Abstract
Introduction
Patients undergoing endovascular therapy for acute ischemic stroke may require general anesthesia to undergo the procedure. At present there is little clinical evidence to guide the choice of anesthetic in this acute setting. The clinical implications of experimental studies demonstrating anesthetic neuroprotection are poorly understood. Here we evaluated the impact of anesthetic treatment on neurologic outcome in experimental stroke.
Methods
Controlled studies of anesthetics in stroke using the filament occlusion model were identified in electronic databases up to December 15, 2015. The primary outcome measures, infarct volume, and neurological deficit score were used to calculate the normalized mean difference (NMD) for each comparison. Meta-analysis of NMD values provided estimates of neuroprotection and contributions of predefined factors: study quality, the timing of treatment, and duration of ischemia.
Results
In 80 retrieved publications anesthetic treatment reduced neurological injury by 28% (95% C.I. 24-32%; P<0.0001). Internal validity was high: publication bias enhanced the effect size by 4% or less, effect size increased with study quality (P=0.0004) and ~70% of studies were adequately powered. Apart from study quality, no predefined factor influenced neuroprotection. Neuroprotection failed in animals with comorbidities. Neuroprotection by anesthetics was associated with pro-survival mechanisms.
Conclusion
Anesthetic neuroprotection is a robust finding in studies using the filament occlusion model of ischemic stroke and should be assumed to influence outcomes in studies using this model. Neuroprotection failed in females and animals with comorbidities suggesting that the results in young male animals may not reflect human stroke.
Introduction
International guidelines support the use of endovascular therapy in selected patients with acute ischemic stroke1. Due to safety concerns such as airway control or patient movement, some patients may require significant sedation or general anesthesia during interventional procedures or during supportive intensive care. Currently, there is little clinical evidence to guide anesthetic choice in the setting of acute stroke. Experimental studies in animals suggest that anesthetics are neuroprotective in the setting of focal cerebral ischemia. However, concerns have been raised that methodological shortcomings of preclinical studies leading to low internal validity (poor quality, selective publication, and small study effects) have exaggerated neuroprotective effects2,3, reducing the reliability of translation of preclinical findings to clinical treatments4. Existing reviews of anesthetic neuroprotection5–7 have not quantified the impact of internal validity on neuroprotection.
The specific aims of the meta-analysis were to quantify the impact, if any, of study quality, publication bias and the timing of anesthetic administration upon neurological outcomes in the middle cerebral artery filament occlusion model of focal ischemia in rodents. The findings could potentially provide guidance for future preclinical and clinical studies – ‘Is neuroprotection by anesthetics a robust finding, can we tell how large the effect is, and under what conditions it occurs?’. Finally, do the findings justify further evaluation in primate or human studies?
The filament model in rats and mice has been used to screen putative neuroprotective agents and the associated mechanisms of action. Investigators using the filament model have expended considerable effort to control confounding variables such as the temperature and physiological state of the animals and the adequacy of the experimental occlusion. Studies that replicate findings from rodents in other mammals, particularly primates, are uncommon, and frequently vary widely in methodology. To justify investigations in animals larger than rodents as a precursor for human studies, it would seem prudent to confirm that results from the studies published to date are not compromised by poor study quality and publication bias2,3. From an analytic perspective, our selection of the filament model in rats and mice was an attempt to reduce heterogeneity by selecting studies that share a relatively consistent set of methodologies.
The project protocol was registered and published online on the CAMARADES (Collaborative Approach to Meta-Analysis and Review of Animal data from Experimental studies) website:CAMARADES.
Materials and Methods
This systematic review identified controlled studies of the impact of anesthetics on neurologic injury following focal ischemia using the middle cerebral artery filament occlusion method in rats or mice. The primary outcome measures were infarct volume (IV) and/or neurological deficit score (NDS). Secondary outcomes included evidence of biological pathways involved in ischemic tolerance induced by anesthetics.
Search Strategy
Studies were identified by searching the electronic databases MEDLINE and EMBASE up to and including December 15, 2015, using the search strategy detailed below (Box 1). There were no language restrictions. Eligibility assessment and study quality evaluation were performed in duplicate in an unblinded, independent standardized manner by two authors (RA, AW), with a third author (DPA) mediating disagreements. Data extraction was performed in duplicate (DPA, AW). Relevant articles were selected from the overall search results by scanning the titles and abstracts of retrieved publications. Abstracts of scientific meetings from 2013 to 2015 of the International Stroke Conference, the European Stroke Conference, the Annual Meeting - Society for Technology in Anesthesia, the Annual Meeting of the Society for Neuroscience in Anesthesiology and Critical Care, the American Society of Anesthesiologists Annual Meeting, the Canadian Anesthesiologists Society Annual Meeting, the Japanese Society of Neuroanesthesia and Critical Care, the Anaesthetic Research Society Meeting were reviewed by hand. All authors reviewed and agreed with the inclusion of the full-text publications.
Box 1 Search Terms Applied in Medline and EMBASE Databases.
<stroke$.tw,kw> OR
<stroke$.ti,kw.> OR
<brain ischemia$.tw,kw.> OR
<transient ischemic attack$.tw,kw>OR
<middle cerebral artery.tw.kw>OR
<middle cerebral artery.tw.kw>OR
<cerebral infarction$.tw,kw> OR
<neuroprotective agent$.tw,kw>
AND
<propofol.mp.> OR
<ketamine.mp> OR
<sevoflurane.mp.> OR
<isoflurane.mp> OR
<etomidate.mp.> OR
<halothane.mp.>
The Medline 'field' restriction was used to isolate retracted publications and was applied July 16, 2016.
Inclusion and Exclusion Criteria
Studies were included if they reported results of controlled comparisons of the effect of anesthetic administration on primary outcome measures in rats or mice subjected to focal cerebral ischemia induced by filament occlusion of the middle cerebral artery. In addition to the search terms in Box 1, anesthetics retrieved by the search strategy and included in the analysis were: desflurane, xenon, pentobarbital, dexmedetomidine, fentanyl, remifentanil, and thiopental. Studies included temporary (60, 90, and 120 minutes) and permanent focal cerebral ischemia. We excluded studies of thrombotic or embolic focal ischemia, forebrain ischemia, global cerebral ischemia, occlusion methods requiring craniotomy and cellular/tissue models of ischemia. Studies were also excluded if the effect size of the anesthetic intervention could not be expressed as a mean and standard deviation, if no control intervention group was investigated and when the number of experimental animals could not be determined.
Quality Assessment
Methodological quality was assessed independently by two investigators using a published individual study quality checklist8.
Data Extraction
Data coding included reference identification (authors, year of publication, source), nature of animals (species/strain, age, weight, sex), anesthetic information (dose, timing of administration, dose-response design, control drug), ischemic model (timing of intervention, duration of ischemia, confirmation of ischemia by cerebral blood flow measurement, location of occlusion and infarct), and the time of outcome measurement.
