Abstract
Background:
Community pharmacy has become a major access point for several types of vaccinations. Despite the success of vaccination programs like influenza, pneumococcal, and herpes zoster, the rates of human papillomavirus vaccination continue to lag.
Objectives:
The primary objective is to describe and report on the impact of a multimodal series of pharmacist-led educational interventions on human papillomavirus vaccination rates in a community pharmacy setting. The primary outcome of this study was change in pharmacist-delivered human papillomavirus vaccination throughout a corresponding 8-week period in 2014 and 2015.
Methods:
A single-center, quasi-experimental interrupted time series mixed-methods pilot study was used to investigate a pharmacist-led, multimodal educational intervention approach to improve human papillomavirus vaccination rates in the community.
Results:
During the 2014 control period, there were no human papillomavirus vaccines dispensed or administered according to the internal prescription dispensing software. In 2015, a total of 10 patients indicated that they were vaccinated, with 9 patients receiving their first dose and 1 patient receiving his or her second dose at the pharmacy. Pharmacist recommendation was the most reported education method for increasing patient awareness of the human papillomavirus vaccine (n = 10).
Conclusion:
This study demonstrates pharmacist designed, educational interventions may impact human papillomavirus vaccination rates in the community. Further community-based research with larger sample sizes is warranted to verify these results. Due to the unique barriers to human papillomavirus vaccination, a multimodal and inter-professional approach such as the one presented here is warranted.
Keywords: Human papillomavirus, vaccination, human papillomavirus vaccination, pharmacist
The community pharmacy has become a major access point for several types of vaccinations. Despite the success of vaccination programs such as influenza, pneumococcal, and herpes zoster, the rates of human papillomavirus (HPV) vaccination continue to lag. HPV is a group of more than 150 viruses, including approximately 40 types affecting both males and females. It is commonly known for causing genital warts, but is also responsible for several types of cancer including genitourinary cancer. It is most commonly acquired and transmitted through sexual intercourse.
The Food and Drug Administration (FDA) has approved three vaccine products for the prevention of HPV; all of these vaccines are comprised of a series of three separate intramuscular injections administered in the deltoid region of the upper arm or in the anterolateral area of the thigh. The Advisory Committee on Immunization Practices (ACIP) recommends to start HPV vaccination series at age 11 or 12 years. The series may be started as early as age 9 and as late as age 26 for females and 21 for males.1 The series is given over a period of 6 months with the second injection given 2 months after the first, and the third after 4 months. Completion of the series is necessary to receive 70% (Gardasil® and Cervarix®) or 90% (Gardasil 9) protection from cervical cancer and 90% protection from genital warts. Since 2006, when the first HPV vaccine was licensed, usage has lagged, especially for second and third doses.2 The Centers for Disease Control and Prevention (CDC) estimates every year that the HPV three-dose series is delayed, 4400 women will develop cervical cancer and an additional 53,000 cases will develop over the lifetime of females younger than 13 of age.3 Also, HPV vaccination rates continue to be well below the established Healthy People Goals for 2020 of 80%: in 2014, only 40% of females and 21% of males 13–15 years had received the full HPV series. Possible barriers include gaps in knowledge on the part of patients, parents, and prescribers.4 Pharmacists have been suggested as an important component of an interdisciplinary team to improve HPV vaccination rates, and, currently most states (80%) allow pharmacist to administer HPV.5
In 2013, the American Pharmacists Association (APhA) reported while 88% and 75% of pharmacies administered influenza and pneumococcal vaccines, respectively, only 37% administered HPV vaccinations. A recent report from the President’s Cancer Panel on Accelerating HPV Vaccine Uptake has specifically called on the pharmacy profession as part of multifaceted solution to improving the vaccination of adolescents and young adults.6 However, despite national calls for inclusion, pharmacist continues to be underutilized as HPV vaccine providers, and third-party insurance coverage of the vaccine at the pharmacy remains low. A multimodal approach to overcoming barriers to HPV vaccinations was recently suggested and includes screening, educating, recommending, and reminding.4 The aim of this pilot project is to implement and explore the impact of a pharmacist-led multimodal approach to improve HPV vaccination rates in the community.
Objectives
The primary objective is to describe and report on the impact of a multimodal series of pharmacist-led educational interventions on HPV vaccination rates in a community pharmacy setting. The primary outcome of this study was change in pharmacist-delivered HPV vaccination throughout a corresponding 8-week period in 2014 and 2015. A secondary outcome was patient self-reported data collected via a paper survey. This included the following: how did you hear about the vaccine, why did you decide to get the vaccine, and vaccination status.
