Abstract
Purpose
Delay performance of adjuvant chemotherapy (AC) after surgery has been presented to affect survival of breast cancer patients adversely, but the risk factors for delay in initiation remain controversial. Therefore, we conducted this systematic review of the literature and meta-analysis aiming at identifying the risk factors for delay of adjuvant chemotherapy (DAC) in non-metastatic breast cancer patients.
Methods
The search was performed on PubMed, Embase, Chinese National Knowledge Infrastructure and Wanfang Database from inception up to July 2016. DAC was defined as receiving AC beyond 8-week after surgery. Data were combined and analyzed using random-effects model or fixed-effects model for risk factors considered by at least 3 studies. Heterogeneity was analyzed with meta-regression analysis of year of publication and sample size. Publication bias was studied with Egger’s test.
Results
A total of 12 observational studies including 186982 non-metastatic breast cancer patients were eligible and 12 risk factors were analyzed. Combined results demonstrated that black race (vs white; OR, 1.18; 95% CI, 1.01–1.39), rural residents (vs urban; OR, 1.60; 95% CI, 1.27–2.03) and receiving mastectomy (vs breast conserving surgery; OR, 1.35; 95% CI, 1.00–1.83) were significantly associated with DAC, while married patients (vs single; OR, 0.58; 95% CI, 0.38–0.89) was less likely to have a delay in initiation. No significant impact from year of publication or sample size on the heterogeneity across studies was found, and no potential publication bias existed among the included studies.
Conclusions
Risk factors associated with DAC included black race, rural residents, receiving mastectomy and single status. Identifying of these risk factors could further help decisions making in clinical practice.
Introduction
Breast cancer is the most common type of malignant tumor and second leading cause of death in women worldwide. It is estimated that there will be 249,260 new cases and 40,890 deaths in United States in 2016 [1], which places a heavy burden on the healthcare system. Surgery is the “gold standard” treatment for early breast cancer [2] and adjuvant chemotherapy (AC) has been proved to have a significant survival benefit [3]. Although the appropriate time interval from surgery to the start of AC has not been defined, many studies demonstrated that shorter time interval was associated with better survival outcomes [4–7]. A more recent meta-analysis reported that a 4-week increase in time to initiation of AC led to a significant increase in the risk of death [8]. The initiation of AC was regularly suggested within 8 to 12 weeks after surgery [9].
While worse survival outcome from delay of adjuvant chemotherapy (DAC) has been well established, the risk factors for DAC remain unknown. Since the risk factors could not be evaluated by randomized controlled trails, evidence from numerous observational studies demonstrated that the risk factors associated with DAC included demographics, clinical characteristics, pathologic characteristics and surgical approaches[5–7, 10–18]. However, their impact on DAC remain inconsistent.
Due to a lack of understanding of the risk factors, we therefore conducted this systematic review and meta-analysis to identify the impact of risk factors on DAC.
Materials and methods
Search strategy
A systematic review was conducted to identify all studies concerning the risk factors for DAC in non-metastatic breast cancer patients by searching PubMed, Embase, Chinese National Knowledge, and Wanfang Database from inception up to July 2016. Two investigators (XFH and BCZ) independently carried out the search using the following keywords simultaneously: (1) breast cancer or breast carcinoma or breast neoplasm or breast tumor; (2) adjuvant treatment or adjuvant chemotherapy; (3) delay chemotherapy or delayed chemotherapy. The reference lists of the selected articles were also reviewed for additional relevant studies.
Eligibility criteria
The inclusion criteria were as follow: the time interval between surgery and administration of AC was defined; at least one risk factor concerning DAC was investigated; odds ratio (OR) or risk ratio (RR), and associated 95% confidence intervals (CI) were available or could be calculated from the original articles. Only full-report in English was included. For duplicated cases, the most comprehensive one was eligible for inclusion. Articles were excluded if they did not meet the above criteria, or the information provided was insufficient for the outcome data extraction or quality assessment.
