Abstract
One of the functions of the cerebellum in motor learning is to predict and account for systematic changes to the body or environment. This form of adaptive learning is mediated by plastic changes occurring within the cerebellar cortex. The strength of cerebellar-to-cerebral pathways for a given muscle may reflect aspects of cerebellum-dependent motor adaptation. These connections with motor cortex (M1) can be estimated as cerebellar inhibition (CBI): a conditioning pulse of transcranial magnetic stimulation delivered to the cerebellum before a test pulse over motor cortex. Previously, we have demonstrated that changes in CBI for a given muscle representation correlate with learning a motor adaptation task with the involved limb. However, the specificity of these effects is unknown. Here, we investigated whether CBI changes in humans are somatotopy specific and how they relate to motor adaptation. We found that learning a visuomotor rotation task with the right hand changed CBI, not only for the involved first dorsal interosseous of the right hand, but also for an uninvolved right leg muscle, the tibialis anterior, likely related to inter-effector transfer of learning. In two follow-up experiments, we investigated whether the preparation of a simple hand or leg movement would produce a somatotopy-specific modulation of CBI. We found that CBI changes only for the effector involved in the movement. These results indicate that learning-related changes in cerebellar–M1 connectivity reflect a somatotopy-specific interaction. Modulation of this pathway is also present in the context of interlimb transfer of learning.
SIGNIFICANCE STATEMENT Connectivity between the cerebellum and motor cortex is a critical pathway for the integrity of everyday movements and understanding the somatotopic specificity of this pathway in the context of motor learning is critical to advancing the efficacy of neurorehabilitation. We found that adaptive learning with the hand affects cerebellar–motor cortex connectivity, not only for the trained hand, but also for an untrained leg muscle, an effect likely related to intereffector transfer of learning. Furthermore, we introduce a novel method to measure cerebellar–motor cortex connectivity during movement preparation. With this technique, we show that, outside the context of learning, modulation of cerebellar–motor cortex connectivity is somatotopically specific to the effector being moved.
Keywords: adaptation, cerebellum, connectivity, somatotopy, transcranial magnetic stimulation
Introduction
The cerebellum is known to play an important role in adaptive motor learning, an error-based process in which the brain learns to compensate for systematic movement errors. For example, in a visuomotor rotation, a cursor is rotated relative to hand movement such that the cursor's movement direction no longer matches that of the hand. This creates a mismatch between the predicted and actual sensory outcome of a movement, which drives error reduction in healthy individuals (Tseng et al., 2007; Shadmehr et al., 2010). The cerebellum is thought to be critical for learning error-based adaptation tasks (Martin et al., 1996; Diedrichsen et al., 2005; Chen et al., 2006) because patients with cerebellar degeneration show a marked impairment in such learning (Weiner et al., 1983; Martin et al., 1996; Smith and Shadmehr, 2005). In addition, acquisition of a visuomotor adaptation is enhanced when anodal transcranial direct current stimulation, a form of noninvasive excitatory stimulation, is applied over the cerebellum during training (Galea et al., 2011; Block and Celnik, 2013).
Although it is recognized that the cerebellum is crucial for adaptation, possible physiological mechanisms have only recently been explored (Medina and Lisberger, 2008; Carey, 2011; Schonewille et al., 2011; Gao et al., 2012; Yang and Lisberger, 2014). Transcranial magnetic stimulation (TMS) has been used to assess these neurophysiological responses in humans: a paired-pulsed TMS technique is used to stimulate the cerebellum just before stimulating the contralateral motor cortex (M1) (Ugawa et al., 1995; Pinto and Chen, 2001; Daskalakis et al., 2004). It is well established that dentate nucleus of the cerebellum projects to M1 (Allen and Tsukahara, 1974; Hoover and Strick, 1999) in a disynaptic excitatory pathway via the ventrolateral thalamus (Shinoda et al., 1985; Dum and Strick, 2003; Evrard and Craig, 2008). Therefore, this TMS technique is thought to measure the inhibitory projection from the cerebellar cortex to the dentate, which reduces M1 activity via the dentate-thalamus-cortical pathway (cerebellar inhibition; CBI). We have shown recently that the level of CBI to a leg muscle, the tibialis anterior (TA), decreases when healthy subjects learn a cerebellum-dependent locomotor adaptation task (Jayaram et al., 2011). Interestingly, those participants experiencing a larger magnitude of adaptation (i.e., greater degree of learning) expressed greater reduction in CBI. A similar reduction of CBI has also been observed in a hand muscle, the first dorsal interosseous (FDI), in subjects learning a visuomotor adaptation hand task (Schlerf et al., 2012) and in response to observing or performing a finger sequence task (Torriero et al., 2011). These findings suggest a change in cerebellar excitability associated with learning. However, it is not known whether these learning-associated changes are somatotopically specific to the trained effector.
To determine whether motor learning-related changes in cerebellar–M1 connectivity are somatotopy specific or global enough to affect untrained effectors, we examined CBI in both a hand and leg muscle (FDI and TA, respectively) before, during, and after participants learned a visuomotor rotation with the hand. Because this type of learning can transfer to untrained limbs (Sainburg and Wang, 2002; Criscimagna-Hemminger et al., 2003; Wang and Sainburg, 2004; Morton and Bastian, 2006; Savin and Morton, 2008; Balitsky Thompson and Henriques, 2010; Joiner et al., 2013), modulation of CBI in an untrained limb could be related to interlimb transfer (i.e., reflecting a somatotopy-specific plastic mechanism) or it could be a nonspecific response. Therefore, we also investigated CBI in the context of movement preparation in the absence of motor learning. We predicted that modulation of CBI would be somatotopy specific in both situations, meaning that, in the motor learning task, any CBI changes in the untrained effector could be related to transfer of learning.
Materials and Methods
Subjects
In total, 32 subjects (mean age 23.9 years; 19 men) participated in three experiments (10 in Experiment 1, 10 in Experiment 2, and 12 in Experiment 3). All subjects reported that they did not have conditions that would exclude them from noninvasive brain stimulation, including previous history of migraines, diabetes, seizures, or any brain or peripheral nerve diseases. Exclusion criteria also included the use of nicotine, alcohol, and recreational drugs and the absence of prescribed medication affecting the CNS, all of which may alter plasticity and motor learning. All subjects reported that they were right-handed and neurologically healthy. All subjects gave informed consent approved by the Johns Hopkins Institutional Review Board.
Experiment 1: CBI changes due to visuomotor adaptation
Experimental protocol.
