently, LAM and/or COL4, COL4 from human placenta (Sigma Aldrich) and LAM from Engelbreth-Holm-Swarm sarcoma (Life Technologies) were fluorescently labeled with AlexaFluor 488 and AlexaFluor 633 (Life Technologies) according to the manufacturer’s protocol, via reaction of succinimidyl ester groups on the dyes with primary amines in the proteins. SIA was then performed using a flat, featureless polydimethylsiloxane (PDMS) stamps to transfer the ECM proteins onto the COL1 film by adapting previously published methods.[23] Briefly, featureless (flat) PDMS stamps ~1 cm2 were sonicated in a 50% ethanol solution for 60 minutes, dried using a nitrogen gun and incubated with a mixture of COL4 and LAM (200 μL of 50 μg/mL each in PBS) or just COL4 (50 μg/mL in PBS), mixed 1:1 fluorescently-labeled to unlabeled protein (Figure 1b). The stamps were then rinsed, dried and brought into conformal contact, ECM side down, onto poly(N-isopropylacrylamide) (PIPAAm)(10% in butanol, Polysciences) coated coverslips for 1 hour to transfer the ECM sheet to the PIPAAm (Figure 1c). Gelatin coated glass slides were prepared by dipping glass slides in to a warm 20% gelatin solution followed by drying for 5 minutes. The coverslips prepared above were placed ECM side onto the gelatin (Figure 1d) and immersed in room temperature distilled water to trigger dissolution of the PIPAAm and the transfer of the ECM sheet to the gelatin (Figure 1e). The gelatin was cut with a scalpel around the ECM sheet, peeled off of the glass with forceps and placed ECM down onto the dried COL1 film (Figure 1f), and then placed in a humidified incubator at 37 °C for 45 minutes to melt the gelatin and complete transfer of the ECM sheet onto the COL1. These completed EBMs were then rinsed twice with warm PBS, incubated in warm PBS for an additional 45 minutes, and then again rinsed twice with warm PBS to remove any gelatin residue (Figure 1g). Two controls were used, COL1 films and COL1 films that had gelatin (with no ECM sheet) melted over them (COL1+gelatin). Finally, for cell seeding the top of 15 mL centrifuge tubes were cut off and sealed around the samples using vacuum grease to restrict the seeding area for cells. Samples were placed in 6 well plates, covered with PBS and sterilized with the lid on under UV light for 15 minutes. Transfer of the fluorescent protein sheet at each step was confirmed using confocal laser scanning microscopy (Nikon AZ100 Laser Scanning Confocal Microscope).
Structural analysis of engineered basement membranes
Atomic force microscopy (AFM) was used to image the surface topography of the COL4+LAM assembled on PIPAAm, COL1 control films, COL4 assembled on PIPAAm and COL4+LAM and COL4 EBMs. All samples were imaged on a MFP-3D-BIO AFM (Asylum Research) using AC mode in air with AC160TS cantilevers (Olympus) and scan sizes 1 and 10 μm. The Zsensor data was used and post-processed in IgorPro (WaveMetrics), using a first order flatten, before analysis. Three spots per sample and three samples each of COL1, COL4+LAM on PIPAAm and COL4+LAM EBMs at each scan sized were averaged to determine the root mean square (RMS) roughness and 3 samples per condition were used to determine the mean RMS roughness. The RMS roughness data was statistically analyzed by one-way ANOVA followed by Tukey’s multiple pairwise comparison (p<0.05). One sample each of COL4 on PIPAAm and COL4 EBM were imaged to compare to the COL4+LAM data.
Laser scanning multiphoton microscopy (Leica TCS SP5) was used to analyze the layered structure of the COL4+LAM and COL4 EBMs compared to COL1 controls. The COL1 fibers in both the COL1 films and EBM samples were imaged using second harmonic imaging. The Alexa Fluor 488 labeled COL4 was imaged simultaneously by splitting the signal at 491 nm with a dichroic mirror and sending it through a FITC emission filter. Samples were imaged with a 25x water immersion objective and Z-stacks were post-processed using AutoQuant and Imaris image analysis software (Bitplane, Inc.).