Pre-defined factors that could affect neurological injury were: the quality of the study, direct confirmation of the induction of ischemia, the duration of ischemia, the timing of administration of anesthetic relative to the ischemic episode (before vs during/after), the nature of the anesthetic (if any) received by the control group (‘neuroprotective’ (isoflurane, sevoflurane, halothane, barbiturates), ‘neutral’ (α-chloralose, chloral hydrate), ‘awake’, and study anesthetic class (volatile anesthetics-halothane, isoflurane, desflurane, sevoflurane, xenon; intravenous hypnotics-barbiturates, propofol, opioids; ketamine). We assigned studies to the ‘treatment before ischemia’ category when this timing was stated in the study design and confirmed by the presence of an appropriate control group. Studies that compared outcomes with different anesthetics for the surgical procedure and the ischemic period were included with those that explicitly compared anesthetics administered during ischemia/reperfusion; these studies made up the ‘treatment during/after ischemia’ category. These variables were coded as moderators and evaluated as covariates in meta-regression models.
We performed three post hoc analyses: a meta-analysis of neuroprotection in female animals, aged animals and animals with comorbid conditions, a meta-analysis to compare remote exposures (1 or more days before the ischemic insult) to anesthetic exposures immediately preceding ischemia and a qualitative comparison of the biological pathways associated with neuroprotection induced by two structurally similar anesthetics, isoflurane, and sevoflurane.
Data Handling in Meta-Analysis
Meta-analysis and regression were performed with commercial meta-analysis software, Comprehensive Meta-analysis V3, available online at Comprehensive Meta-Analysis V3). Neurological outcomes were reported as IV and/or NDS. When the numerical values of outcomes were not reported, digital versions of the manuscripts were interrogated by extracting calibrated digitized ‘snapshots’ of figures using Engauge Digitizer 5.2 software (GitHub, San Francisco, CA, USA). Authors were contacted and data requested if data was missing or illegible figures prevented data extraction. If a response was not received or the data was unavailable, the study was excluded (2 studies). Data reported as individual values were evaluated for normality using the Shapiro-Wilk test. Data failing the Shapiro-Wilk test or reported as median values were converted to mean and standard deviation9. When applicable, composite mean and standard deviations were calculated as described in the Cochrane Handbook (Cochrane Handbook). Unless otherwise indicated, results are expressed as mean ± 95% confidence intervals (C.I.). The number of animals in a study group is represented n; the number of comparisons contributing to a meta-analysis is represented by k.
Statistical Analysis
The contribution of study quality to effect size was evaluated with meta-regression; an inverse relationship between study quality and effect implies that results from poor quality studies have exaggerated the effect size estimate 8. Bias introduced by studies of low power was evaluated by calculating the power of each study to detect the summary effect in the meta-regression analysis3.
Evidence for publication bias was sought by constructing funnel plots, performing Orwin’s fail-safe N test10, and determining Egger’s regression intercept11. Quantification of the impact of publication bias on summary effects was estimated with “trim and fill”12.
The summary effect resulting from the meta-analysis is a weighted average of the treatment effects in the included studies. For each reported outcome, we calculated the normalized mean difference (NMD) by dividing the mean difference in outcome between the control and treated groups by the mean outcome value in the control group13. This index of effect size enables outcomes that are measured on different scales (for example, infarct volume and neurological deficit score) to be combined in the same meta-analysis. The NMD has the additional advantage that the result is expressed in terms of % reduction in neurological injury, which may be easier to grasp than another common index of effect size, the standardized mean difference. Values for NMD, 95% confidence limits and the number of animals in the treated and control groups were entered into the meta-analysis software as continuous variables under the category of raw difference in means and confidence intervals for independent groups. For data that were extracted from comparisons of many treatment groups with one control group, the number of animals in the control group was divided by the number of study comparisons to provide a more realistic estimate of the confidence intervals in the measurement13. This Bonferroni correction was applied to 5/93 calculations. For data extracted from dose-response studies, a dose suitable for general anesthesia was selected to be representative of the entire study. (Other doses of anesthetic commonly evaluated were suitable for sedation or for maximal suppression of electroencephalographic activity.) Five studies compared outcomes in normal control animals to animals with comorbid conditions. The control animals were included in the ‘all-included-studies’ meta-analysis; subgroup analysis was conducted to evaluate the impact of comorbidities.
Meta-regression analysis was performed using a random effects model with the restricted maximum likelihood method for estimating variance between studies and the Knapp-Hartung method14 for significance testing. The summary effect and test of the effect size were obtained by performing the meta-regression without covariates. The predefined factors described above were used as incremental covariates in the meta-regression models. Each regression model was evaluated by testing that all coefficients (except the intercept) were zero, testing that the unexplained variance was zero (goodness of fit), estimating the total between-study variance (τ2) and the proportion of between-study variance explained by the model (R2 analog). The order of covariates in the model was adjusted to maximize the reduction in between-study variance.
Variation in effect sizes or heterogeneity was evaluated with the ratio of the observed variation to the within-study error (Q), the estimated variance of the true effect size (τ2) and the proportion of the observed variance that reflects real differences in effect size (I2). The range of true effects was estimated to be the summary effect ± 2τ.
Results
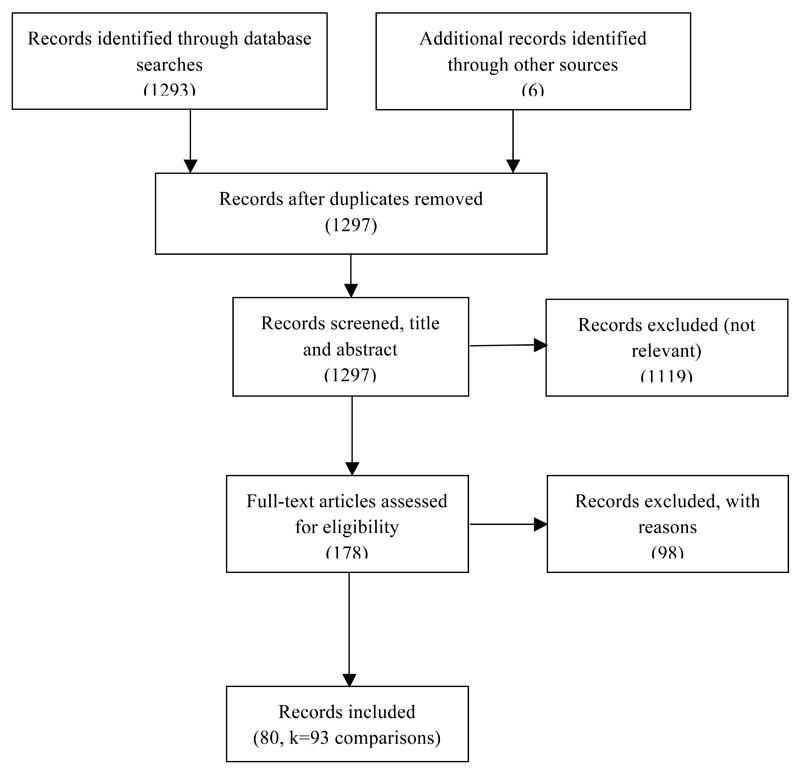
Our search strategy is shown in Figure 1. The characteristics of the individual studies including species, strain, sex, ischemia duration, anesthetics for treated and control groups and outcome measures are summarized (see Table, Supplemental Digital Content 1). Reasons for exclusion of studies identified in the search are shown (see Table, Supplemental Digital Content 2), and the reasons for excluding specific studies commonly found in narrative reviews are provided (see Table, Supplemental Digital Content 3). There were no retracted publications in this group as of July 17, 2016. The search of the Proquest® Global Thesis databank with search terms “focal cerebral ischemia AND anesthetic preconditioning OR postconditioning AND mouse OR rat” failed to identify any relevant dissertations. With this strategy, we retrieved 80 publications, which included 93 comparisons of the primary outcomes between treated (n=1040) and control (n=1053) animals. Within these 93 comparisons, 58 reported both IV and NDS, 30 IV only and 5 NDS only. When experiments examining molecular mechanisms are included, the total number of experimental animals in the included studies was 6982 (mice: 1389, rats: 5598) where rats were the subject in the majority of comparisons (70/93).