Data extraction
All the searched articles were independently reviewed by two investigators (XFH and BCZ). After reading the titles and abstracts, the full texts were retrieved for those potentially included articles to achieve further assessment for inclusion. Discordance in selection was solved through discussion. For the included studies, following data were extracted: author details, year of publication, data source if available, study location, sample size, age of participants, TNM stage, AC regimens if available, cut-off categorical value of time interval, any information about quality assessment under the guideline of the Newcastle-Ottawa Scale, any risk factor investigated, OR, RR and associated 95% CIs. The accuracy of extracted data was ultimately confirmed by a third investigator (FY).
Statistical analysis
Data were combined and analyzed when the risk factor was adequately considered by at least 3 studies. Because all the included studies were observational, multivariate estimates were preferentially used. If not available, univariate estimates were extracted. When the OR, RR and associated 95%CIs were not present in the original article, we calculated OR by assessing the total number of events and total number of patients in each group. The 8-week delay was determined as the cut-off time point. For studies having different time points, the closest one to the 8-week was used. We measured the inter-study heterogeneity by using I2 statistic. Substantial heterogeneity was defined if an I2 value exceeded 50%. Forest plots were carried out to estimate the pooled ORs using the random-effects model when I2 value exceeded 50%, or the fixed-effects model when I2 value not exceeding 50%. Meta-regression analysis was performed to assess the impact of year of publication and sample size on the effect on the inter-study heterogeneity. The publication bias was assessed by Egger’s test. A two-tailed p-value < 0.05 was considered statistically significant. All the statistical analyses were conducted by Stata software (Stata SE 12.0). This systematic review and meta-analysis was performed under the guidelines of MOOSE [19].
Results
Study selection
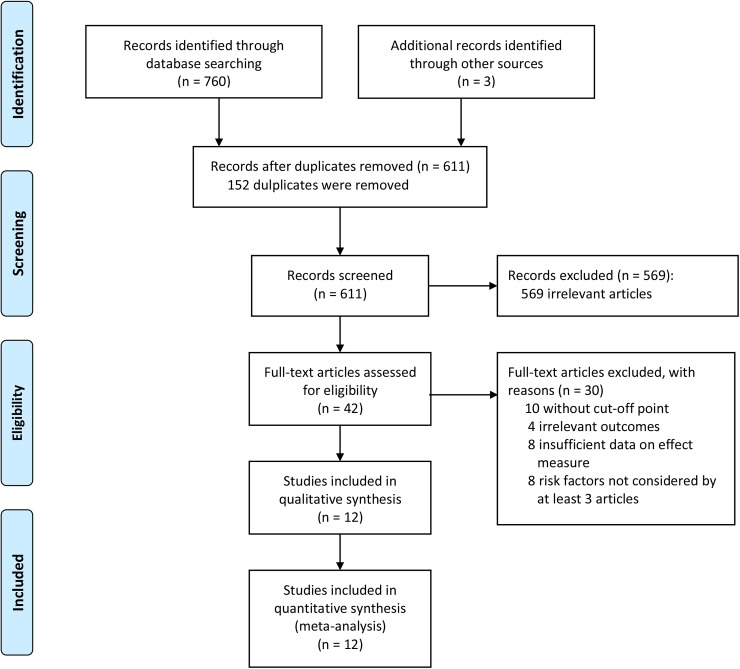
The search and selection process for eligible studies was shown in Fig 1. A total of 760 potentially relevant articles were identified, and 3 additional articles were included by manually screening the reference lists. 152 duplicates were found and removed. After reading the titles and abstracts, 569 irrelevant studies were excluded and the remaining 42 articles were reviewed in full text. Of these, 30 studies were excluded because of various reasons. Ultimately, a total of 12 articles were included for meta-analysis after detailed assessments [5–7, 10–18].
Fig 1. Flowchart of search and selection process for eligible studies.
Study characteristics
Table 1 summarized the characteristics of the eligible studies. A total of 186982 patients with stage I, II, or III breast cancer were encompassed between 2006 and 2014. The cutoff values of DAC were from 45 to 90 days. Most of the studies were carried out in United States, with one in New Zeeland and one in Canada. No prospective studies were included.