CE cell isolation and culture
Bovine CE cells were isolated from fresh whole bovine eyes and expanded on a soft COL4 coated PDMS substrate with elastic modulus of 50 kPa, as previously described.[22] Briefly, corneas were excised from whole globes (Pel Freez Biologicals), soaked briefly in PBS containing penicillin (100 IU/mL), streptomycin (100 μg/mL), gentamicin (50 μg/mL), amphotericin B (2.5 μg/mL) and glucose (1 g/L), and then incubated endothelial side up in a 12-well ceramic spot plate with TrypLE Express (200 μL) at 37 °C for 20 minutes. The corneas were scraped with a rubber scalpel to release the CE cells, the TrypLE Express, containing CE cells, was pipetted from the corneas, combined and centrifuged for 5 minutes at 1500 rpm to collect the cells. The cells were resuspended in low-glucose DMEM supplemented with 10% FBS, containing antibiotics (as above), seeded into a T-25 flask with a COL4 coated 50 kPa PDMS bottom (passage 0) and passaged 1:3 into new flasks upon reaching confluence. Once confluent at passage 1, bovine CE cells were released with TrypLE Express, resuspended at a density of 75,000 cells mL−1, and 1 mL was seeded on each of the three sample types: COL1, COL1+gelatin and EBM. We used a reducer with a 14.5 mm diameter placed within the well of the 6-well plate around the samples to reduce the seeding area to 165 mm2, making the final seeding density 455 cells mm−2. Nine samples per type were used for each time point of 1, 2 and 4 weeks (except for COL1 at 4 weeks, which had 8 samples due to loss of 1 sample during staining prep).
Human CE cells were isolated from the corneas of 4 different donors (obtained through National Disease Research Interchange) using a modified version of the method published by Peh et al.[32] First the corneas were rinsed in PBS containing penicillin, streptomycin, amphotericin B, gentamicin and glucose as described above. Next Descemet’s membrane and CE cells were manually stripped from the cornea with forceps, digested in collagenase I-S (1mg mL−1) (Sigma Aldrich) in Human Endothelial SFM media (Life Technologies) for 3 hours, 37 °C, and centrifuged for 5 min at 1100 RPM. The supernatant was removed and the pellet was washed with PBS to remove excess collagenase and centrifuged again for 5 min at 1100 RPM. The supernatant was again removed; the pellet was resuspended in TrypLE Express and incubated for 5 minutes at 37 °C, and then centrifuged for 5 min at 1100 RPM. The human CE cell pellet was resuspended in 1 mL Human Endothelial SFM media with 5% FBS, with antibiotics (as above without gentamicin), and FGF2 (10 ng/mL) and seeded onto a COL4+LAM EBM or a COL4 EBM. Human CE cells were cultured on the EBM for at least 14 days. Table 1 details the donor age, sex and race as well as the CE cell density and number of corneas seeded per EBM.
Immunofluorescent staining, imaging and analysis
At each time point samples were removed from culture, rinsed in PBS containing calcium and magnesium (PBS++) and fixed in 4% formaldehyde in PBS++ with 0.05% Triton-X100 for 15 minutes. Samples were rinsed twice in PBS++ for 5 minutes and incubated for 5 minutes with DAPI NucBlue (Life Technologies). Samples were rinsed one time in PBS++ before incubation with mouse anti-ZO-1 (1:100 dilution, Life Technologies) and rabbit anti-laminin (1:100 dilution, Sigma Aldrich) or AlexaFluor 488 Phalloidin (1.5:100 dilution, Life Technologies) in PBS++ for 2 hours at 37 °C. Following incubation, samples were rinsed 3 times for 5 minutes in PBS++ and each sample that was labeled with anti-ZO-1 and anti-laminin was incubated with a AlexaFluor 555 goat anti-mouse (1.5:100 dilution) and AlexaFluor 488 goat anti-rabbit secondary antibodies (1.5:100 dilution, Life Technologies) for 2 hours at 37 °C. Samples were rinsed again and mounted on glass slides using Prolong Anti-Fade (Life Technologies). A Zeiss confocal microscope was used to image 5 random areas per bovine cell sample (Zeiss LSM 700) and 3 areas per sample for the human cell samples. Cell density was calculated using ImageJ (National Institutes of Health) from the DAPI channel using the multi-point selection tool to manually count the number of nuclei per image and dividing by the image area. The cell densities for the images collected for each confluent sample were averaged to determine the cell density of each sample. For the bovine cell samples, the average cell density for each sample type per time point was then calculated by averaging all 9 samples. The bovine cell density data at each time point was statistically analyzed using One-way ANOVA followed by Tukey’s multiple pairwise comparison (1 and 4 weeks) or One-way ANOVA on ranks with Tukey’s test (2 week).