Figure 1.
Results of the Search Strategy (There were 93 comparisons between treated and control animals nested in the 80 included publications)
The majority (71/93) of the comparisons involved a study design in which molecular mechanisms for neuroprotection were the main purpose of the investigation. This study design can be likened to an ‘if A: then B’ statement, in which the investigation of mechanisms (B) was contingent on demonstration of a change in histologic or behavioral outcome (A).
The range of evidence in the included studies8 is shown in Table 1. We retrieved only one study that directly compared neuroprotection between the sexes15. No studies evaluating the effects of treatment before ischemia in animals with comorbid conditions were retrieved. The overall methodological quality is summarized in Table 2, where on the ten items, the median score was 7, 1st, 3rd quartiles: 6,8, range 2 to 9. Studies were compliant with the majority of the items; exceptions were ‘blinded allocation to ischemia’ (12%), sample size calculation/pilot study data (35%) and ‘Conflict of Interest Statement’ (33%).
Table 1.
Range of Evidence in Included Studies (80 manuscripts)
| Experimental Criterion | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment before ischemia | Number of Studies by Criterion | |||||||||
| Inhaled agents | ||||||||||
| Sevoflurane | 12 | 12 | yes | 1/11 | 0 | 0 | 12 | 3 | 10 | 2 |
| Isoflurane | 15 | 16 | yes | 2/13 | 0 | 0 | 15 | 2 | 14 | 1 |
| Desflurane | 1 | 1 | no | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Intravenous agents | ||||||||||
| Propofol | 2 | 2 | yes | 1/2 | 0 | 0 | 1 | 0 | 3 | 0 |
| Remifentanil | 1 | 1 | no | 0/1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Dexmedetomidine | 1 | 0 | no | 0/1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Treatment during/after ischemia | ||||||||||
| Inhaled agents | ||||||||||
| Sevoflurane | 6 | 8 | yes | 1/5 | 3 | 3 | 8 | 2 | 7 | 1 |
| Isoflurane | 16 | 26 | yes | 2/23 | 2 | 3 | 26 | 2 | 19 | 7 |
| Desflurane | 2 | 2 | yes | 0/2 | 0 | 0 | 2 | 2 | 2 | 0 |
| Xenon | 2 | 2 | yes | 0/2 | 0 | 0 | 2 | 2 | 2 | 0 |
| Intravenous agents | ||||||||||
| Propofol | 11 | 12 | yes | 3/9 | 0 | 0 | 8 | 2 | 12 | 0 |
| Ketamine | 1 | 2 | yes | 0/2 | 0 | 1 | 1 | 0 | 1 | 0 |
| Barbiturates | 2 | 2 | yes | 0/2 | 0 | 0 | 2 | 2 | 2 | 0 |
Assessment of functional (1) or histological (2) outcome; replicated in two or more laboratories (3), tested with permanent and temporary occlusion, (4) tested in females and males (5), tested in animals with comorbidities (diabetes, hypertension) (6) , clinically appropriate route of administration(7), dose-response relationship investigated (8), assessment in acute phase(9), assessment in chronic phase (10). Range of Evidence from Sena et al.8
Table 2.
Quality Checklist8 for Included Studies (80 manuscripts)
| Quality Category | Number of Studies with Quality (% of total) |
|---|---|
| (1) Monitoring of blood pressure and blood gases | 62 (77) |
| (2) Peer-reviewed publication | 80 (100) |
| (3) Control of temperature | 79(98) |
| (4) Random allocation to treatment | 59 (73) |
| (5) Blinded induction of ischemia | 10 (12) |
| (6) Blinded assessment of (functional) outcome | 63 (78) |
| (7) Non-neuroprotective anesthetic | 58 (72) |
| (8) Pilot data, sample size calculation | 28(35) |
| (9) Animal welfare compliance | 76(96) |
| (10) Conflict of interest statement | 27(33) |
Methods used to evaluate the primary outcome measures evolved over the time during which the studies were conducted. There were 63 comparisons that reported NDS values. Six methods were used in 50 of the studies: Rogers et al16 (21 studies), Garcia et al. 1995 17(11 studies), Longa et al. 18(10 studies), Bederson et al.19(4 studies), Bonilla et al.20, Hara et al.21 (2 studies each). Eighty-eight comparisons involving infarct volume were reported, one by magnetic resonance imaging and 87 by tissue staining techniques: 2,3,5-triphenyltetrazolium chloride (TTC) (65 studies), hematoxylin and eosin (H&E) (12 studies), Nissl (5 studies), cresyl violet (3 studies), immunohistochemical stains (2 studies).
Reduction of Neurologic Injury by Anesthetics
The average IV in the control animals, corrected for species and strain of rodent was 39 % (35 to 42%) of the contralateral (non-ischemic) hemisphere. This represents a severe injury, approximately 35% larger than that previously reported4. There were 58 comparisons that reported both IV and NDS. With treatment, the changes in NDS (NMD between treated and control) correlated with the changes in IV (Pearson product-moment correlation coefficient: 0.6, P< 0.0001, k=58).
