Abstract
Objectives:
Exercise improves endothelial dysfunction, the key manifestation of cardiovascular and cerebrovascular disease, and is recommended in both cardiovascular and cerebrovascular rehabilitation. Disagreement remains, however, on the role of intensity of exercise. The purpose of this review was to gather current knowledge on the effects of high-intensity training versus moderate-intensity continuous exercise on endothelial function in cardiovascular and cerebrovascular patients.
Methods:
A systematic review was performed in PubMed database, Embase and Cochrane libraries and on PEDro using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Studies were restricted to cardiovascular and cerebrovascular patients, and healthy subjects as general reference. Interventions comprised of high-intensity training alone, high-intensity training compared to moderate-intensity continuous exercise, or no training, with endothelial function as outcome measure. Endothelial function was measured either physiologically by flow-mediated dilatation and/or by systemic biomarkers. Data were analyzed descriptively due to non-comparability for a meta-analysis to be performed.
Results:
A total of 20 studies were included in the review. Although there was great heterogenecity in design, population and exercise protocols, all studies found high-intensity training to be safe. High-intensity training was equal to moderate-intensity continuous exercise through improvement in endothelial function in 15 of the 20 selected studies, as measured by flow-mediated dilatation, nitric oxide bioavailability and circulating biomarkers. Only a few studies examined high-intensity training in cerebrovascular patients, none with endothelial function as outcome.
Conclusion:
High-intensity training is promising as a time-efficient exercise strategy in cardiovascular rehabilitation, but data on endothelial effects in cerebrovascular rehabilitation are warranted. Agreement on a more uniform exercise protocol is essential to further investigate the optimal exercise mode for cerebrovascular rehabilitation.
Keywords: Vascular endothelium, endothelial function, high-intensity training, interval training, high-intensity exercise, aerobic exercise, flow-mediated dilatation
Introduction
Exercise reduces risk factors of cardiovascular disease by targeting different organ systems simultaneously (Figure 1), thus reducing the mortality after stroke or cardiovascular events.1 One mechanism by which exercise may induce beneficial effects is improvement of endothelial function.
Figure 1.
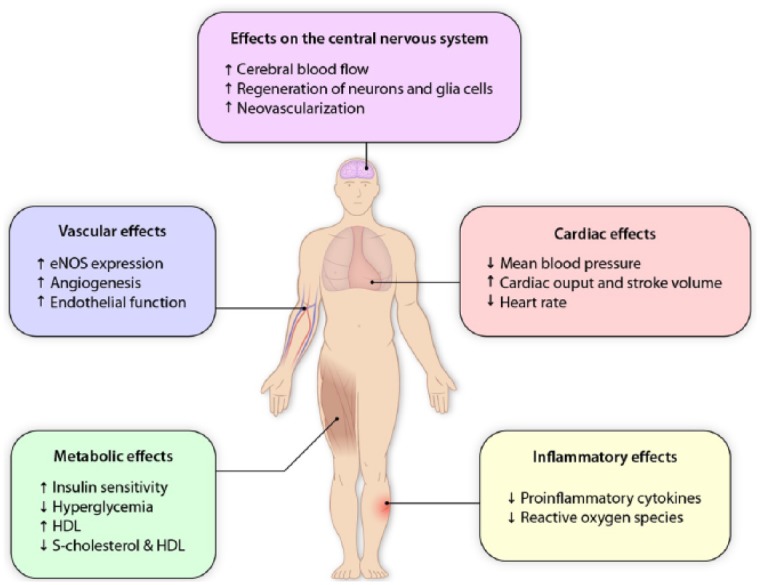
The general effects of exercise. The effect of exercise targets various organ systems thus improving cardiovascular, endothelial, cerebral and metabolic function, all parameters important to improve rehabilitation and reduce stroke risk. The figure depicts the various physiological systems affected. Increased flow in the vessels induced by exercise activates endothelial mechanoreceptors initiating synthesis of nitric oxide (NO) through activation of endothelial nitric oxide synthase (eNOS). Nitric oxide release is involved in both processes of vasodilation and inflammation. Dysfunction of the endothelium increases risk of platelet aggregation and leukocyte infiltration through adhesion molecules. Furthermore, exercise improving muscle and cardiac function improves the metabolism of glucose and cholesterol.
Illustration is created with inspiration from Schmidt et al.2
A main function of the endothelium is the regulation of vascular tone modulation of platelet activity and leukocyte adhesion in inflammatory processes, in addition to angiogenesis by production of nitric oxide (NO) and endothelial progenitor cells (EPCs).3 Endothelial dysfunction results in reduced NO bioavailability, causing reduced vasodilatation and increased expression of adhesion molecules inducing low-grade inflammation and platelet aggregation. The generation of reactive oxygen species (ROS) enhances inflammation by interacting with NO. This “host defence response” augments the risk of atherosclerosis and thereby cardiovascular and cerebrovascular disease if sustained.3,4 Increasing age, high body mass index (BMI), smoking, hypertension and metabolic diseases, such as diabetes and dyslipidemia, are known risk factors of endothelial dysfunction.5 Various repairing processes of the endothelium take place both locally by replication of adjacent cells and systemically by circulating EPCs recruited from bone marrow.3 Exercise enhances these repairing processes.2,6,7
Measurement and clinical estimations of vascular endothelial function can be carried out by either physiological tests of vasoreactivity or by circulating biomarkers. Of the physiological tests, flow-mediated dilation (FMD) is the most widely used. FMD is measured by reference to the brachial or carotid artery diameter before and after induction of shear stress. Vascular shear stress is induced by the release of an inflated cuff producing reactive hyperemia.8 The response appears to be predictive of cerebrovascular and cardiovascular events in asymptomatic individuals, as well as patients with established disease.9–11 Another physiological test is the reactive hyperemia index (RHI), which is reported to be significantly decreased in patients with cardiovascular disease.12
Circulating endothelial cells and endothelial microparticles (EMPs) are used as biomarkers since they are released during apoptosis of endothelial cells, and induce pro-inflammatory responses. Also, EPCs are used because the NO-dependent release of EPC from bone marrow may reflect the capacity of the body for endothelial repair. Plasma concentration of NO or nitrites are not useful markers since values can be confounded by other sources of NO, in particular dietary sources.3,13
The protective effect of exercise on endothelial function has been proved in studies of cardiovascular patients14 regardless of the etiology of the disease. Such findings may be extended to endothelial function in cerebrovascular disease since these groups of patients share pathophysiological mechanisms.2,15,16 Additionally, early initiation of training post-stroke is often recommended.17 Exercise intervention can be subdivided into two groups according to intensity: moderate-intensity continuous exercise (MICE) and high-intensity training (HIT). MICE is defined as exercise at approximately 60% of maximum heart rate (HR) or maximum capacity for aerobic work (VO2max) for a longer duration, for example, 45–60 min, whereas HIT is composed of short bursts of exercise at >85% of maximum HR or VO2max, separated by periods of rest. Although primarily seen in the training procedures of elite athletes, HIT is now gaining an increasing role in rehabilitation, particularly in cardiovascular patients.2,18
Quite extensive work has been carried out examining the effect of various kinds of exercise on cardiovascular patients.14,19 However, no systematic review has previously compared the effect of HIT on endothelial function in both cardiovascular and cerebrovascular patients. The following systematic review therefore aims to gather the published literature on the effect of HIT on endothelial function in cerebrovascular and cardiovascular patients and to compare with MICE or no training.