Supplementary Material
Acknowledgments
This work was supported by an Ocular Tissue Engineering and Regenerative Ophthalmology (OTERO) postdoctoral fellowship from the Louis J. Fox Center for Vision Restoration at UPMC to R.N.P. and the National Institutes of Health (NIH) Director’s New Innovator Award Program, [grant number DP2HL117750] to A.W.F.
References
- 1.a) Hagedorn EJ, Sherwood DR. Curr Opin Cell Biol. 2011;23:589. doi: 10.1016/j.ceb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) LeBleu VS, MacDonald B, Kalluri R. Exp Biol Med. 2007;232:1121. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]; c) Yurchenco PD, Amenta PS, Patton BL. Matrix Biol. 2004;22:521. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Wiradjaja F, DiTommaso T, Smyth I. Birth Defects Res Part C. 2010;90:8. doi: 10.1002/bdrc.20172. [DOI] [PubMed] [Google Scholar]
- 3.a) Joyce NC. Prog Retinal Eye Res. 2003;22:359. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]; b) Zhu C, Joyce NC. Invest Ophthalmol Vis Sci. 2004;45:1743. doi: 10.1167/iovs.03-0814. [DOI] [PubMed] [Google Scholar]
- 4.Engelmann K, Bednarz J, Valtink M. Exp Eye Res. 2004;78:573. doi: 10.1016/s0014-4835(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 5.2014 Eye Banking Statistcal Report. EBAA; 2015. [Google Scholar]
- 6.Elhalis H, Azizi B, Jurkunas UV. Ocul Surf. 2010;8:173. doi: 10.1016/s1542-0124(12)70232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JCR, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, SundarRaj N, Timpl R, Virtanen I, Ljubimov AV. Invest Ophthalmol Vis Sci. 2007;48:4989. doi: 10.1167/iovs.07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Weller JM, Zenkel M, Schlotzer-Schrehardt U, Bachmann BO, Tourtas T, Kruse FE. Invest Ophthalmol Vis Sci. 2014;55:3700. doi: 10.1167/iovs.14-14154. [DOI] [PubMed] [Google Scholar]
- 8.a) Johnson DH, Bourne WM, Campbell RJ. Arch Ophthalmol. 1982;100:1948. doi: 10.1001/archopht.1982.01030040928012. [DOI] [PubMed] [Google Scholar]; b) Murphy C, Alvarado J, Juster R. Invest Ophthalmol Vis Sci. 1984;25:1402. [PubMed] [Google Scholar]
- 9.a) Murphy C, Alvarado J, Juster R, Maglio M. Invest Ophthalmol Vis Sci. 1984;25:312. [PubMed] [Google Scholar]; b) Nucci P, Brancato R, Mets MB, Shevell SK. Arch Ophthalmol. 1990;108:247. doi: 10.1001/archopht.1990.01070040099039. [DOI] [PubMed] [Google Scholar]
- 10.a) Melles GR, Lander F, van Dooren BT, Pels E, Beekhuis WH. Ophthalmology. 2000;107:1850. doi: 10.1016/s0161-6420(00)00253-0. [DOI] [PubMed] [Google Scholar]; b) Price MO, Gorovoy M, Price FW, Benetz BA, Menegay HJ, Lass JH. Ophthalmology. 2013;120:246. doi: 10.1016/j.ophtha.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Price MO, Price FW., Jr Int Ophthalmol Clin. 2010;50:137. doi: 10.1097/IIO.0b013e3181e21a6f. [DOI] [PubMed] [Google Scholar]; d) Terry MA, Ousley PJ. Cornea. 2001;20:239. doi: 10.1097/00003226-200104000-00001. [DOI] [PubMed] [Google Scholar]; e) Terry MA, Ousley PJ. Cornea. 2001;20:14. doi: 10.1097/00003226-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tan DTH, Dart JKG, Holland EJ, Kinoshita S. Lancet. 2012;379:1749. