Abstract
A previous study revealed that, although monkeys with bilateral lesions of either the orbitofrontal cortex (OFC) or the amygdala could learn an action–outcome task, they could not adapt their choices in response to devalued outcomes. Specifically, they could not adjust their choice between two actions after the value of the outcome associated with one of the actions had decreased. Here, we investigated whether OFC needs to interact functionally with the amygdala in mediating such choices. Rhesus monkeys were trained to make two mutually exclusive actions on a touch-sensitive screen: “tap” and “hold.” Taps led to the availability of one kind of food outcome; holds produced a different food. On each trial, monkeys could choose either a tap or a hold to earn the corresponding food reward. After consuming one of the two foods to satiety, monkeys were then tested on their ability to adapt their choices in response to the updated relative valuation of the two predicted outcomes. Whereas intact (control) monkeys shifted their choices toward the action associated with the higher value (nonsated) food, monkeys with crossed surgical disconnection of the amygdala and OFC did not. These findings demonstrate that amygdala–OFC interactions are necessary for choices among actions based on the updated value of predicted outcomes and they also have a bearing on the idea that OFC specializes in stimulus- or object-based choices in contrast to action- or response-based choices.
SIGNIFICANCE STATEMENT Dysfunctional interactions between orbitofrontal cortex (OFC) and the amygdala underlie several mental health disorders, often related to value-based decision making. Understanding the underlying neural circuitry may help to develop therapies for those suffering from mood and anxiety disorders and provide insight into addiction. Here, we investigated whether the amygdala must interact with OFC to make adaptive choices. Monkeys learned to perform two different actions, “tap” for one kind of food reward and “hold” for another, and then one of the two foods was devalued temporarily. Intact monkeys shifted their choice to whichever action produced the higher-value food; monkeys with crossed surgical disconnection of OFC and the amygdala did not. Therefore, OFC and the amygdala must interact functionally to mediate adaptive choices.
Keywords: action value, decision making, medial frontal cortex, orbital frontal cortex, reward value
Introduction
Studies of reward-based decision making in macaques and humans have pointed to contrasting specializations for medial and orbital components of the frontal lobe. Orbital frontal cortex (OFC) has been implicated in stimulus–outcome associations and therefore in object- or stimulus-based choices, whereas medial frontal cortex (MFC) has been implicated in action–outcome associations and therefore in action- or response-based choices (Rushworth et al., 2007; Rudebeck et al., 2008; Camille et al., 2011; Chudasama et al., 2013). For economy of reference, we will refer to this idea as the “stimulus–action dichotomy.” The amygdala also plays a role in reward-based decision making; when multiple food outcomes are available, the basolateral amygdala is essential for registering changes in the value of rewards (Wellman et al., 2005).
To examine the neural basis of action–outcome associations, Rhodes and Murray (2013) devised an action–outcome task in which monkeys chose between two mutually exclusive actions performed on a touch-sensitive screen. The two actions led to delivery of different food rewards (outcomes). After training monkeys to perform this task, the investigators used a devaluation procedure, selective satiation, to change the relative value of the two potential outcomes. They found that monkeys with either bilateral amygdala lesions or bilateral OFC lesions were impaired in adapting their choices based on updated valuations. Although the disruptive effects of amygdala lesions had been expected, those after OFC lesions had not. Until recently (Gourley et al., 2013; Gremel and Costa, 2013; Bradfield et al., 2015), most studies of choices among actions and action–outcome learning pointed to MFC, not OFC (Ostlund and Balleine, 2007; Rudebeck et al., 2008; Camille et al., 2011), as the critical substrate. At first glance, the findings of Rhodes and Murray (2013) seem to contradict the idea that OFC specializes in representing stimulus–outcome associations as opposed to action–outcome associations.
To follow up on these findings, we investigated whether OFC needs to interact functionally with the amygdala in mediating adaptive, action-based choices. A positive finding after crossed disconnection of the amygdala and OFC would bolster our previous findings regarding the neural basis for action-based choices. In addition, it is well established that a choice between two objects based on the value of predicted outcomes requires amygdala–OFC interaction (Baxter et al., 2000). If we obtained a similar finding for choices between two actions, then this would provide additional support for the idea that OFC is important for adaptive choices based on predicted outcomes generally regardless of whether the choice is between actions or between objects. A negative finding would indicate that the amygdala and OFC do not need to interact functionally to enable macaques to make such choices despite their reciprocal anatomical connections. That result would be more consistent with the stimulus–action dichotomy.
Materials and Methods
Subjects
A total of 13 male rhesus monkeys (Macaca mulatta), weighing between 4.9 and 12.5 kg at the beginning of the experiment, were used (Table 1). Four monkeys received crossed lesions of the amygdala and OFC (AMG × OFC). Nine monkeys served as unoperated controls; seven were historical controls (Rhodes and Murray, 2013) and two were tested concurrently with the operated group. Surgery was conducted before training.
Table 1.