Method
Literature search
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews. The PubMed database (which includes literature from MEDLINE, journals and online books) was searched using the search strategy in Table 1 in the Data Supplement. Additionally, the Embase and Cochrane libraries, and PEDro were searched in a similar way with adaptations where necessary. Reference lists were also searched for relevant studies. The final search was conducted in January 2016. Table 1 shows search strategy and search strings.
Table 1.
PubMed search strategy.
| Patients | Intervention | Comparison | Outcome |
|---|---|---|---|
| “High-intensity training” | Endothelium, Vascular [Mesh] | ||
| “High intensity training” | “Endothelium, Vascular” | ||
| “Interval training” | “endothelial function” | ||
| “Interval exercise training” | |||
| “High intensity exercise” | |||
| “High intensity aerobic interval training” | |||
| “Aerobic exercise” |
Studies were included if they met inclusion criteria (see below) and the quality of the study was evaluated while reading the full-text articles.
The abbreviation for high-intensity training, HIT, is more widely used for “heparin-induced thrombocytopenia” and yielded no useful results in the search, but was included for the sake of completeness.
To broaden the search in PubMed, the MeSH term “endothelium, vascular” was used combined with the text “endothelium, vascular” and “endothelial function” (see the Data Supplement).
Search strings
Patients <Restriction on “patients” not necessary to limit search>
Comparison <Restriction on “comparison” not necessary to limit search>
Intervention
(“High-intensity training” OR “High intensity training” OR “Interval training” OR “Interval exercise training” OR “High intensity exercise” OR “High intensity aerobic interval training” OR “Aerobic exercise” OR “High intensity interval exercise” OR “HIT”)
AND
Outcome
(Endothelium, Vascular [Mesh] OR “Endothelium, Vascular” OR “endothelial function”)
Results: 258 hits
Study selection
This review examines the effect of exercise on endothelial dysfunction, regardless of etiology and with broad inclusion criteria to reflect the patient population for whom such training is targeted. The inclusion criteria for this review were as follows:
Randomized controlled studies, non-randomized controlled studies and non-controlled studies.
Participants aged 19+ years diagnosed with and/or treated for either cerebrovascular or cardiovascular disease, such as stroke, heart failure (HF) and coronary artery disease (CAD).
Studies in healthy subjects were included as a general reference since the effect of HIT might only be seen in participants with no endothelial dysfunction.
Interventions including intense aerobic intervals by >85% of maximum HR or VO2max.
Intervention of either a single bout of HIT compared to MICE/baseline or a HIT group exercising for several weeks compared to MICE and/or a control group.
Outcome measured by endothelial function assessed by FMD and/or circulating markers of endothelial function such as EMP, EPCs or vascular endothelial growth factors (VEGFs).
Articles were initially selected based on headlines, then further selected based on abstract and, finally, the relevant studies were selected after reading full-text articles. Search strategy was checked and repeated by second author (R.S.K.) who also screened titles and abstracts for relevance. Both reviewers examined all potentially relevant full-text articles for consistency with the inclusion criteria. Any disagreement was planned to be settled by the third author (C.K.); however, there were no disagreements between reviewers.
Non-English studies and animal studies were excluded. Groups with only endothelial risk factors such as aging, diabetes mellitus, hypertension and obesity, but no clinical signs of cerebrovascular or cardiovascular disease were excluded. Studies examining individuals with disease in other organ systems (cancer, kidney, hematological, thyroid or rheumatic diseases) and specific genetic mutations were also excluded. Studies with multimodal interventions or studies on nutrition were excluded. Studies exclusively examining the impact on aerobic capacity, VO2max, were not included.
Data analysis
All studies varied significantly in design, population and exercise intervention, and therefore, meta-analysis was not appropriate. A descriptive synthesis of the data was undertaken instead.
Details about participants and exercise interventions are summarized in Table 2 and results are summarized in Table 3. A flow diagram of the selection process can be seen in Figure 2.
Table 2.