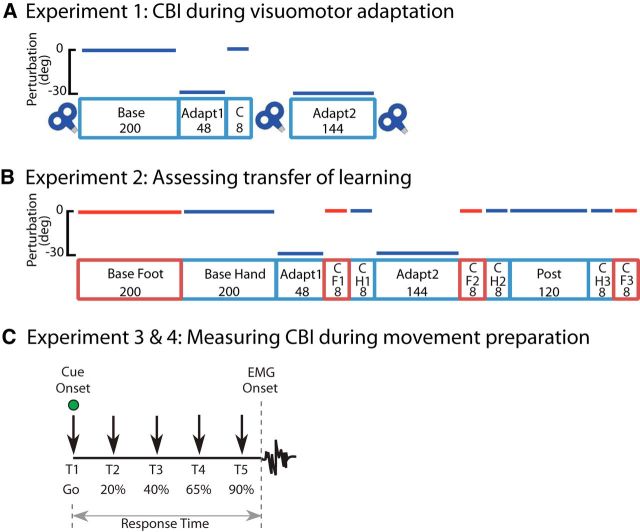
Each subject participated in an experiment testing changes in CBI in the right hand (FDI) and right leg (TA) before and during right hand visuomotor adaptation (Fig. 1A). The experimental session consisted of five training blocks. The Baseline block consisted of 200 trials with the right hand. After the baseline block, a 30° clockwise perturbation (CW) was applied to the cursor display (Adapt1, 48 trials). To assess how much the individual learned, each participant underwent a quick eight-trial segment with the 30° clockwise perturbation turned off (Catch). Participants then completed another 144 trials with the visuomotor rotation turned back on (Adapt2) to allow them to fully correct for the perturbation. Before Baseline and immediately after Catch and Adapt2, CBI measurements were recorded for right FDI and right TA.
Figure 1.
Experimental design. A, Experiment 1 consisted of five behavioral blocks and three physiological measurements. In Adapt1 and Adapt2, subjects were exposed to a 30° CW cursor rotation; cursor movement was veridical in the remaining blocks. Cerebellar-motor cortex connectivity (CBI) was assessed before Base and after Catch and Adapt2. The numbers in each block represent the number of trials. B, Experiment 2 consisted of 11 behavioral blocks. Adapt1 and Adapt2 had a 30° CW rotation; other blocks were veridical. Red, Right foot movements; blue, right hand movements. There were no physiological measurements for this experiment. C, In Experiment 3, premovement CBI for the FDI was assessed at five different timings (T1–T5) before movement initiation with either the index finger or foot, with T1 at cue onset and T5 being the closest to movement onset. Timings were adjusted to individual RTs. Experiment 4 mirrored the setup of Experiment 3, but premovement CBI was assessed for the TA muscle.
Behavioral tasks.
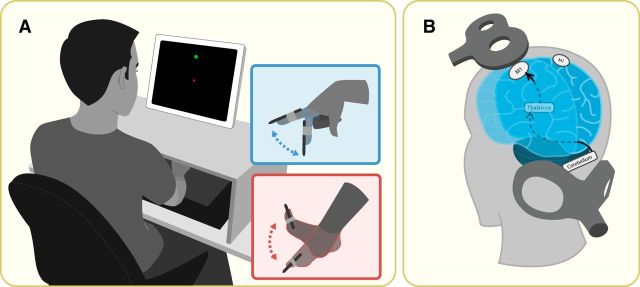
Subjects were seated in front of a vertical computer screen (Fig. 2A). A wireless digitizing pen (Wacom) was attached to their dominant index finger with a co-flex bandage. Subjects were instructed to move their finger over a digitizing tablet (Wacom) positioned horizontally at waist level. Subjects viewed feedback of their finger movements as a white dot (2 mm) displayed on the computer screen. Subjects were instructed to move to the starting position, a white square (3 mm) at the center of the screen, and then to “shoot” through one of eight targets that appeared 10 cm radially from the starting position.
Figure 2.
Methods. A, Setup for the behavioral task used in all three experiments. Participants viewed the vertical computer monitor for visual feedback about the task and trained with the right hand before switching to the right foot to assess hand-to-foot transfer. For foot movements, the tablet was placed vertically and the foot was propped up. B, Coil placement to measure CBI. This technique requires paired-pulse stimulation in which one TMS coil is placed over M1 (test) and the other over the cerebellum (condition). To determine CBI for the right hand muscle (FDI), the conditioning pulse was delivered over the right cerebellum 5 ms before the test pulse was applied over the left M1 representation of FDI. The same procedure was followed to determine CBI for the right leg muscle (TA), with the test pulse applied over the left M1 representation of TA.
The 2D position of the digitizing pen was recorded continuously at 75 Hz using a custom MATLAB program (The MathWorks). All kinematic data were filtered at 10 Hz with a low-pass Butterworth filter and differentiated numerically to calculate velocity. The onset of each movement was determined as the point at which radial velocity crossed 5% of peak velocity. Once subjects had moved 10 cm from the starting position (10 cm circular boundary), their final position was recorded.
To ensure consistency in movement duration, subjects received auditory feedback at the end of each movement: a low-pitched or high-pitched tone if they were too slow (>375 ms) or too fast (<275), respectively. Subjects' vision of their hands or the digitizing tablet was blocked. Targets were displayed pseudorandomly such that every set of eight consecutive trials included one of each of the target positions. For each trial, subjects' performance was quantified as the angular end point error, which was defined as the angle between the starting position to the center of the target and the imaginary line connecting the starting position to the end point (Hadipour-Niktarash et al., 2007; Galea et al., 2011). Epochs were created by binning eight consecutive trials. For each block, the initial amount of error (mean error) was determined by averaging over consecutive epochs (Krakauer et al., 2005).
EMG recordings and TMS protocol.
EMG was recorded with Ag/AgCl EMG electrodes placed over the training FDI muscle and ipsilateral TA muscle (Experiment 1: right FDI and TA, Experiment 3: right FDI). EMG data were stored for offline analysis using Signal software (CED). M1 excitability of the right FDI (Experiments 1 and 3) and TA (Experiment 1 only) muscles was assessed using a 70-mm-diameter figure-eight TMS coil (Magstim) over the motor representation of the muscle. To localize the stimulation site and to maintain consistency of responses, we used a Brain Sight neuronavigation system (Rogue Research). After determining the resting motor thresholds for these muscles using standard procedures (Rossini et al., 1994), we determined the stimulator output intensity needed to elicit motor evoked potentials (MEPs) of ∼1 mV (SI1 mV) at rest and before movement onset (Experiment 3 only). TMS before movement onset was triggered via a customized MATLAB function using the peri-triggering capability of Signal software.
CBI.
We measured the level of cerebellar–M1 connectivity or CBI for right FDI and TA using a standard paired-pulse TMS paradigm (Ugawa et al., 1995; Werhahn et al., 1996; Pinto and Chen, 2001; Daskalakis et al., 2004). First, we determined the brainstem motor threshold using a double cone coil (Magstim) over the inion. This is defined as the minimal intensity (to the nearest 5% of stimulator output) required to elicit five 50 μv MEPs of the target muscle (Rossini et al., 2015). Second, we tested CBI by delivering a conditioning stimulus (CS) 3 cm lateral to the inion and 5 ms before a test stimulus (TS) targeting the contralateral M1 representation of FDI or TA (Galea et al., 2009; Jayaram et al., 2011; Schlerf et al., 2015).