Weights of individual monkeys at the beginning of training
| Monkey | Weight (kg) |
|---|---|
| AMYG × OFC | |
| Case 1 | 7.0 |
| Case 2 | 5.3 |
| Case 3 | 6.2 |
| Case 4 | 5.9 |
| Mean | 6.1 |
| Controls | |
| Case 1 | 4.9 |
| Case 2 | 7.1 |
| Case 3 | 8.9 |
| Case 4 | 8.6 |
| Case 5 | 8.2 |
| Case 6 | 12.5 |
| Case 7 | 8.3 |
| Case 8 | 8.7 |
| Case 9 | 9.1 |
| Mean | 8.5 |
All monkeys were housed individually in a temperature- and humidity-controlled room on a 12 h light/dark cycle (lights on at 7:00 A.M.) and testing occurred during the light period. During the study, the monkeys were given controlled access to primate chow supplemented with fruit to ensure sufficient motivation to respond in the test apparatus.
Surgery
Four monkeys received surgery in two stages to produce crossed surgical disconnection of the amygdala and OFC; that is, removal of the amygdala in one hemisphere and of OFC in the other hemisphere. Two monkeys received amygdala lesions as their first surgery followed by the OFC lesion, whereas the remaining two monkeys received operations in the reverse order. The site of lesions was also balanced across hemispheres.
Lesions of the amygdala and OFC were produced by injection of the excitotoxin ibotenic acid. Aseptic procedures were used. In each case, anesthesia was induced with ketamine hydrochloride (10 mg/kg, i.m.) and maintained with isoflurane (1.0–3.0%, to effect). Heart rate, respiration rate, blood pressure, expired CO2, and body temperature were monitored during surgery and isotonic fluids were given throughout. After completing the series of ibotenate injections, the surgical site was closed in anatomical layers with sutures. The preoperative and postoperative treatment regimen consisted of dexamethasone sodium phosphate (0.4 mg/kg, i.m.) and cefazolin antibiotic (15 mg/kg, i.m.) for 1 d before surgery and 1 week after surgery to reduce swelling and prevent infection, respectively. At the end of surgery and for 2 additional days, the monkeys received the analgesic ketoprofen (10–15 mg, i.m.), followed by ibuprofen (100 mg) for the following 5 d. Operations were separated by a minimum of 2 weeks.
Monkeys receiving an amygdala lesion were anesthetized and then placed in a stereotaxic frame. A large bone flap was turned over the appropriate portion of the cranium. The injection sites were calculated based on landmarks that were visible on MRI scans obtained before surgery. The sagittal sinus served as a landmark for the mediolateral coordinates and the interaural plane (ear bars) served as a landmark for the anteroposterior and dorsoventral coordinates. The monkeys received between 17 and 23 injections to sites located ∼2 mm apart in each plane. Each injection consisted of 0.6–1.0 μl of ibotenic acid (10–15 μg/μl; 0.2 μl/min; Sigma-Aldrich) administered via a 30-gauge Hamilton syringe held in a David Kopf Instruments manipulator. Before lowering the needle, small slits were made in the dura to allow the needle to pass unobstructed into the brain. The needle remained in place 2–3 min after each injection to limit diffusion of the toxin up the needle track. As shown in Figures 1 and 2, the intended lesion encompassed the entire amygdala including the basolateral and centromedial nuclear groups. On closing, 30 ml of mannitol (25%, 1 ml/min, i.v.) was administered to control edema.
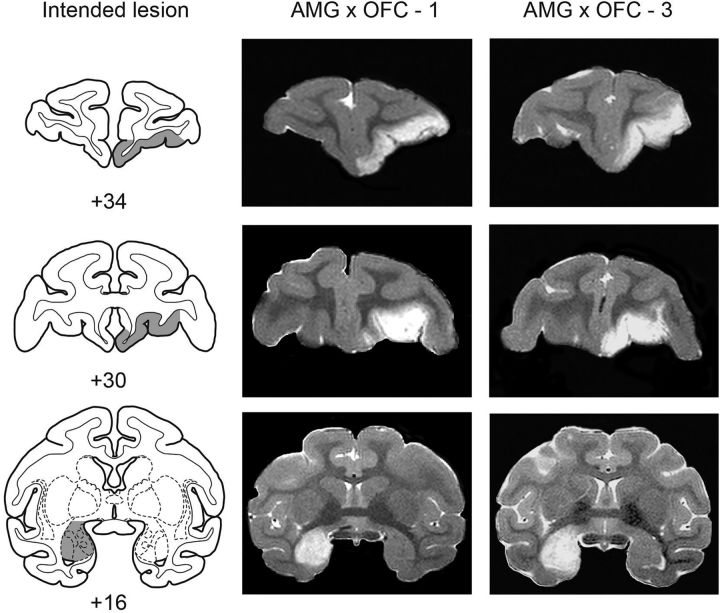
Figure 1.
Crossed surgical disconnection of the amygdala and OFC: cases 1 and 3. Intended lesion (gray-shaded region) are shown on standard sections of a rhesus monkey brain (left column), together with postoperative T2-weighted MR images from two monkeys with crossed lesions (central and right columns). The MR images are selected to match the anteroposterior levels shown for the intended lesion. The white areas are regions of hypersignal due to edema resulting from injection of excitotoxins and are taken as a reflection of the extent of the lesion. Numerals indicate distance in millimeters from the interaural plane (0). Compare and contrast line drawings of the intended lesion (left column) with MR sections of the cases illustrated (center and right columns).