Overview of participants and exercise interventions.
| Reference | Design | Blinded | Participants number and diagnosis | Exercise intervention |
||
|---|---|---|---|---|---|---|
| Mode: HIT, MICE, control | Protocol | Duration (weeks) | ||||
| Yamamoto et al.20 | Non-randomized trial | No | 10 healthy participants | HIT | • A single bout of HIT • Exercise at 90% of anaerobic threshold for 20 min • Cycle ergometer |
0 |
| Wisløff et al.21 | RCT | Yes | 27 participants with post-infarction HF | MICE, HIT, control | • 3 MICE/HIT sessions per week either or control • HIT: 4 × 4 min at 90%–95% max HR, 3-min active recovery at 50%–70% max HR between intervals • MICE: continuously at 70%–75% of max HR for 47 min • Control: standard advice regarding physical activity • “Uphill” treadmill walking |
12 |
| Rognmo et al.22 | Non-randomized trial | No | 10 athletes 7 healthy controls |
HIT | • A single bout of HIT session • 5 × 5-min intervals, the last 3 min of every bout >90% of max HR • 2-min active recovery at 60%–70% of max HR between intervals • Treadmill running |
0 |
| Rakobowchuk et al.23 | RCT | No | 20 healthy untrained participants | HIT, MICE | • HIT session 3 times per week or MICE 5 times per week • HIT: 30-s Wingate test, ⇑no. of tests; 4 tests during weeks 1–2, 5 tests during weeks 3–4 and 6 tests during weeks 5–6. Active recovery for 4–5 min between intervals. • MICE: 65% of VO2max, increasing duration; 40 min in weeks 1–2, 50 min in weeks 3–4 and 60 min in weeks 5–6 • Performed on bicycle ergometer |
6 |
| Munk et al.24 | RCT | Yes | 40 participants after PCI—implantation of bare metal stent or drug eluting stent. | HIT, control | • 3 HIT sessions per week or control • HIT: 4 × 4-min intervals at 80%–90% of max HR. 3-min active recovery at 60%–70% of max HR between intervals. • Controls: usual care • Bicycle ergometer or running |
24 |
| Hermann et al.25 | RCT | Yes | 27 long-term heart transplant recipients | HIT, control | • 3 HIT sessions per week or control • Bicycle intervals of 4 min/2 min/30 s at 80%, 85% and 90% of VO2max, recovery periods of 30 s • Finally, 10-min staircase running at 80% of VO2max and recovery walking down at 50% of VO2max • Bicycle ergometer + staircase running |
8 |
| Moholdt et al.26 | RCT | Yes | 107 CAD patients | HIT, MICE | • 2 weekly exercise sessions • HIT: 4 × 4 min intervals at 85%–95% of max HR, 3-min active recovery between intervals at 70% of max HR • MICE: 35-min “vigorous” continuous exercise • Treadmill running |
12 |
| Currie et al.27 | Within-subject repeated-measures design | No | 10 CAD patients | HIT, MICE | • 2 exercise sessions separated by an average of 11 days. Order of condition was randomized. • HIT: 10 × 1 min at 88% of VO2max with 1-min active recovery (10% of VO2max) between intervals • MICE: 30 min at 50% of VO2max • Bicycle ergometer |
0 |
| Rakobowchuk et al.28 | Controlled study | No | 20 healthy participants | HIT | • Heavy metabolic stress intervals: 30 s at 120% of VO2max and 60 s of active recovery, increasing duration: 30 min in weeks 1–2, 35 min in weeks 3–4 and 40 min in weeks 5–6 • Moderate metabolic stress intervals: 10 s exercise at 120% VO2max and 20-s active recovery. Increasing duration in the same fashion as above. • Assignment to either condition is controlled, matched by initial VO2max • Bicycle ergometer |
6 |
| Morikawa et al.29 | Non-randomized study | No | 26 patients with rest or effort angina | HIT | • 3 successive days of exercise • 4 × 3-min intervals at 75%–85% of max HR with 3-min active recovery between intervals at 30%–60% of max HR • Treadmill |
0 |
| Guiraud et al.30 | Within-subject repeated-measures design | No | 19 CAD patients | HIT, MICE | • 2 exercise sessions separated by 14 days • HIT: 2 sets of 10 min composed of 15 s at 100% of VO2peak and 15-s passive recovery. 4-min passive recovery between the two sets. • MICE: exercise at 70% of VO2peak. Duration adjusted to match the total energy expenditure of the HIT session (isocaloric exercise session). Mean duration: 28.7 min. • Bicycle ergometer |
0 |
| Isaksen et al.31 | Non-randomized study | Yes | 38 patients with ICD | HIT, control | • 3 sessions per week • HIT: 4 × 4-min intervals at 85% of max HR with 3-min active recovery between at 60%–70% of max HR • Controls: standard care? • Bicycle ergometer or treadmill running |
12 |
| Angadi et al.32 | RCT | Yes | 19 HFpEF patients. | HIT, MICE | • 3 sessions per week of either HIT or MICE • HIT: 4 × 4 min at 85%–90% of max HR with 3-min active recovery between intervals • MICE: 30 min continuously at 70% of max HR • Treadmill running |
4 |
| Wahl et al.33 | Within-subject repeated-measures design | No | 12 triathletes/cyclists | HIT, MICE | • One session of 3 different protocols with 1 week between • High volume: 120 min at 55% of peak power output (PPO) • HIT-A: 4 × 4-min intervals at 95% of PPO. Active recovery between intervals (45% of PPO) • HIT-B: 4 × 30 s with maximal effort (“all out”). Active recovery between intervals (45% of PPO). • Bicycle ergometer |
0 |
| Conraads et al.34 | RCT | Yes | 200 CAD participants. (SAINTEX-CAD study) |
HIT, MICE | • 3 sessions per week of either HIT or MICE • HIT: 4 × 4-min intervals at 90%–95% of max HR. Active recovery at 50%–70% of max HR between each interval. • MICE: 37 min at 65% of max HR • Bicycle ergometer • 12 weeks intervention was followed by instructions and monitored home training (cycling/running) |
12 |
| Dall et al.35 | RCT | Yes | 16 heart transplant recipients | HIT, MICE | • 3 sessions per week of either HIT or MICE • HIT: alternating intervals of 4, 2 and 1 min duration at >80% of VO2peak for a total 16 min of interval training. 2-min active recovery between each interval • MICE: biking for 45 min at 60%–70% of VO2peak • Bicycle ergometer |
12 |
| Van Craenenbroeck et al.36 | RCT | Yes | 200 CAD participants. (SAINTEX-CAD study) |
HIT, MICE | • See Conraads et al.34 | 12 |
HIT: high-intensity training; MICE: moderate-intensity continuous exercise; HF: heart failure; HR: heart rate; CAD: coronary artery disease; RCT: randomized controlled trial; ICD: implantable cardioverter defibrillator; VO2max: maximum capacity for aerobic work; HFpEF: heart failure with preserved ejection fraction; PPO: peak power output; PCI: percutaneous coronary intervention.