Table 1. Main characteristics of included studies.
| Study | Location | Data source | No. of patients | Age/years | Stage | Chemotherapy regimen a | Delay cutoff |
|---|---|---|---|---|---|---|---|
| Hershman, 2006 7 | United States | SEER | 5007 | >65 | Ⅰ, Ⅱ | N/S | 3 months |
| Lohrisch, 2006 5 | Canada | Breast Cancer Outcomes Unit Database of the British Columbia Cancer Agency | 2594 | 47(median) | Ⅰ, Ⅱ | AC, CEF, FAC/CAF, CMF | 12weeks |
| Jara Sanchez, 2007 10 | United States | El A´ lamo | 2782 | 21–93 | Ⅰ, Ⅱ, Ⅲ | CMF, A-based, T-based, TA | 9 weeks |
| Alderman, 2010 11 | United States | N/S | 3643 | N/S | Ⅰ, Ⅱ, Ⅲ | N/S | 8weeks |
| Fedewa, 2010 12 | United States | National Cancer Data Base | 107587 | 18–99 | Ⅰ, Ⅱ, Ⅲ | N/S | 90 days |
| Balasubramanian, 2012 13 | United States | New Jersey State Medicaid Files | 365 | 20–64 | Ⅰ, Ⅱ, ⅢA | CAF-based | 3 months |
| Simon, 2012 14 | United States | Henry Ford Health System | 2234 | 61.2 (average) | Ⅰ, Ⅱ, Ⅲ | N/S | 60 days |
| Freedman, 2013 15 | United States | SEER | 54592 | ≥66 | Ⅰ, Ⅱ, Ⅲ | N/S | 90 days |
| Sheppard, 2013 16 | United States | N/S | 359 | 25–89 | N/S | N/S | 90 days |
| Barry, 2014 17 | United States | N/S | 70 | 30–65 | Ⅰ, Ⅱ | N/S | 45 days |
| Gagliato Dde, 2014 6 | United States | Breast Medical Oncology Institutional Database | 6827 | 19–85 | Ⅰ, Ⅱ, Ⅲ | A-based, TA-based, or other type. | 60 days |
| Seneviratne, 2014 18 | New Zealand | Waikato breast cancer register | 922 | N/S | Ⅰ, Ⅱ, Ⅲ | N/S | 60 days |
Abbreviation: SEER: Surveillance, Epidemiology, and End Results Program; N/S: not stated.
a AC = doxorubicin + cyclophosphamide; CEF = cyclophosphamide + epirubicin + fluorouracil; FAC/CAF = fluorouracil + doxorubicin + cyclophosphamide; CMF = cyclophosphamide + methotrexate + fluorouracil; A-based = anthracycline-based; T-based = taxane-based; TA = anthracycline + taxane; CAF-based = cyclophosphamide, doxorubicin/ epirubicin, 5-fluorouracil, or a combination of these agents.
Quality assessment
To assess the quality of the observational studies, selection of participants, study comparability, and ascertainment of exposure were examined for all the included studies based on the Newcastle-Ottawa Scale [20] (shown in Table 2). A maximum of 9 starts could be obtained as the highest quality. The scores assessed for the eligible studies were ranged from 6 to 9, all of which were identified as very good or good in quality [21].
Table 2. Methodological quality of studies included in the meta-analysis.
| Study | Case definition adequate | Representativeness of the cases | Selection of controls | Definition of controls | Control for important factors a | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | Total quality scores |
|---|---|---|---|---|---|---|---|---|---|
| Hershman, 2006 7 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Lohrisch, 2006 5 | ☆ | — | — | ☆ | ☆ | ☆ | ☆ | ☆ | 6 |
| Jara Sanchez, 2007 10 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Alderman, 2010 11 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Fedewa, 2010 12 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Balasubramanian, 2012 13 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Simon, 2012 14 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Freedman, 2013 15 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Sheppard, 2013 16 | ☆ | — | — | ☆ | ☆ | ☆ | ☆ | ☆ | 6 |
| Barry, 2014 17 | ☆ | — | — | ☆ | ☆☆ | ☆ | ☆ | ☆ | 7 |
| Gagliato Dde, 2014 6 | ☆ | — | — | ☆ | ☆☆ | ☆ | ☆ | ☆ | 7 |
| Seneviratne, 2014 18 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
a A maximum of 2 stars could be awarded for this item. Studies that controlled for age received one star, whereas studies that controlled for other factors received an additional star.