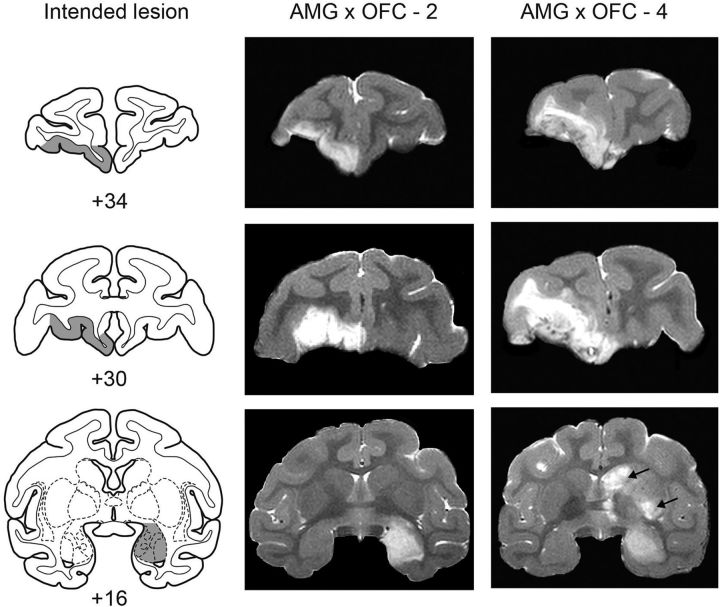
Figure 2.
Crossed surgical disconnection of the amygdala and OFC: cases 2 and 4. Intended lesion (gray-shaded region) are shown on standard sections of a rhesus monkey brain (left column), together with postoperative T2-weighted MR images from two monkeys with crossed lesions (central and right columns). Conventions are as in Figure 1. Note that case 4 sustained inadvertent damage to parts of the caudate nucleus and putamen (black arrows), presumably as a result of infarction associated with the amygdala injections.
Monkeys receiving an OFC lesion were anesthetized and then placed in a custom head holder. At the beginning of surgery, monkeys were given 30 ml of mannitol (25%, 1 ml/min, i.v.) to increase access to the orbital surface and to control edema. A large bone flap was turned over the dorsal frontal cortex. The dura was opened with a crescent-shaped cut and then reflected toward the orbit. Sulcal landmarks on the orbital surface were identified with the aid of an operating microscope. Injections of excitotoxins were made into sites located ∼2 mm apart. Each injection consisted of 1.0 μl of ibotenic acid (10–15 μg/μl; Sigma-Aldrich) injected as a bolus via a hand-held Hamilton syringe with a 30-gauge needle. The monkeys received between 87 and 99 injections. The intended lesion (Figs. 1, 2) encompassed the entire OFC in one hemisphere, corresponding approximately to areas 11, 13, and 14 of Walker (1940); it extended from the fundus of the lateral orbital sulcus laterally to the rostral sulcus on the medial surface of the hemisphere. The rostral boundary of the injections was an imaginary line joining the rostral tips of the medial and lateral orbital sulci. The caudal boundary of the injections was an imaginary line joining the most caudal points of the medial and lateral orbital sulci.
Assessment of lesions
The extent of the amygdala lesions was estimated by examination of T2-weighted MRI scans obtained within 4–5 d of surgery. For each of the operated monkeys, MR images (1 mm slices) through the amygdala were matched to drawings of coronal sections of a representative rhesus monkey brain spaced at 1 mm intervals. Then, for each level, the region of the hypersignal present in the scan was plotted onto the drawings (for more details on the lesion assessment method, see Izquierdo and Murray, 2004). Similar methods were used to assess the extent of the OFC lesions. Finally, the volume of the lesions was determined using a digitizing tablet (Wacom). Figures 1 and 2 show postoperative MRI images from the monkeys that received crossed disconnection of the amygdala and OFC.
The volume of the lesions was expressed as a percentage of the overall volume of the structure in a representative monkey brain. We estimated that the lesions affected, on average, 92.5% of the volume of the amygdala (range, 88.5–100%), and 82.5% of the volume of OFC (range, 69.1–97.2%). Therefore, the lesions were largely as intended.
Each of the operated monkeys sustained some inadvertent damage to adjacent structures. The unintended damage, which was typically unilateral and minor in extent, was evident in regions adjacent to the targeted structures; slight inadvertent damage occurred in the anterior entorhinal cortex (three monkeys), frontal polar cortex (two monkeys), ventral claustrum (one monkey), and anterior hippocampus (two monkeys). In addition, one monkey sustained some inadvertent damage to the head of the caudate nucleus and putamen apparently due to an infarction associated with the amygdala injections.
Apparatus
Training took place in a sound- and light-attenuating chamber into which a monkey test cage was wheeled and securely fastened. The chamber was illuminated by a 15 W bulb, which allowed enough light for operation of a closed-circuit television camera. A fan mounted in the ceiling of the chamber provided ventilation and masked extraneous noise. Stimuli were presented on a 19-inch monitor with a touch-sensitive screen that was positioned on the back wall of the chamber 15 cm in front of the monkey cage. Two images (5 × 5 cm) that differed in pattern and color were used as stimuli during both touch screen training and preliminary training for the tap/hold task. A single stimulus, a solid red square (5 × 5 cm), was used for later phases of training (see below) and for the main task. The food rewards were two different foods selected from peanuts, M&Ms, and Skittles (Mars Foods). Rewards were dispensed from automated dispensers (Med Associates) that were mounted on top of the testing chamber and delivered into a cup located to the left of the monitor. All tasks were controlled and behavioral data collected by a computer using custom software (Ryklin Software).