Table 3.
Overview of results.
| Reference | Outcome | p-value | Conclusions |
|---|---|---|---|
| Yamamoto et al.20 | • No significant difference in serum NOx products pre- or post-exercise | N.S. | ⇓HIT |
| Wisløff et al.21 | • HIT + MICE: ⇑FMD (HIT >> MICE) | <0.05 | HIT > MICE > controls |
| Rognmo et al.22 | • HIT: transient ⇓FMD, ⇑bioavailability of NO after HIT session • Normalization of FMD within 24 h, but ⇑NO levels in athletes after 24 h • Inverse relationship between resting vessel diameter before exercise and FMD |
<0.02 <0.05 <0.05 |
⇓HIT |
| Rakobowchuk et al.23 | • HIT + MICE: ⇑Popliteal artery distensibility, ⇑absolute FMD in popliteal artery, ⇑relative FMD • Changes in carotid artery distensibility = N.S |
<0.05 0.06 0.05 |
HIT = MICE |
| Munk et al.24 | • HIT: ⇑FMD (HIT >>controls) | 0.01 | HIT > controls |
| Hermann et al.25 | • HIT: ⇑FMD (HIT >>controls) | 0.024 | HIT > controls |
| Moholdt et al.26 | • HIT + MICE: ⇑FMD • 0 difference between HIT and MICE |
N.S. | HIT = MICE |
| Currie et al.27 | • HIT + MICE: ⇑Brachial FMD | <0.05 | HIT = MICE |
| Rakobowchuk et al.28 | • Heavy and moderate metabolic stress intervals: FMD-change = 0, ⇑low-flow-mediated constriction • Change in EPC = 0a |
0.33 <0.048 |
⇓HIT |
| Morikawa et al.29 | • HIT: ⇑FMD | <0.001 | ⇑HIT |
| Guiraud et al.30 | • HIT + MICE: 0 change in EMP, nitrites and nitrates • HIT + MICE: inverse relationship between [EMP] before and following exercise |
N.S. <0.001 |
⇓HIT, MICE |
| Isaksen et al.31 | • HIT: ⇑Brachial artery FMD • Controls: ⇓Brachial artery FMD |
0.019 | HIT > controls |
| Angadi et al.32 | • HIT + MICE: change FMD = 0 | N.S. | ⇓HIT, MICE |
| Wahl et al.33 | • HIT-A and HIT-B: ⇑VEGF, ⇑Hepatocyte growth factor • HVT: no change • ⇓Migration inhibitory factor at 60 and 180 min after exercise • All three interventions: ⇓EMP |
<0.004 and <0.006 <0.001 and 0.01 <0.0001 |
HIT = MICE |
| Conraads et al.34 | • HIT + MICE: ⇑FMD • Baseline FMD inversely correlated to FMD change after intervention |
<0.001 <0.001 |
HIT = MICE |
| Dall et al.35 | • HIT + MICE: change in RHI = 0 • No statistical difference between groups |
N.S. | ⇓HIT, MICE |
| Van Craenenbroeck et al.36 | • HIT + MICE: change in numbers of EPC and EMP = 0 • Baseline EMP was related to the magnitude of change in FMD following exercise, however |
N.S
0.001 |
⇓HIT, MICE |
HIT: high-intensity training; MICE: moderate-intensity continuous exercise; FMD: flow-mediated dilatation; N.S.: not significant; <: better than; ⇑/⇓: positive effect/no change; [EMP]: concentration of EMP; EMP: endothelial microparticle; EPC: endothelial progenitor cells; VEGF: vascular endothelial growth factor; HVT: high-volume training; RHI: reactive hyperemia index.
Except in two participants.
Figure 2.
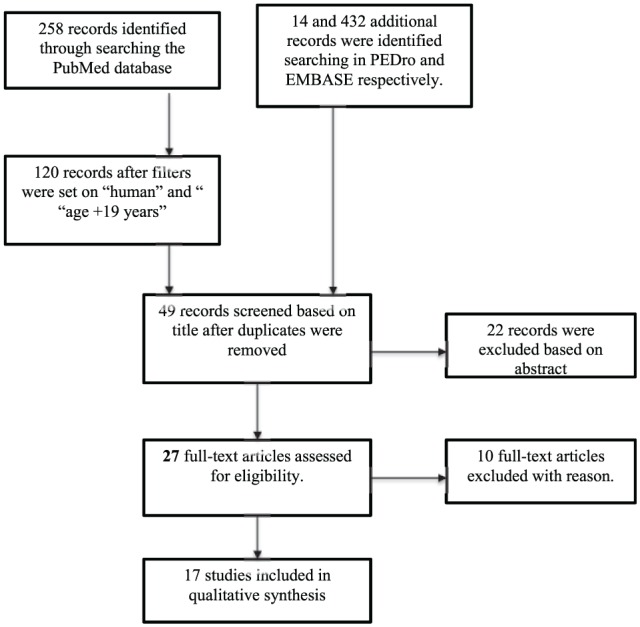
Flow diagram of selection process.
Results
Study selection
The search yielded 704 records (i.e. articles, abstracts, research protocols etc) (PubMed: 258 records; EMBASE: 432 records; PEDro: 14 records). In PubMed, filters were subsequently set to “human” and “age +19 years” reducing number of records to 120. Following setting of filters and removing duplicates, 49 studies were screened based on title. Of these, 22 studies were excluded, leaving 27 full-text for assessment. Of the 27 studies, 23 were retrieved from the PubMed database, whereas 4 articles were found using PEDro19,32 and EMBASE,35,36 respectively. In total, 10 studies were excluded after reading full-text articles. Causes of exclusion included the following: VO2max as outcome, exercise with only moderate intensity, not including the population of interest and inappropriate definition of HIT.