CS intensity was set at 5% below the brainstem motor threshold to the cerebellum. This elicits maximum recruitment of the cerebellar–M1 connections, whereas lower intensities results in less inhibition of this pathway (Werhahn et al., 1996; Pinto and Chen, 2001; Daskalakis et al., 2004; Galea et al., 2009). Test stimulus over M1 was adjusted to elicit MEPs with average peak-to-peak amplitude of ∼1 mV (SI1 mV), which is ideal for CBI assessment (Ugawa et al., 1995; Pinto and Chen, 2001; Daskalakis et al., 2004). Ten paired pulses (cerebellum + M1) and 10 single pulses (M1 only) were intermixed randomly and delivered at 5 s intervals. CBI is expressed as the average MEP amplitude evoked by the cerebellar-conditioned stimulation relative to the average MEP amplitude evoked by the unconditioned TMS pulses over M1.
Experiment 2: Transfer from right hand to right leg
Experimental protocol.
Each subject completed 11 behavioral blocks. No physiological measurements were tested in this experiment. (Fig. 1B). A total of 200 trials with the right foot (Baseline Foot) were followed by 200 trials with the right hand (Baseline Hand). As in Experiment 1, a screen–cursor 30° CW transformation was applied during Adapt1 and Adapt2 (48 trials each, right hand) to elicit adaptive learning. Immediately after Adapt1, the transformation was removed and eight trials in the foot (Catch Foot1) and hand (Catch Hand1) were performed to determine the presence of transfer of learning to the foot. Transfer was again assessed after Adapt2 with another nonperturbed eight trials with the foot and hand (Catch Foot2 and Catch Hand2). Participants then completed a final 120 hand movements without perturbation to wash out the learning (Post). Finally, another eight trials with the foot and hand were assessed to determine whether any previously seen transfer would wash out as well (Catch Foot3 and Catch Hand3). No physiological measurements were made.
Behavioral tasks.
Participants were seated in front of a computer screen as in Experiment 1. In addition, a bench was placed between the computer station and chair. The right leg was supported comfortably such that the foot was lifted a few inches of the floor, with the leg remaining parallel to the floor. For experimental blocks using the leg, the digitizing tablet was clamped vertically in front of the right foot. The digitizing pen was attached with a co-flex as an extension to the inner side of the bare foot. Feedback (both visual and auditory) of foot movements was the same as described for hand movements. To reduce the complexity of the performance with the foot, only four targets (up, down, left and right from the center) of the possible eight were presented; the order of appearance of these targets was random. The hand was exposed to the eight different target locations as described in Experiment 1.
Experiments 3 and 4: Premovement CBI changes due to simple reaction time task
Experimental protocol.
Each subject participated in two sessions testing CBI in the right hand (FDI; Experiment 3) or the right leg (TA; Experiment 4) during a simple reaction time task. In Experiment 3 only, a CBI recruitment curve for the FDI muscle was assessed at rest. In Experiment 4, a double-cone coil was used over M1 to elicit MEPs at rest for the TA muscle. For each experiment, subjects performed abduction of the right index finger in one session and dorsiflexion of the right foot in the other session, with session order counterbalanced across subjects. Paired- and single-pulse TMS was applied at intervals during movement preparation.
Behavioral tasks.
Subjects were seated in front of a computer monitor and instructed to respond to a visual (green circle) go signal by lifting either the right index finger or lifting the right foot in separate sessions. The go signal appeared at random intervals (5–7 s). Before the appearance of the go signal, subjects were instructed to remain relaxed and avoid anticipation of the cue. Response Time (RT) was defined as the interval between the go signal and the onset of the EMG burst in FDI or TA (Fig. 1C). At the beginning of each session, subjects were familiarized with the simple reaction time paradigm and 30 trials were performed to characterize each subject's individual RT to the go signal in the absence of TMS. A total of 120 trials were completed per session. Trials in which background EMG was detected before TMS onset were excluded from analysis.
EMG recordings and TMS protocol were identical to those in Experiment 1, with the following exceptions. EMG was recorded from either the right FDI in Experiment 3 or the right TA in Experiment 4 and TMS measures were made for the M1 representation of right FDI (Experiment 3) or right TA (Experiment 4). In addition, the stimulator output intensity needed to elicit MEPs of ∼1 mV (SI1 mV) both at rest and before movement onset was calculated. TMS before movement onset was triggered via a customized MATLAB function using the peri-triggering capability of Signal software.
The CBI protocol was identical to that in Experiment 1 except that the level of cerebellar–M1 connectivity for right FDI in Experiment 3 and for the right TA in Experiment 4 were measured. In addition, CS intensity for Experiment 3 was based on a CBI recruitment curve (RCCBI). In Experiment 4, the CS intensity was set at 5% below the brainstem motor threshold to the cerebellum. This intensity was selected because cerebellar–M1 connections for the TA muscle show reduced inhibition compared with the FDI muscle (Jayaram et al., 2011).
RCCBI was computed at rest before beginning the Experiment 3 behavioral session. This was done by decreasing cerebellar CS intensity by −5% steps below brainstem threshold using four different CS intensities (−5%, −10%, −15%, and −20% brainstem threshold). For each subject, the CS intensity inflection point collected from RCCBI was used for premovement CBI measurements (CBImove) to evaluate whether movement preparation elicits further inhibition or disinhibition.
For both Experiments 3 and 4, CBImove was measured at five different time intervals throughout the course of the simple reaction time paradigm in a pseudorandomized order (Fig. 1C; T1–T5). To do this, 12 paired pulses (cerebellum + M1) and 12 single pulses (M1 only) were measured for each time interval. As described previously, the different time intervals were tuned to each subject's RT of the task (Murase et al., 2004; Duque et al., 2005; Hummel et al., 2009), where T1 corresponded to cue onset and T2–T5 reflects 20%, 40%, 65%, and 90% of subject RT, respectively.
Statistical analysis
Statistical analysis was performed using SPSS software. For each block in Experiments 1 and 2, apart from catch trial blocks, initial error (mean error) was determined by averaging across epochs (Krakauer et al., 2005). Repeated-measures ANOVA (ANOVARM) was used to compare changes in CBI across time points (Baseline, Adapt1, and Adapt2) and muscles (Right FDI vs Right TA), and separately to compare mean movement error across time points (Baseline and Catch epochs) and limbs (hand vs foot in Experiment 2).