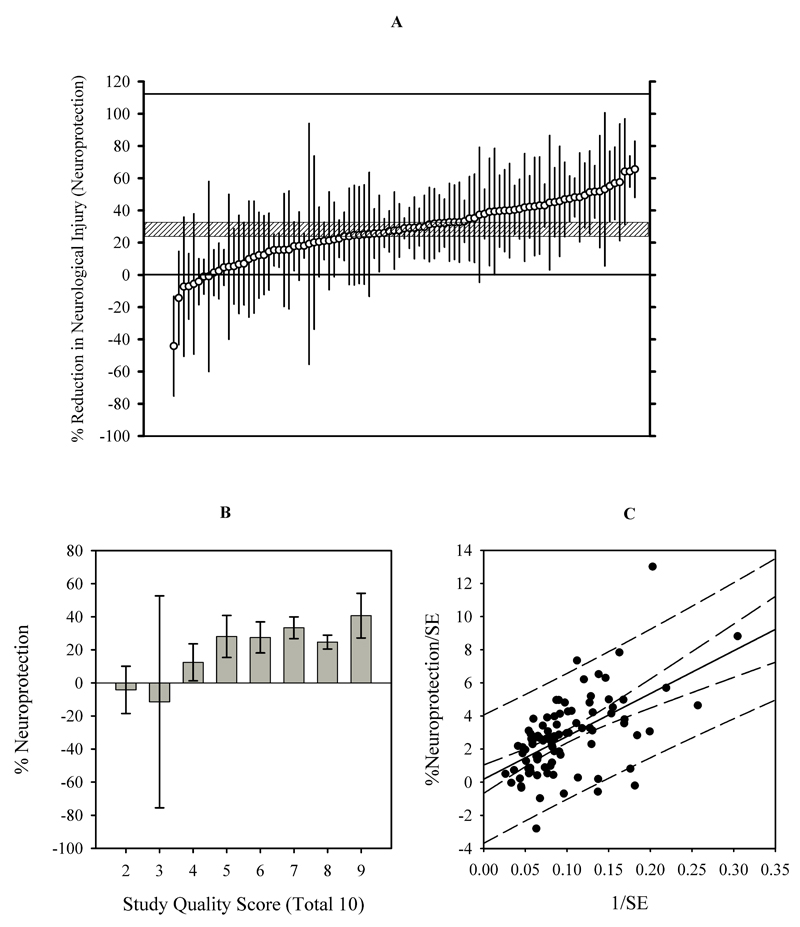
Anesthetics reduced neurological injury (IV and NDS combined) by 28% (24 to 32, Z= 14, P<0.0001) (Figure 2A) with considerable heterogeneity of the estimates (Q= 330, I2=72%, τ=15). Of the pre-defined moderators that apply to all included studies, only study quality was a significant covariate for the effect size (coefficient = 5, 2 to 7; P=0.0003) and accounted for 18% of the between-studies variance. None of the other pre-defined factors significantly improved the regression model (Table 3). In fact, the decrease in R2 with the addition of other moderators indicates a decrease in variance explained by the model, likely because of interaction with ‘study quality’. When analysis was restricted to comparisons from studies with a quality score > 5 (k=77), anesthetics reduced neurological injury by 30% (26 to 34%) (Z=15, P<0.0001); τ = 14, giving an estimated range of true effects from 3% to 58% (Q=250, P<0.0001; I2=70%).
Figure 2.
A. Neuroprotective effects of all included comparisons (k=93) showing individual normalized mean differences and 95% C.I. The summary effect was 28% (24 to 32%) reduction in neurological injury. B. Neuroprotective effects were greater in high quality studies (meta-regression coefficient = 5, (C.I. 2 to 7, 1-sided P value =0.0003). Bars represent the summary effect ± 95% C.I. C. Egger’s regression intercept was 0.8 (C.I. 0.4 to 2.0%, P=0.2030), indicating a failure to detect bias. Long-and short-dash curves represent the 95% confidence and prediction intervals respectively.
Table 3.
Incremental evaluation of contributions of covariates to regression models of all comparisons (k=93), Random Effects (Restricted Maximum Likelihood), Knapp Hartung, Normalized Mean Difference
| Current Model | Test of Model (a) | Goodness of fit (b) | Change from prior(c,d) | Test of change (c) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Set | Covariate | Tau2 | R2 | F | df1 | df2 | P-value | Q | df | P-value | Tau2 | R2 | F | df1 | df2 | P-value |
| Intercept | 213 | 0 | ||||||||||||||
| Study Quality | 175 | 0.18 | 13 | 1 | 91 | 0.0005 | 296 | 91 | <0.0001 | -38 | 0.18 | 13 | 1 | 91 | 0.0005 | |
| Timing of Treatment | 174 | 0.18 | 7 | 2 | 90 | 0.0019 | 289 | 90 | <0.0001 | -0.8 | 0 | 0.6 | 1 | 90 | 0.4568 | |
| Duration of Ischemia* | 60 | 178 | 0.16 | 4 | 3 | 89 | 0.0060 | 281 | 89 | <0.0001 | 4 | 0 | 0.0 | 1 | 89 | 0.8494 |
| 90 | 179 | 0.16 | 3 | 4 | 88 | 0.0122 | 279 | 88 | <0.0001 | 0.6 | 0 | 0.4 | 1 | 88 | 0.5380 | |
| 120 | 182 | 0.14 | 3 | 5 | 87 | 0.0244 | 278 | 87 | <0.0001 | 3 | 0 | 0.2 | 1 | 87 | 0.6791 | |
This is a tabulation of statistics from a series of separate models. The first row is a model with no covariates, the second row has one covariate, and so on. The results evaluate the impact of each covariate when prior covariates in the model are held constant. (a) Simultaneous test that all coefficients up to and including the current row are zero. (b) Test that all with covariates up to and including the current row in the model, the residual is zero. (c) Change from the prior row to the current due to the added covariate. (d) The row-to-row increase in Tau2 and decrease in R2 reflects sampling error. The reference groups for timing of intervention and duration of ischemia were treatment during/afer ischemia and permanent ischemia respectively. *When considered as a set, transient ischemia was not a significant covariate in the model (F=0.19, df1=3, df2=87, p=0.8996). Table format adapted from Comprehensive Meta-analysis®. * - minutes. Abbreviations: df – degrees of freedom, k – number of comparisons between treatment and control; Timing of Treatment: before vs during/after ischemia.
The influence of confirmation of ischemia with laser Doppler flowmetry on effect size was one of the covariates evaluated by meta-regression of results from studies of transient ischemia. The results for this model (Study Quality, Doppler flowmetry, k=80 studies) show that while study quality was a significant covariate (P=0.0004), confirmation of ischemia was not (P=0.0930).
Meta-regression of results from studies of anesthetics administered before ischemia (k=41) showed that anesthetic exposure one or more days before ischemia (k=17) provided greater neuroprotection than exposure in the immediate pre-ischemia period (3h or less before ischemia) (regression coefficient = 13, C.I. 2 to 25, P=0.0099).
Risk of bias in individual studies – Quality and Small Studies Effects
Estimates of neuroprotection increased with study quality (Figure 2B), indicating that results from poor quality studies did not exaggerate the summary estimates. Among the included studies, the average power to detect a 30% difference in outcome measures between the treated and control groups was 0.70 (0.67 to 0.80) and 0.70 (0.64 to 0.77) for IV and NDS respectively. Using a meta-regression model that included effect size and study quality, the sample size of the individual studies was not a significant covariate (regression coefficient= 0.2, C.I. -0.4 to 0.8, P=0.2107), suggesting that small, underpowered studies did not exert an inappropriate influence on the effect size estimates.