Risk factors extracted for meta-analysis
In total, 12 risk factors were extracted from the included studies, including age at diagnosis (<70 vs ≥70 years), race (white vs black), county (urban vs rural), comorbidity status (Charlson score 0 vs ≥1), marital status (single vs married), TNM stage (I + II vs III), hormone receptor status (estrogen receptor [ER] and progesterone receptor [PR] negative vs ER and/or PR positive), histological grade (well and/or moderately differentiated vs poorly differentiated), surgical approach (breast conserving surgery [BCS]vs mastectomy), number of involved nodes (0–9 vs ≥ 10), tumor size (0-5cm vs > 5cm), and lymphatic vascular invasion status (absent vs present).
Risk factors contributing to DAC
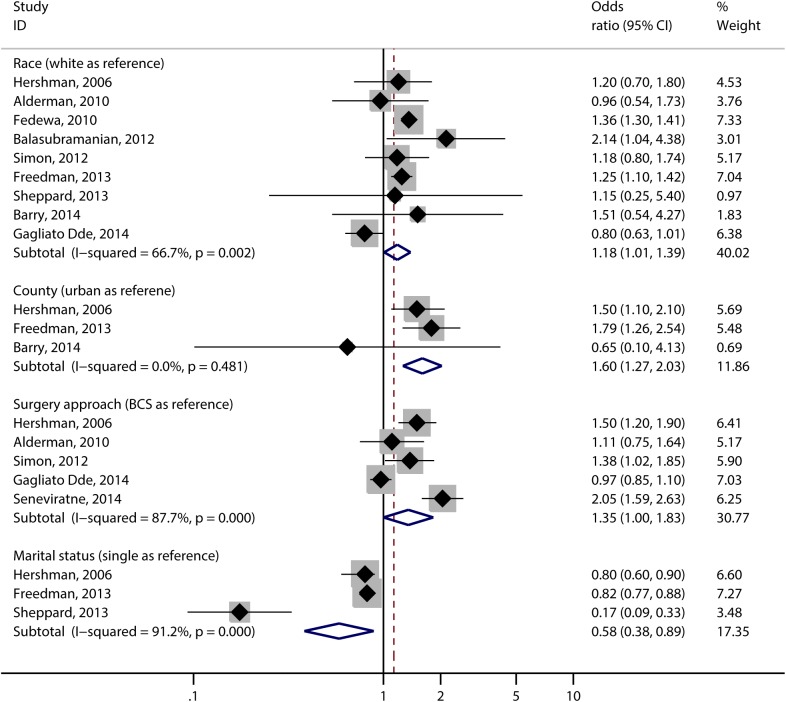
The pooled results demonstrated that an 18% increased risk of DAC for black race compared with white race (OR, 1.18; 95% CI, 1.01–1.39; I2 = 66.7%), a 60% higher risk for rural residents than urban residents (OR, 1.60; 95% CI, 1.27–2.03; I2 = 0.0%), and a 35% higher risk for patients receiving mastectomy than patients receiving BCS (OR, 1.35; 95% CI, 1.00–1.83; I2 = 87.7%). While married patients were less likely to have a delay in initiation compared with single patients (OR, 0.58; 95% CI, 0.38–0.89; I2 = 91.2%, Fig 2).
Fig 2. Forrest plots of risk factors that were contributed to DAC.