Setting
This study was performed at Reeves-Sain Pharmacy, an independent community pharmacy in Murfreesboro, TN. The pharmacy mainly serves population representing suburban and semirural demographics. According to the US Census Bureau, 2010–2014, 5-year American Community Survey, Murfreesboro has a population of 126,118, a median annual household income of US$50,337, and mean annual household income of US$65,682. The Murfreesboro area contains 20 other pharmacies, 6 independent and 14 chain. Of the 20 pharmacies, 85% are known to provide immunizations.
Methods
Practice description
The pharmacy provides several cognitive pharmacy services (CPS) including point-of-care testing (POCT), medication therapy management (MTM), pharmacogenomic testing, disease state management (DSM), and vaccination services. Over 5000 vaccinations are given at Reeves-Sain annually, with HPV vaccination representing a small fraction.
Intervention
A list of community pharmacist-initiated modes of intervention was developed by a group of pharmacists and pharmacy leadership, including a post graduate year 1 (PGY-1) Community Pharmacy Resident, at the intervention pharmacy. The interventions were to meet the following requirements: (1) reproducible (2) scalable, and (3) integrated into current staff workload. The interventions were developed and implemented jointly by pharmacy leadership, pharmacists, and marketing staff. The educational interventions targeted three main audiences (see Figure 1):
Figure 1.
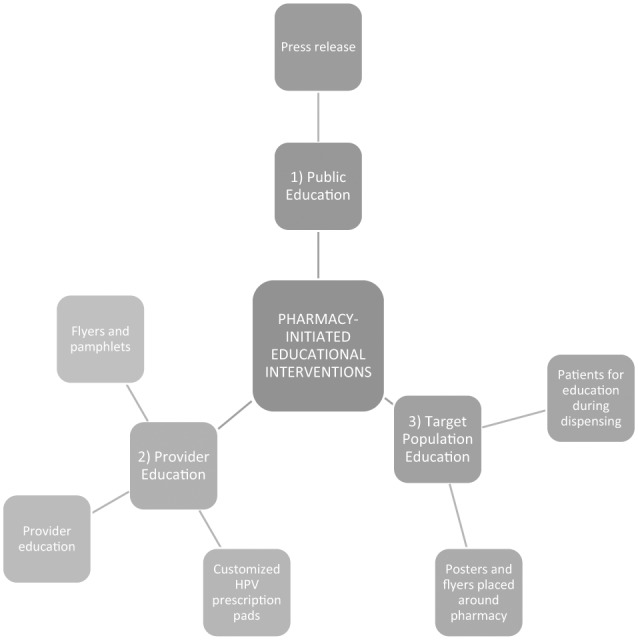
Pharmacist-led educational interventions on HPV vaccinations.
Target patient population at the pharmacy.
Local physician offices.
Public.
Design
This is a single-center, quasi-experimental interrupted time series mixed-methods pilot study. Data were collected during an 8-week intervention period between 20 March 2015 and 15 May 2015 and compared to an identical 8-week historic control period in 2014. The research was approved by the University of Tennessee Health Science Center Institutional Review Board.
The target patient population at the pharmacy was identified through the use of the prescription dispensing software and pharmacist assessment. Pharmacists were alerted to potential candidates for HPV vaccination via a software rule that flagged patients filling acne or birth-control prescriptions at the study pharmacy. The customized software rule was created based on the available technology in order to capture patients within the indicated age range for the vaccine. After the alert, the pharmacist would then assess the flagged patient within dispensing workflow using demographic information found within the software platform to determine whether the HPV vaccine was indicated for the patient. Additionally, flyers and posters about the HPV vaccine were also placed around the pharmacy.
In March, local physician offices near the pharmacy were visited by the pharmacy staff. The purpose of these visits was to establish consent to perform the study, provide customized prescription pads specific for the HPV vaccine at the study pharmacy, and provide flyers with patient education materials on the HPV vaccine. Physician office specialties included obstetrics and gynecology, family medicine, and pediatrics.
In order to target the general public, a one-time press release regarding the HPV vaccine was published in the local city newspaper. The release contained information about HPV infection and the HPV vaccine, including its indications, adverse effects, and contraindications.