Behavioral testing
Monkeys with crossed surgical disconnection of the amygdala and OFC and unoperated controls learned to perform two different actions (“tap” and “hold”) on a touch-sensitive screen to earn two different food rewards. Which action was paired with which corresponding food reward was consistent for each monkey throughout training, but was counterbalanced across groups. Monkeys underwent training to build up the tap and hold actions individually. Once acquired, monkeys were given the choice between the two different action options on every trial. We then evaluated each monkey's action preference after changing the value of the food reward. We used a selective satiation procedure to produce a devaluation of the food reinforcer. In this procedure, monkeys were given the opportunity to consume one of the foods to satiety. The test given after this selective satiation procedure is often termed the “reinforcer devaluation test” or simply the “devaluation test” for short. Reinforcer devaluation tests were performed under extinction conditions.
Tap/hold task
Detailed procedures for training monkeys on this action–outcome task have been described previously (Rhodes and Murray, 2013). Briefly, monkeys learned to initiate touch screen actions and were then trained in the tap/hold task in sequential stages, as follows.
Phase 1.
The aim of this phase was to train the monkeys to perform the two different actions: tap (6 touches to the appropriate zone on the screen within 2 s) and hold (steady contact with the appropriate zone on the screen for 2 s). Monkeys proceeded through a series of stages that were customized to the individual monkey until they acquired the mutually exclusive tap and hold actions. Tap and hold led to the delivery of different foods; the action–food assignment was balanced within and across groups and was fixed throughout the experiment for individual monkeys. Tap and hold actions were trained in separate 50 trial sessions conducted back to back with the order alternated across days.
Phase 2.
During this phase, monkeys were trained to make the tap and hold actions when reward was delivered on a probabilistic, random-ratio (RR) schedule (sequentially reducing from RR1 to RR4). Use of the RR schedule is a standard approach intended to promote goal-directed behavior (Dickinson et al., 1983), one that encourages responding during test sessions for which there is no reward delivery (i.e., extinction). Tap and hold actions were trained in separate 50 trial sessions conducted back to back with the order alternated across days.
Tap/hold main task.
In the main task, unlike the training phases, both action options were available for choice on every trial. The tap and hold action zones, which were identical red rectangles, were presented in the same position on the screen (tap on the left, hold on the right) as in previous training stages, but now the action zones were offered simultaneously. On each trial, the monkey was able to choose whether to make the tap or the hold action. In addition, during this phase, the number of rewards delivered for each action was adjusted, if necessary, to balance between tap and hold actions. Monkeys received one 50 trial session per day.
Devaluation test
Monkeys underwent tests on four separate days to assess their action preferences after devaluation of the food reward associated with one of the two actions. On each test day, monkeys were fed one food to satiety in a selective satiation procedure intended to decrease the value of that food temporarily. The monkeys were then tested under extinction for tap/hold actions in the choice format. Each test session was composed of 15 discrete trials. These tests were meant to ascertain whether the monkeys would alter the pattern of their actions based on the current value of the food outcome. Between test sessions, monkeys received retraining on the main task to overcome any possible reduction in baseline responding resulting from extinction testing.
Food preference test
Directly after each devaluation test, monkeys were given a choice between the same two foods used in the training phase, one each on the experimenter's outstretched hands. The monkey was allowed to choose and consume only one item and a record was made of that choice. A total of four trials was administered per food preference test.
Statistics
Sessions to criterion, actions on the devaluation test, number of omissions, and amounts of food eaten during selection satiation were subjected to repeated-measures ANOVA. If Mauchly's test indicated that the assumption of sphericity had been violated, then degrees of freedom were estimated using the Greenhouse–Geisser procedure. An α level of 0.05 was used for all statistical tests.
Results
Before training, four rhesus monkeys received crossed surgical disconnection of the amygdala and OFC; nine additional rhesus monkeys served as unoperated controls. To summarize our experimental procedures, the operated and control monkeys were trained to perform two different instrumental actions, tap and hold, which they performed on a touch-sensitive screen to earn two different foods, one associated with tap and the other associated with hold. In later testing, monkeys consumed one of these two foods to satiety and were then tested for action selection under extinction conditions. We expected that controls would show a reduction in the action associated with the devalued food (i.e., a devaluation effect).
Tap/hold acquisition
Monkeys required a mean of 18.6, 11.2, and 12.3 sessions to complete the three training stages, phase 1, phase 2, and the main task, respectively. A 2 × 3 mixed ANOVA on the number of sessions to criterion with factors of group (CON, AMG × OFC) and training stage (phase 1, phase 2, main task) revealed no main effect of training stage (F(2,22) = 1.94, p = 0.17, Greenhouse–Geisser corrected p = 0.19) and no main effect of group (F(1,11) = 3.25, p = 0.10). In addition, there was no interaction between group and training stage (F(2,22) = 0.11, p = 0.90, Greenhouse–Geisser corrected p = 0.78), indicating that both groups performed comparably on each stage.