Risk factors not contributing to DAC
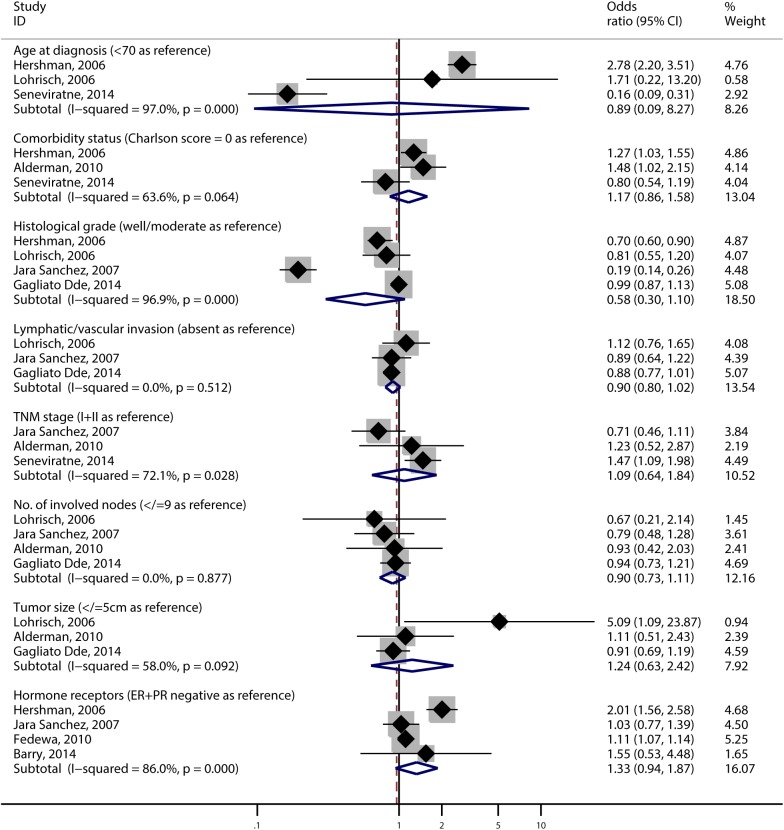
Older than 70 years (OR, 0.89; 95% CI, 0.09–8.27; I2 = 97.2%), worse comorbidity status (OR, 1.17; 95% CI, 0.86–1.58; I2 = 63.6%), poorer histological differentiation (OR, 0.58; 95% CI, 0.30–1.10; I2 = 96.9%), presence of lymphatic vascular invasion (OR, 0.90; 95% CI, 0.80–1.02; I2 = 0.0%), higher TNM stage (OR, 1.09; 95% CI, 0.64–1.84; I2 = 72.1%), involved nodes ≥10 (OR, 0.90; 95% CI, 0.73–1.11; I2 = 0.0%), tumor size > 5cm (OR, 1.24; 95% CI, 0.63–2.42; I2 = 58.0%) and ER / PR positive status (OR, 1.33; 95% CI, 0.94–1.87; I2 = 86.0%, Fig 3) were not correlated with an increased risk of DAC.
Fig 3. Forrest plots of risk factors that were not contributed to DAC.
Meta-regression analysis and publication bias assessment
Meta-regression analysis suggested that year of publication and sample size did not have a significant impact on the heterogeneity across studies for each factor. Egger’s test demonstrated that no potential publication bias existed among the included studies for various factors (shown in Table 3).
Table 3. Meta-regression analysis and Egger’s test for various factors.
| No. of studies | Meta-regression analysis a | Egger’ s test (P value) | ||
|---|---|---|---|---|
| Year of publication (P value) | Sample size (P value) | |||
| Race | 9 | 0.546 | 0.389 | 0.283 |
| County | 3 | 0.914 | 0.549 | 0.455 |
| Surgical approach | 5 | 0.615 | 0.156 | 0.257 |
| Marital status | 3 | 0.685 | 0.616 | 0.372 |
| Age | 3 | 0.078 | 0.159 | 0.599 |
| Comorbidity status | 3 | 0.504 | 0.396 | 0.738 |
| Histological grade | 4 | 0.892 | 0.669 | 0.555 |
| Lymphatic vascular invasion | 3 | 0.587 | 0.605 | 0.311 |
| TNM stage | 3 | 0.231 | 0.593 | 0.661 |
| No. of involved nodes | 4 | 0.766 | 0.824 | 0.277 |
| Tumor size | 3 | 0.346 | 0.484 | 0.288 |
| Hormone receptors | 4 | 0.945 | 0.740 | 0.439 |
a Adjustment for both year of publication and sample size were performed when number of studies were at least 4.
Discussion
In this meta-analysis, data on 186982 non-metastatic breast cancer patients from 12 studies were analyzed in characterizing the risk factors related to DAC. Combined results demonstrated that black race, rural residents and receiving mastectomy had significantly higher likelihood of experiencing DAC, while married patients were at lower risk. To the best of our knowledge, this is the first systematic review and meta-analysis evaluating the previously reported risk factors associated with DAC.