Study characteristics
An overview of study characteristics is given in Table 2. In total, 17 studies fulfilled the inclusion criteria. Publication year ranged from year 2007 to 2015. One study in angina patients used a HR at 75%–85% of maximum.29 This was accepted as HIT since the HR of approximately 85% was to consider as their maximum capacity. Most studies (8 of 17) were randomized controlled studies (RCT), when considering the two studies from the SAINTEX-CAD study in 2015 as one. Four studies were non-randomized trials, three within-subject repeated-measures designs and only one was a controlled study. In total, 9 of the 17 studies included were blinded.
Patient characteristics
The majority of the studies (12 of 17) included cardiovascular patients with stable CAD, HF, heart transplant recipients or patients implanted with an implantable cardioverter defibrillator (ICD). In all studies, male subjects outnumbered female subjects. Two separate studies originated from the SAINTEX-CAD study but focused on different outcome measures and were analyzed individually.34,36 The remaining five studies investigated healthy untrained subjects and/or athletes. There were no studies examining HIT and endothelial function in stroke patients or other cerebrovascular patients.
Intervention characteristics
All exercise sessions were initiated by approximately 10 min warm-up and finished with 10–15 min cool-down. Four parameters varied between interventions:
Intensity of exercise;
Duration of intervals;
Mode of recovery (active/passive);
Duration of exercise session and recovery between intervals.
The most frequently used HIT protocol (5 out of 17 studies) was 4 × 4 min of exercise with 3-min active recovery between intervals. This was first described in the study by Wisløff et al.21 One study used a protocol with 4 × 3 min of exercise,29 and one study used alternating duration of exercise of 4, 2 or 1 min.35 Longer duration of exercise sessions was seen in two studies, one using 5 × 5 min intervals22 and one with a single 20-min interval.20 The remaining seven studies chose a HIT protocol with short-duration exercise ranging from 10 to 30 s. In general, the duration of HIT sessions was 30–40 min including warm-up and cool-down, while the duration of MICE sessions was 40–60 min.
All training sessions were supervised and performed on either a treadmill, as staircase running or on a bicycle ergometer. Number of sessions per week varied between two and seven times per week. The majority of studies (10 out of 17) had protocols with two to three exercise sessions per week.
The studies either investigated the acute effect on endothelial function of a single bout of high- or moderate-intensity exercise (6 studies) or the long-term effect of regular HIT, compared to regular moderate-intensity exercise or usual care (11 studies) with duration varying from 4 to 52 weeks. The majority of these studies (10 studies) took place over a period of 4–12 weeks.
Characteristics of outcome measurements
An overview of outcome measurements and results is given in Table 3. The primary physiological outcome measure for endothelial function was FMD measured in the brachial artery or the carotid artery, which was assessed in 14 studies. One study assessed FMD in the popliteal artery. The study by Dall et al.35 used RHI measured in the fingertip before and after occlusion of the upper arm as the endothelial function outcome measure. The most common biomarker of endothelial function investigated was NO bioavailability. Rakobowchuk et al.28 included measurement of EPCs, Guiraud et al.30 used EMPs as a biomarker and Van Craenenbroeck et al.36 included both EPC and EMP. Furthermore, Wahl et al.33 also included each of VEGF, hepatocyte growth factor and migration inhibitory factor as outcome measures for endothelial function and pro-angiogenic status, in addition to EMP. Serum NO was reported in two studies.20,22
Other outcome measures relating to endothelial function—pro-inflammatory markers, improvement of aerobic capacity, VO2max,22–25,28,30,31,32,34 or “quality of life”21,34,35—are not described in this review as they do not directly monitor endothelial function.
Impact of HIT on endothelial function
Of the 17 studies included in this review, 6 studies showed no change in endothelial function related to exercise, whereas the remaining 11 studies showed improvement of endothelial function following either moderate- or high-intensity exercise. Table 3 shows an overview of the results. No studies reported adverse effects or showed any evidence of damage to vessel wall or negative effect on endothelial function after exercise—regardless of intensity.
Five studies included healthy subjects: either untrained or athletes. All these studies found HIT to be safe, but results on endothelial function varied. One study examining serum NOx products (nitrate and nitrite) before and after exercise found no significant difference.20 In the study by Rognmo et al.,22 FMD measured 1 h after HIT was significantly reduced in both athletes and healthy controls, reaching baseline level within 24 h. In contrast, the bioavailability of NO and antioxidant-status improved 1 h after exercise and was normalized within 24 h—though slower in the athletes.22 Rakobowchuk et al.28 also found FMD to remain unchanged by training. In addition, EPC levels did increase transiently in a few participants performing heavy metabolic stress intervals; in general, though, there were no findings to indicate an increase in the number of EPCs after exercise.
The two remaining studies including healthy subjects found HIT to be equal to MICE concerning changes in FMD and EMP, and HIT was suggested to be superior in promoting pro-angiogenic conditions.23,33 All studies concluded that future research needs to investigate HIT in other populations, including the long-term impact of HIT intervention.
Acute response
Early initiation of training post-stroke is often recommended; hence, this review included studies examining the acute effect of exercise on endothelial function (i.e. exercise for 0 weeks). This was examined in six studies; three within-subject repeated-measures design27,30,33 and three non-randomized trials.22,26,29 Three studies used healthy subjects31,32,35 and three studies examined CAD patients.28,30,33 Three of the studies found no acute effect of HIT on endothelial function measured by FMD serum nitrate or nitrite or EMP,20,22,30 while the remaining three studies found improvement in FMD27,29 and reduced number of EMP33 after a single session27 or three successive sessions29 of HIT.
Longitudinal studies
Longer duration of HIT intervention was examined in 11 studies; 9 RCTs,21,23–26,32,34–36 1 non-randomized trial31 and 1 controlled study.28 Duration of training protocol varied between 4,32 623,28 and 8 weeks25 up to 1221,26,31,34–36 and 24 weeks.24
In total, 10 of the 11 studies compared HIT to either MICE (7 studies) or to controls (3 studies) receiving standard advice on physical activity. One study compared each of HIT, MICE and controls.21 FMD and/or the chosen biomarkers were measured at baseline and at the end of the intervention program. In the studies where exercise intervention in hospital was followed by supervised home training, measurements were carried out both when participants finished in-hospital intervention and at the end of the training program.