Selective satiation
Control monkeys ate a mean of 225.0 g of food during each of the four selective satiation sessions, whereas AMG × OFC monkeys ate a mean of 121.1 g. The 2 × 4 mixed ANOVA on the amount of food eaten before sessions conducted under extinction revealed no main effect of devaluation day (F(3,33) = 0.20, p = 0.89) and no interaction of devaluation day and group (F(3,33) = 0.21, p = 0.89). There was, however, a main effect of group (F(1,11) = 10.38, p = 0.008); the control monkeys ate more food during the selective satiation procedure than the operated group. This likely reflects the greater average size of monkeys in the control group. The weights of the individual monkeys are provided in Table 1. The potential influence on devaluation effects of the amount of food eaten during the selective satiation procedure is considered at the end of the next section.
Devaluation test
Our main measure was derived from actions performed during the devaluation tests administered immediately after the selective satiation procedure. On each trial, monkeys chose between the action associated with the devalued food and the action associated with the nondevalued (i.e., currently and temporarily higher-value) food. The mean number of actions of each type from the 4 devaluation sessions were subjected to a 2 × 2 mixed ANOVA with factors of group (CON, AMG × OFC) and action type (devalued, nondevalued).
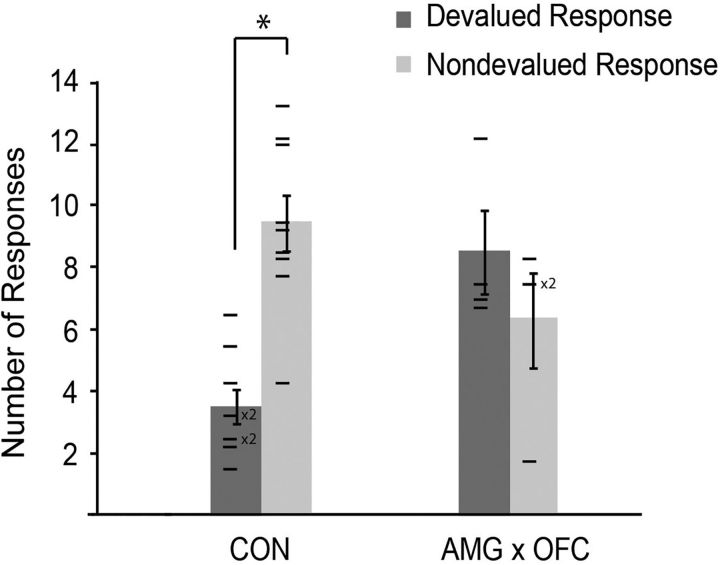
Results are summarized in Figure 3. ANOVA revealed no main effect of either action type (F(1,11) = 1.99, p = 0.19) or group (F(1,11) = 1.94, p = 0.19). However, there was a significant interaction of action type and group (F(1,11) = 9.31, p = 0.011), indicating that the selective satiation procedure produced different patterns of actions across the two groups. Post hoc paired samples t tests revealed a statistically significant difference in the number of actions associated with the devalued versus nondevalued foods in the controls (t(8) = 4.65, p = 0.002; left pair of bars in Fig. 3), but not in the operated group (t(3) = 0.76, p = 0.50; right pair of bars in Fig. 3). Therefore, as expected, controls showed a devaluation effect; after selective satiation, they produced a significantly greater number of actions associated with the nondevalued food relative to the devalued food. Monkeys with AMG × OFC lesions made comparable numbers of actions associated with the devalued and nondevalued foods, indicating that the lesion disrupted the devaluation effect in this group. Importantly, there were virtually no action errors, for example, tap responses performed in the hold response zone or vice versa, nor was there a degradation in the ability to perform the actions. During both the devaluation tests and the retraining sessions, all monkeys continued to perform the two actions in a routine manner.
Figure 3.
Mean number of actions (±SEM) made under extinction conditions averaged across the four devaluation tests. There were 15 trials per session and, on each trial, there were two action options available to the monkey: a tap or hold associated with the devalued reward (devalued action) and a tap or hold associated with the nondevalued reward (nondevalued action). CON, Unoperated control monkeys. *Significant difference between the number of devalued and nondevalued actions performed during the devaluation tests. Horizontal lines show scores of individual monkeys.
The average number of omissions (e.g., failure to perform either a tap or a hold) per devaluation test was 1.49. A 2 × 4 mixed ANOVA with factors of group (CON, AMG × OFC) and devaluation session (1–4) revealed no main effect of session (F(3,33) = 0.13, p = 0.94). Therefore, extinction did not become more pronounced across subsequent devaluation sessions. The ANOVA also revealed no main effect of group (F(1,11) = 1.94, p = 0.19) and no interaction between devaluation session and group (F(3,33) = 0.44, p = 0.73), indicating that there was no influence of the lesion on the number of omissions.
The average latency for an action after the onset of the red prompting squares was 6.03 s. This latency was examined using a 2 × 2 mixed ANOVA with factors of group (CON, AMG × OFC) and action type (devalued, nondevalued). The ANOVA revealed no main effect of action type (F(1,11) = 1.25, p = 0.29) and no main effect of group (F(1,11) = 2.78, p = 0.12). There was also no significant interaction between action type and group (F(1,11) = 0.74, p = 0.41). Therefore, latency was influenced by neither the action type (devalued or nondevalued) nor the lesion.