In Experiments 3 and 4, each participant's RTs were averaged in bins of 30 trials and a paired-sample t test was used to compare the first 30 RT trials to the last 30 trials to determine whether participants were improving their performance throughout the task. The magnitude of CBImove at T1–T5 was expressed relative to CBI recorded at rest [e.g., CBImove T5 modulation = (CBI at T5)/(CBI at rest) × 100%] to better characterize changes in CBI relative to rest. Subsequently, to compare changes in CBImove across sessions (finger-movements, foot-movements), ANOVARM with premovement timing (T1–T5) was used as a within-subjects factor. For Experiment 3, ANOVARM was also used to compare the effect of changing CS intensity (−5%, −10%, −15%, and −20% below brainstem threshold) on CBI (RCCBI).
For all experiments, when ANOVAs yielded significant results, post hoc analyses were conducted using Tukey's HSD tests.
Results
Experiment 1: CBI decreases after visuomotor adaptation
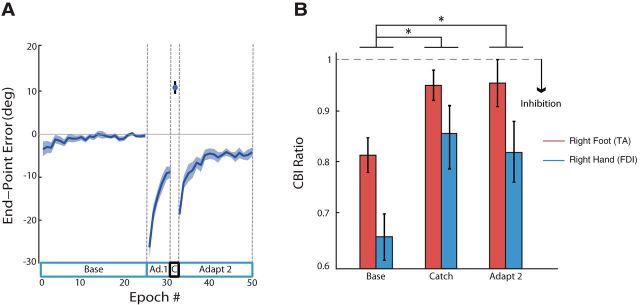
When the 30° CW visuomotor transformation was applied in Adapt1, large initial errors were observed (first epoch mean ± SE was −26.45 ± 2.24°; Fig. 3A). When the visuomotor transformation was removed for a Catch epoch after 48 movements, subjects displayed partial learning of the rotation, as indicated by counter-CW errors (11.08 ± 0.97°). When the perturbation resumed at the beginning of Adapt2, error reduction continued and, by the end of Adapt2, subjects had compensated for 25.92 ± 1.03° of the original 30° perturbation.
Figure 3.
Experiment 1 Results. A, End point error (blue line) with SEs (shaded region) during baseline (Base), adaptation (Adapt1 and Adapt2), and catch trials (C). Negative values indicate clockwise deviations caused by the visuomotor perturbation. B, Physiological measure of CBI for both the right FDI (blue) and TA (red). CBI was recorded before any movements (Base) and immediately after catch trials (Catch) and late adaptation (Adapt2). *CBI decreased significantly for both muscle effectors after early perturbation exposure.
The magnitude of CBI for both the FDI and TA muscles (hand and leg) was reduced after adaptation with the right hand, indicating disinhibition (Fig. 3B). An ANOVARM on CBI ratio values revealed a time effect (F(2,36) = 9.785; p < 0.01), but no effect on muscle group (F(1,18) = 2.74; p = 0.12) and, importantly, no interaction (F(2,36) = 0.16; p = 0.90). Post hoc tests revealed that CBI after Catch and after Adapt2 was significantly different from baseline CBI (p < 0.01; p = 0.01, respectively), suggesting that visuomotor adaptation with the hand results in a significant reduction in CBI for both FDI and TA muscles.
Experiment 2: Right hand learning transfers to right foot movements
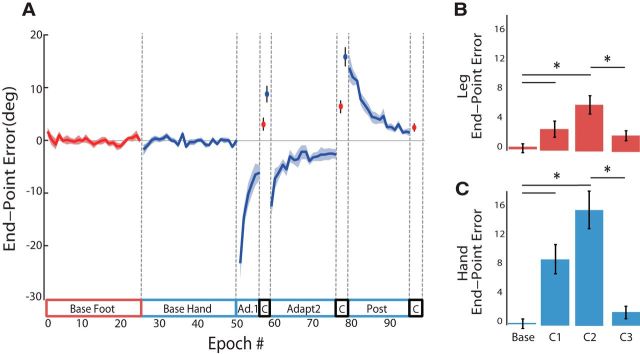
As in Experiment 1, subjects in Experiment 2 were able to almost completely adjust their hand movements to the 30° CW visuomotor rotation (mean error of final epoch in Adapt2 was −2.32 ± 1.11°; Fig. 4). When comparing behavioral blocks across right hand and right leg (Baseline, Catch1, Catch2, and Catch3), ANOVARM revealed a significant effect of time (F(2,36) = 24.94; p < 0.01), limb (F(1,36) = 9.44, p = 0.01) and a limb × time interaction effect (F(2,36) = 5.63; p < 0.01). Post hoc analysis revealed that movement error in Catch Foot1 and Catch Foot2 were each different from Base Foot (p = 0.05, 0.01), suggesting that the right hand's adaptation transferred to the foot. Critically, Post hoc analysis also revealed a difference in Catch Hand1 and Catch Hand2 being different from Base Hand (both p < 0.01). A comparison of foot aftereffects (Catch Foot 1–2) with hand aftereffects (Catch Hand 1 and the first epoch of Post 1) indicated that the amount of transfer at Foot Catch1 was 42.3%, and 42.2% at Foot Catch2. Post hoc tests also showed that Catch Foot2 and Catch Foot3 were different from each other (p < 0.01), suggesting that transfer of learning from hand to foot had degraded after washout of learning from the hand.
Figure 4.
Experiment 2 Results. A, End point error and SEs for right hand (blue) and right leg (red) movements. Negative values indicate clockwise deviation. B, C, Mean end-point errors in degrees (±SEM) for right leg (red) and right hand (blue) for the baseline and three catch trial epochs. Post hoc analysis revealed significant changes in error for both effectors, indicating hand-to-foot transfer.
Experiments 3 and 4: CBI changes in a somatotopy-specific manner
RTs
Participants did not improve their RT throughout the experiment. For Experiment 3, the baseline average RT for the hand (176.1 ± 6.2 ms) was not significantly different from RT from the last 30 hand trials (174.0 ± 5.6 ms; t(11) = 1.242, p = 0.24). Baseline foot RT (191.9 ± 8.7 ms) was also not different from the last 30 foot trials (190.3 ± 7.5 ms; t(11) = 0.8, p = 0.44). Similarly, in Experiment 4, baseline foot RT (196.0 ± 5.7 ms) was not different from the last 30 foot trials (193.3 ± 5.4 ms; t(7) = 0.971, p = 0.36) and baseline hand RT (181.2 ± 7.3 ms) was not different from the last 30 hand trials (179.9 ± 6.7 ms; t(7) = 0.759, p = 0.47).
CBI recruitment curve (Experiment 3 only)
ANOVARM comparing the CBI ratio across CS intensity revealed a significant effect for CS intensity (F(1,11) = 7.926, p < 0.01). Critically, TS MEP amplitudes were not significantly different across different CS conditions (F(1,11) = 0.029, p = 0.87), suggesting that the CBI changes were due to changing CS intensities. As a result, the mean CS intensity set for CBImove was 72.9 ± 0.74% of the stimulator output, yielding a rest CBI ratio response of 0.82 ± 0.07.