Risk of bias across studies
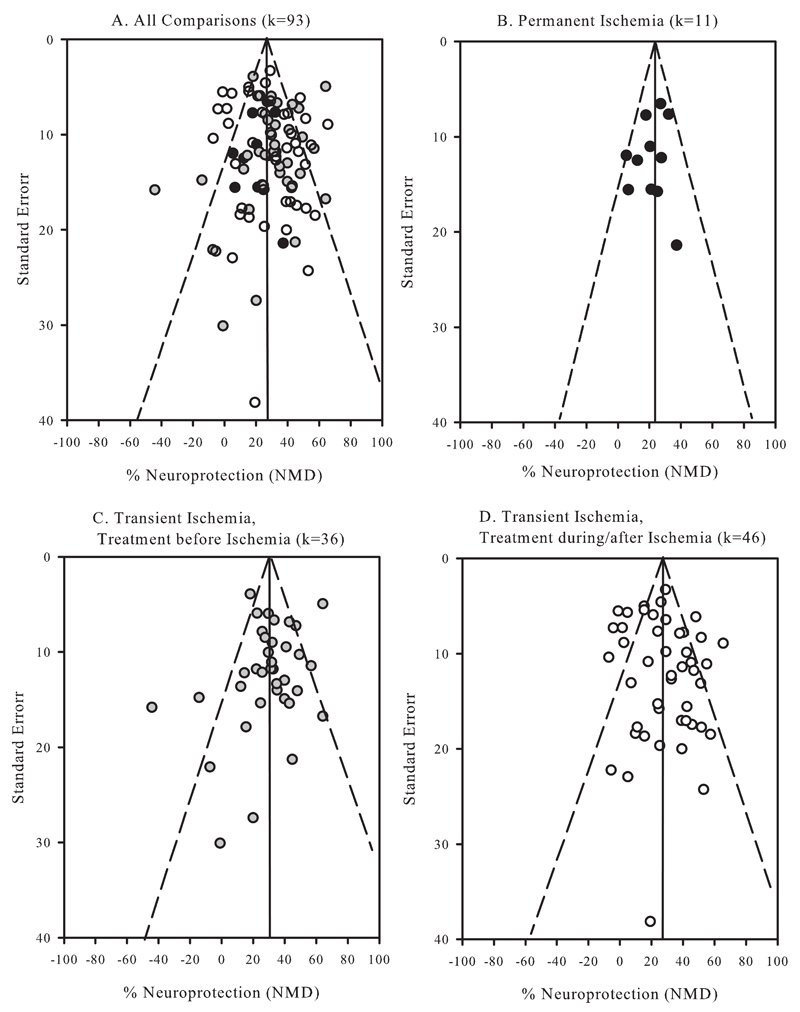
Egger’s regression analysis for the entire data set did not provide evidence for significant publication bias in the results (Figure 2C, intercept = 0.2, C.I. -0.7 to 1.0, P=0.4072). The funnel plot for the dataset is asymmetric, with 8 studies imputed to the left of the mean. Applying ‘trim and fill’, the adjusted value was 25% reduction in injury compared to the observed value: 28%. Publication bias is unlikely to account for the treatment effect – Orwin’s fail-safe method estimates that assuming that a 10% difference is trivial, 158 studies showing no difference between treated and control would be required to bring the point estimate to 10%. Results of subgroup meta-analysis (Figure 3) were similar to those provided by meta-regression.
Figure 3.
Funnel plots of results grouped according to duration of ischemia (permanent vs transient) and timing of treatment before or during/after the onset of ischemia. Vertical lines represent the summary effects; dashed lines denote the 95% confidence intervals.
Posthoc Analysis of Neuroprotection in Animals with Co-morbid Conditions
Five comparisons evaluated neuroprotection in animals with differing pre-ischemic physiological conditions or sex: male versus female, high fat versus normal diet, obese versus normal weight, diabetic versus non-diabetic, and aged versus young. The studies evaluated sevoflurane (3 comparisons) or isoflurane (2 comparisons). When female animals and animals with comorbidity were pooled, the neurologic injury was 45% worse than in control male animals (95% C.I. 31 to 59%, P=0.0000, τ=0.00). Subgroup meta-analysis revealed that neuroprotection failed in animals with comorbidities (NMD: 9% (C.I. -2. to 19%) when compared with 30% (C.I. 16 to 43%) neuroprotection observed in animals without comorbidities (P=0.0155)).
Mechanisms and Molecular Targets
A wide range of molecular targets important for modulation of cerebral ischemia was associated with anesthetic neuroprotection. The most frequently studied pathways involved cell death inhibition (k=20), activation of pro-survival pathways (k=11), excitotoxicity (k=10), signal transduction (k=8), oxidative stress, (k=6) and inflammation (k=6). A summary of the pathways and molecular target investigated is provided in Table 4.
Table 4.
Molecular pathways and proteins identified in the studies included in the meta-analysis.
| Volatile Anesthetic | Molecular Pathway | Proteins | First Author, Reference |
|---|---|---|---|
| Isoflurane (treatment before ischemia) | ↑cell survival, cell proliferation, neuroprotection | Akt – neuroprotection absent in female and Akt-/- mice | Kitano H. J Cereb Blood Flow Metab 2007;27:1377 |
| ↓nuclear transcription of cytokines, inflammatory mediators | ↓NF-κβ, IL-1β, IL-6 | Li H. Neurobiol Dis 2013;54:216 | |
| ↑cell survival, cell proliferation, neuroprotection | ↑pAkt via EEAT3, glutamate, PI3K, ERK, pSer9-GSK3β ↓GSK3β | Li L. Brain Res Bull 2013;98:23 | |
| In penumbra, ↓ cytochrome c release from mitochondria, ↓activation of caspase 3 | ↑Bcl-2 | Li L. et al. Eur J Pharmacol 2008;586:106 | |
| ↓apoptosis and microglial activation |
↓Hsp60, TLR4, MyD88, IL-1β, TNFα, Bax ↑Iκβ-α, Bcl-2 |
Sun M. Scientific Reports 2015;5:11445 | |
| ↑SUMO enzymatic cascade | ↑Ubc9 | Tong L. Mol Neurobiol 2015;51:1221 | |
| ↓inflammation | ↓ expression of TLR4, MyD88, NF-κB | Xiao Z. Mol Med Rep 2015;12:675 | |
| Activation of sphingosine signalling | ↑activity of SPK2, neuroprotection absent in SPK2-/- animals and with SPK2 block | Yung L. Stroke 2012;43:199 | |
| Isoflurane (treatment during/after ischemia) | ↓Up-regulation of inflammatory pathway, ↓edema | ↓IL-6, IL-1β, | Bleilevens C. Exp Brain Res 2013;224:155 |
| Mitochondrial protection | activation and opening of mitochondrial KATP channels | Lee J. Anesthesiology 2008:108:1055 | |
| ↓activation of NF-κB transcription factor | ↓NF-κB, IL-1β and IL-6 | Li H. Neurobiol Dis 2013;54:216 | |
| ↑HIF-α and iNOS gene expression | ↑HIF-α, iNOS | Li Q. et al. Brain Res 2012;1451:1 | |
| ↓cell survival, cell proliferation, neuroprotection, absent with high-fat diet. | ↓ of ischemia-induced Akt signaling | Yu H. Obesity 2014; 22: 2396 | |
| Sevoflurane (treatment before ischemia) |
↓mitochondrial permeability via activation of Akt signaling ↓ endogenous inhibition of Akt |
↑phosphorylation of GSK-3β ↓CTMP |
Chen Y. Br J Anaesth 2015;114:327 |