Because, on average, the control subjects consumed more food during the selective satiation procedures than did the operated subjects, we reran the ANOVA adding in the amount of food eaten as a covariate. This modified ANOVA, like the original, revealed an interaction of group and action type (F(1,10) = 15.53, p = 0.003), which indicates that the group difference in amount of food consumed cannot account for the results of the devaluation tests.
Retraining
Between devaluation tests, all monkeys underwent brief periods of retraining on the main task. This procedure was performed to counter any effects of the devaluation tests, which were run in extinction conditions, thereby ensuring that the monkeys remained motivated to perform the task. Table 2 provides the number of days spent in each retraining period for each monkey. Visual inspection of the data shows extensive overlap between groups in the number of retraining days. A 2 × 3 mixed ANOVA with factors of group (CON, AMG × OFC) and retraining interval (1–3) confirmed no effect of group (F(1,11) = 0.07, p = 0.80), no effect of retraining interval (F(2,22) = 1.97, p = 0.16), and no significant interaction between group and retraining interval (F(2,22) = 0.22, p = 0.80), indicating that there was no influence of the lesion on the amount of retraining required between devaluation tests.
Table 2.
Number of retraining days administered between devaluation tests
| Monkey | Interval | ||
|---|---|---|---|
| AMYG × OFC | 1 | 2 | 3 |
| Case 1 | 3 | 6 | 3 |
| Case 2 | 3 | 5 | 2 |
| Case 3 | 3 | 3 | 2 |
| Case 4 | 4 | 2 | 3 |
| Mean | 3.25 | 4.00 | 2.50 |
| Controls | |||
| Case 1 | 4 | 10 | 4 |
| Case 2 | 3 | 1 | 2 |
| Case 3 | 2 | 2 | 6 |
| Case 4 | 2 | 2 | 2 |
| Case 5 | 2 | 2 | 2 |
| Case 6 | 2 | 4 | 3 |
| Case 7 | 2 | 4 | 2 |
| Case 8 | 3 | 3 | 2 |
| Case 9 | 4 | 6 | 2 |
| Mean | 2.67 | 3.78 | 2.78 |
Interval number corresponds to the retraining interval between the first and second devaluation tests (interval 1), the second and third tests (interval 2), etc.
Food preference test
Across all food preference tests, which were always conducted using the same two foods as produced by the tap and hold actions, there were eight of 208 occasions on which a monkey chose the devalued food. This occurred six times spread over two different subjects in the AMG × OFC group and twice in one control monkey. A one-sample two-tailed t test conducted on the percentage chosen of the nondevalued food during food preference tests for all monkeys indicated that selection of the more highly valued (i.e., nonsated) food was significantly different from chance (t(12) = 21.12, p < 0.001). A t test of groups conducted for all four food preference tests combined revealed no differences between operated and control monkeys (t(11) = 1.85, p = 0.09) in the extent to which they chose the nondevalued food. Therefore, for all monkeys, operated and control alike, satiety mechanisms were intact and satiety transferred from the home cage to the test apparatus.
Discussion
We found that crossed surgical disconnection of the amygdala and OFC, like bilaterally symmetrical lesions of either structure (Rhodes and Murray, 2013), severely disrupted devaluation effects on an action–outcome task. Therefore, monkeys with crossed disconnection of the amygdala and OFC were impaired in their ability to link a planned action with the current, updated value of a predicted outcome and to choose an action on that basis.
Crossed disconnection versus bilateral lesions
It might seem unremarkable that degrading amygdala–OFC interactions would cause the same impairment as bilateral lesions of OFC. However, bilateral lesions of frontal areas can cause less selective effects than crossed disconnection lesions involving the same areas (Parker and Gaffan, 1998). One possible reason for this is that bilateral OFC lesions entirely remove its specialized representations, whereas after crossed disconnection, one hemisphere retains them. In this view, a disconnection lesion provides a more specific understanding of amygdala–OFC interactions than do bilateral lesions of either structure. Consistent with this idea, the crossed disconnections produced some different results than did our previous bilateral lesions (Rhodes and Murray, 2013). Monkeys with bilateral amygdala lesions showed more omissions and a longer latency to perform actions associated with the devalued food. The crossed disconnection lesion did not cause these effects, presumably because the remaining amygdala interacted with structures other than OFC to forestall them.
Given the importance of amygdala–OFC interactions in our task, a question remains about whether this interplay has “directionality” in terms of encoding and retrieval functions. Pharmacological inactivations at different times during devaluation tests have addressed this issue to an extent (Wellman et al., 2005; West et al., 2012; Murray et al., 2015), but future studies using the “crossed temporal disconnection” design of Parkes and Balleine (2013) might prove valuable.
Alternative interpretations
We can rule out several alternative interpretations of the impairment. For instance, operated monkeys acquired the two instrumental actions at the same rate as controls. In addition, there were virtually no action errors either during the devaluation tests or at any other time after initial training. Therefore, the deficit did not result from difficulty in learning or performing the actions. Furthermore, the two groups were equally motivated during the devaluation tests, as indicated by the lack of a group difference in omissions. Moreover, after selective satiation, both operated and control monkeys chose the higher value food when given a visual choice between the two foods. Therefore, the monkey's food preferences shifted in response to satiety, the selective satiation transferred from the home cage to the test apparatus, and the monkeys could discriminate the foods. These observations show that the impairment is specific to representing the updated value of predicted food outcomes for different action choices.