Evaluation
To assess the impact that pharmacist educational interventions had on targeted patients within the pharmacy, a paper survey was given to patients prior to dispensing or administering the vaccine. Inclusion criteria for the survey consisted of males and females 9–26 years of age who had been targeted and subsequently counseled on the HPV vaccine. The questionnaire was designed by site pharmacists, pharmacy leadership, and faculty at a local college of pharmacy. The survey was piloted on a convenience sample prior to launch. The survey’s aim was to assess comfort level with the vaccine, identify patient sources of education about the vaccine, and to identify the source of influence for receiving the vaccine. For those under 18 years of age, a parent or legal guardian was given the questionnaire to complete. Statistics were analyzed using SPSS 23.
Results
During the 2014 control period, there were no HPV vaccines dispensed or administered according to the internal prescription dispensing software. During the 2015 intervention period, there were a total of 21 questionnaire respondents. A total of 10 patients indicated that they were vaccinated, with 9 patients receiving their first dose and 1 patient receiving his or her second dose at the pharmacy. Results are found in Table 1. Pharmacist recommendation was the most reported education method for patient awareness (n = 10). Pharmacist recommendation was also the most cited reason for obtaining vaccination (n = 6). Physician recommendation and media outreach were also influential in HPV vaccine awareness and uptake. Patients were more likely to choose the pharmacy as a vaccination site due to “no appointment being necessary” (n = 8) and convenience of hours (n = 4). The majority of patients (n = 9) receiving the vaccine were on the first dose of the HPV series. Of patients who chose not to receive the vaccine, the most common reasons included cost (n = 6) and insurance coverage (n = 5).
Table 1.
HPV vaccination questionnaire results.
| Description | n (%) |
|---|---|
| How did you hear about the HPV vaccine? | |
| Pharmacist recommendation | 10 (48) |
| Doctor recommendation | 5 (24) |
| Saw flyer at pharmacy | 2 (10) |
| Friend | 1 (5) |
| Doctor did not offer vaccine | 1 (5) |
| TV commercial | 1 (5) |
| Unanswered | 1 (5) |
| Why did you decide to get vaccinated (may choose more than one)? | |
| Unanswered | 7 (30) |
| Pharmacist recommendation | 6 (26) |
| Doctor recommendation | 4 (17) |
| Parent recommendation | 2 (9) |
| Friends and family | 2 (9) |
| Pharmacy flyers/posters | 1 (4) |
| Patient had breast cancer | 1 (4) |
| TV advertisement | 0 (0) |
| Why did you decide to get vaccinated at this pharmacy (may choose more than one)? | |
| Unanswered | 9 (40) |
| No need to make appointment | 8 (36) |
| Convenient hours | 4 (18) |
| Doctor’s office did not offer | 1 (5) |
| What dose in the HPV series is your shot today? | |
| Unanswered | 11 (52) |
| First dose | 9 (43) |
| Second dose | 0 (0) |
| Third dose | 1 (5) |
| If you do not choose to receive the HPV vaccine today, what is the reason (may choose more than one)? | |
| Cost | 6 (32) |
| Lack of insurance coverage | 5 (26) |
| No time | 2 (11) |
| Older than 26 years of age | 2 (11) |
| Wanted more information | 1 (5) |
| Said there was a lack of information | 1 (5) |
| Reaction to vaccine | 1 (5) |
| Due to possible side-effects | 1 (5) |
HPV: human papillomavirus.
Discussion
Historically, pharmacy’s impact on HPV vaccination rates has been low due to the uniqueness of the patient population, legal constraints, social barriers, and insurance coverage.5,7 Despite these barriers, this study demonstrates that pharmacists can have an impact both directly and indirectly in improving HPV vaccination. This is the first study to date investigating such pharmacist-initiated interventions on HPV vaccination rates, and aligns with prior published evidence, which found that educational interventions for vaccinations can improve vaccine rates and acceptance for influenza, pneumococcal, and herpes zoster vaccines.8–10
Previously published literature has only suggested that pharmacists can play a key role in improving HPV vaccine rates through direct (vaccine administration) and indirect (screening, educating, recommending, and reminding) roles.4,11 It postulates that even in states where legal barriers (e.g. scope of practice) prevent the pharmacist from vaccinating, educational interventions are still possible. However, the degree to which these interventions affect HPV vaccine rates had not been determined. The project found that these recommended and simple interventions, such as patient screening and education, prescriber outreach, and public service announcements, are indeed a viable approach to improving HPV care. This study also shows preliminary evidence that a pharmacist’s recommendation is a key factor in a patient’s decision to pursue HPV vaccination. Additionally, this study found that the community pharmacy offers advantages over other healthcare settings due to convenient hours and ability to receive a vaccine without an appointment. The interventions themselves were performed without the need for additional pharmacy personnel and were integrated into the workflow of the pharmacy. Therefore, we also believe that the implementation of these interventions may be scalable and sustainable in similar community pharmacy practice settings.