Results from 9 subset studies of our meta-analysis suggested that black race was associated with an 18% increased risk of DAC compared with white race, which was consistent with the conclusions of previous studies [22, 23]. However, the pooled result should be interpreted cautiously because the magnitude of race disparity on DAC was quite modest (18%) and high heterogeneity of 66.7% was observed across studies. African American women were the major component of black race in our study. The reasons for them to have a higher risk of DAC might result from following aspects: low education level, disadvantaged socioeconomic status (SES), unavailability of transportation and a lack of insurance [24–27]. Since the disparity of SES between black and white race would affect their decision on the initiation of AC after surgery [28], hence, we further divided these 9 studies into two groups: SES unknown between black and white race (U-SES), and lower SES for black race than white race (L-SES). The meta-analysis for these two groups (shown in S1 Fig) demonstrated that black race in L-SES group had a 35% increased risk of DAC, which was higher than the pooled result (18% increased risk) of the 9 studies, while there was no significant difference in U-SES group. This could partially explain that the lower SES of black race might push them to start AC administration later than white race. More work is warranted to further address this issue.
In addition, our combined result from 4 studies demonstrated that mastectomy was associated with an 83% increased risk of DAC compared with BCS. Because the extent of mastectomy is larger than that of BCS, it is more likely for patients receiving mastectomy to suffer greater complications, including surgical site infections, wound dehiscence and skin flap necrosis [29, 30], which could result in a longer recovery period and so delaying AC administration. A recent meta-analysis suggested that mastectomy with immediate breast reconstruction did not necessarily delay the initiation of AC compared with mastectomy only [31]. However, our study did not analyze the effect of mastectomy with immediate breast reconstruction on DAC, because no sufficient data could be extracted from the included studies. Therefore, more future studies evaluating mastectomy with immediate breast reconstruction and BCS on impact of DAC are warranted to further address this issue.
Besides, three studies of the current meta-analysis documented DAC in rural residents, which was consistent with the previously reported studies and the reasons has been well interpreted that rural residents had less access to comprehensive hospitals and difficult transportation to the long-distant qualified hospitals [14, 24]. Otherwise, we also observed that married patients were 42% less likely to delay the AC than single patients, since married patients usually gain more support from family members to accept clinician’s recommendation and start treatment [32, 33]. It is noted that several risk factors evaluated in our meta-analysis did not have significant association with DAC as mentioned in the results, which might be attributed to few studies included and inconsistent findings across included studies.
The greatest strengths of the current study are the large sample sizes of over 180000 non-metastatic breast cancer patients and wide range of evaluated risk factors. The study indicated that black race, receiving mastectomy, rural residents and single status were significantly associated with DAC, which could be helpful for clinicians to identify the specific population groups and to start AC early. Furthermore, our work would promote the health system to pay extra attention to improve the medical conditions for patients at increased risk of delay of treatment. Of note, our meta-analysis did not focus on the survival outcomes caused by DAC. One reason is that we could not extract sufficient data from the eligible studies, since there were only 4 included studies referring to the survival outcomes. Another reason is that many previous studies and meta-analysis have demonstrated that longer time interval was associated with worse survival outcomes. Nevertheless, we did not deny that in some cases, DAC was not associated with increased risk of mortality, such as in a cohort of postmenopausal, ER-positive breast cancer patients following adjuvant endocrine therapy [34, 35].
Several potential limitations of our meta-analysis should be considered. First, data were extracted from observational studies, so the inherent potential bias caused by unmeasured and uncontrolled confounders were inevitable. Second, high heterogeneity across studies was identified, although meta-regression analysis was performed to estimate the impact of year of publication and sample size and no statistically significant result was found. Thus, the interpretation of our results should be with caution. Besides, the cutoff time point of DAC was not uniform among the eligible studies, ranging from 45 to 90days, which might probably result in variability across studies and so could distort our findings.
In conclusion, our meta-analysis of the current literature demonstrated that black race, rural residents, receiving mastectomy and single status led to significantly increased risk of experiencing DAC in non-metastatic breast cancer patients. Identification of these factors could be helpful for personalized treatment planning.