It might be argued that our results arise from unilateral removal of either structure rather than their interaction, which the crossed-disconnection design tests. In an earlier study, we found that monkeys with removal of the amygdala and OFC in one hemisphere had a mild and transient impairment on an object-based version of the task (Izquierdo and Murray, 2004, 2010). Given the extended period of postoperative testing used here, we doubt that within-hemisphere amygdala–OFC lesions would have caused an impairment of the degree and duration that we observed. This assumption remains to be tested, however.
Comparison with the object-based devaluation task
With the present results, our findings from an action-based devaluation task are fully consistent with those on the object-based version. Bilateral lesions of either OFC or the amygdala (Málková et al., 1997; Izquierdo et al., 2004; Rhodes and Murray, 2013) and crossed disconnection of the amygdala and OFC (Baxter et al., 2000; present study) cause impairments on both versions of the task. Together, these results indicate that amygdala–OFC cooperation plays a necessary role in choices based on the updated value of predicted outcomes for choices among either objects or actions.
This conclusion gains support from studies of the amygdala in rodents, which show that it contributes to choices among actions based on outcome valuations. Rats with bilateral lesions of the basolateral amygdala are impaired on instrumental devaluation tests regardless of whether the lesions are made before (Balleine et al., 2003; Corbit and Balleine, 2005) or after (Ostlund and Balleine, 2008) training and these findings are independent of whether reinforcer devaluation is achieved by conditioned taste aversion or selective satiation (Johnson et al., 2009).
Likewise for OFC, studies in mice (Gourley et al., 2013; Gremel and Costa, 2013) and rats (cf. Ostlund and Balleine, 2007; Bradfield et al., 2015) support a role in the selection of action based on outcome valuations. Note, however, that the area called OFC in rodents is not homologous with the granular OFC areas removed here, which are primate innovations (Preuss and Goldman-Rakic, 1991; Passingham and Wise, 2012).
Subdivisions of OFC
OFC in macaques is composed of multiple cytoarchitectonic areas (11, 13, and 14) and subareas. Object-based choices depend on lateral OFC (areas 11 and 13), but not medial OFC (area 14) (Rudebeck and Murray, 2011). Recently, a study in rodents identified a role for medial OFC in retrieval of outcome-specific information to guide action (Bradfield et al., 2015). Because the present study involved lesions that included both lateral and medial OFC, perhaps only part of OFC is critical for the present results. Furthermore, the posterolateral part of OFC (area 13) makes its contribution during the selective satiation procedure, when food value is being updated, but not afterward, when monkeys use these updated valuations to make choices (Murray et al., 2015). It seems likely that the same conclusions apply to choices among actions, but this hypothesis remains to be tested.
Stimulus–action dichotomy
The stimulus–action dichotomy holds that OFC specializes in stimulus–outcome or object–outcome representations, whereas MFC specializes in action–outcome or response–outcome representations. On the surface, our findings seem to contradict this idea because an impairment in action–outcome behavior (Fig. 3) might not be expected to follow amygdala–OFC disconnections.
We can reconcile our findings with the stimulus–action dichotomy in two ways. First, we designed the tap/hold task to match action–outcome tests used with rodents. Just as rodents might press separate levers, our monkeys contacted two response zones on a touch-sensitive screen. The spatial separation of these zones could have led to the monkeys learning a place–action–outcome association instead of a pure action–outcome association.
Second, our results support a stimulus–outcome specialization of OFC provided that the stimulus in question is understood to be the food outcome itself. The term “outcome” covers a broad range of concepts. An outcome often corresponds to an unconditioned stimulus (US), which triggers an unconditioned response (UR). Another concept of outcomes corresponds closely with reinforcement or rewards (goals). In yet other usages, the term outcome refers to some sensory feature(s) of a reward, what it is worth to an animal at any given time, or the probability of reward availability, among other decision variables. An outcome can be general, such as caloric or common currency valuations, or specific. For the latter, an outcome's specificity might be related to visual, olfactory, gustatory, or visceral sensations. In anthropoid primates, color vision and shape vision play an especially important role in the representations of outcomes in part because the olfactory system regressed during haplorhine evolution (Heritage, 2014) and in part because foveal and trichromatic vision evolved in haplorhines and anthropoids, respectively (Murray et al., 2017).
Some features of the food outcomes, such as gustatory and olfactory ones, serve as USs that trigger autonomic responses, oral and gastric secretions, and visceral sensations (URs). Other features of food outcomes, such as their color and shape, can serve as conditioned stimuli (CSs). Accordingly, a food's visual features can contribute to outcome representations in some stimulus–outcome associations and to stimulus representations in others.
From this perspective, our results do not contradict the stimulus–action dichotomy at all. In the object-based version of the task, monkeys learn associations between objects and food outcomes, including the visual sensory features of such outcomes. When a monkey confronts a choice between two objects, each potential choice generates a prediction, from memory, about the outcome that should follow, which in turn elicits a readout of its current value. Amygdala–OFC interactions mediate these linkages.