The three studies comparing HIT to controls24,25,31 found HIT to be superior to controls on improvement of endothelial function measured by FMD.
In the remaining studies, examining the long-term effect of HIT compared to MICE and/or controls, results varied. Some studies found HIT to be equal to MICE in the improvement of endothelial function measured by FMD and NO bioavailability23,26,34 or by decrease in EMP.33 One study found improvement of FMD to be greater in the HIT group compared to MICE and controls, concluding that intensity in exercise is an important factor of endothelial function.21 The remaining longitudinal studies found no change in endothelial function after weeks in either mode of exercise.32,35,36
With regard to the duration of intervals, there seems to be no difference between HIT protocol and results. No change in endothelial function was reported in studies with short-interval HIT protocol28,30 as well as with long duration of intervals.22,32,35,36 The remaining studies that found HIT to be equal to MICE, or superior to controls, showed large variations in their exercise protocols regarding duration of intervals, type of recovery between intervals and duration of training intervention.
Positive results were seen across different patient populations—improvement of endothelial function was seen in CAD and HF patients as well as heart transplant recipients and patients with an ICD unit.
Discussion and conclusion
This systematic review retrieved 17 studies, all of which found HIT to be safe, time-efficient and well-tolerated by healthy subjects as well as patients with cardiovascular disease. The effect of both a single bout of HIT and interventions of longer duration was studied. The majority of all studies found HIT to be equal to MICE or usual care with regard to the improvement of endothelial function. The improvements were seen regardless of interval mode, patient population and duration of intervention—with exception of cerebrovascular patients of which this review found no studies.
Limitations
There are limitations in several of the studies included in this review, in particular concerning selection and characterization of the participants. Few studies specify their inclusion criteria of participants or detailed information about physical and physiological status.22,27 Likewise, prior exercise habits, comorbidities or previous participation in rehabilitation programs were not presented.30 One could speculate that a possible cofounder could be that those motivated and physically fit for HIT are exclusively selected for the trials, reflecting a non-generalizable minority of patients. Of note, there is a general, lack of information on whether the participants were provided—or not—with additional advice concerning diet, smoking habits, daily physical activity and so on. Regarding choice of exercise protocol, none of studies explained their choice of HIT mode or duration of intervals other than by referring to previous protocols. This is reflected in the great variation between studies, making it difficult to compare results in a proper meta-analysis. Also, the outcome measurement protocols have limitations as FMD is observer dependent. Furthermore, the lack of consensus on a protocol of measurement of EMPs and EPCs contributes to significant variability between research centers.37 It is therefore possible that assays for monitoring circulating biomarkers need to be further developed in order to be a valid measurement of changes in endothelial function.
Only 9 of the 17 selected studies were blinded when evaluating outcome measures (see Table 2), including the 2 studies based on “the SAINTEX-CAD study” from 2015. Of the nine studies, six concluded HIT to be equal to or better than MICE or control,21,24–26,31,32,34 while three found MICE to be superior to HIT or no significant effect by either of the two training modalities regarding an effect on endothelial function.32,35,36
Another major limitation is the relatively small number of included studies in this review. However, this does emphasize the need for further research within this subject.
Of the studies included, sample sizes of the studies were generally small. The number of participants in the 17 studies included in this review adds up to 79 healthy adults and 529 cardiovascular patients, a total sum of only 608 participants. The RCTs included only approximately 20–40 participants in total, as previously discussed.19 Studies with a larger population size and a more well-defined inclusion criteria and background of participants are better placed to verify the positive impact of HIT.
Since both significant and non-significant results in favor of both HIT and MICE were presented in the current studies, it is reasonable to assume that there are no concerns of publication bias in this review.
The impact of HIT on endothelial function
Not all studies support the finding that HIT improves endothelial function: Yamamoto et al.20 did not find a significant change in serum nitrate or nitrite, but only in serum concentration of NO; thus, nitrate or nitrite appears be a non-useful biomarker of endothelial function.3 Angadi et al.,32 examining patients with HF with preserved ejection fraction, found no change of FMD after exercise, explaining this by too short a training protocol or the fact that baseline FMD was not impaired in the participants. Dall et al.,35 examining heart transplant recipients, also found no change of RHI after 12 weeks of exercise which could be explained by RHI, which focus on small vessels, not being as sensitive as FMD measurements which mainly focus on large arteries. Also suggested is a detrimental effect of the immunotherapy used in this patient group on the vascular function.
The shortening of recovery time enhances the intensity of the exercise session, and passive recovery allows a greater number of interval session repetitions.14 Hence, multiple combinations of HIT protocols are available, which are reflected in the greater variety of HIT modes in the selected studies. In addition, there is general lack of agreement on when to initiate HIT in rehabilitation. Future studies examining different HIT protocols and active versus passive recovery are needed to examine the role and importance of interval duration and recovery mode in different populations. Furthermore, longitudinal studies are needed to investigate optimal initiation time and duration of exercise programs in rehabilitation. Additionally, results may differ when considering only immediate effects and not including long-term effects; both early acquisition of changes and late phase recovery should be addressed.
HIT and cerebrovascular patients
This systematic review aimed to investigate the effect of HIT on endothelial dysfunction in cardiovascular as well as cerebrovascular patients. However, no studies fulfilled the inclusion criteria and investigated the impact of HIT on endothelial function in stroke rehabilitation. So far, seven studies have investigated HIT in stroke rehabilitation38–44—all with positive results. Nevertheless, the HIT protocols used varied quite a lot and used gait speed and VO2max as outcome measure. One study has investigated endothelial function and MICE in sub-acute stroke.21 In this study, exercise intensity was at 50%–70% of maximum HR with duration of 30 min. After 8 weeks of intervention, FMD and bilateral brachial artery diameter improved significantly in all subjects, though this effect was not repeatable at the 1-month follow-up.