Premovement CBImove
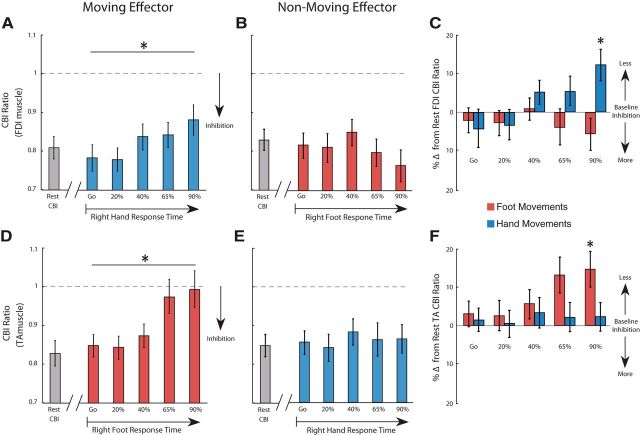
To assess whether CBI changes are somatotopy specific, we measured CBImove for the FDI (Experiment 3) and TA (Experiment 4) muscle representation when participants were asked to either move the right index finger or right foot. In Experiment 3, when participants made movements with their finger, ANOVARM revealed an effect of FDI CBImove for RT (F(4,44) = 8.192, p = 0.02; Fig. 5A). Conversely, when FDI CBImove was recorded in preparation of foot movements, ANOVARM failed to find the same effect (F(4,44) = 3.214, p = 0.22; Fig. 5B). These results indicate that, in the absence of learning, CBI changes of the FDI muscle occurs during the preparation of finger movements, but not the foot. In Experiment 4, when participants made movements with the right foot, ANOVARM on TA CBImove values revealed a time effect (F(4,28) = 4.920; p < 0.01; Fig 5D). Conversely, when TA CBImove was recorded in preparation of finger movements, ANOVARM did not reveal the same effect (F(4,28) = 0.241, p > 0.50; Fig. 5E). Together, the results from Experiment 3 and 4 suggest a somatotopic effect of CBI changes during movement preparation.
Figure 5.
CBImove. A, B, The x-axis represents CBImove for the right FDI in preparation to moving the hand (A) and foot (B). FDI CBImove measured at five timings (T1–T5) with respect to individual mean response times separately for the hand (blue) and foot (red). *CBI was reduced significantly only in preparation of hand movements. C, CBImove was calculated as the percentage difference from FDI CBI obtained at rest. D, E, The x-axis represents CBImove for the right TA in preparation to moving the foot (D) and hand (E). F, Percentage difference from TA CBI obtained at rest. Positive values indicate disinhibition and negative values increased inhibition. *Only hand movements at 90% RT (T5) modulated CBImove. Data are shown as mean ± SEM.
Furthermore, to compare directly the changes in FDI CBImove between preparation of finger movements and foot movements, we subtracted the amount of CBI measured at rest for each session. Here, ANOVARM revealed an effect of FDI CBImove for the group (finger, foot; F(1,22) = 6.214, p = 0.02) and RT (Go, T1, …, T5) × group interaction (F(4,88) = 4.713, p = 0.01; Fig. 5C). Specifically, CBImove recorded during movement preparation at 90% RT (T5) was significantly different from CBI assessed at cue representation (p = 0.03), indicating that specific FDI CBI changes occur just before movement onset. We performed the same analysis to compare the changes in TA CBImove. Similar to the results of Experiment 3, ANOVARM showed an effect of TA CBImove for group (foot, finger; F(1,14) = 7.911, p = 0.02) and the RT (Go, T1, …, T5) × group interaction (F(4,56) = 3.210, p = 0.02; Fig. 5F).
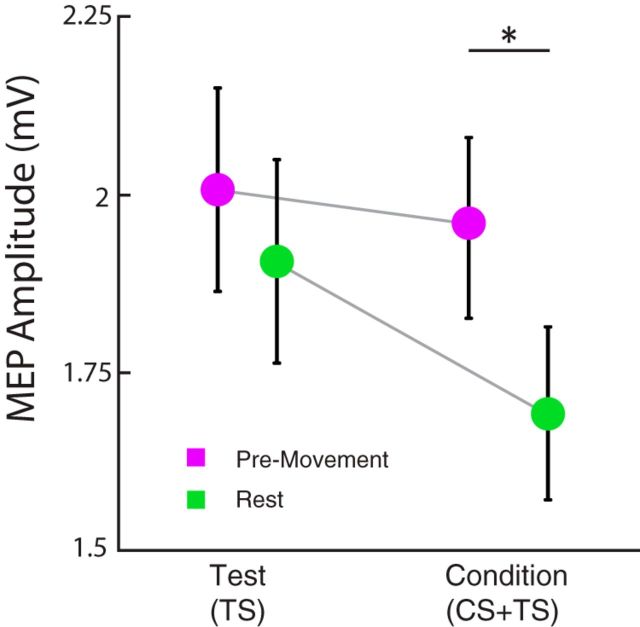
Importantly, we determined that modulation of CBI were due to changes in conditioned (CS + TS) responses from the cerebellum and not due to changes in TS responses from M1. Although ANOVARM revealed a significant effect of TS MEP amplitude during finger movement preparation (Go, T1, …, T5; F(4,44) = 14.823, p = 0.001), we controlled for this confound by measuring FDI CBI at rest with a matched TS MEP amplitude observed at the 90% RT of CBImove (TS ∼2 mV; Fig. 6). When we compared TS and CS+TS MEP amplitudes between rest and premovement measurements using matched TS responses, two-way ANOVARM showed a significant interaction of MEP amplitudes for state (rest, premovement) × condition (TS, CS+TS; F(1,22) = 6.295, p = 0.043). Specifically, CS + TS MEP amplitude for premovement was different from rest (p = 0.02) despite having comparable TS MEP amplitude (p = 0.458). This indicates that the changes in FDI CBImove observed at 90% RT are due to changes in cerebellar excitability (CS + TS), not to higher M1 excitability.
Figure 6.
Rest and premovement TS and CS + TS MEP amplitudes. For each participant, we assessed CBI at rest matching the TS MEP amplitudes obtained during CBImove at 90% of RT (TS). CS + TS MEP amplitude (CBI) was only present at rest (green), not when assessed in the context of movement (purple). This indicates that the reduction of CBImove is not due to increased excitability in M1.
Discussion
Here, we addressed the question of whether changes in cerebellar–M1 connectivity (CBI) are somatotopy specific in the presence or absence of adaptive motor learning. When individuals learned a visuomotor rotation with the hand, CBI changed for both the involved hand and idle ipsilateral foot. The transfer of learning that we observed between hand and foot could explain why CBI changed in both representations. We disentangled this issue by measuring CBI in the context of movement preparation of a well characterized behavior with no learning. We found that CBI for the hand only changed when participants prepared to make hand movements and not foot movements. Similarly, CBI measured for the foot only changed with preparation of foot movements. This indicates that, in the absence of learning and transfer of learning, CBI only modulates for the effector involved in the movement. These results address an important physiological question, showing that modulation of cerebellar–M1 connectivity responses reflect a somatotopy-specific mechanism.