We propose a similar account for the action-based version of the task, which differs from the object-based version in that it requires linking the representation of a planned action with a predicted food outcome. Despite this difference, both versions involve associations between the CS-like features of a food outcome and updated valuations. When a monkey chooses between two actions, both motor plans generate a prediction about a food outcome, which elicits its updated valuation. This interpretation agrees with a role for amygdala–OFC interactions in registering updated valuations during the satiation procedure. In this view, both action- and object-based choices rely on a common mechanism for predicting outcomes and their sensory features, along with reading out their updated valuation based on current biological states and needs.
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Mental Health–National Institutes of Health (Grant ZIAMH002887). We thank Richard Saunders and Emily Moylan for surgical assistance and Ben Basile and Steven Wise for comments on an earlier version of the manuscript.
The authors declare no competing financial interests.
References
- Balleine BW, Killcross AS, Dickinson A (2003) The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci 23:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA (2000) Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci 20:4311–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield LA, Dezfouli A, van Holstein M, Chieng B, Balleine BW (2015) Medial orbitofrontal cortex mediates outcome retrieval in partially observable task situations. Neuron 88:1268–1280. 10.1016/j.neuron.2015.10.044 [DOI] [PubMed] [Google Scholar]
- Camille N, Tsuchida A, Fellows LK (2011) Double dissociation of stimulus-value and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. J Neurosci 31:15048–15052. 10.1523/JNEUROSCI.3164-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Daniels TE, Gorrin DP, Rhodes SE, Rudebeck PH, Murray EA (2013) The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cereb Cortex 23:2884–2898. 10.1093/cercor/bhs266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW (2005) Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci 25:962–970. 10.1523/JNEUROSCI.4507-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Nicholas DJ, Adams CD (1983) The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. QJEP 35:35–51. 10.1080/14640748308400912 [DOI] [Google Scholar]
- Gourley SL, Olevska A, Zimmermann KS, Ressler KJ, Dileone RJ, Taylor JR (2013) The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci 38:2382–2388. 10.1111/ejn.12239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM (2013) Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 4:2264. 10.1038/ncomms3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage S. (2014) Modeling olfactory bulb evolution through primate phylogeny. PLoS One 9:e113904. 10.1371/journal.pone.0113904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA (2004) Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol 91:2023–2039. 10.1152/jn.00968.2003 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA (2010) Functional interaction of medial mediodorsal thalamic nucleus but not nucleus accumbens with amygdala and orbital prefrontal cortex is essential for adaptive response selection after reinforcer devaluation. J Neurosci 30:661–669. 10.1523/JNEUROSCI.3795-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA (2004) Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci 24:7540–7548. 10.1523/JNEUROSCI.1921-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC (2009) The basolateral amygdala is critical to the expression of Pavlovian and instrumental outcome-specific reinforcer devaluation effects. J Neurosci 29:696–704. 10.1523/JNEUROSCI.3758-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Gaffan D, Murray EA (1997) Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci 17:6011–6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Moylan EJ, Saleem KS, Basile BM, Turchi J (2015) Specialized areas for value updating and goal selection in the primate orbitofrontal cortex. eLife 4:e11695. 10.7554/eLife.11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Wise SP, Graham KS (2017) The evolution of memory systems: ancestors, anatomy, and adaptations. Oxford: OUP. [Google Scholar]
- Ostlund SB, Balleine BW (2007) Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci 27:4819–4825. 10.1523/JNEUROSCI.5443-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW (2008) Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci 28:4398–4405. 10.1523/JNEUROSCI.5472-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, Gaffan D (1998) Memory after frontal/temporal disconnection in monkeys: conditional and non-conditional tasks, unilateral and bilateral frontal lesions. Neuropsychologia 36:259–271. 10.1016/S0028-3932(97)00112-7 [DOI] [PubMed] [Google Scholar]
- Parkes SL, Balleine BW (2013) Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J Neurosci 33:8753–8763. 10.1523/JNEUROSCI.5071-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Wise SP (2012) The neurobiology of the prefrontal cortex. Oxford: OUP. [Google Scholar]
- Preuss TM, Goldman-Rakic PS (1991) Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol 310:429–474. 10.1002/cne.903100402 [DOI] [PubMed] [Google Scholar]
- Rhodes SE, Murray EA (2013) Differential effects of amygdala, orbital prefrontal cortex, and prelimbic cortex lesions on goal-directed behavior in rhesus macaques. J Neurosci 33:3380–3389. 10.1523/JNEUROSCI.4374-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA (2011) Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci 31:10569–10578. 10.1523/JNEUROSCI.0091-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF (2008) Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci 28:13775–13785. 10.1523/JNEUROSCI.3541-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME (2007) Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 11:168–176. 10.1016/j.tics.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Walker AE. (1940) A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol 73:59–86. 10.1002/cne.900730106 [DOI] [Google Scholar]
- Wellman LL, Gale K, Málková L (2005) GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci 25:4577–4586. 10.1523/JNEUROSCI.2257-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Forcelli PA, Murnen AT, McCue DL, Gale K, Málková L (2012) Transient inactivation of basolateral amygdala during selective satiation disrupts reinforcer devaluation in rats. Behav Neurosci 126:563–574. 10.1037/a0029080 [DOI] [PMC free article] [PubMed] [Google Scholar]