|
↓nuclear translocation ↓apoptosis |
↓NDRG2 | Li X. Anesthesiology 2014;121:549 | |
| ↓apoptosis | ↓ miR-15b (target: 3’-UTR of Bcl-2), ↑Bcl-2 | Shi H. CNS Neurol Dis Drug Targets 2013;12:381 | |
| ↑neuroprotection via activation of TREK-1 K+ channels in CNS | ↑TREK-1 | Tong L. Br J Anaesth. 2014;113:157 | |
| ↓inflammation | Suppression of NF-κB and p38 MAPK, ↓COX2, iNOS,TNF-α, Il-1α, IL-1β | Wang H. Front Biosci 2011;E3:604 | |
|
↓apoptosis ↑NOTCH signaling |
↑Notch-1, HES-1, HES-5 | Yang Q. Anesthesiology 2012;117:996 | |
| ↑anti-oxidant capacity | ↑glutathione peroxidase and catalase activities | Yang Q. Anesth Analg 2012;112:931 | |
| ↑ mitochondrial function | ↓H2O2 production, ↓ opening of MPTP | Ye R. Crit Care Med 2012;40:2685 | |
| ↑opening/activation of mitochondrial KATP channels | ↑translocation of PKCε to plasma membranes | Ye Z. Mol Biol Rep 2012;39:5049 | |
| ↑opening/activation of mitoKATP channels | ↑ p38 MAPK activation | Ye Z. Neurol Sci 2012;33:239 | |
| Sevoflurane (treatment during/after ischemia) | ↓apoptosis | ↑Bcl-2,↓Bax | Dong P. Neuroscience 2014;275:2 |
| ↑tolerance to oxygen deprivation and cell survival ↓mitochondrial permeability |
↑p-AKT, NQO1, Nrf2, HO-1 ↑binding activity of Nrf2 to ARE |
Li B. Int J Devl Neurosci 2014;38: 79 | |
| ↓apoptosis | ↑Bcl-2,↓ Bax | Wang J-K. Brain Res. 2010;1357:142 | |
| ↓apoptosis via ↑opening/activation of mitochondrial KATP channels |
↑Kir6.2 (mitochondrial KATP channel component) inducing uptake of K+ into mitochondrial matrix ↓Ca2+ overload ↓opening of mPTP |
Wang J-K. Neurol Res 2015;37:77 | |
| ↑opening/activation of mitochondrial KATP channels | ↑PKC, MAPK, adenosine receptors, Kir6.2 | Yang Z. Mol Med Rep 2014;9:843 | |
| Activation of PI3/Akt pathway | ↑HIF-α and HO-1 gene expression | Ye Z. Brain Res 2012;1463:63 | |
| ↓inflammation | ↓serum concentrations of inflammatory mediators | Zhang Y. Molecules 2012;17:341 |
Abbreviations:
AKT, protein kinase B; Bax, Bcl-2-like protein 4; Bcl-2, B-cell lymphoma 2; cJNK, c-Jun-N-terminal kinase; COX, cyclooxygenase; CTMP, carboxy-terminal protein; EEAT3, excitatory amino acid transporter 3; ERK, extracellular signal-related kinases; GSK-3β, glycogen synthase kinase 3-β, HES, hairy and enhancer of split;HIF, hypoxia-inducible factor, Hsp, heat shock protein; H2O2, hydrogen peroxide; ICAM, intercellular adhesion molecule; IL, interleukin; MAPK, Mitogen-activated protein kinase; miRNA, micro RNA; MMP, matrix metalloproteinases; mPTP, mitochondrial permeability transition pore; MyD88, myeloid differentiation primary response gene 88; NDRG2, n-myc downstream regulated gene 2; NF, nuclear factor; NICD, Notch intracellular domain; NO, nitric oxide; NOS, nitric oxide synthase; Nrf2, nuclear factor erythroid 2-related factor, NQ01, quinidine oxidoreductase 1; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; Prok2, prokineticin 2; SPK2, sphingosine kinase 2; TIMP, tissue inhibitor of metalloproteinase; TNFα, tumor necrosis factor alpha; TREK, TWIK-related (2-pore domain) K+ channel, Ubc9, ubiquitin conjugase 9; 3’UTR, three prime untranslated region; VCAM, vascular cell adhesion protein, VEGF, Vascular endothelial growth factor.
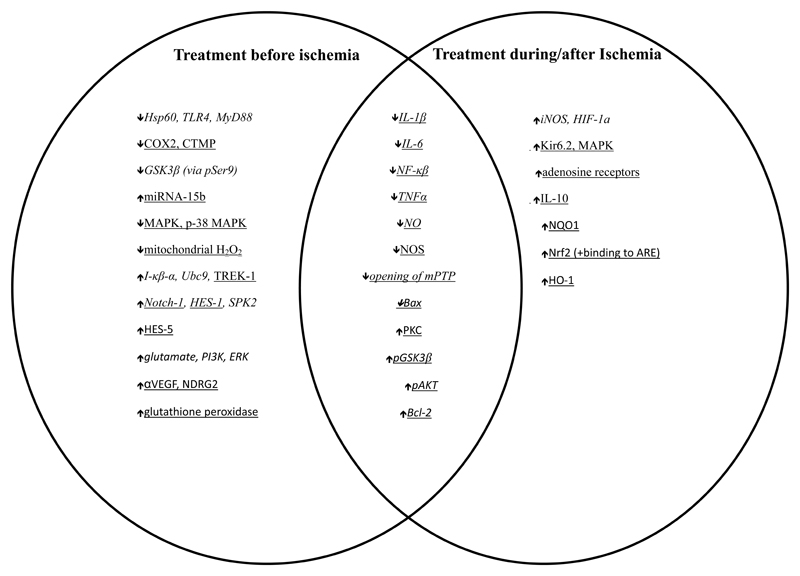
We sought evidence of common neuroprotective pathways according to drug class and timing of administration by comparing results from isoflurane and sevoflurane, two anesthetics with similar molecular structure and pharmacokinetic properties. Figure 4 shows that IL-1β, IL-6, NF-κB, Bax, NO, and NOS are down-regulated and the opening of mPTP, PKC, pGSK3β, pAKT, and Bcl-2 are up-regulated in models of neuroprotection where animals are exposed to either isoflurane or sevoflurane irrespective of the timing of anesthetic exposure. Further analysis shows that molecular targets have been identified that are unique to before vs after/during exposures to anesthetic agents (Figure 4). A micro-ribonucleic acid (RNA) species, miRNA-15b, has been shown to be down-regulated before sevoflurane exposure and upregulated before isoflurane exposure, respectively, suggesting that post-transcriptional gene regulation by RNA interference may also be playing a role in anesthetic neuroprotection.
Figure 4.
Venn diagram of molecular proteins associated with neuroprotection in isoflurane (italics) and sevoflurane (underlined) in the filament model of focal cerebral ischemia in rats and mice. Abbreviations as for Table 4.