CBI changes for multiple representations are related to a somatotopy-specific mechanism
Previously, we showed that CBI changes for the trained hand in a visuomotor adaptation task (Schlerf et al., 2012), as well as for the trained leg during locomotor adaptation (Jayaram et al., 2011). The present study extends these findings by showing that adaptive learning with the hand can produce changes in CBI for muscle representations not involved in the task. Interestingly, our second experiment showed transfer of learned movements experienced with the right hand to the right foot, which we would expect to cause CBI changes in both representations. Therefore, the nonspecific changes in cerebellar excitability observed in our first experiment are likely related to a transfer of learning from hand to leg. Results from our final two experiments show that, in the absence of learning, CBI modulates specifically for the muscle involved in movement preparation. Together, these findings indicate that cerebellar–M1 connectivity changes are somatotopy specific.
Premovement CBI Changes versus Learning-Induced CBI changes
We observed CBI changes during both movement preparation and after adaptive learning, but interpret these results to be driven by different mechanisms; premovement CBI is likely due to activity patterns of Purkinje cells (PCs) and deep cerebellar nuclei (DCN), whereas learning-induced changes in CBI reflect cerebellar plastic changes.
Although the physiology of cerebellar TMS remains poorly understood, it is possible that stimulation results in the parallel-fiber-mediated activation of PCs, which inhibit the DCN (Celnik, 2015). The reduced CBI closer to movement initiation in this study may therefore represent a decrease of inhibition from hand- or leg-affiliated PCs that activate hand- or leg-affiliated DN cells, respectively. Animal studies have shown burst activity of DCN during preparation of limb movement, in which inactivation of the cerebellum results in delay of M1 activity and delay in the initiation of movements (Brooks, 1975; Miller and Brooks, 1982). In addition, suppression of optogenetically modified PCs in rodents can activate dentate cells (Heiney et al., 2014), suggesting that the onset of activity in DCNs results from disinhibition by PCs. Furthermore, a recent study in monkeys showed that, before wrist movement onset, wrist-affiliated PCs were suppressed whereas wrist-affiliated DN cells showed concurrent burst activity without prior suppression (Ishikawa et al., 2014). Therefore, during the preparation of a specific muscle movement, the cerebellar cortex may reduce its inhibition to M1 via the cerebello-thalamo-M1 pathway, consistent with the results found in this study.
In contrast, we interpret the CBI changes in learning to reflect the plastic changes in cerebellar output that are responsible for changing motor behavior during adaptation. Although the cerebellum contains multiple sites and forms of plasticity (Boyden et al., 2004; Jörntell and Hansel, 2006; Gao et al., 2012), two plasticity sites have been shown to be important for motor learning: long-term potentiation (LTP) of mossy fibers and interneurons in the cerebellar cortex (D'Angelo, 2005; Grasselli and Hansel, 2014) and long-term depression (LTD) of parallel fiber–PC synapses. In particular, animal studies have associated LTD in PCs with adaptive learning, triggered by climbing fiber inputs driven by inaccurate movements (Gilbert and Thach, 1977; Medina and Lisberger, 2008; Yang and Lisberger, 2014). Because errors are prevalent early in adaptive learning, we interpret our CBI changes to reflect reduced PCs activity. Accordingly, if PCs are less excitable, then a conditioning stimulus would be less likely to engage the cerebellar-dentate-thalamic pathway, which would result in less M1 inhibition, consistent with the results of this study. However, it is important to consider that LTP of parallel fibers and inhibitory interneurons can result in the same net effect as LTD of parallel fiber–PC synapses (D'Angelo, 2014; Jörntell, 2017). Therefore, it is possible that multiple plasticity mechanisms throughout the cerebellum operate in learning a new behavior (Medina and Mauk, 2000; Jörntell and Ekerot, 2003; Yang and Lisberger, 2014; Mapelli et al., 2015).
The novel finding of this study is that learning-related changes in CBI are somatotopic specific. As described previously (Morton and Bastian, 2004; Savin and Morton, 2008), we found that adaptive learning transfers between the arm and leg. In addition to this behavioral finding, we show a similar effect using a cerebellar physiological measure. Therefore, the transfer in CBI changes found in Experiment 2 appears related to the transfer of learning because CBI follows a somatotopy-specific pattern when assessed in the context of movement preparation.
Hand-to-leg transfer may be mediated by interactions between overlapping cerebellar representations
Several neuroimaging studies have demonstrated gross motor somatotopy for upper and lower limb representations within the cerebellar cortex (Nitschke et al., 1996; Rijntjes et al., 1999; Grodd et al., 2001; Stoodley and Schmahmann, 2009; Schlerf et al., 2010; Buckner et al., 2011) and the dentate nucleus (Dimitrova et al., 2006; Küper et al., 2012). In addition, studies in nonhuman primates have reported that dentate nucleus somatotopic representation of the lower limb is located more rostrally compared with the upper limb (Allen et al., 1978; Rispal-Padel et al., 1982; van Kan et al., 1994). Indeed, retrograde axonal transport of neurotropic viruses injected in different body representations of M1 demonstrated a rostral–caudal output organization of leg, arm, and face representations in the dentate nucleus (Dum and Strick, 2003; Lu et al., 2007). However, there is also evidence of anatomical overlap for these representations. For example, electrical stimulation of the cerebellar nuclei can cause concurrent movement of lower and upper limbs (Rispal-Padel et al., 1982) and, in some cases, dentate neurons reacted to both lower and upper limb movements (van Kan et al., 1994). In humans, fMRI studies have suggested that there is extensive overlap between finger and foot movement activation when looking at group analysis of cerebellar cortex (Rijntjes et al., 1999) and dentate nucleus (Küper et al., 2012) activation.
It is not known to what extent the overlapping arm and leg representations can interact with each other, but the microarchitecture of the cerebellar cortex raises the possibility that such interactions may occur within the cerebellum and serve as a substrate of ipsilateral transfer. A single mossy fiber may have synaptic contacts with 448 cerebellar granule cells (Ito, 1984) and the parallel fibers of each granule cell branch and excite hundreds of Purkinje cells up to several millimeters from the branch point (Fox and Barnard, 1957). Climbing fibers also branch and, although each Purkinje cell receives only one climbing fiber input, each climbing fiber may synapse with 10 Purkinje cells (Eccles et al., 1966). Therefore, it is possible that some Purkinje cells that stimulate the leg representation receive both parallel fiber and climbing fiber inputs from the arm and are therefore able to undergo plastic modifications in response to visuomotor learning with the ipsilateral arm, leading to transfer. An alternative possibility is that, within a cerebellar hemisphere, arm and leg representations interact with each other through the large network of inhibitory interneurons present throughout cerebellar cortex (Ito, 1984). For example, a single Golgi cell receives ∼228 mossy fiber and ∼4788 parallel fiber inputs, in addition to inputs from climbing fibers, Purkinje collaterals, and other interneurons, and inhibits up to 5700 granule cells (Ito, 1984). Golgi cells and other cerebellar interneurons are thought to be involved in the specificity and modulation of cerebellar cortical computations (Ito, 1984).