This emphasizes the need for research on the effect of HIT on endothelial function in stroke patients. Stroke rehabilitation poses additional issues, including unsteady ambulation, cognitive impairment, pain, memory deficits and severely reduced motor function. This greatly narrows the target group of early HIT intervention in stroke rehabilitation. Patients suffering from lacunar stroke may be more capable of participating in HIT exercise in an early phase of rehabilitation since this group of patients suffers from less severe neurological deficiencies with relatively preserved motor function. Of note, individuals with lacunar infarction have a significantly impaired brachial FMD compared to other stroke subtypes11 making exercise intervention, and more knowledge of its impact on endothelial function, highly relevant. It is possible that other patients with large-vessel stroke subtypes could gain positive effects from HIT after general rehabilitation and regaining of motor function.
Application of HIT in a clinical setting
This review found HIT safe and the majority of the selected studies found it equal to MICE in cardiac rehabilitation. Three studies were excluded as they did not fulfill the selected definition of HIT,45–47 of note, no clear definition yet exists on HIT in a clinical setting. One borderline study29 was accepted for analysis; it may be questioned whether the definition used in healthy persons (i.e. 85% of maximum HR or VO2max) is indeed applicable to patients with motor deficits, or whether surrogate markers such as shortness of breath should be applied instead.
In a recent study, patients actually preferred HIT to MICE, which makes patients more likely to adhere to newly introduced or continued training where HIT is applied.14 Such improved compliances are important given that a patient’s adherence to exercise after discharge is a well-known challenge.48 A recent study shows that any improvement of endothelial function may gradually diminish if not maintained on a regular basis.35 With this in mind, HIT might be a more conceivable and feasible exercise strategy in the rehabilitation of stroke patients. In this respect, the goal in rehabilitation must be adherence to exercise post-discharge in order to improve endothelial function, reduce risk factors and prevent stroke recurrent. In general, it is important that training protocol fulfills the recommended guidelines for training and testing, for example, the standards from the American Heart Association.49
Conclusion
In conclusion, HIT is safe and highly relevant in patients with cardiovascular disease and may also be so in patients with cerebrovascular disease—though current research primarily involves the former patient population. In the majority of studies fulfilling the inclusion criteria, HIT shows positive impact on endothelial function when measured by FMD and circulating biomarkers. This review found no studies on the effect of HIT on endothelial function in cerebrovascular patients, though three studies showed feasibility of HIT training in stroke but did not investigate endothelial function. Future research must investigate the effect of HIT on endothelial function in cerebrovascular patients and also determine whether the current definition of HIT is applicable in a clinical setting with physically impaired patients.
Acknowledgments
The author(s) are grateful to Musa Büyükuslu for contributing to the design of illustrations and Steve Mackison for revision of language.
Footnotes
Declaration of conflicting interests: R.S.K. was funded by grant from Herlev Hospital Research Council.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Sacco RL, Wolf PA, Kannel WB, et al. Survival and recurrence following stroke. The Framingham study. Stroke 1982; 13(3): 290–295. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt W, Endres M, Dimeo F, et al. Train the vessel, gain the brain: physical activity and vessel function and the impact on stroke prevention and outcome in cerebrovascular disease. Cerebrovasc Dis 2013; 35(4): 303–312. [DOI] [PubMed] [Google Scholar]
- 3. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007; 115(10): 1285–1295. [DOI] [PubMed] [Google Scholar]
- 4. Vita JA. Endothelial function. Circulation 2011; 124(25): e906–e912. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 2004; 109(5): 613–619. [DOI] [PubMed] [Google Scholar]
- 6. Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 2003; 107(25): 3152–3158. [DOI] [PubMed] [Google Scholar]
- 7. De Biase C, De Rosa R, Luciano R, et al. Effects of physical activity on endothelial progenitor cells (EPCs). Front Physiol 2013; 4: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011; 300(1): H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Billinger SA, Mattlage AE, Ashenden AL, et al. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther 2012; 36(4): 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stenborg A, Terent A, Lind L. Endothelium-dependent vasodilatation in forearm is impaired in stroke patients. J Intern Med 2006; 259(6): 569–575. [DOI] [PubMed] [Google Scholar]
- 11. Chen PL, Wang PY, Sheu WH, et al. Changes of brachial flow-mediated vasodilation in different ischemic stroke subtypes. Neurology 2006; 67(6): 1056–1058. [DOI] [PubMed] [Google Scholar]
- 12. Moerland M, Kales AJ, Schrier L, et al. Evaluation of the EndoPAT as a tool to assess endothelial function. Int J Vasc Med 2012; 2012: 904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yong PJ, Koh CH, Shim WS. Endothelial microparticles: missing link in endothelial dysfunction? Eur J Prev Cardiol 2013; 20(3): 496–512. [DOI] [PubMed] [Google Scholar]
- 14. Guiraud T, Nigam A, Gremeaux V, et al. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7): 587–605. [DOI] [PubMed] [Google Scholar]
- 15. Lennon O, Carey A, Gaffney N, et al. A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil 2008; 22(2): 125–133. [DOI] [PubMed] [Google Scholar]
- 16. Tang A, Marzolini S, Oh P, et al. Feasibility and effects of adapted cardiac rehabilitation after stroke: a prospective trial. BMC Neurol 2010; 10: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011; 377(9778): 1693–1702. [DOI] [PubMed] [Google Scholar]
- 18. Hussain SR, Macaluso A, Pearson SJ. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol Rev 2016; 24: 273–281. [DOI] [PubMed] [Google Scholar]