In comorbidity studies, some putative mechanisms appeared to be ‘necessary’ for a neuroprotective effect. In diabetic rats22 and rats with diet-induced obesity23, failure of anesthetic neuroprotection was associated with decreased expression of the pro-survival mitochondrial KATP channel. Lack of anesthetic neuroprotection in aged rats was associated with a failure to reverse ischemia-induced suppression of Bcl-2 pro-survival proteins in the apoptosis pathway24. Male mice showed neuroprotection with isoflurane treatment before ischemia whereas their female counterparts did not, an effect that was androgen receptor-dependent15. In female animals in the latter study and in mice with high-fat diets25, isoflurane failed to induce the pro-survival Akt pathway as it did in neuro-protected animals. Taken together, the findings suggest that the presence of co-morbid conditions can reduce the neuroprotective effect of sevoflurane and isoflurane through interference with expression of pro-survival pathways.
Discussion
The main finding of the present systematic review and meta-analysis is that exposure to anesthetics, regardless of drug or timing, is associated with a 22-30% reduction in neurological injury in a rodent model of focal cerebral ischemia. The neuroprotection observed was not significantly enhanced by publication bias, small study effects or poor study design. The only predefined covariate that influenced the extent of neuroprotection was study quality: the greater the study quality, the greater the neuroprotection observed. Post hoc analysis showed that in female animals or animals with comorbidity, the neuroprotective effect evident in young male animals was not observed.
As could be anticipated from the wide variety of drugs and study designs, there was substantial heterogeneity in the estimates: more than 70% of the variability in the results was estimated to derive from differences between the studies rather than sampling error (I2=72%). Using multivariate meta-regression models we were not able to identify covariates other than study quality that could explain the observed heterogeneity. In studies with a dose-response design, we judged that methodological differences in anesthetic dose and timing of administration were too great to justify meta-analysis.
Neuroprotective effects in the range of 20-35% have been previously reported in experimental stroke studies 8,26,27. In a review of 1,026 experimental treatments in focal ischemia4 the average level of neuroprotection was 24-31%, irrespective of the primary hypothesized mechanism of drug action. The authors speculated that meta-analysis and regression of pooled data for different drugs might help to determine whether these experimental models are characterized by a “baseline” level of neuroprotection4.
The present review is an observational study using pooled data from studies of diverse anesthetics. The effect size appears to be predictable from the sample size: the average number of animals in each group was 12. The effect size determined by the meta-analysis is consistent with selection of studies with a group size of 10-12 that fulfill the requirements of nominal statistical significance (P<0.05 and power=0.8). This provides a possible explanation for the present (implausible) findings that the effect size was unaffected by the choice of drug, the timing of administration or the duration of ischemia – the sample sizes were underpowered to detect any difference less than 20%. The calculated effect size may be misleadingly large because it is derived from studies that were drawn from a biased sample: those studies that demonstrated a positive neuroprotective effect with small sample sizes, usually less than 20 animals3. The selection bias reflects the practical requirement that investigators choose to ‘use a model that works’ when investigating mechanisms of action. The quantification of the effect size does not add much value to the dichotomous statistical significance judgment (P<0.05). We propose that the biased sample is the source of the “baseline” level of neuroprotection suggested previously by O’Collins and colleagues4. This does not negate the findings, but restricts the generalization of the results to the conditions of the model, in this case, young adult male rodents.
We identify three implications our results may have for anesthetic neuroprotection in animal and clinical stroke research. First, the majority of studies in the present review were too small to provide reliable estimates of the true neuroprotective effect. This is not a criticism; the studies were designed to investigate the mechanism(s) of neuroprotection, not the effect size. The corollary is that clinicians should not be ‘impressed’ by a 30% improvement in outcome, which, as described above, is mostly a function of study design. Although the 95% confidence intervals for neuroprotective effect were narrow (26-34%), the estimated range of true effects was 3% to 58%. Large sample sizes allow the identification of small effect sizes which may not be clinically interesting. Before proceeding with clinical investigations, preclinical animal studies designed with sample sizes sufficient to provide precise estimates of effect size should replicate the findings of promising small screening studies.
The second implication is that investigation of young, normal male animals may not be a useful model for human stroke. We hesitate in presenting this conclusion because it is based upon a small number of studies and a post hoc analysis. Nevertheless, replication in diverse species and in animals with comorbidities is thought to be useful8, and the present results show that anesthetic neuroprotection failed in female and aged animals and animals with comorbidities. Failure of neuroprotection in the latter studies was associated with lack of activity in protective pathways present in young male animals. We speculate that replication of neuroprotective effects in animals with comorbidities as a precursor to clinical investigations may improve the translation of findings from the laboratory to the clinic.
The third implication of our findings is that most anesthetics will have some neuroprotective effect in stroke models. This makes “avoidance of anesthetics with intrinsic neuroprotective effects”8 difficult or impossible. It appears to us to be inappropriate to assume that because the duration of anesthesia may be brief, there is little interaction with putative therapeutic agents under investigation. Even an experimental design in which both control and treatment limbs of an experiment receive the anesthetic assumes that anesthetic effects are simply additive to those of the drug under investigation. The presence of interaction could be determined with dose-response data of the drugs, individually and in combination28.
Overall, we feel that the main challenge for clinical extension of the findings in the present review does not derive from methodological shortcomings or internal validity of the included studies. Two obstacles to clinical application of the results are that we do not have a precise estimate of the neuroprotective effect (range: 3-53% improvement) and that there is a suggestion in the data that anesthetics are not neuroprotective in female or aged animals and animals that have pre-existing risk factors for stroke. Both of these challenges should be met before proceeding with studies of other species.
Are the findings of the meta-analysis the result of bias? As described in the Results, the influence of traditionally described forms of bias on the summary effect was minimal. Was the positive methodology (if A: then B) used in the study design a form of bias? In the studies included in the present review, we speculate that some investigators adjusted their sample size to that required to achieve nominal statistical significance (α < 0.05, power= 0.80), not ‘chasing P values’ but to minimize the number of animals in the demonstration of a biological positive result. Inoannidis29 has defined bias as “the combination of various design, data analysis, and presentation factors that tend to produce research findings when they should not be produced”. The selective publication of results from a ‘working’ preclinical stroke model as we propose occurred in some of the studies included in the present review, does not (to us) satisfy this definition of bias.
In studies of animals in which neuroprotection failed, the injury was 45% greater in controls with comorbidity than in young males. This raises the possibility that neuroprotection was not detectable at the chosen group size in the presence of a more severe injury. It also appeared that pro-survival mechanisms operative in young male animals were not functional in females, aged animals and animals with comorbidity. The latter observation leads us to the hypothesis that the risk for stroke that accompanies disease states such as diabetes derives not only from an increased burden of vascular disease but also a reduced capacity for cellular compensation during ischemia. The phenomenon of “co-opted robustness” has been used to describe the alterations in biological systems that maintain complex disease states such as diabetes and cancer30. The wide variety of protective molecular mechanisms that were identified in the included studies suggests that neuroprotection depends on a complex evolvable biologic system. Identification of causality in this setting may be highly unlikely31. Instead of hoping for a “silver bullet”, we may, at best, stumble upon a “silver shotgun” 32, one that probably involves interaction with multiple complex genetic pathways.