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 12.a) Koizumi N, Sakamoto Y, Okumura N, Okahara N, Tsuchiya H, Torii R, Cooper LJ, Ban Y, Tanioka H, Kinoshita S. Invest Ophthalmol Vis Sci. 2007;48:4519. doi: 10.1167/iovs.07-0567. [DOI] [PubMed] [Google Scholar]; b) Levis HJ, Peh GSL, Toh KP, Poh R, Shortt AJ, Drake RAL, Mehta JS, Daniels JT. Plos One. 2012:7. doi: 10.1371/journal.pone.0050993. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Levis HJ, Peh GSL, Toh KP, Poh RWY, Drake R, Mehta JS, Daniels JT. J of Tissue Eng Regener Med. 2012;6:136. [Google Scholar]; d) Mimura T, Yamagami S, Yokoo S, Usui T, Tanaka K, Hattori S, Irie S, Miyata K, Araie M, Amano S. Invest Ophthalmol Vis Sci. 2004;45:2992. doi: 10.1167/iovs.03-1174. [DOI] [PubMed] [Google Scholar]; e) Vrana NE, Builles N, Justin V, Bednarz J, Pellegrini G, Ferrari B, Damour O, Hulmes DJS, Hasirci V. Invest Ophthalmol Vis Sci. 2008;49:5325. doi: 10.1167/iovs.07-1599. [DOI] [PubMed] [Google Scholar]; f) Yamagami S, Mimura T, Yokoo S, Usui T, Tanaka K, Hattori S, Irie S, Miyata K, Araie M, Amano S. Invest Ophthalmol Vis Sci. 2004;45:U521. doi: 10.1167/iovs.03-1174. [DOI] [PubMed] [Google Scholar]
- 13.a) Kimoto M, Shima N, Yamaguchi M, Hiraoka Y, Amano S, Yamagami S. Invest Ophthalmol Vis Sci. 2014;55:2337. doi: 10.1167/iovs.13-13167. [DOI] [PubMed] [Google Scholar]; b) Lai JY, Ma DHK, Lai MH, Li YT, Chang RJ, Chen LM. Plos One. 2013;8 doi: 10.1371/journal.pone.0054058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu PL, Lai JY, Ma DHK, Hsiue GH. J Biomater Sci, Polym Ed. 2008;19:1. doi: 10.1163/156856208783227695. [DOI] [PubMed] [Google Scholar]
- 15.a) Gao XS, Liu WS, Han BQ, Wei XJ, Yang CZ. J Mater Sci:Mater Med. 2008;19:3611. doi: 10.1007/s10856-008-3508-0. [DOI] [PubMed] [Google Scholar]; b) Liang Y, Liu WS, Han BQ, Yang CZ, Ma Q, Song FL, Bi QQ. Colloids Surf, B. 2011;82:1. doi: 10.1016/j.colsurfb.2010.07.043. [DOI] [PubMed] [Google Scholar]; c) Ozcelik B, Brown KD, Blencowe A, Daniell M, Stevens GW, Qiao GG. Acta Biomater. 2013;9:6594. doi: 10.1016/j.actbio.2013.01.020. [DOI] [PubMed] [Google Scholar]; d) Wang TJ, Wang IJ, Chen S, Chen YH, Young TH. Colloids Surf, B. 2012;90:236. doi: 10.1016/j.colsurfb.2011.10.043. [DOI] [PubMed] [Google Scholar]; e) Young TH, Wang IJ, Hu FR, Wang TJ. Colloids Surf, B. 2014;116:403. doi: 10.1016/j.colsurfb.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 16.a) Bayyoud T, Thaler S, Hofmann J, Maurus C, Spitzer MS, Bartz-Schmidt KU, Szurman P, Yoeruek E. Curr Eye Res. 2012;37:179. doi: 10.3109/02713683.2011.644382. [DOI] [PubMed] [Google Scholar]; b) Choi JS, Williams JK, Greven M, Walter KA, Laber PW, Khang G, Soker S. Biomaterials. 2010;31:6738. doi: 10.1016/j.biomaterials.2010.05.020. [DOI] [PubMed] [Google Scholar]; c) Fu Y, Fan XQ, Chen P, Shao CY, Lu WJ. Cells Tissues Organs. 2010;191:193. doi: 10.1159/000235680. [DOI] [PubMed] [Google Scholar]
- 17.a) Amano S, Mimura T, Yamagami S, Osakabe Y, Miyata K. Jpn J Ophthalmol. 2005;49:448. doi: 10.1007/s10384-005-0245-5. [DOI] [PubMed] [Google Scholar]; b) Proulx S, Audet C, Uwamaliya JD, Deschambeault A, Carrier P, Giasson CJ, Brunette I, Germain L. Tissue Eng, Part A. 2009;15:1709. doi: 10.1089/ten.tea.2008.0208. [DOI] [PubMed] [Google Scholar]; c) Proulx S, Bensaoula T, Nada O, Audet C, Uwamaliya JD, Devaux A, Allaire G, Germain L, Brunette I. Invest Ophthalmol Vis Sci. 2009;50:2686. doi: 10.1167/iovs.08-2793. [DOI] [PubMed] [Google Scholar]