Supporting information
(TIF)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by funds from the National Natural Science Foundation of China (81472575, 81272514, 81372133), the Key Program of the National Natural Science Foundation of China (31030061), the Science and Technology Planning Project of Guangdong and Guangzhou (2013B060300009, 2014J4100169). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. Epub 2016/01/09. [DOI] [PubMed] [Google Scholar]
- 2.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2015;26(8):1533–46. Epub 2015/05/06. PubMed Central PMCID: PMC4511219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (London, England). 2005;365(9472):1687–717. Epub 2005/05/17. [DOI] [PubMed] [Google Scholar]
- 4.Colleoni M, Bonetti M, Coates AS, Castiglione-Gertsch M, Gelber RD, Price K, et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18(3):584–90. Epub 2000/02/02. [DOI] [PubMed] [Google Scholar]
- 5.Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(30):4888–94. Epub 2006/10/04. [DOI] [PubMed] [Google Scholar]
- 6.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(8):735–44. Epub 2014/01/29. PubMed Central PMCID: PMC3940536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast cancer research and treatment. 2006;99(3):313–21. Epub 2006/04/04. 10.1007/s10549-006-9206-z [DOI] [PubMed] [Google Scholar]
- 8.Raphael MJ, Biagi JJ, Kong W, Mates M, Booth CM, Mackillop WJ. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast cancer research and treatment. 2016;8:8. [DOI] [PubMed] [Google Scholar]
- 9.Cold S, During M, Ewertz M, Knoop A, Moller S. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). British journal of cancer. 2005;93(6):627–32. Epub 2005/09/02. PubMed Central PMCID: PMC2361615. 10.1038/sj.bjc.6602734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alderman AK, Collins ED, Schott A, Hughes ME, Ottesen RA, Theriault RL, et al. The impact of breast reconstruction on the delivery of chemotherapy. Cancer. 2010;116(7):1791–800. Epub 2010/02/10. PubMed Central PMCID: PMC2847068. 10.1002/cncr.24891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balasubramanian BA, Demissie K, Crabtree BF, Strickland PA, Pawlish K, Rhoads GG. Black Medicaid beneficiaries experience breast cancer treatment delays more frequently than whites. Ethnicity & disease. 2012;22(3):288–94. Epub 2012/08/09. [PubMed] [Google Scholar]
- 12.Barry PN, Riley EC, Pan J, Crew JB, Lee K, Jain D, et al. Delay of adjuvant chemotherapy after elective mastectomy and immediate reconstruction in breast-conservation candidates: a matched-pair analysis. American journal of clinical oncology. 2014;37(6):575–9. Epub 2013/03/08. 10.1097/COC.0b013e318280d79f [DOI] [PubMed] [Google Scholar]
- 13.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(27):4135–41. Epub 2010/08/11. [DOI] [PubMed] [Google Scholar]
- 14.Freedman RA, He Y, Winer EP, Keating NL. Racial/Ethnic differences in receipt of timely adjuvant therapy for older women with breast cancer: are delays influenced by the hospitals where patients obtain surgical care? Health services research. 2013;48(5):1669–83. Epub 2013/05/15. PubMed Central PMCID: PMC3796107. 10.1111/1475-6773.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jara Sanchez C, Ruiz A, Martin M, Anton A, Munarriz B, Plazaola A, et al. Influence of timing of initiation of adjuvant chemotherapy over survival in breast cancer: a negative outcome study by the Spanish Breast Cancer Research Group (GEICAM). Breast cancer research and treatment. 2007;101(2):215–23. Epub 2006/07/11. 10.1007/s10549-006-9282-0 [DOI] [PubMed] [Google Scholar]