Supplementary Material
Acknowledgments
The authors wish to thank the following individuals from the University of Calgary: Helen Lee Robertson, Health Sciences Librarian; from the Department of Anesthesia : Drs. J. Mikhayel, MD and C. Applewhaite, MD for their assistance in data extraction; Drs. B. Wang, MD, and Dr. J.M. Davies, MD for translation of Chinese manuscripts, and review of the manuscript respectively.
Partial salary support for DPA, AMW, JJM, and RMA during the project was provided by the Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
This study was supported in part (SKM) by the UK National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) infrastructure award: ivSyRMAF– the CAMARADES–NC3Rs in vivo systematic review and meta-analysis facility (NC/L000970/1), Edinburgh, UK
Footnotes
The authors have no conflicts of interest to declare.
Presented, in part, at the Canadian Anesthesiologists Annual Meeting, Vancouver, June 24-27, 2016
References
- 1.Wahlgren N, Moreira T, Michel P, Steiner T, Jansen O, Cognard C, Mattle HP, van Zwam W, Holmin S, Tatlisumak T, Petersson J, et al. Mechanical thrombectomy in acute ischemic stroke. Int J Stroke. 2016;11:134–47. doi: 10.1177/1747493015609778. [DOI] [PubMed] [Google Scholar]
- 2.Sena ES, van der Worp HB, Bath PMW, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8(3):e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nature Rev Neurosci. 2013;14:365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 4.O'Collins VE, Macleod MM, Donnan GA, Horky LA, van der Worp HB, Howells DW. 1,026 Experimental treatments in acute stroke. Ann Neurol. 2006;59:467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 5.Ishida K, Berger M, Nadler J, Warner DS. Anesthetic neuroprotection: antecedents and an appraisal of preclinical and clinical data quality. Current Pharm Des. 2014;20:5751–65. doi: 10.2174/1381612820666140204111701. [DOI] [PubMed] [Google Scholar]
- 6.Deng J, Lei C, Chen Y, Fang Z, Yang Q, Zhang H, Cai M, Shi L, Dong H, Xiong L. Neuroprotective gases-Fantasy or reality for clinical use? ProgNeurobiol. 2014;115:210–45. doi: 10.1016/j.pneurobio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Warner DS, James ML, Laskowitz DT, Wijdicks EF. Translational research in acute central nervous system injury. Lessons learned and the future. JAMA. 2014;71:1311–8. doi: 10.1001/jamaneurol.2014.1238. [DOI] [PubMed] [Google Scholar]
- 8.Sena ES, van der Worp HB, Howells DW, Macleod MM. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30(9):433–9. doi: 10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13–23. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orwin RG, Boruch RF. RRT meets RDD; statistical strategies for assuring privacy in telephone surveys. Public Opin Q. 1983;46:560–7. doi: 10.1086/268752. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. Br Med J. 1997;315:629–42. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval S, Tweedie R. A nonparametric 'trim and fill' method for accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 13.Vesterinen HM, Sena WS, Egan KJ, Hirst TC, Churlov L, Currie GL, Antonic A, Howells DW, Macleod MR. Meta-analysis of data from animal studies: A practical guide. J Neurosci Methods. 2014;221:92–102. doi: 10.1016/j.jneumeth.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 14.IntHout J, Ioannidis JPA, Borm GF. The Hartun-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Method. 2014;14:25–37. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemai. J Cereb Blood Flow Metab. 2007;27:1377–86. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;1997(28):2060–5. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributatble to midlle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–34. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middlle cerebral artery occulusion without craniotomy in rats. Stroke. 1989;20:84–9. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 20.Bonilla C, Zurita M, Otero L, Aguayo C, Rico MA. Failure of delayed intravenous administration of bone marrow stromal cells after traumatic brain injury. J Neurotrauma. 2012;29:394–400. doi: 10.1089/neu.2011.2101. [DOI] [PubMed] [Google Scholar]
- 21.Hara H, Huang PL, Pananian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–11. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Huang B, Liu J, Li L, Li X. Decreased brain KATP channel contributes to exacerbation of injury and the failure of neuroprotection by sevoflurane post-conditioning in diabetic rats. PLos One. 2013;8(8):e73334. doi: 10.1371/journal.pone.0073334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z, Chen Y, Zhang Y, Jiang Y, Fang X, Xu J. Sevoflurane postconditioning against cerebral ischemic neuronal injury is abolished by diet-induced obesity: Role of mitochondrial KATP channels. Mol Med Rep. 2014;9:843–50. doi: 10.3892/mmr.2014.1912. [DOI] [PubMed] [Google Scholar]
- 24.Dong P, Zhao J, Zhang Y, Dong J, Zhang L, Li D, Li L, Zhang X, Yang B, Lei W. Aging causes exacerbated ischemic brain injury and failure of sevoflurane post-conditioning: role of B-cell lymphoma-2. Neuroscience. 2014;275:2–11. doi: 10.1016/j.neuroscience.2014.05.064. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Deng J, Zuo Z. High-fat diet reduces neuroprotection of isoflurane post-treatment: role carboxyl-terminal modulator protein-Akt signaling. Obesity. 2014;22(11):2396–405. doi: 10.1002/oby.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCann SK, Irvine C, Mead GE, Sena E, Currie GL, Egan K, Macleod MR, Howells DW. Efficacy of antidepressants in animal models of ischemic stroke. Stroke. 2014;45:3055–63. doi: 10.1161/STROKEAHA.114.006304. [DOI] [PubMed] [Google Scholar]
- 27.Pedder H, Vesterinen HM, Macleod MR, Wardlaw JM. Systematic review and meta-analysis of interventions tested in animal models of lacunar stroke. Stroke. 2014;45:563–70. doi: 10.1161/STROKEAHA.113.003128. [DOI] [PubMed] [Google Scholar]
- 28.O’Collins VE, Macleod MR, Donnan GA, Howells DW. Evaluation of combination therapy in animal models of cerebral ischemia. J Cereb Blood Flow Metabol. 2012;32:585–597. doi: 10.1038/jcbfm.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannidis JPA. Why most published research findings are false. PLoS Medicine. 2005;2:e124–e9. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitano H. Biological robustness. Nat Rev Genet. 2004;5:826–37. doi: 10.1038/nrg1471. [DOI] [PubMed] [Google Scholar]
- 31.Ellis GFR. Top-down causation and emergence: some comments on mechanisms. Interface Focus. 2012;2:126–40. doi: 10.1098/rsfs.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi H, Sun B, Zhang J, Lu S, Zhang P, Wang H, Zhang P, Wang H, Yu Q, Stetler RA, Vosler PS, et al. miR-15b suppression of Bcl-2 contributes to cerebral ischemic injury and is reversed by sevoflurane preconditioning. CNS Neurol Disord Drug Targets. 2013;12(3):381–91. doi: 10.2174/1871527311312030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.