Future directions
In addition to demonstrating that cerebellar–M1 connectivity is organized somatotopically, we describe a novel method to measure cerebellar–M1 connectivity physiology during movement preparation. This is important because previous investigations have only provided evidence that the cerebellum exerts an influence on M1 at rest or in an indirect manner. For example, premovement facilitation of MEPs normally observed in response to M1 TMS is reduced in patients with spinocerebellar degeneration (Nomura et al., 2001) and unilateral cerebellar stroke (Battaglia et al., 2006) and this has been linked with deficits in motor preparation and motor imagery in these populations. Therefore, the paradigm used in this study could be used in future research to assess context-dependent (premovement) physiological changes in patients to further understand the role of the cerebellum in movement preparation. Furthermore, future investigations will need to address what aspects of behavioral transfer are associated with changes in cerebellar–M1 connectivity.
Footnotes
This work was supported by the National Institute of Child Health, and Development, National Institutes of Health (Grants R01HD053793 and 1F31HD078130-01A1). We thank Tziporah Thompson for helping with the experimental design and figures.
The authors declare no competing financial interests.
References
- Allen GI, Tsukahara N (1974) Cerebrocerebellar communication systems. Physiol Rev 54:957–1006. [DOI] [PubMed] [Google Scholar]
- Allen GI, Gilbert PF, Yin TC (1978) Convergence of cerebral inputs onto dentate neurons in monkey. Exp Brain Res 32:151–170. [DOI] [PubMed] [Google Scholar]
- Balitsky Thompson AK, Henriques DY (2010) Visuomotor adaptation and intermanual transfer under different viewing conditions. Exp Brain Res 202:543–552. 10.1007/s00221-010-2155-0 [DOI] [PubMed] [Google Scholar]
- Battaglia F, Quartarone A, Ghilardi MF, Dattola R, Bagnato S, Rizzo V, Morgante L, Girlanda P (2006) Unilateral cerebellar stroke disrupts movement preparation and motor imagery. Clin Neurophysiol 117:1009–1016. 10.1016/j.clinph.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Block H, Celnik P (2013) Stimulating the cerebellum affects visuomotor adaptation but not intermanual transfer of learning. Cerebellum 12:781–793. 10.1007/s12311-013-0486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Raymond JL (2004) Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci 27:581–609. 10.1146/annurev.neuro.27.070203.144238 [DOI] [PubMed] [Google Scholar]
- Brooks VB. (1975) Roles of cerebellum and basal ganglia in initiation and control of movements. Can J Neurol Sci 2:265–277. 10.1017/S0317167100020369 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345. 10.1152/jn.00339.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MR. (2011) Synaptic mechanisms of sensorimotor learning in the cerebellum. Curr Opin Neurobiol 21:609–615. 10.1016/j.conb.2011.06.011 [DOI] [PubMed] [Google Scholar]
- Celnik P. (2015) Understanding and modulating motor learning with cerebellar stimulation. Cerebellum (London, England) 14:171–174. 10.1007/s12311-014-0607-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hua SE, Smith MA, Lenz FA, Shadmehr R (2006) Effects of human cerebellar thalamus disruption on adaptive control of reaching. Cereb Cortex 16:1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R (2003) Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89:168–176. [DOI] [PubMed] [Google Scholar]
- D'Angelo E. (2005) Synaptic plasticity at the cerebellum input stage: mechanisms and functional implications. Arch Ital Biol 143:143–156. [PubMed] [Google Scholar]
- D'Angelo E. (2014) The organization of plasticity in the cerebellar cortex: from synapses to control. Prog Brain Res 210:31–58. 10.1016/B978-0-444-63356-9.00002-9 [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R (2004) Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol 557:689–700. 10.1113/jphysiol.2003.059808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R (2005) Neural correlates of reach errors. J Neurosci 25:9919–9931. 10.1523/JNEUROSCI.1874-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova A, de Greiff A, Schoch B, Gerwig M, Frings M, Gizewski ER, Timmann D (2006) Activation of cerebellar nuclei comparing finger, foot and tongue movements as revealed by fMRI. Brain Res Bull 71:233–241. 10.1016/j.brainresbull.2006.09.015 [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL (2003) An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol 89:634–639. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG (2005) Transcallosal inhibition in chronic subcortical stroke. Neuroimage 28:940–946. 10.1016/j.neuroimage.2005.06.033 [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinás R, Sasaki K (1966) The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol 182:268–296. 10.1113/jphysiol.1966.sp007824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC, Craig AD (2008) Retrograde analysis of the cerebellar projections to the posteroventral part of the ventral lateral thalamic nucleus in the macaque monkey. J Comp Neurol 508:286–314. 10.1002/cne.21674 [DOI] [PubMed] [Google Scholar]
- Fox CA, Barnard JW (1957) A quantitative study of the Purkinje cell dendritic branchlets and their relationship to afferent fibres. J Anat 91:299–313. [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P (2009) Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29:9115–9122. 10.1523/JNEUROSCI.2184-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P (2011) Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21:1761–1770. 10.1093/cercor/bhq246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, van Beugen BJ, De Zeeuw CI (2012) Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci 13:619–635. 10.1038/nrn3312 [DOI] [PubMed] [Google Scholar]
- Gilbert PF, Thach WT (1977) Purkinje cell activity during motor learning. Brain Res 128:309–328. 10.1016/0006-8993(77)90997-0 [DOI] [PubMed] [Google Scholar]
- Grasselli G, Hansel C (2014) Cerebellar long-term potentiation: cellular mechanisms and role in learning. Int Rev Neurobiol 117:39–51. 10.1016/B978-0-12-420247-4.00003-8 [DOI] [PubMed] [Google Scholar]
- Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M (2001) Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13:55–73. 10.1002/hbm.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R (2007) Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci 27:13413–13419. 10.1523/JNEUROSCI.2570-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiney SA, Kim J, Augustine GJ, Medina JF (2014) Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J Neurosci 34:2321–2330. 10.1523/JNEUROSCI.4547-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL (1999) The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci 19:1446–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Steven B, Hoppe J, Heise K, Thomalla G, Cohen LG, Gerloff C (2009) Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology 72:1766–1772. 10.1212/WNL.0b013e3181a609c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Tomatsu S, Tsunoda Y, Lee J, Hoffman DS, Kakei S (2014) Releasing dentate nucleus cells from Purkinje cell inhibition generates output from the cerebrocerebellum. PLoS One 9:e108774. 10.1371/journal.pone.0108774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. (1984) The cerebellum and neural control. Baltimore: Raven. [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, Celnik P (2011) Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex 21:1901–1909. 