Several limitations were present in this pilot project. As the survey was only distributed to patients identified within the prescription-filling software, we may not have captured on survey those patients who were referred directly from the physician and those patients who had not filled prescriptions at the pharmacy before. This limitation was due to workflow limitations at the pharmacy that prevented this method of survey distribution. Additionally, it is not possible to directly link the interventions performed by the study pharmacy to the patient self-reported reasons for receiving the vaccine. Physician recommendations, for example, may have occurred even without provider outreach as described in the study methods. The short 8-week window, when the study was conducted, may not have been adequate to fully capture the target population. Use of customized paper prescription pads may not be the best means of increasing prescribing of the HPV vaccine due to the widespread and growing use of electronic prescribing. Moreover, it is not possible to extrapolate these results beyond the studied population of patients within an independent community pharmacy in a suburban community. Future studies should be powered to detect statistical improvements in vaccine rates based on a single pharmacist educational intervention. Timeframes should be at least 6 months in duration, and cover the time around back-to-school and pairing with other age-recommended vaccines such as influenza, Tdap, and meningococcal.
Conclusion
This study demonstrates pharmacist designed, educational interventions may impact HPV vaccination rates in the community. Further community-based research with larger sample sizes is warranted to verify these results. Due to the unique barriers to HPV vaccination, a multimodal and inter-professional approach such as the one presented here is warranted.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the University of Tennessee Health Science Center’s Institutional Review Board (IRB) under IRB Number 14-03503-XP.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
References
- 1. Markowitz LE, Dunne EF, Saraiya M, et al. Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05): 1–30. [PubMed] [Google Scholar]
- 2. Rahman M, Islam M, Berenson AB. Differences in HPV immunization levels among young adults in various regions of the United States. J Community Health 2015; 40(3): 404–408. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and post licensure vaccine safety monitoring, 2006–2013—United States. MMMR Morb Mort Wkly Rep 2013; 62: 591–595. [PMC free article] [PubMed] [Google Scholar]
- 4. Nielsen AR, Hayney MS. Human papillomavirus: a brief overview and recommendations for pharmacists. J Am Pharm Assoc 2014; 54(5): 558–561. [DOI] [PubMed] [Google Scholar]
- 5. Brewer NT, Chung JK, Baker HM, et al. Pharmacist authority to provide HPV vaccine: novel partners in cervical cancer prevention. Gynecol Oncol 2014; 132: S3–S8. [DOI] [PubMed] [Google Scholar]
- 6. US Department of Health and Human Services. Accelerating HPV vaccine uptake: urgency for action to prevent cancer. A report to the president of the United States from the president’s cancer panel. Bethesda, MD: National Cancer Institute, 2014, http://deainfo.nci.nih.gov/advisory/pcp/annualReports/HPV/index.htm (accessed 12 September 2014). [Google Scholar]
- 7. Jennifer M, Sturpe DA, Khanna N. Human papillomavirus vaccine and cervical cancer prevention: practice and policy implications for pharmacists. J Am Pharm Assoc 2008; 48(1): e1–e13. [DOI] [PubMed] [Google Scholar]
- 8. Teeter BS, Garza KB, Stevenson TL, et al. Factors associated with herpes zoster vaccination status and acceptance of vaccine recommendation in community pharmacies. Vaccine 2014; 32(43): 5749–5754. [DOI] [PubMed] [Google Scholar]
- 9. Baker DW, Brown T, Lee JY, et al. A multifaceted intervention to improve influenza, pneumococcal, and herpes zoster vaccination among patients with rheumatoid arthritis. J Rheumatol 2016; 43: 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas RE, Lorenzetti DL. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev 2014; 7: CD005188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah PD, Gilkey MB, Pepper JK, et al. Promising alternative settings for HPV vaccination of US adolescents. Expert Rev Vaccines 2014; 13(2): 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]