- 18.a) Fan TJ, Ma XY, Zhao J, Wen Q, Hu XZ, Yu HZ, Shi WY. Mol Vis. 2013;19:400. [PMC free article] [PubMed] [Google Scholar]; b) Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, Kinoshita S. Invest Ophthalmol Vis Sci. 2004;45:800. doi: 10.1167/iovs.03-0016. [DOI] [PubMed] [Google Scholar]; c) Wu WC, Ye M, Yan WT, Lu F, Qu J, Wang QM, Zhou XT. Curr Eye Res. 2007;32:199. [Google Scholar]
- 19.Kopsachilis N, Tsinopoulos I, Tourtas T, Kruse FE, Luessen UW. Clin Experiment Ophthalmol. 2012;40:187. doi: 10.1111/j.1442-9071.2011.02678.x. [DOI] [PubMed] [Google Scholar]
- 20.Szymanski JM, Ba MC, Feinberg AW. J Mater Chem B. 2015;3:7993. doi: 10.1039/C5TB01003A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Dyrlund TF, Poulsen ET, Scavenius C, Nikolajsen CL, Thogersen IB, Vorum H, Enghild JJ. J Proteome Res. 2012;11:4231. doi: 10.1021/pr300358k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC. Lab Invest. 1995;72:461. [PubMed] [Google Scholar]; c) Poulsen ET, Dyrlund TF, Runager K, Scavenius C, Krogager TP, Hojrup P, Thogersen IB, Sanggaard KW, Vorum H, Hjortdal J, Enghild JJ. J Proteome Res. 2014;13:4659. doi: 10.1021/pr500252r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palchesko RN, Lathrop KL, Funderburgh JL, Feinberg AW. Sci Rep. 2015;5:7955. doi: 10.1038/srep07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinberg AW, Parker KK. Nano Lett. 2010;10:2184. doi: 10.1021/nl100998p. [DOI] [PubMed] [Google Scholar]
- 24.Abrams GA, Schaus SS, Goodman SL, Nealey PF, Murphy CJ. Cornea. 2000;19:57. doi: 10.1097/00003226-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 25.a) Baniasadi M, Minary-Jolandan M. Materials. 2015;8:799. doi: 10.3390/ma8020799. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chernoff EAG, Chernoff DA. J Vac Sci Technol, A. 1992;10:596. [Google Scholar]; c) Gathercole LJ, Miles MJ, Mcmaster TJ, Holmes DF. J Chem Soc Faraday T. 1993;89:2589. [Google Scholar]; d) Schmitt FO, Hall CE, Jakus MA. J Cell Compar Physl. 1942;20:11. [Google Scholar]
- 26.Sun Y, Jallerat Q, Szymanski JM, Feinberg AW. Nat Methods. 2015;12:134. doi: 10.1038/nmeth.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai JY, Chen KH, Hsu WM, Hsiue GH, Lee YH. Arch Ophthalmol. 2006;124:1441. doi: 10.1001/archopht.124.10.1441. [DOI] [PubMed] [Google Scholar]
- 28.Dapena I, Ham L, Melles GRJ. Curr Opin Ophthalmol. 2009;20:299. doi: 10.1097/ICU.0b013e32832b8d18. [DOI] [PubMed] [Google Scholar]
- 29.Anshu A, Price MO, Price FW. Ophthalmology. 2012;119:536. doi: 10.1016/j.ophtha.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 30.a) Kakutani K, Okumura N, Schlotzer-Schrehardt U, Kruse FE, Kinoshita S, Koizumi N. Invest Ophthalmol Vis Sci. 2015:56. doi: 10.1167/iovs.14-15163. [DOI] [PubMed] [Google Scholar]; b) Okumura N, Kakutani K, Numata R, Nakahara M, Schlotzer-Schrehardt U, Kruse F, Kinoshita S, Koizumi N. Invest Ophthalmol Vis Sci. 2015;56:2933. doi: 10.1167/iovs.14-15163. [DOI] [PubMed] [Google Scholar]
- 31.Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. Adv Funct Mater. 2005;15:1762. [Google Scholar]
- 32.Peh GSL, Toh KP, Wu FY, Tan DT, Mehta JS. Plos One. 2011;6 doi: 10.1371/journal.pone.0028310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.