- 19. Cornish AK, Broadbent S, Cheema BS. Interval training for patients with coronary artery disease: a systematic review. Eur J Appl Physiol 2011; 111(4): 579–589. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto K, Kondo T, Kimata A, et al. Lack of effect of aerobic physical exercise on endothelium-derived nitric oxide concentrations in healthy young subjects. Nagoya J Med Sci 2007; 69(3–4): 167–172. [PubMed] [Google Scholar]
- 21. Wisløff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007; 115(24): 3086–3094. [DOI] [PubMed] [Google Scholar]
- 22. Rognmo O, Bjornstad TH, Kahrs C, et al. Endothelial function in highly endurance-trained men: effects of acute exercise. J Strength Cond Res 2008; 22(2): 535–542. [DOI] [PubMed] [Google Scholar]
- 23. Rakobowchuk M, Tanguay S, Burgomaster KA, et al. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol 2008; 295(1): R236–R242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Munk PS, Staal EM, Butt N, et al. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation. A randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J 2009; 158(5): 734–741. [DOI] [PubMed] [Google Scholar]
- 25. Hermann TS, Dall CH, Christensen SB, et al. Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant 2011; 11(3): 536–541. [DOI] [PubMed] [Google Scholar]
- 26. Moholdt T, Aamot IL, Granoien I, et al. Aerobic interval training increases peak oxygen uptake more than usual care exercise training in myocardial infarction patients: a randomized controlled study. Clin Rehabil 2012; 26(1): 33–44. [DOI] [PubMed] [Google Scholar]
- 27. Currie KD, McKelvie RS, Macdonald MJ. Flow-mediated dilation is acutely improved after high-intensity interval exercise. Med Sci Sports Exerc 2012; 44(11): 2057–2064. [DOI] [PubMed] [Google Scholar]
- 28. Rakobowchuk M, Harris E, Taylor A, et al. Heavy and moderate interval exercise training alters low-flow-mediated constriction but does not increase circulating progenitor cells in healthy humans. Exp Physiol 2012; 97(3): 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morikawa Y, Mizuno Y, Harada E, et al. Aerobic interval exercise training in the afternoon reduces attacks of coronary spastic angina in conjunction with improvement in endothelial function, oxidative stress, and inflammation. Coron Artery Dis 2013; 24(3): 177–182. [DOI] [PubMed] [Google Scholar]
- 30. Guiraud T, Gayda M, Juneau M, et al. A single bout of high-intensity interval exercise does not increase endothelial or platelet microparticles in stable, physically fit men with coronary heart disease. Can J Cardiol 2013; 29(10): 1285–1291. [DOI] [PubMed] [Google Scholar]
- 31. Isaksen K, Munk PS, Valborgland T, et al. Aerobic interval training in patients with heart failure and an implantable cardioverter defibrillator: a controlled study evaluating feasibility and effect. Eur J Prev Cardiol 2015; 22: 296–303. [DOI] [PubMed] [Google Scholar]
- 32. Angadi SS, Mookadam F, Lee CD, et al. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985); 2015: 119: 753–758. [DOI] [PubMed] [Google Scholar]
- 33. Wahl P, Jansen F, Achtzehn S, et al. Effects of high intensity training and high volume training on endothelial microparticles and angiogenic growth factors. PLoS ONE 2014; 9(4): e96024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conraads VM, Pattyn N, De Maeyer C, et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol 2015; 179: 203–210. [DOI] [PubMed] [Google Scholar]
- 35. Dall CH, Gustafsson F, Christensen SB, et al. Effect of moderate- versus high-intensity exercise on vascular function, biomarkers and quality of life in heart transplant recipients: a randomized, crossover trial. J Heart Lung Transplant 2015; 34(8): 1033–1041. [DOI] [PubMed] [Google Scholar]
- 36. Van Craenenbroeck EM, Frederix G, Pattyn N, et al. Effects of aerobic interval training and continuous training on cellular markers of endothelial integrity in coronary artery disease: a SAINTEX-CAD substudy. Am J Physiol Heart Circ Physiol 2015; 309(11): H1876–H1882. [DOI] [PubMed] [Google Scholar]
- 37. Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens 2012; 6(2): 85–99. [DOI] [PubMed] [Google Scholar]
- 38. Lau KW, Mak MK. Speed-dependent treadmill training is effective to improve gait and balance performance in patients with sub-acute stroke. J Rehabil Med 2011; 43(8): 709–713. [DOI] [PubMed] [Google Scholar]
- 39. Pohl M, Mehrholz J, Ritschel C, et al. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke 2002; 33(2): 553–558. [DOI] [PubMed] [Google Scholar]
- 40. Gjellesvik TI, Brurok B, Hoff J, et al. Effect of high aerobic intensity interval treadmill walking in people with chronic stroke: a pilot study with one year follow-up. Top Stroke Rehabil 2012; 19(4): 353–360. [DOI] [PubMed] [Google Scholar]
- 41. Munari D, Pedrinolla A, Smania N, et al. High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: preliminary results of a pilot randomized controlled trial. Eur J Phys Rehabil Med 2016. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42. Holleran CL, Rodriguez KS, Echauz A, et al. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J Neurol Phys Ther 2015; 39(2): 95–102. [DOI] [PubMed] [Google Scholar]
- 43. Boyne P, Dunning K, Carl D, et al. Within-session responses to high-intensity interval training in chronic stroke. Med Sci Sports Exerc 2015; 47(3): 476–484. [DOI] [PubMed] [Google Scholar]
- 44. Globas C, Becker C, Cerny J, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabil Neural Repair 2012; 26(1): 85–95. [DOI] [PubMed] [Google Scholar]
- 45. Goto C, Higashi Y, Kimura M, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 2003; 108(5): 530–535. [DOI] [PubMed] [Google Scholar]
- 46. Deljanin M, Ilic S, Lazarevic G, et al. Impact of interval versus steady state exercise on nitric oxide production in patients with left ventricular dysfunction. Acta Cardiol 2009; 64(2): 219–224. [DOI] [PubMed] [Google Scholar]
- 47. Rankovic G, Djindjic N, Rankovic-Nedin G, et al. The effects of physical training on cardiovascular parameters, lipid disorders and endothelial function. Vojnosanit Pregl 2012; 69(11): 956–960. [PubMed] [Google Scholar]
- 48. Slovinec D’Angelo ME, Pelletier LG, Reid RD, et al. The roles of self-efficacy and motivation in the prediction of short- and long-term adherence to exercise among patients with coronary heart disease. Health Psychol 2014; 33(11): 1344–1353. [DOI] [PubMed] [Google Scholar]
- 49. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013; 128(8): 873–934. [DOI] [PubMed] [Google Scholar]