- 16.Simon MS, Lamerato L, Krajenta R, Booza JC, Ruterbusch JJ, Kunz S, et al. Racial differences in the use of adjuvant chemotherapy for breast cancer in a large urban integrated health system. International journal of breast cancer. 2012;2012:453985 Epub 2012/06/13. PubMed Central PMCID: PMC3363414. 10.1155/2012/453985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheppard VB, Isaacs C, Luta G, Willey SC, Boisvert M, Harper FW, et al. Narrowing racial gaps in breast cancer chemotherapy initiation: the role of the patient-provider relationship. Breast cancer research and treatment. 2013;139(1):207–16. Epub 2013/04/17. PubMed Central PMCID: PMC3662254. 10.1007/s10549-013-2520-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seneviratne S, Campbell I, Scott N, Kuper-Hommel M, Round G, Lawrenson R. Ethnic differences in timely adjuvant chemotherapy and radiation therapy for breast cancer in New Zealand: a cohort study. BMC Cancer. 2014;14:839 10.1186/1471-2407-14-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. Epub 2010/07/24. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 21.Takahashi N, Hashizume M. A systematic review of the influence of occupational organophosphate pesticides exposure on neurological impairment. BMJ open. 2014;4(6):e004798 Epub 2014/06/26. PubMed Central PMCID: PMC4078784. 10.1136/bmjopen-2014-004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwyn K, Bondy ML, Cohen DS, Lund MJ, Liff JM, Flagg EW, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100(8):1595–604. Epub 2004/04/10. 10.1002/cncr.20169 [DOI] [PubMed] [Google Scholar]
- 23.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Archives of internal medicine. 2006;166(20):2244–52. Epub 2006/11/15. 10.1001/archinte.166.20.2244 [DOI] [PubMed] [Google Scholar]
- 24.Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer practice. 1997;5(6):361–6. Epub 1997/12/16. [PubMed] [Google Scholar]
- 25.Safer MA, Tharps QJ, Jackson TC, Leventhal H. Determinants of three stages of delay in seeking care at a medical clinic. Medical care. 1979;17(1):11–29. Epub 1979/01/01. [DOI] [PubMed] [Google Scholar]
- 26.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(9):1357–62. Epub 2006/03/22. [DOI] [PubMed] [Google Scholar]
- 27.Breen N, Kessler LG, Brown ML. Breast cancer control among the underserved—an overview. Breast cancer research and treatment. 1996;40(1):105–15. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 28.Herndon JE 2nd, Kornblith AB, Holland JC, Paskett ED. Effect of socioeconomic status as measured by education level on survival in breast cancer clinical trials. Psychooncology. 2013;22(2):315–23. Epub 11 Oct 20. 10.1002/pon.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeevan R, Browne JP, Pereira J, Caddy CM, Sheppard C, van der Meulen JH, et al. Socioeconomic deprivation and inpatient complication rates following mastectomy and breast reconstruction surgery. The British journal of surgery. 2015;102(9):1064–70. Epub 2015/06/16. 10.1002/bjs.9847 [DOI] [PubMed] [Google Scholar]
- 30.Olsen MA, Chu-Ongsakul S, Brandt KE, Dietz JR, Mayfield J, Fraser VJ. Hospital-associated costs due to surgical site infection after breast surgery. Archives of surgery (Chicago, Ill: 1960). 2008;143(1):53–60; discussion 1. Epub 2008/01/23. [DOI] [PubMed] [Google Scholar]
- 31.Xavier Harmeling J, Kouwenberg CA, Bijlard E, Burger KN, Jager A, Mureau MA. The effect of immediate breast reconstruction on the timing of adjuvant chemotherapy: a systematic review. Breast cancer research and treatment. 2015;153(2):241–51. Epub 2015/08/20. PubMed Central PMCID: PMC4559567. 10.1007/s10549-015-3539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neal RD, Allgar VL. Sociodemographic factors and delays in the diagnosis of six cancers: analysis of data from the "National Survey of NHS Patients: Cancer". British journal of cancer. 2005;92(11):1971–5. Epub 2005/05/19. PubMed Central PMCID: PMC2361785. 10.1038/sj.bjc.6602623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipscomb J, Gillespie TW, Goodman M, Richardson LC, Pollack LA, Ryerson AB, et al. Black-white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast cancer research and treatment. 2012;133(1):285–96. Epub 2012/01/27. PubMed Central PMCID: PMC4698875. 10.1007/s10549-011-1916-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ejlertsen B. Adjuvant chemotherapy in early breast cancer. Dan Med J. 2016;63(5).(pii):B5222 [PubMed] [Google Scholar]
- 35.Ejlertsen B, Jensen MB, Mouridsen HT. Excess mortality in postmenopausal high-risk women who only receive adjuvant endocrine therapy for estrogen receptor positive breast cancer. Acta Oncol. 2014;53(2):174–85. Epub 2013 Nov 13. 10.3109/0284186X.2013.850738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.