10.1093/cercor/bhq263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Brayanov JB, Smith MA (2013) The training schedule affects the stability, not the magnitude, of the interlimb transfer of learned dynamics. J Neurophysiol 110:984–998. 10.1152/jn.01072.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H. (2017) Cerebellar physiology: links between microcircuitry properties and sensorimotor functions. J Physiol 595:11–27. 10.1113/JP272769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Ekerot CF (2003) Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J Neurosci 23:9620–9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Hansel C (2006) Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 52:227–238. 10.1016/j.neuron.2006.09.032 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF (2005) Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci 25:473–478. 10.1523/JNEUROSCI.4218-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küper M, Thürling M, Stefanescu R, Maderwald S, Roths J, Elles HG, Ladd ME, Diedrichsen J, Timmann D (2012) Evidence for a motor somatotopy in the cerebellar dentate nucleus–an FMRI study in humans. Hum Brain Mapp 33:2741–2749. 10.1002/hbm.21400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Miyachi S, Ito Y, Nambu A, Takada M (2007) Topographic distribution of output neurons in cerebellar nuclei and cortex to somatotopic map of primary motor cortex. Eur J Neurosci 25:2374–2382. 10.1111/j.1460-9568.2007.05482.x [DOI] [PubMed] [Google Scholar]
- Mapelli L, Pagani M, Garrido JA, D'Angelo E (2015) Integrated plasticity at inhibitory and excitatory synapses in the cerebellar circuit. Front Cell Neurosci 9:169. 10.3389/fncel.2015.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT (1996) Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119:1183–1198. 10.1093/brain/119.4.1183 [DOI] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG (2008) Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci 11:1185–1192. 10.1038/nn.2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Mauk MD (2000) Computer simulation of cerebellar information processing. Nat Neurosci 3:1205–1211. 10.1038/81486 [DOI] [PubMed] [Google Scholar]
- Miller AD, Brooks VB (1982) Parallel pathways for movement initiation of monkeys. Exp Brain Res 45:328–332. 10.1007/BF01208592 [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ (2004) Prism adaptation during walking generalizes to reaching and requires the cerebellum. J Neurophysiol 92:2497–2509. 10.1152/jn.00129.2004 [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ (2006) Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26:9107–9116. 10.1523/JNEUROSCI.2622-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG (2004) Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55:400–409. 10.1002/ana.10848 [DOI] [PubMed] [Google Scholar]
- Nitschke MF, Kleinschmidt A, Wessel K, Frahm J (1996) Somatotopic motor representation in the human anterior cerebellum. A high-resolution functional MRI study. Brain 119:1023–1029. 10.1093/brain/119.3.1023 [DOI] [PubMed] [Google Scholar]
- Nomura T, Takeshima T, Nakashima K (2001) Reduced pre-movement facilitation of motor evoked potentials in spinocerebellar degeneration. J Neurol Sci 187:41–47. [DOI] [PubMed] [Google Scholar]
- Pinto AD, Chen R (2001) Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res 140:505–510. 10.1007/s002210100862 [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Buechel C, Kiebel S, Weiller C (1999) Multiple somatotopic representations in the human cerebellum. Neuroreport 10:3653–3658. 10.1097/00001756-199911260-00035 [DOI] [PubMed] [Google Scholar]
- Rispal-Padel L, Cicirata F, Pons C (1982) Cerebellar nuclear topography of simple and synergistic movements in the alert baboon (Papio papio). Exp Brain Res 47:365–380. [DOI] [PubMed] [Google Scholar]
- Rossini PM, et al. (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application: an updated report from an I.F.C.N. Committee. Clin Neurophysiol 126:1071–1107. 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J (2002) Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145:437–447. 10.1007/s00221-002-1140-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin DN, Morton SM (2008) Asymmetric generalization between the arm and leg following prism-induced visuomotor adaptation. Exp Brain Res 186:175–182. 10.1007/s00221-007-1220-9 [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Verstynen TD, Ivry RB, Spencer RM (2010) Evidence of a novel somatopic map in the human neocerebellum during complex actions. J Neurophysiol 103:3330–3336. 10.1152/jn.01117.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Bastian AJ, Celnik PA (2012) Dynamic modulation of cerebellar excitability for abrupt, but not gradual, visuomotor adaptation. J Neurosci 32:11610–11617. 10.1523/JNEUROSCI.1609-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Spampinato D, Celnik PA (2015) Laterality differences in cerebellar-motor cortex connectivity. Cereb Cortex 25:1827–1834. 10.1093/cercor/bht422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille M, Gao Z, Boele HJ, Veloz MF, Amerika WE, Simek AA, De Jeu MT, Steinberg JP, Takamiya K, Hoebeek FE, Linden DJ, Huganir RL, De Zeeuw CI (2011) Reevaluating the role of LTD in cerebellar motor learning. Neuron 70:43–50. 10.1016/j.neuron.2011.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW (2010) Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33:89–108. 10.1146/annurev-neuro-060909-153135 [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Futami T, Kano M (1985) Synaptic organization of the cerebello-thalamo-cerebral pathway in the cat. II. Input-output organization of single thalamocortical neurons in the ventrolateral thalamus. Neurosci Res 2:157–180. 10.1016/0168-0102(85)90010-0 [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R (2005) Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol 93:2809–2821. 10.1152/jn.00943.2004 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44:489–501. 10.1016/j.neuroimage.2008.08.039 [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Ferlazzo F, Caltagirone C, Petrosini L (2011) Changes in cerebello-motor connectivity during procedural learning by actual execution and observation. J Cogn Neurosci 23:338–348. 10.1162/jocn.2010.21471 [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ (2007) Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98:54–62. 10.1152/jn.00266.2007 [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I (1995) Magnetic stimulation over the cerebellum in humans. Ann Neurol 37:703–713. 10.1002/ana.410370603 [DOI] [PubMed] [Google Scholar]
- van Kan PL, Horn KM, Gibson AR (1994) The importance of hand use to discharge of interpositus neurones of the monkey. J Physiol 480:171–190. 10.1113/jphysiol.1994.sp020351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2004) Interlimb transfer of novel inertial dynamics is asymmetrical. J Neurophysiol 92:349–360. 10.1152/jn.00960.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH (1983) Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33:766–772. 10.1212/WNL.33.6.766 [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Taylor J, Ridding M, Meyer BU, Rothwell JC (1996) Effect of transcranial magnetic stimulation over the cerebellum on the excitability of human motor cortex. Electroencephalogr Clin Neurophysiol 101:58–66. 10.1016/0013-4694(95)00213-8 [DOI] [PubMed] [Google Scholar]
- Yang Y, Lisberger SG (2014) Role of plasticity at different sites across the time course of cerebellar motor learning. J Neurosci 34:7077–7090. 10.1523/JNEUROSCI.0017-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]